Abstract
Even though recent advances in liver surgery have led to a more efficient approach to hilar cholangiocarcinoma (HC), resection with curative intent remains a challenge for hepatobiliary surgeons [1]. There is strong evidence to show better survival and long-term outcomes when microscopically tumor-free surgical margins are obtained in these patients [2]. En-bloc resections of liver parenchyma with the extrahepatic bile duct is mandatory to manage tumors with direct hepatic invasion, as well as to accomplish an R0 resection on tumors that frequently extend longitudinally out to involve the hepatic ducts [3–5].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
22.1 Introduction
Even though recent advances in liver surgery have led to a more efficient approach to hilar cholangiocarcinoma (HC), resection with curative intent remains a challenge for hepatobiliary surgeons [1]. There is strong evidence to show better survival and long-term outcomes when microscopically tumor-free surgical margins are obtained in these patients [2]. En-bloc resections of liver parenchyma with the extrahepatic bile duct is mandatory to manage tumors with direct hepatic invasion, as well as to accomplish an R0 resection on tumors that frequently extend longitudinally out to involve the hepatic ducts [3–5].
Due to the close relation between the bile duct bifurcation and the vascular structures that enter the liver, tumor growth might rapidly compromise these structures. The achievement of negative surgical margins in these patients is technically demanding, requiring combined vascular resection and reconstruction [1–6]. Currently, resection of the portal vein (PV) is considered a routine procedure when it is compromised, concomitant resection of the hepatic artery remains controversial since it is associated with a higher operative mortality rate (33–55 %) [5, 7–10]. Several reasons might explain for these poor results, such as a greater duration of liver ischemia due to simultaneous portal vein resection in most cases, and pre-existing liver dysfunction due to obstructive jaundice and persistent cholangitis [9, 10]. These conditions are the key factors for the development of postoperative liver failure [7, 8, 11, 12].
In HC Bismuth-Corlette type IIIb, the tumor invades the left bile duct, and in some circumstances, compromises liver vascular structures [13]. Given the relationship of the right hepatic artery (RHA) is set in most cases behind the common hepatic duct and near the bifurcation, extension of the tumor posteriorly and to the right involves the RHA and the PV or its branches. Since HC type IIIb often requires a left hepatectomy extended to the anterior segments of the right liver, invasion of the RHA usually is a contraindication to perform these procedures with curative intent [2, 7, 11]. This leads to different treatment options to solve this problem.
Even though Majno et al. [14] consider that after resection of the hepatic artery, the remaining liver can function adequately with normal flow of the PV and collateral arteries, though the absence of arterial blood flow may cause liver necrosis, hepatic abscess formation and an increased risk of complications to the biliary anastomosis [14, 15]. Wang et al. [12] presented two patients with HC type IIIb with contralateral arterial invasion. They performed a left hepatectomy combined with RHA resection without reconstruction. The early postoperative results were satisfactory, however one patient developed liver necrosis and abscess 3 months later. Young et al. [16] reported using arterialization of the PV as a salvage procedure when arterial reconstruction was not possible. Miura et al. [17] and Yasuda et al. [18] reported resection of locally advanced HC after stepwise arterial embolization used to stimulate the development of neo-arterialization of the right liver so that the RHA could be resected without reconstruction.
As arterial vascular reconstruction is preferable to any of the techniques described above, other authors reported the use of arterial vascular reconstruction to increase the resectability of HC when tumor involvement of the hepatic artery became an obstacle to negative resection margins [7, 8, 19, 20]. In these series, arterial reconstruction was performed as the last step after the portal vein was reconstructed. When arterial reconstruction is performed at the end of the procedure, the surgeon may find it too late to realize that the distal artery of the future liver remnant (FLR) does not have enough length for reconstruction when oncological resection was completed. This is a critical situation which is not easy to solve [16].
Since liver failure is the leading cause of death in these patients [8], to dissociate arterial and portal ischemia during vascular reconstruction to ensure the arterial blood supply to the remnant liver in the first surgical step might have a positive impact on early and late postoperative outcomes.
In this chapter we describe a novel surgical technique that allows an oncological clearance in patients with HC type IIIb with contralateral arterial invasion by performing arterial reconstruction as the first step of the surgical procedure.
22.2 Surgical Technique
We routinely use laparoscopic staging to rule out the presence of liver metastases or peritoneal carcinomatosis. After laparotomy, mobilization of the left liver is performed. Dissection of the hepatic pedicle starts from the duodenopancreas to the hilum. Complete lymphadenectomy of the celiac axis and its branches, the PV and the gastrohepatic ligament is then performed. This maneuver facilitates identification of the elements in the pedicle. During surgery, dissection should always be carried out away from the tumor. Once the presence of a HC type IIIb with contralateral arterial invasion is confirmed by palpation, visualization and intraoperative color-doppler ultrasound (IOUS), the next step is to isolate the posterior branch of the RHA (segments 6 and 7) inside the Rouviere’s sulcus (Fig. 22.1). This artery, in most cases is free and away from the tumor and it can then be dissected easily. Once this step is accomplished, the distal common bile duct is transected as close as possible to its entrance into the pancreas. The edge of the transected duct is sent for fresh frozen pathological examination. As the left hepatic artery usually runs on the left edge of the hepatic pedicle and in most of the time it is not involved by the tumor, it can be mobilized and isolated along its full length. This allows enough length of the artery to reach the Rouviere’s sulcus to perform an arterial reconstruction with the posterior branch of the RHA. The left hepatic artery is rotated 90° anti-clockwise in order to carry out an anastomosis with the arterial branch of segments 6 and 7 (Fig. 22.2). We prefer to perform the anastomosis using a microscope. It is important whenever possible to use the left hepatic artery because it has a similar diameter to the right posterior artery. Occasionally, the RHA is invaded by tumor and its proximal stump may also be used for arterial reconstruction with the posterior branch of the RHA (Fig. 22.3). Once the anastomosis is finished arterial blood supply to the FLR is ensured. Segment 1 is then mobilized to be included in the resection specimen. The left PV is divided and the liver parenchyma is transected (left trisectionectomy or extended left hepatectomy) (Fig. 22.4). If PV resection is required, it can be done after transection of liver parenchyma is completed, with the assurance that the remnant liver can tolerate portal clamping because it has good arterial vascularization. After vascular reconstruction is accomplished, the middle and left hepatic veins are divided and sutured. Finally the specimen is removed and biliary reconstruction is performed using a Roux-en-Y loop. At the end of the procedure, IOUS should always be done to confirm the adequacy vascularization of the liver remnant.
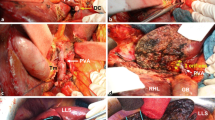
(a) Once cholecystectomy is performed, the posterior branch of the right hepatic artery (RHA) is isolated within the Rouviere’s sulcus. (b) The common bile duct (CBD) is divided distally and turned away from the operative field with the specimen. TM tumor, LHA left hepatic artery, RPSA right posterior segment artery, HA hepatic artery, PV portal vein, RPSBD right posterior segment bile duct
(a) The right hepatic artery (RHA) and the left hepatic artery (LHA) are divided with tumor-free margins. (b) After this, the LHA is rotated to the right side of the porta hepatis. This maneuver allows arterial reconstruction between the LHA and the right posterior segment artery (RPSA). LPV left portal vein, TM tumor, CBD common bile duct (Reproduced with permission from HPB: The Official Journal of the International Hepato Pancreato Biliary Association [21])
This picture shows two possible intraoperative scenarios of arterial compromise and details the reconstructive alternatives with the right posterior segment artery (RPSA). (a) The right hepatic artery (RHA) is nearly totally invaded by the tumor before its bifurcation. (b) The reconstruction demands dividing the left hepatic artery (LHA) and its transfer to the right edge of the porta hepatis. By this means, the LHA can reach the posterior branch of the RHA and the anastomosis is performed. (c) The RHA is distally invaded by the tumor. (d) In this situation the reconstruction can be achieved with the proximal remnant of the common RHA
After vascular reconstruction is completed and arterial blood flow is restored, the liver parenchyma is transected. TM tumor, RHA right hepatic artery, LHA left hepatic artery, RPSA right posterior segment artery, PV portal vein, RPSBD right posterior segment bile duct (Reproduced with permission from HPB: The Official Journal of the International Hepato Pancreato Biliary Association [21])
22.3 Conclusion
We described a novel surgical technique that allows performing an oncological resection in patients with HC type IIIb and contralateral arterial invasion that might otherwise be considered as unresectable. The technique is clinically and technically feasible [21]. In this technique, arterial anastomosis is carried out as the first surgical step to provide adequate arterial blood supply to the FLR before parenchymal transection. If additional PV resection is required, it can be done at the end of transection of the liver parenchyma. This ensures that the remnant liver can tolerate portal clamping because it has been arterially vascularizated. This novel approach offers these patients a hope of cure and might improve their outcomes by lessening the negative effects of prolonged liver ischemia.
References
Hemming AW, Reed AI, Fujita S, et al. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005;241:693–9.
Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–17.
Kosuge T, Yamamoto J, Shimada K, et al. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg. 1999;230:663–71.
Sano T, Shimada K, Sakamoto Y, et al. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surg. 2006;244:240–7.
Neuhaus P, Jonas S, Settmacher U, et al. Surgical management of proximal bile duct cancer: extended right lobe resection increases resectability and radicality. Langenbecks Arch Surg. 2003;388:194–200.
Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–18.
Nagino M, Nimura Y, Nishio H, et al. Hepatectomy with simultaneous resection of the portal vein and hepatic artery for advanced perihilar cholangiocarcinoma: an audit of 50 consecutive cases. Ann Surg. 2010;252:115–23.
Miyazaki M, Kato A, Ito H, et al. Combined vascular resection in operative resection for hilar cholangiocarcinoma: does it work or not? Surgery. 2007;141:581–8.
Madariaga JR, Iwatsuki S, Todo S, et al. Liver resection for hilar and peripheral cholangiocarcinomas: a study of 62 cases. Ann Surg. 1998;227:70–9.
Gerhards MF, van Gulik TM, de Wit LT, et al. Evaluation of morbidity and mortality after resection for hilar cholangiocarcinoma—a single center experience. Surgery. 2000;127:395–404.
Clary B, Jarnigan W, Pitt H, et al. Hilar cholangiocarcinoma. J Gastrointest Surg. 2004;8:298–302.
Wang WL, Tang XF, Yao MY, et al. Safety and efficiency of left hemihepatectomy combined with hepatic artery resection for hilar cholangiocarcinoma with artery infiltration: report of 2 cases. Can J Surg. 2008;51:305–7.
Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140:170–8.
Majno PE, Pretre R, Mentha G, et al. Operative injury to the hepatic artery. Consequences of a biliary-enteric anastomosis and principles for rational management. Arch Surg. 1996;131(2):211–5.
Miyazaki M, Ito H, Nakagawa K, et al. Unilateral hepatic artery reconstruction is unnecessary in biliary tract carcinomas involving lobar hepatic artery: implications of interlobar hepatic artery and its preservation. Hepatogastroenterology. 2000;47:1526–30.
Young AL, Prasad KR, Adair R, et al. Portal vein arterialization as a salvage procedure during left hepatic trisectionectomy for hilar cholangiocarcinoma. J Am Coll Surg. 2008;207:e1–6.
Miura T, Hakamada K, Ohata T, et al. Resection of a locally advanced hilar tumor and the hepatic artery after stepwise hepatic arterial embolization: a case report. World J Gastroenterol. 2008;14:3587–90.
Yasuda Y, Larsen PN, Ishibashi T, et al. Resection of hilar cholangiocarcinoma with left hepatectomy after pre-operative embolization of the proper hepatic artery. HPB (Oxford). 2010;12:147–52.
Lee SG, Song GW, Hwang S, et al. Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Sci. 2010;17:476–89.
Sakamoto Y, Sano T, Shimada K, et al. Clinical significance of reconstruction of the right hepatic artery for biliary malignancy. Langenbecks Arch Surg. 2006;391:203–8.
de Santibañes E, Ardiles V, Alvarez FA, et al. Hepatic artery reconstruction first for the treatment of hilar cholangiocarcinoma bismuth type IIIB with contralateral arterial invasion: a novel technical strategy. HPB (Oxford). 2012;14(1):67–70.
Acknowledgements
We thank Dr. Axel Beskow for the drawings included in this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht and People's Medical Publishing House
About this chapter
Cite this chapter
de Santibañes, E., Ardiles, V., Alvarez, F.A. (2013). The Hepatic Artery Reconstruction First Approach in Hilar Cholangiocarcinoma Type IIIb. In: Lau, W. (eds) Hilar Cholangiocarcinoma. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6473-6_22
Download citation
DOI: https://doi.org/10.1007/978-94-007-6473-6_22
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6472-9
Online ISBN: 978-94-007-6473-6
eBook Packages: MedicineMedicine (R0)