Abstarct
A simple and novel amperometric biosensor for glucose detection is proposed. It is based on the immobilization of glucose oxidase in a poly(vinyl alcol) matrix drop casted on a platinum electrode surface (Pt/GOx-PVA). The composite material GOx-PVA has been characterized by UV-vis spectroscopy to verify the preservation of enzyme structural integrity and of the enzymatic activity in PVA membrane. X-ray Photoelectron Spectroscopy (XPS) characterization revealed a homogeneous film deposited on Pt whose structure is preserved under operative conditions. Glucose was determined in the absence of a mediator used to transfer electrons between the electrode and the enzyme. Amperometric characterization has been performed at ?400 mV by using pulsed amperometric detection (PAD). Under the selected optimal conditions, the biosensor showed wide dynamic range (0.1–37 mM) yielding a low limit of detection (10 ?M). Biosensor performance was satisfactory also in terms of repeatability, reproducibility and anti-interference ability.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Wide Dynamic Range
- Amperometric Response
- Glucose Biosensor
- Pulse Amperometric Detection
- Glucose Detection
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
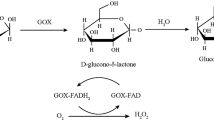
Enzyme-based biosensors for glucose detection continue to attract nowadays much interest due to the clinical importance of glucose and thus to the necessity of developing new rapid and easy procedures for its monitoring, especially in analysis in vivo [1]. In this contest, amperometric enzyme electrodes based on glucose oxidase (GOx) have played a leading role in the move to simple easy-to-use blood sugar testing [2]. Most methods of glucose detection are based on the use of enzymatic biosensors in which an electrons transfer mediator is applied to shuttle the electrons between the redox centers of the enzyme and the electrode [3]. On the contrary, in mediators free enzyme biosensors the electron is transferred directly GOx to the electrode via the active site of the enzyme [4]. In this way, it is possible to work at low operating potential, close to that of the redox potential of the enzyme, thus leading to a very high selectivity as the interference of other electroactive species is drastically reduced.
In our work, a novel mediator-free glucose biosensor is proposed. It is fabricated by a simple immobilization procedure of GOx into a biocompatible polymer, poly(vinyl alcohol) (PVA), deposited on a Pt electrode (Pt/GOx-PVA). The choice of PVA relies in its recognized properties as an ideal enzyme immobilization material mimicking the enzyme’s natural environment and able to stabilize enzymatic activity [5]. The very low operating potential (?400 mV versus SCE) as well as the successful glucose detection in the absence of oxygen evidence the mediator-less electron transport occurring [2]. The composite material GOx-PVA has been characterized by UV-vis spectroscopy to verify the preservation of enzyme structural integrity and of the enzymatic activity in PVA membrane. Moreover, XPS characterization revealed a homogeneous film deposited on Pt substrate whose structure is not altered under operative conditions.
2 Experimental
GOx (type VII from Aspergillus niger; 179,000 units/g), ?-d-glucose and all other chemicals were obtained from Sigma. All reagents were analytical grade. 1 M stock glucose solutions (prepared every 2 weeks in a phosphate buffer solution (PBS) pH 7.0, I = 0.2) were allowed to mutarotate at room temperature overnight before use.
All electrochemical experiments were performed on a PalmSens electrochemical workstation (PalmSens instruments BV, The Netherlands) using a conventional three-electrode cell with a Pt disk (surface area, 0.0314 cm2), a Pt wire and a saturated calomel electrode (SCE) as working, counter and reference electrodes, respectively. All electrochemical experiments were carried out in PBS (pH 7.0, I = 0.2). The buffer was purged with high-purity nitrogen prior to each electrochemical measurement and a nitrogen environment was kept over solution during experiments to prevent the solution from oxygen.
Modified electrodes were prepared by drop casting depositing on the electrode surface a drop of modifier solution, prepared as follows. Firstly, 3 mg of GOx (500 units/mL) were dissolved in 1 mL aqueous solution of 10% PVA in ultrasonic bath for 5 min. Afterwards 2 ?L of GOx-PVA solution were deposited by casting on the Pt surface and left to evaporate for 45 min at room temperature. The same protocol was followed to prepare XPS samples onto Pt foils (area 3 cm2). XPS analysis was carried out by using a Leybold LHS10 spectrometer equipped with an unmonochromatized AlK? source (operating at 10 kV and 17 mA) and a SPECS multi-channel detector. For UV-vis experiments 200 ?L of GOx-PVA mixture was deposited on a quartz slide and left evaporate for 1 h at room temperature. UV-vis spectra were recorded using a Varian Cary 50 UV-vis spectrophotometer in the wavelength range from 300 to 700 nm.
3 Results and Discussion
Glucose detection has been performed amperometrically at ?400 mV by using pulsed amperometric detection (PAD). Figure 53.1 reports calibration curves on Pt/GOx-PVA, bare Pt and Pt/PVA (glucose 0.1–37 mM). In the inset Pt/GOx-PVA amperometric response to glucose 0.1–2 mM is shown. In this range a linear relationship was observed (R2 = 0.9913) with a sensitivity of 9.66 ?AmM?1. A linear relationship between the reciprocal of current response (1/I) and the reciprocal of glucose concentration (1/C) was obtained in the concentration range 2.0–37 mM (R2 = 0.9944). Biosensor performance was satisfactory in terms of limit detection (10 ?M), repeatability (relative standard deviation (RSD) of 5.6%) and reproducibility (RSD of 5.7%), tested in triplicate on the same film and on three different films, respectively. Moreover, response stability revealed to be another interesting feature of the developed system as it was almost completely recovered 87% after 35 days in air at room temperature.
Moreover, biosensor selectivity was verified. Amperometric response revealed in fact to be specific to glucose without significant interference from other sugars as fructose (Fru) and sorbitol (Sor) or from electroactive species as ascorbic acid (AA) and uric acid (UA) coexisting with glucose in biological fluids (Fig. 53.2).
As a verification of enzyme integrity when it is embedded PVA, UV-vis spectra of GOx in PVA film were acquired. Results collected in Fig. 53.3A show GOx spectra in PVA matrix in the oxidized form (solid line) and its transformation in the reduced state (dotted line) after the addition of glucose 10 mM, according to literature data [6].
XPS analysis on Pt/GOx-PVA film was performed soon after its preparation (Fig. 53.3B(a)) and after being subjected to PAD measurements (Fig. 53.3B(b)) in the presence of glucose 10 mM. The absence of Pt4f signal evidenced a homogeneous deposition on Pt substrate that is preserved after the use of film in glucose detection. The film showed in fact high stability under operative conditions, as shown by the comparison between XPS spectrum of freshly prepared film (a) and of film exposed to glucose (b).
4 Conclusions
A new mediator free glucose biosensor based on the immobilization of GOx in PVA matrix on Pt electrode was proposed. The structural integrity of enzyme was confirmed by UV-vis and film homogeneity was verified by XPS analysis. Electrochemical investigations of the system by PAD in the presence of glucose revealed a wide dynamic range with a low limit of detection. Moreover the biosensor performance was satisfactory also in terms of repeatability, reproducibility and selectivity.
References
O’Neill RD, Lowry JP, Rocchitta G, McMahon CP, Serra PA (2008) Designing sensitive and selective polymer/enzyme composite biosensors for brain monitoring in vivo. Tr Anal Chem 27:78–88
Wang J (2008) Electrochemical glucose biosensors. Chem Rev 108:814–825
Heller A, Feldman B (2008) Electrochemical glucose sensors and their applications in diabetes management. Chem Rev 108:2482–2505
Zhang W, Li J (2004) Third-generation biosensors based on the direct electron transfer of proteins. Anal Sci 20:603–609
Wong F-L, Adbdul-Aziz A (2008) Comparative study of poly(vinyl alcohol)-based support materials for the immobilization of glucose oxidase. J Chem Technol Biot 83:41–46
Massey V (2000) The chemical and biological versatility of riboflavin. Biochem Soc Trans 28:283–296
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media B.V.
About this paper
Cite this paper
Chirizzi, D., Guascito, M.R., Malitesta, C., Mazzotta, E. (2011). Electrochemical and Spectroscopic Characterization of Glucose Oxidase Immobilized in Polyvinyl Alcohol and Applications in Glucose Detection. In: Neri, G., Donato, N., d'Amico, A., Di Natale, C. (eds) Sensors and Microsystems. Lecture Notes in Electrical Engineering, vol 91. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-1324-6_53
Download citation
DOI: https://doi.org/10.1007/978-94-007-1324-6_53
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-1323-9
Online ISBN: 978-94-007-1324-6
eBook Packages: EngineeringEngineering (R0)