Abstract
Vanadium compounds are present in trace amounts in living organisms. In mammals, once absorbed from the gastrointestinal tract, they distribute among tissues and storage mainly in liver, kidney and bone. Vanadium compounds in pharmacological doses exert interesting effects as insulin enhancers, antitumoral and osteogenic agents. This review deals with the more relevant information about the osteogenic effects of vanadium compounds. Their actions on the different components of hard tissue and bone related cells in culture are summarized. Besides, the putative mechanism of action and the effects on in vivo models are also discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Vanadium is a trace element present in biological systems, both in plants and animals. In vertebrates, it enters the organism mainly by the digestive and respiratory tracts through food and inhalation, respectively [1, 2]. Once absorbed, it distributes among tissues and accumulates in different organs, being the greater quantities of vanadium detected in liver, kidney and especially in bone [2].
While vanadium essentiality has been established in lower forms of life and also in higher plants, convincing evidence to support an essential role for this element in humans is still lacking [3].
Although ubiquitous in air, soil, water, and food supply, vanadium is generally found in nanogram or microgram quantities, which makes it difficult to measure. Its role as an essential element in humans remains uncertain due in part to the lack of symptoms associated to its deficiency in man and to the scarce methods strictly suitable to assure the absolute absence of this trace element in vanadium free diets for experimental animal models. However, there is scientific consensus on the putative biochemical role for vanadium compounds in living organisms [4].
In animal models and human beings, pharmacologic amounts of vanadium (i.e., 10–100 times normal intake) affect cholesterol and triglyceride metabolism, stimulate glucose oxidation and glycogen synthesis in the liver [5].
Vanadium’s primary mode of action is as a cofactor that enhances or inhibits enzymes. The inhibition of the activity of Na+-K+-ATPase by vanadate was the first effect of vanadium thoroughly investigated and reported in the literature [6], but currently, many other biological and pharmacological actions have been described for vanadium derivatives [7]. The role of vanadium in regulation of intracellular signaling pathways converts it in a possible therapeutic agent to be use in the treatments of a number of diseases. Different metabolic alterations for carbohydrates, lipids and proteins are markers for Diabetes mellitus. Besides, the disease is also characterized for a group of chronic complications which diminish the quality of life of diabetic patients and, finally, cause their death. Patients with Diabetes mellitus, mainly type 1 diabetic patients develop higher rates of bone resorption and turnover which convey to a decrease bone mineral density compared with healthy controls. Recently, Diabetes mellitus has been shown to be associated with osteoporosis [8, 9]. Several mechanisms seem to underlay bone alteration process in diabetic patients. Both, micro and macro angiopathies cause a decrease of blood flow especially in highly vascularised tissues. As a consequence, different metabolic changes take place in these tissues, including bone. For instance, it is probably that the synthesis and function of anabolic factors for bone development would decrease and, also highly concentrations of advanced glycation end (AGE) products can be formed in the constituents of the extracellular matrix (ECM). Among these components, collagen is one of the more affected proteins which cause a great impact in bone formation and functions [10]. Recently, some vanadium compounds have been reported in the literature as osteogenic agents due to their actions on bone related cells and on collagen formation. Besides, from a chemical point of view, it is well known the possible replacement of phosphorous by vanadium in the apatite lattice of bone tissue [11–14]. This review addresses and summarizes the osteogenic effects of vanadium compounds both in vitro and in vivo models as well as on cell regulatory processes that can be considered as putative mechanisms of action for these compounds. Besides, the possible mechanisms of adverse side effects derived from the vanadium administration are also briefly commented.
2 Hard Tissue
Bone is a dynamic tissue that is constantly being reshaped by osteoblasts, which build bone, and osteoclasts, which resorb bone. Osteoclasts and osteoblasts are instrumental cells in controlling the amount of bone tissue in vertebrates.
Osteoblasts are mononucleate cells responsible for bone formation. The origin of these cells is depicted in Fig.7.1. Osteoblasts derive from osteoprogenitor cells located in the periosteum and the bone marrow. Progenitor osteoblastic cells are immature cells that express the crucial regulatory transcription factor Cbfa1/Runx2. Osteoprogenitors differentiate under the influence of growth factors, in particular the bone morphogenetic proteins (BMPs) [15]. Moreover, other growth factors including fibroblast growth factor (FGF), platelet-derived growth factor (PDGF) and transforming growth factor beta (TGF-β) may promote the proliferation and differentiation of osteoblast precursors and potentially induce the mineralization process (osteogenesis). Mature osteoblasts are highly specialized cells which synthesize the major constituents of the osteoid, that is the unmineralized, organic extracellular matrix (ECM), which is composed mainly of Type I collagen and noncollagenous proteins. Osteoblasts are also responsible for mineralization of the osteoid. In fact, the mature osteoblasts promote the formation of the biological hydroxyapatite, the main hard tissue mineral phase component. These cells also regulate their content of mineral ions through the activity of different enzymes such as alkaline phosphatase (ALP) which is anchored in the osteoblastic membranes and plays a key role in the mineralization process. Aside from ALP, the organic matrix exerts a great degree of crystallographic control over the nucleation and growth of mineral particles. In the different steps of mineralization, type I collagen, the predominant protein in the ECM of bone and teeth, plays a key role [16]. Osteoblasts that are trapped in the bone matrix become osteocytes. They cease to generate osteoid and mineralized matrix, and instead act in a paracrine manner on active osteoblasts.
The other relevant cells in hard tissues are the osteoclasts. They remove bone tissue through a process known as bone resorption. Osteoclasts are multinucleate cells formed by the fusion of cells of the monocyte-macrophage cell line (Fig.7.1).
Osteoclasts are characterized by high expression of tartrate resistant acid phosphatase (TRAP) and cathepsin K [17].
3 Vanadium Compounds Interactions with Hard Tissue Components
3.1 With Hydroxyapatite
Hydroxyapatite is the major mineral component of the mineral phase of bones and hard tissues in mammals. It is thermodynamically more stable than other minerals which form during the development of the mineralization process. Hydroxyapatite is synthesized via intermediate precursors such as amorphous calcium phosphate (ACP), octacalcium phosphate, or dicalcium phosphate dehydrate [18–20].
The chemical nature and the open lattice of hydroxyapatite promote substitution events both for cations and anions (Fig.7.2, Taken from reference [21]).
Hydroxyapatite lattice. Taken from reference [21]
The most common substitutions involve carbonate, fluoride and chloride for hydroxyl groups, while defects can also exist resulting in deficient hydroxyapatite. The substitution and the vacancy effects are very important since these processes in the apatite lattice contribute to a greater solubility of this phase and in consequence, favor its behavior as an ion reservoir. Physiologically, the mineralization process takes place with a non random distribution. It occurs inside matrix vesicles (MVs) which are extracellular, membrane-invested particles selectively located at sites of initial calcification in cartilage, bone, and predentin [22].
Matrix vesicle biogenesis occurs by polarized budding and pinching-off of vesicles from specific regions of the outer plasma membranes of differentiating growth plate chondrocytes. The first crystals of apatite bone mineral are formed within the MVs [16]. This is followed by propagation of hydroxyapatite into the ECM and its deposition between collagen fibrils.
When vanadium is absorbed in vertebrates, it distributes among different tissues and is storage mainly in bone [23].
The skeletal retention and bone effects of vanadium are of particular research interest [24]. Vanadate ions can be incorporated into hydroxyapatite lattice possibly for its analogy to phosphate [25, 26]. For these reasons, special attention was focused on the interaction of vanadium species with hydroxyapatite and with some components of the ECM such as chondroitin sulfate A, N-acetyl-D-galactosamine and D-Glucuronic acid. The structural and spectroscopic effects of the incorporation of VO 3−4 in the hydroxyapatite lattice were investigated as a model for the process of incorporation of vanadium in bone [27]. The substitution of PO 3−4 by VO 3−4 is facilitated for the structural behavior of the hydroxyapatite lattice which lends itself for substitution. Nevertheless, it is required that the apatite would be in an amorphous form for the incorporation of vanadate to be accomplished since it could not be observed when the apatite lattice is in the crystalline state. The incorporation of small quantities of VO 3−4 in the PO 3−4 sites produces only slight distortions in the macroscopic and microscopic structure of the mineral phase of bones. This fact could be determined by X-ray diffraction studies of substituted hydroxyapatite powder samples. Based on these results, it can be assumed that bone has an active role in the detoxification process when the organisms are exposed to high vanadium concentrations, comparable to that known for other toxic trace elements that can be also incorporated in bone. On the contrary, the substitution of calcium by other cations in the hydroxyapatite lattice affects these bonds more strongly [28, 29]. Besides, it was also demonstrated that VO2+ could not be incorporated into the apatite lattice although it is strongly adsorbed on its surface [30]. All together these studies showed that hydroxyapatite is a good model to study the incorporation of vanadium in bone.
3.2 With ECM Components
To continue with the understanding of the effects of vanadium species on hard tissues we investigated the interactions of vanadium with chondroitin sulfate A (CSA), an acid mucopolysaccharide present in connective tissue and other mineralized systems [31]. Results from different spectroscopic determinations suggested the coordination of oxovanadium(IV) to the carboxylate group and the glycosidic oxygen of the D-glucuronate moieties [32]. These results are relevant for the interaction of VO2+ with collagen, a system in which interactions of oxovanadium(IV) with nitrogen has been clearly established [33, 34].
3.3 With Bone Cells in Culture
The relevance of vanadium in bone tissue arises from the studies performed to establish the essentiality of this element in animals and human beings [23, 35].
As bone is quantitatively the main tissue for vanadium storage bone accumulation is twice than the accumulation in kidney and tenfold the liver accumulation [36] it is worthy to try to understand the effects of vanadium compounds in bone related cells. Cells in culture are a useful system to investigate many different biological events. In particular, considering the biology and biochemistry of hard tissues, it is interesting to thoroughly study events such as cell proliferation, differentiation and mineralization, as well as the intracellular mechanisms by which vanadium derivatives exert their biological actions.
In particular, we have studied the effects of vanadium compounds in two osteoblast-cell lines of murine origin Fig.7.3. [37–40].
MC3T3E1 osteoblast-like cells. The osteoblasts were cultured in DMEM at 37°C for 24 h, fixed and stained with Giemsa for microscopy observation (Magnification: 100×) (Left panel). UMR106 osteoblast-like cells. Osteoblasts were cultured in DMEM 37°C, 24 h, fixed and stained with Giemsa for microscopy observation (Magnification: 100×) (Right panel)
MC3T3E1 osteoblasts are derived from mouse calvaria. These cells display the features of typical fibroblasts [41].
MC3T3E1 cell line is a model of preosteoblasts that can differentiate to mature osteoblasts in culture. The different maturation stages of this cell line in vitro resemble that of the physiological process in vivo (proliferation, differentiation and mineralization), providing an adequate system for biological studies referred to bone tissues.
In the proliferative stage, MC3T3E1 cells do not express a great level of alkaline phosphatase (ALP) specific activity. After 10 days of culture in the presence of ascorbic acid and β glycerophosphate, they expressed specific markers of mature osteoblast phenotype. The differentiation step correlates with the expression of different proteins such as ALP and collagen accumulation in the ECM. The mineralization of ECM begins approximately at 15–20 days of culture. After that, the cells programme their death by apoptosis [42].
UMR106 osteoblast-like cells are derived from a rat osteosarcoma induced by 32P. This immortalized cell line exhibits the characteristic osteoblast phenotype. The cells express high levels of specific ALP activity and produce type I collagen but they are unable to synthesize bone mineral phase in culture [39].
This simple model of osteoblast-like cells in culture allows the investigation of pharmacological effects of many vanadium compounds. Specially, complexes of vanadyl(IV) cation with simple sugars (mono- and disaccharides, and related compounds such as polyhols and acids) were investigated [43, 44].
Practically all the vanadyl(IV)-sugar complexes synthesized in our laboratory were evaluated for their bioactivity on osteoblast cell lines in culture. This approach has allowed us to find an outstanding compound among this series: the complex of vanadyl(IV) cation with the disaccharide trehalose (TreVO) which displayed insulin mimetic activity [45]. Besides, in long term cultures carried out with the MC3T3E1 cell line [40], this complex revealed as a good osteogenic compound since it promoted type I collagen production and the mineralization of the ECM in the cultures (See Fig.7.4) [40]. Moreover, it could also be established the mechanism of action of this compound which exerted its mitogenic effect, at low doses, through the activation of the extracellular regulated kinase (ERK) pathway. On the other hand, it was demonstrated the participation of oxidative stress in the cytotoxic actions of this complex at high concentrations [39, 40].
Moreover, the bioactivity of vanadyl(IV) derivatives with flavonoids and related compounds have been also investigated with the aid of this cellular in vitro model to assess their osteogenic ability. In this context, a complex of vanadyl(IV) cation with the flavonoid Quercetin, also showed promissory osteogenic activity in osteoblasts in culture. Flavonoids are polyphenolic compounds obtained from plants. Recently they have aroused a great scientist interest due to their broad pharmacological activity. They present antioxidant, antitumoral and antibacterial properties [46–49].
The complex of Quercetin and vanadyl(IV) cation, QuerVO, was studied in cultures of MC3T3E1 and UMR106 cells. Cell proliferation was evaluated by the crystal violet bioassay. For studying cellular differentiation, two markers of osteoblast phenotype (ALP and collagen production) were analyzed. Finally, to get an insight into the putative mechanisms of action, studies on the effect of this complex on the activation of ERK pathway were performed and reported [50]. QuerVO displayed interesting osteogenic actions such as induction of the synthesis of collagen type I. The complex caused stimulation of ERK phosphorylation and this activation seems to be one of the possible mechanisms used by the complex to exert its biological effects.
Another interesting osteogenic oxovanadium (IV) derivative thoroughly studied for our group was the vanadyl(IV) complex with ascorbic acid (VOAsc) [51]. This complex significantly stimulated collagen production in osteoblasts and inhibited ALP activity in UMR106 cells. Besides, after 3 weeks of culture, VOAsc (5–25 μM) increases the formation of mineralization nodules in the ECM of MC3T3E1 cultures. At higher concentrations this complex stimulated cellular apoptosis in osteoblast cell lines.
On the other hand, the effects of vanadium(V) compounds such as metavanadate and decavanadate in bone fish cells have been investigated by Aureliano Alves and his group [52]. Short- and long-term studies were performed in VSa13, a fish bone-derived cell line. Metavanadate in short periods was less toxic than decavanadate. In long term studies the effects of both vanadium derivatives were similar. They stimulated cell proliferation but strongly impaired the mineralization process.
The reported results suggest that vanadium(IV) complexes display more effective osteogenic properties in osteoblasts in culture than vanadium(V) derivatives, although more research is required in this sense to get a deeper insight into the osteogenic properties of vanadium.
As bone homeostasis is the result of the balance between bone resorption and bone formation, we have studied the activation of macrophages because these cells are related with bone resorption. We investigated the effect of a complex of vanadyl(IV) cation with the non steroideal anti-inflammatory drug Aspirin, VOAspi, on a culture of murine macrophage RAW 264.7. VOAspi caused the activation of macrophages by a mechanism dependent on L-type calcium channel and the generation of nitric oxide. On the contrary, free vanadyl(IV) cation exerted cytoxic effects by a mechanism independent of calcium channel and nitric oxide generation [53]. The studies on vanadium actions in osteoclasts and related cells are very scarce and merits more attention in the future.
4 Mechanism of Action
The putative mechanisms for the osteogenic actions of vanadium compounds are currently under exhaustive investigation. In fact, we have carried out different experiments in osteoblasts in culture to achieve this aim [37, 40, 54]. As an overview, at low concentrations, most vanadium derivatives behave as weak mitogens and promote osteoblast differentiation in a way similar to the insulin. Vanadium derivatives regulate osteoblastic proliferation, differentiation and stimulated the glucose consumption [55, 56]. Extracellular regulated kinases-1,2 (ERKs) and phosphatidyl inosytol-3 kinase (PI3-K) are the main intracellular transduction pathways used by vanadium to exert its biological effects. These pathways are stimulated as a result of the inhibition that vanadium derivatives cause on the protein tyrosine phosphatases (PTPases) [57, 58].
The model of osteoblastic cells in culture has allowed us to demonstrate the ability of low doses of TreVO to promote cell proliferation and to stimulate ERK phosphorylation in the MC3T3E1 osteoblastic cell line. This effect was totally abrogated by an inhibitor of MEK (PD98059) and wortmannin (an inhibitor of the PI3-K), but not by a mixture of free radical scavengers (vitamins E and C) [45]. These results suggest that low doses of the complex, which are mitogenic for MC3T3E1 cells, could act though the PI3K-MEK-ERK pathway and by a mechanism independent of free radicals. On the contrary, at higher doses, the vanadium complex inhibited cell proliferation of MC3T3E1 osteoblasts and the osteosarcoma UMR106 cell line. This effect was not blocked by neither wortmannin, nor PD98059 or by the mixture of vitamins C and E. However, high concentrations of the complex strongly increased ERK phosphorylation, an effect that was partially blocked by wortmannin, PD98059 or a mixture of vitamins E and C. In addition, the combination of these inhibitors showed an additive effect over the inhibition of ERK activation but not over the inhibition of cell proliferation [45]. Altogether these results indicate that, although high doses of vanadium stimulate ERK phosphorylation through the PI3K-MEK-dependent pathway and also through an oxidative mechanism, the inhibition of cell proliferation does not seem to be associated with the activation of these pathways.
On the other hand, there are some other mechanisms that may be involved in the osteogenic effects of vanadium such as the direct activation of a cytosolic protein tyrosine kinase [55, 59]. Results reported by our laboratory are in agreement with the latter observations. Insulin and TreVO stimulated the glucose consumption in osteoblast-like cells, but the PI3-K inhibitor wortmannin did not abrogate this effect. In addition, staurosporine at concentrations that do not affect PKC, was a potent inhibitor of the glucose consumption simulated by this vanadium(IV) complex. [45]. In addition, the inhibition of glycogen synthase kinase-3 (GSK-3) is important for the activation of glycogen synthase [60]. Vanadium(IV) complexes inhibit GSK-3 through phosphorylation and in turn activate glycogen synthase promoting glucose consumption on osteoblats. In the presence of staurosporine, the vanadium derivatives failed to stimulate GSK-3 phosphorylation [44]. Altogether these results suggest that the cytosolic tyrosine protein kinase and GSK-3 may be involved in the insulin mimetic activity of the vanadyl(IV) compounds on osteoblast cell in culture.
5 In Vivo Osteogenic Effects of Vanadium
Several studies suggest that vanadium compounds ameliorate diabetic-related bone disorders, primarily by improving the diabetic state [61, 62].
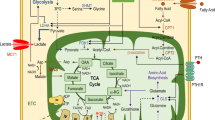
Treatment in control and streptozotocin-induced diabetic female Wistar rats with bis(ethylmaltolato)oxovanadium(IV) (BEOV), was effective in incorporating vanadium into bone. In all treated groups, BEOV increased osteoid volume. In non-diabetic rats, BEOV increased cortical bone toughness, mineralization and bone formation [61] (Fig.7.5).
In this figure it can be observed two of the most important signaling transduction pathways of insulin as well as the sites used by vanadium as an insulin enhancer. Both pathways regulate the expression of genes related to bone cell proliferation and differentiation as well as their action on proteins of the cytoskeleton which are related to cellular morphological changes. Moreover, vanadium compounds participate in ionic exchange with phosphate favoring its accumulation in bone tissue and potentiate effects on this tissue
Following the long-term administration of vanadyl acetylacetonate, trabecular thickness, mineral apposition rate and plasma osteoclacin in diabetic rats were either improved or normalized, however reduced bone mineral density was not increased [62].
In higher animals, vanadium has been demonstrated to be essential. After their mothers had been fed carefully formulated vanadium-deficient diets, second-generation goat kids suffered skeletal damage and died within 3 days of parturition [5]. In human beings vanadium essentiality has not been proven yet. Pharmacologic amounts of vanadium (i.e., 10–100 times normal intake) affect cholesterol and triglyceride metabolism, influence the shape of erythrocytes, and stimulate glucose oxidation and glycogen synthesis in the liver [5]. Moreover, clinical studies carried out in normal or diabetic patients indicated that orally administered vanadium(IV) showed low or no toxicity [63–65].
6 Risk Assessment for the Use of Vanadium Compounds as Potential Therapeutics Drugs: Cytotoxicity and Genotoxicity Studies
Despite the potential use of vanadium compounds either as insulinomimetic, osteogenic or anticancer drugs, its possible harmful effects argue against its clinical use. Understanding of the mechanisms underlying vanadium compounds toxicity is important in evaluating its use as a therapeutic drug.
The toxic effects of vanadium are related to the dose, cell line and vanadium nature (oxidation state, free or complexed). Studies concerning the mechanisms of action of vanadate showed that this compound induces gene expression, oxidative burst, changes in cytosolic calcium, tyrosine phosphorylation, enzyme activation or inhibition and also, morphological changes and cytoskeletal alterations [6, 66–71].
Several studies suggest that hydrogen peroxide is involved in vanadate-induced cell growth arrest and cell death [72–75].
However, a more recently study suggested that vanadate toxicity occurs by two distinct pathways, one dependent on and one independent of H2O2 production [76]. Vanadate concentrations that reduced cellular viability to approximately 60–70% of the control (10 μmol/L) did not induce H2O2 formation. A second hypothesis raised by these authors proposed that peroxovanadium compounds, produced once vanadate enters into the cells, are responsible for the cytotoxicity.
On the other hand, decreased cell viability induced by vanadyl sulphate in tumorogenic and non-carcinogenic cells has been also previously reported [77]. Moreover, we have also previously reported the cytotoxicity of a vanadium(V) derivative with salicylaldehyde semicarbaxone in osteoblasts in culture [78]. This complex caused cytotoxicity in the osteoblastic cells mainly through oxidative stress and through the activation of ERK pathways which, although it is classically recognized as a key transducer in the signal cascade mediating the cell proliferation, and differentiation [79], some investigations have shown that the activation of ERK pathway can mediate cell cycle arrest and apoptotic and non apoptotic cell death [80].
On the other hand, even though vanadyl(IV) is less toxic than vanadium(V), it is always crucial to investigate the toxicity of vanadium(IV) derivatives intended to be used in therapeutics. Recently, we have reported an exhaustive study on a vanadyl(IV) complex with a multidentate oxygen donor oda=oxodiacetate, VO(oda) in osteoblast cell lines in culture [81]. The complex increased the level of ROS which correlated with a decreased in GSH/GSSG ratio, dissipation of the mitochondria membrane potential and promoted an increase in ERK cascade phosphorylation, which is involved in the regulation of cellular death and survival.
Considerable attention has attracted the assessment of the genotoxicity of vanadium compounds in recent years. Adverse effects of pentavalent and tetravalent vanadium compounds on chromosome integrity and segregation have been observed in vitro. In particular, we have studied the genotoxic effect of VO(oda) in two osteoblast cell lines (UMR106 and MC3T3E1) that revealed a twofold increase in the micronucleus frequency at 25 μM compared with control in UMR106 and at 10 μM in MC3T3E1. An example of a binucleated osteoblastic cell containing a micronucleus is depicted in Fig.7.6 for both cell lines. Besides we have recently reported preliminary results on the increase in DNA strand breaks in a human colon adenocarcinoma cell line (Caco-2 cells) induced by VO(oda) [82]. In agreement with our results, vanadium(IV) compounds proved to be genotoxic in several in vitro systems, inducing micronuclei and chromosomal aberrations in human peripheral blood cells [83–85], and DNA strand breaks through the generation of free radicals [86, 87].
Pentavalent vanadium compounds have been also reported to induce genotoxic actions stimulating the increase in micronucleus frequency, DNA strand breaks, sister chromatid exchanges (SCE) and chromosomal aberrations in different in vitro systems [83, 84, 88–91].
These positive findings were confirmed in some in vivo studies in mice using acute intraperitoneal or intragastric administrations [92–94].
Moreover, the genotoxic potential in vivo by a subacute oral exposure of vanadyl sulphate has been evaluated. Effects on chromosome integrity and segregation, visualized as micronuclei, and primary DNA lesions detectable by comet assay, have been assessed in somatic and germ cells [95]. In summary, taken in consideration the multiple biological activities exerted by vanadium on critical cell mechanisms, even in the very low dose range [96, 97], it is suggested to maintain a cautious approach in the safety evaluation of vanadium compounds.
7 Concluding Remarks
The potential therapeutics actions of vanadium compounds at hard tissues have attracted the attention of scientists in the field of diabetes mellitus, bone disease, aging and cancer since vanadium compounds are store mainly in bones. The use of osteogenic primary or cloned lines in culture is a useful strategy to investigate the osteogenic properties of vanadium compounds. Although in vitro experiments showed that the toxicity actions of vanadium compounds seem to be related to the oxidation state of the element as well as the nature of the ligands in the coordination sphere and also, on the cell type in culture, in vivo reports show little, if any, toxicity in long-term administration. Vanadium compounds have demonstrated several interesting effects on biological processes such as cell proliferation, differentiation and mineralization of the ECM in bone cells. These effects convey to vanadium consideration as a potential therapeutic agent for several diseases. Finally, it is worthy to mention that further investigations are needed to completely elucidate the osteogenic mechanism of action of vanadium compounds, even though, currently most evidence point to the role of ERK pathway activation through the inhibitory effect on Protein tyrosine phosphatase (PTPase) activity, calcium channel and the expression of genes related to osteoblast differentiation. Moreover, although there are very few experimental works on this topic in vivo, some studies recently develop suggest the potential relevance of vanadium derivatives in the treatment of bone diseases.
References
Myron DR, Zimmerman Shuler TR, Klevay LM, Lee DE, Nielsen FH (1978) Intake of nickel and vanadium by humans: a survey of selected diets. Am J Clin Nutr 31:527–531
Nielsen FH (1995) Vanadium in mammalian physiology and nutrition. In: Sigel H, Sigel A (eds) Metal ions in biological systems, Vanadium and its role in life. Marcel Dekker, New York
Nielsen FH (1984) Ultratrace elements in nutrition. Annu Rev Nutr 4:21–41
Nriagu JO (1998) Vanadium in the environment, Part 2: health effects. Wiley, New York/Chitester/Weinheim/Brisbane/Singapore/Toronto
Harland BF, Harden-Williams BA (1994) Is vanadium of human nutritional importance yet? J Am Diet Assoc 94:891–894
Cantley LC Jr, Josephson L, Warner R, Yanagisawa M, Lechene C, Guidotti G (1977) Vanadate is a potent (Na, K)-ATPase inhibitor found in ATP derived from muscle. J Biol Chem 252:7421–7423
Tsiani E, Fantus IG (1997) Vanadium compounds biological actions and potential as pharmacological agents. Trends Endocrinol Metab 8:51–58
Schwartz AV (2993) Diabetes mellitus: does it affect bone? Calcif Tissue Int 73:515–519
de Paula FJ, Horowitz MC, Rosen CJ (2010) Novel insights into the relationship between diabetes and osteoporosis. Diabetes Metab Res Rev 26:622–630
Schwartz AV (2003) Diabetes mellitus: does it affect bone? Calcif Tissue Int 73:515–519
Stankiewicz PJ, Tracey AS, Crans DC (1995) Inhibition of phosphate-metabolizing enzymes by oxovanadium(V) complexes. Met Ions Biol Syst 31:287–324
Plass W (2002) Transition metal centers in biological matrices: structure and function of vanadate in vanadium haloperoxidases and as phosphate analog. In: Rollnik H, Wolf D (eds) NIC symposium 2001, Proceedings, John von Neumann Institute for Computing, Jülich, NIC Series
Crans DC (2005) Fifteen years of dancing with vanadium. Pure Appl Chem 77:1497–1527
Steens N, Ramadan AM, Parac-Vogt TN (2009) When structural and electronic analogy leads to reactivity: the unprecedented phosphodiesterase activity of vanadates. Chem Commun (Camb) 28:965–967
Agata H, Asahina I, Yamazaki Y, Uchida M, Shinohara Y, Honda MJ, Kagami H, Ueda M (2007) Effective bone engineering with periosteum-derived cells. J Dent Res 86:79–83
Anderson HC (2003) Matrix vesicles and calcification. Curr Rheumatol Rep 5:222–226
Nijweidi Ehb PJ, Feyen JHM (1986) Cells of bone: proliferation, differentiation, and hormonal regulation. Physiol Rev 66:855–886
Kartsogiannis V, Ng KW (2004) Cell lines and primary cell cultures in the study of bone cell biology. Mol Cell Endocrinol 228:79–102
Eanes ED (2001) Amorphous calcium phosphate. Monogr Oral Sci 18:130–147
Eanes ED, Gillessen IH, Posner A (1970) A note on the crystal growth of hydroxyapatite precipitated from aqueous solutions. Mater Res Bull 5:377–383
Etcheverry SB, Ferrer EG, Gonzalez-Baró AC, Parajón-Costa BS, Williams PAM (2009) Vanadis´charms: from the mithology to the bioinorganic chemistry. J Arg Chem Soc 97:127–150
Anderson HC (1995) Molecular biology of matrix vesicles. Clin Orthop Rel Res 314:266–280
Nielsen FH (1995) Vanadium and its role in life. In: Sigel H, Sigel A (eds) Metal ions in biological systems. Marcel Dekker, New York
Barrio DA, Etcheverry SB (2010) Potential use of vanadium compounds in therapeutics. Curr Med Chem 17:3632–3642
Gresser MJ, Tracey AS (1990) Vanadates as phosphate analogs in biochemistry. In: Chasteen ND (ed) Vanadium in biological systems. Kluwer Academic, Dordrecht
Crans DC (1994) Enzyme interactions with labile oxovanadates and other oxometalates. Comm Inorg Chem 16:35–76
Etcheverry SB, Apella MC, Baran EJ (1984) A model study of the incorporation of vanadium in bone. J Inorg Biochem 20:269–274
Apella MC, Etcheverry SB, Baran EJ (1981) Untersuchung der symmetrischen Phosphat-Valenzschwingung in gemischten Calcium-Strontium-Apatiten. Z Naturforsch 36b:1190–1192
Narda GE, Pedregosa C, Etcheverry SB, Baran EJ (1990) Schwingungsspektroskopische Untersuchung einiger gemischter Calcium/Cadmium-Hydroxylapatite. Z Naturforsch 45b: 1133–1135
Narda GE, Apella MC, Etcheverry SB, Baran EJ (1984) Hydrolytisches und thermisches Verhalten von Sn3PO4F3. Z Anorg Allg Chem 515:207–212
Etcheverry SB, Williams PAM, Baran EJ (1994) The interaction of the vanadyl(IV) cation with chondroitin sulfate A. Biol Trace Elem Res 42:43–52
Etcheverry SB, Williams PAM, Baran EJ (1996) Synthesis and characterization of a solid vanadyl (IV) complex of D-glucuronic acid. J Inorg Biochem 63:285–289
Etcheverrry S, Williams PAM, Baran EJ (1996) A spectroscopic study of the interaction of the VO2+ cation with the two components of chondroitin sulfate. Biol Trace Elem Res 51:169–176
Etcheverrry S, Williams PAM, Baran EJ (1996) A spectrophotometric study of the interaction of VO2+ with cytosine nucleotides. Biol Trace Elem Res 51:169–176
Anke M, Groppel B, Krause U (1991) The essentiality of the toxic elements aluminium and vanadium. In: Momcilovic B (ed) Trace elements in man and animals. IMI, Zagreb
Setyawati IA, Thompson KH, Yuen VG, Sun Y, Battell M, Lyster DM, Vo C, Ruth TJ, Zeisler S, McNeill JH, Orvig C (1998) Kinetic analysis and comparison of uptake, distribution, and excretion of 48V-labeled compounds in rats. J Appl Physiol 84:569–575
Etcheverry SB, Cortizo AM (1998) Bioactivity of vanadium compounds on cells in culture. In: Nriagu JO (ed) Vanadium in the environment. Advances in environmental science and technology; Part A. Wiley, New York
Cortizo AM, Etcheverry SB (1995) Vanadium derivatives act as growth factor-minetic compounds upon differentiation and proliferation of osteoblast-like UMR106 cells. Mol Cell Biochem 145:97–102
Barrio DA, Etcheverry SB (2006) Vanadium and bone development: putative signalling pathways. Can J Physiol Pharmacol 84:677–686
Etcheverry SB, Barrio DA (2007) Vanadium and bone. Relevance of vanadium compounds in bone cells. In: Kustin K, Costa Pesoa J, Crans DC (eds) Vanadium: the versatile element, vol 15, American chemical society series. American Chemical Society, Washington, DC, p 974
Sálice VC, Cortizo AM, Gómez Dumm CL, Etcheverry SB (1999) Tyrosine phosphorylation and morphological transformation induced by four vanadium compounds on MC3T3E1 cells. Mol Cell Biochem 198:119–128
Quarles LD, Yohay DA, Lever LW, Caton R, Wenstrup RJ (1992) Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J Bone Miner Res 7:683–692
Williams PAM, Barrio DA, Etcheverry SB, Baran EJ (2004) Characterization of oxovanadium(IV) complexes of D-gluconic and D-saccharic acids and their bioactivity on osteoblast-like cells in culture. J Inorg Biochem 98:333–342
Barrio DA, Cattáneo ER, Apezteguía MC, Etcheverry SB (2006) Vanadyl(IV) complexes with saccharides. Bioactivity in osteoblast-like cells in culture. Can J Physiol Pharmacol 84: 765–775
Barrio DA, Williams PA, Cortizo AM, Etcheverry SB (2003) Synthesis of a new vanadyl(IV) complex with trehalose (TreVO): insulin-mimetic activities in osteoblast-like cells in culture. J Biol Inorg Chem 8:459–468
Rice-Evans CA, Packer L (1998) Flavonoids in health and disease. Marcel Dekker, New York
Bravo A, Anacona JR (2001) Metal complexes of the flavonoid quercetin: antibacterial properties. Transit Met Chem 26:20–23
Cornard JP, Merlin JC (2001) Structural and spectroscopic investigation of 5-hydroxyflavone and its complex with aluminium. J Mol Struct 569:129–138
Kandaswami C, Lee LT, Lee PP, Hwang JJ, Ke FC, Huang YT, Lee MT (2005) The antitumor activities of flavonoids. In Vivo 19:895–909
Ferrer EG, Salinas MV, Correa MJ, Naso L, Barrio DA, Etcheverry SB, Lezama L, Rojo T, Williams PAM (2006) Synthesis, characterization, antitumoral and osteogenic activities of quercetin vanadyl(IV) complexes. J Biol Inorg Chem 11:791–801
Cortizo AM, Molinuevo MS, Barrio DA, Bruzzone L (2006) Osteogenic activity of vanadyl(IV)-ascorbate complex: evaluation of its mechanism of action. Int J Biochem Cell Biol 38:1171–1180
Tiago DM, Laizé V, Cancela ML, Aureliano M (2008) Impairment of mineralization by metavanadate and decavanadate solutions in a fish bone-derived cell line. Cell Biol Toxicol 24:253–263
Molinuevo MS, Etcheverry SB, Cortizo AM (2005) Macrophage activation by a vanadyl-aspirin complex is dependent on L-type calcium channel and the generation of nitric oxide. Toxicology 210:205–212
Etcheverry SB, Williams PA, Barrio DA, Salice VC, Ferrer EG, Cortizo AM (2000) Synthesis, characterization and bioactivity of a new VO2+/aspirin complex. J Inorg Biochem 80:169–171
Cortizo AM, Salice VC, Vescina CM, Etcheverry SB (1997) Proliferative and morphological changes induced by vanadium compounds on Swiss 3T3 fibroblasts. Biometals 10:127–133
Etcheverry SB, Crans DC, Keramidas AD, Cortizo AM (1997) Insulin-mimetic action of vanadium compounds on osteoblast-like cells in culture. Arch Biochem Biophys 338:7–14
Swarup G, Cohen S, Garbers DL (1982) Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem Biophys Res Commun 107:1104–1109
Tracey AS, Gresser MJ (1986) Interaction of vanadate with phenol and tyrosine: implications for the effects of vanadate on systems regulated by tyrosine phosphorylation. Proc Natl Acad Sci USA 83:609–613
Shisheva A, Shechter Y (1993) Role of cytosolic tyrosine kinase in mediating insulin-like actions of vanadate in rat adipocytes. J Biol Chem 268:6463–6469
Eldar-Finkelman H (2002) Glycogen synthase kinase 3: an emerging therapeutic target. Trends Mol Med 8:126–132
Facchini DM, Yuen VG, Battell ML, McNeill JH, Grynpas MD (2006) The effects of vanadium treatment on bone in diabetic and non-diabetic rats. Bone 38:368–377
Zhang SQ, Chen GH, Lu WL, Zhang Q (2007) Effects on the bones of vanadyl acetylacetonate by oral administration: a comparison study in diabetic rats. J Bone Miner Metab 25:293–301
Mukherjee B, Patra B, Mahapatra S, Banerjee P, Tiwari A, Chatterjee M (2004) Vanadium—an element of atypical biological significance. Toxicol Lett 150:135–143
Hirano S, Suzuki KT (1996) Exposure, metabolism, and toxicity of rare earths and related compounds. Environ Health Perspect 104:85–95
Fawcett JP, Farquhar SJ, Thou T, Shand BI (1997) Oral vanadyl sulphate does not affect blood cells, viscosity or biochemistry in humans. Pharmacol Toxicol 80:202–206
Yin X, Davidson AJ, Tsang SS (1992) Vanadate-induced gene expression in mouse C127 cells: roles of oxygen derived active species. Mol Cell Biochem 115:85–96
Fantus IG, Kadota S, Deragon G, Foster B, Posner B (1989) Pervanadate [peroxide(s) of vanadate] mimics insulin action in rat adipocytes via activation of the insulin receptor tyrosine kinase. Biochemistry 28:8864–8871
Trudel S, Pâquet MR, Grinstein S (1991) Mechanism of vanadate induced activation of tyrosine phosphorylation and of the respiratory burst in HL60 cells. Role of reduced oxygen metabolites. Biochem J 276:611–619
Secrist JP, Burns LA, Karnitz L, Koretzky GA, Abraham RT (1993) Stimulatory effects of the protein tyrosine phosphatase inhibitor, pervanadate, on T-cell activation events. J Biol Chem 268:5886–5893
Huyer G, Liu S, Kelly J et al (1997) Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J Biol Chem 272:843–851
Capella LS, Alcantara JSM, Moura-Neto V, Lopes AG, Capella MAM (2000) Vanadate is toxic to adherent- growing multidrug-resistant cells. Tumour Biol 21:54–62
Bay BH, Sit KH, Paramanantham R, Chan YG (1997) Hydroxyl free radicals generated by vanadyl[IV] induce cell blebbing in mitotic human Chang liver cells. Biometals 10:119–122
Huang C, Zhang Z, Ding M et al (2000) Vanadate induces p53 transactivation through hydrogen peroxide and causes apoptosis. J Biol Chem 275:32516–32522
Torres M, Forman HJ (2002) Vanadate inhibition of protein tyrosine phosphatases mimics hydrogen peroxide in the activation of the ERK pathway in alveolar macrophages. Ann N Y Acad Sci 973:345–348
Capella LS, Gefe MR, Silva EF et al (2002) Mechanisms of vanadate induced cellular toxicity: role of cellular glutathione and NADPH. Arch Biochem Biophys 406:65–72
Capella MAM, Capella LS, Valente RC, Gefé M, Lopes AG (2007) Vanadate-induced cell death is dissociated from H2O2 generation. Cell Biol Toxicol 23:413–420
Holko P, Ligeza J, Kisielewska J, Kordowiak AM, Klein A (2008) The effect of vanadyl sulphate (VOSO4) on autocrine growth of human epithelial cancer cell lines. Pol J Pathol 59:3–8
Rivadeneira J, Barrio DA, Arrambide G, Gambino D, Bruzzone L, Etcheverry SB (2009) Biological effects of a complex of vanadium(V) with salicylaldehyde semicarbazone in osteoblasts in culture: Mechanism of action. J Inorg Biochem 103:633–642
Wang ZI, Bonner JC (2000) Mechanism of extracellular signal-regulated kinase ERK-1 and ERK-2 activation by vanadium pentoxide in rat pulmonary myofibroblasts. Am J Respir Cell Mol Biol 22:590–596
Blázquez C, Galve-Roperh I, Guzmán M (2000) The novo synthesized ceramide signals apoptosis in astrocytes via extracellular signal- regulated kinase. FASEB J 14:2315–2322
Rivadeneira J, Di Virgilio AL, Barrio DA, Muglia CI, Bruzzone L, Etcheverry SB (2010) Cytotoxicity of a vanadyl(IV) complex with a multidentate oxygen donor in osteoblast cell lines in culture. Med Chem 6:9–23
Di Virgilio AM, Rivadeneira J, Reigosa MA, Etcheverry SB (2010) XIV Congreso Latinoamericano de Genética, VIII Congreso de la Asociación Latinoamericana de mutagénesis, carcinogénesis y teratogénesis ambiental, XLIII de la Sociedad de Genética de Chile, XXXIX Confreso de la Sociedad Argentina de Genética. Book of Abstracts, pp 691, 2010
Migliore L, Bocciardi R, Macri C, Lo Jacono F (1993) Cytogenetic damage induced in human lymphocytes by four vanadium compounds and micronucleus analysis by fluorescence in situ hybridization with a centromeric probe. Mutat Res 319:205–213
Migliore L, Scarpato R, Falco P (1995) The use of fluorescence in situ hybridization with a β-satellite DNA probe for the detection of acrocentric chromosomes in vanadium-induced micronuclei. Cytogenet Cell Genet 69:215–219
Rodriguez-Mercado JJ, Roldan-Reyes E, Altamirano-Lozano M (2003) Genotoxic effects of vanadium(IV) in human peripheral blood cells. Toxicol Lett 144:359–369
Shi X, Jiang H, Mao Y, Ye J, Saffiotti U (1996) Vanadium (IV)- mediated free radical generation and related 2′-deoxyguanosine hydroxylation and DNA damage. Toxicology 106:27–39
Bay B, Sith K, Paramanamtham R, Chan Y (1997) Hydroxyl free radicals generated by vanadyl (IV) induce cell bleb bing in mitotic human Chang liver cells. Biometals 10:119–122
Owusu-Yaw J, Choen MD, Fernando SY, Wei CI (1990) An assessment of the genotoxicity of vanadium. Toxicol Lett 50:327–336
Rojas E, Valverde M, Herrera LA, Altamirano-Lozano M, Ostrosky-Wegman P (1996) Genotoxicity of vanadium pentoxide evaluated by the single cell gel electrophoresis assay in human lymphocytes. Mutat Res 359:77–84
Ramirez P, Eastmond DA, Laclette JP, Ostrosky-Wegman P (1997) Disruption of microtubule assembly and spindle formation as a mechanism for the induction of aneuploid cells by sodium arsenite and vanadium pentoxide. Mutat Res 386:291–298
Ivancsits S, Pilger A, Diem E, Schaffer A, Rudiger HW (2002) Vanadate induces DNA strand breaks in cultured human fibroblasts at doses relevant to occupational exposure. Mutat Res 519:25–35
Ciranni R, Antonetti M, Migliore L (1995) Vanadium salts induce cytogenetic effects in in vivo treated mice. Mutat Res 343:53–60
Altamirano-Lozano M, Valverde M, Alvarez-Barrera L, Rojas E (1996) Reprotoxic and genotoxic studies of vanadium pentoxide in male mice. Teratog Carcinog Mutagen 16:7–17
Mailhes JB, Hilliard C, Fuseler JW, London SN (2003) Vanadate, an inhibitor of tyrosine phosphatases, induced premature anaphase in oocytes and aneuploidy and polyploidy in mouse bone marrow cells. Mutat Res 538:101–107
Villani P, Cordelli E, Leopardi P, Siniscalchi E, Veschetti E, Fresegna AM, Crebelli R (2007) Evaluation of genotoxicity of oral exposure to tetravalent vanadium in vivo. Toxicol Lett 170:11–18
Sakurai H (1994) Vanadium distribution in rats and DNA cleavage by vanadyl complex: implication for vanadium toxicity and biological effects. Environ Health Perspect 102:1–4
Barceloux DG (1999) Vanadium. J Toxicol Clin Toxicol 37:265–278
Acknowledgements
SBE, DAB and ALDV are members of the Carrera del Investigador CONICET (Argentina). This work was partially supported by grants from Universidad Nacional de La Plata (Argentina) (X345 and X 554), CONICET (PIP 1125) and the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) (PICT 2218).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Etcheverry, S.B., Di Virgilio, A.L., Barrio, D.A. (2012). Vanadium Effects on Bone Metabolism. In: Michibata, H. (eds) Vanadium. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0913-3_7
Download citation
DOI: https://doi.org/10.1007/978-94-007-0913-3_7
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-0912-6
Online ISBN: 978-94-007-0913-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)