Abstract
Purpose of Review
The physiological control of mineralized tissue development is mediated by two processes: mineralization, such as bone formation due to osteoblast activity, and mineralized tissue destruction by osteoclast bone resorption. In this system, nutritional status, including vitamin intake, influences each regulatory processes, although definite responding mechanisms in target cells vary according to each compound.
Recent Findings
In contrast with water-soluble vitamins that constant supply is required, fat-soluble vitamins such as vitamin D and K are stored in the liver and fat tissue for long time. They are metabolized into congeneric compounds with various activities to participate in the local mineralization process in the body.
Summary
During physiological or non-physiological mineralization, the local actions of vitamin D and K are regulated by nutrient factor derived from dietary supply, and influenced by systemic calcium metabolism and homeostasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Calcium is a major component of mineralized tissue; systemic and local calcium metabolisms responding to vitamin actions are predominantly involved in mineralized tissue development. Vitamin D was discovered as an antirachitic agent capable of preventing bone formation and mineralization failures [1]. Administration of vitamin D to rachitic animals and humans cures impaired bone mineralization. Although it has been postulated that vitamin D directly stimulates osteoblastic bone formation and mineralization, there was no direct in vivo genetic evidence to support this hypothesis until recently.
Among member of fat-soluble vitamins, vitamin K is also associated with mineralizing process in either soft tissue such as blood vessel or bone in addition to blood coagulation which is recognized as a representative function [2]. Vitamin K acts as cofactor of γ-glutamyl carboxylase (GGCX) to stimulate gamma-carboxyglutamic acid (Gla) proteins including bone component proteins, such as osteocalcin (OC), by adding a carboxyl group to the gamma position of glutamine [3]. Accordingly, vitamin D and K are generally considered key players in the pathophysiology of mineralizing defect. Experimental investigations on the effects of vitamins on the various molecular pathways of mineralization are required, as well as to assess clinical significance.
Vitamin D
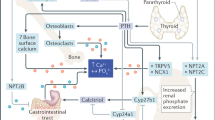
The development of mineralized tissue is influenced by calcium and phosphate homeostasis. Intestinal absorption is crucial in maintaining calcium and phosphate homeostasis in order to preserve normal or positive calcium balance. In this system, active transcellular calcium transport provides efficient intestinal absorption, and this is, to a large extent, regulated by vitamin D.
Vitamin D is metabolized in the liver by 25-hydroxylases into 25-hydroxyvitamin D3 [25(OH)D3], which is further metabolized in the kidney to either 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3] by 1α-hydroxylase (CYP27B1) or 24,25-dihydroxyvitamin D3 [24,25(OH)2D3] by 24-hydroxylase (CYP24A1) [4]. Of these two dihydroxy metabolites, 1,25(OH)2D3 has been recognized as the active form of vitamin D3 [5]. The physiological function of active vitamin D is affected when it binds to the vitamin D receptor located in target organs.
Role of Vitamin D in Calcium and Bone Homeostasis
In laboratory animals, such as rodents, the paracellular pathway is predominantly active immediately after birth but is gradually replaced by a 1,25(OH)2D3-dependent mechanism, which becomes active after weaning [6]. During the first 3–4 weeks after birth, intestinal calcium absorption was sufficient in mice lacking intestinal VDR (intestinal VDR-null mice) due to the paracellular pathway, which is unrelated to 1,25(OH)2D3-dependent transcellular transport [7••, 8]. In the post-weaning period, however, calcium absorption in intestinal VDR-null mice decreased, a phenomenon that was partially mediated by a reduction in the number of calcium channels (TRPV6) and calcium-binding protein (calbindin-D9K) expression, which is directly induced by 1,25(OH)2D3. The regulatory system of the gene encoding major epithelial calcium channels has been investigated, and this defined a direct mechanism whereby 1,25(OH)2D3-VDR signaling promotes TRPV6 expression.
Bone mineralization defects appearing in mice lacking 1,25(OH)2D3-VDR signaling are due to impaired intestinal calcium absorption, which results in altered mineral and hormonal metabolism. As a treatment for these defects, either intravenous calcium injections to patients with vitamin D resistance [9] or dietary calcium supplementation or the reintroduction of VDR activity to the intestine in VDR-null mice [10] efficiently prevents bone abnormalities and impaired mineral homeostasis. These results indicate that the enhancement of intestinal calcium absorption is the most critical role of 1,25(OH)2D3, whereas 1,25(OH)2D3 signaling in bone cells is not a prerequisite for bone development and homeostasis, provided intestinal calcium transport is guaranteed.
The physiological role of 1,25(OH)2D3 depends on dietary calcium intake and calcium balance in the body. When the internal calcium balance is normal, the major target of 1,25(OH)2D3 is the intestine. The active intestinal calcium transport mechanism is mainly induced by 1,25(OH)2D3-VDR signaling. In contrast, when the calcium balance is negative, 1,25(OH)2D3 activities in the bone become dominant.
Mice with systemic VDR inactivation display impaired bone mineralization due to their negative calcium balance as a consequence of low intestinal calcium absorption. Hyperparathyroidism was induced, followed by hypocalcemia, which in turn increased plasma 1,25(OH)2D3 levels. In general, 1,25(OH)2D3 and PTH signals support bone resorption by increasing the expression of the receptor activator of NF-κB ligand (RANKL) in osteoblasts, which stimulates the differentiation of osteoclasts. However, an increase in both plasma PTH and 1,25(OH)2D3 in VDR-null mice does not promote significant osteoclastogenesis. Previous in vitro studies have demonstrated that treatment with either 1,25(OH)2D3 or PTH induces RANKL expression independently and that 1,25(OH)2D3 signaling is not required during osteoclastogenesis when PTH is present [11]. In fact, PTH levels are highly elevated in VDR-null mice; however, the number of osteoclasts is not dramatically increased [12,13,14]. The reason for these differences between the in vitro data and in vivo phenotype of VDR-null mice can be explained as follows: the impairment of osteoclastogenesis by severe hyperparathyroidism is due to a blunted skeletal response to PTH [15]. Another factor is the lack of 1,25(OH)2D3 signaling in osteoblasts. In RANKL transcription, a distal region located over 70 kb upstream of the transcriptional start site of the murine RANKL gene contains a functional cAMP-binding domain that mediates PTH signals [16] as well as domains containing a vitamin D-responsive element [16]. The responsiveness of this region involves independent requirement and/or cross-talk between the two signals; therefore, 1,25(OH)2D3 signaling may be required for the maximal induction of osteoclastogenesis supported by PTH in vivo [17, 18].
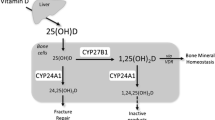
In addition to bone resorption, 1,25(OH)2D3 inhibits calcium deposition into bone and thereby mediates plasma calcium homeostasis. This bone mineralization inhibitory mechanism appears to be due to several 1,25(OH)2D3-responsive genes encoding proteins involved in the regulation of matrix mineralization. One mechanism involves the secretion of acidic serine- and aspartate-rich motif-containing SIBLING proteins, such as osteopontin, a mineralization inhibitor [19]. Another mechanism resulting in decreased mineralization is the accumulation of pyrophosphates (PPi) because PPi inhibit hydroxyapatite formation. The cellular generation of PPi is induced by ectonucleotide pyrophosphate phosphodiesterase (ENPP) and progressive ankylose (ANK), which then transports PPi to the extracellular fluid. 1,25(OH)2D3-VDR signals directly induce the transcription of ENPP-1, -3, and ANK genes. This inhibition of hydroxyapatite formation may have implications for normal physiology because PPi prevents ectopic calcification in soft tissues. In a similar fashion, the abnormal accumulation of PPi inhibits hydroxyapatite formation in the bone. The 1,25(OH)2D3-dependent upregulation of both ENPP and ANK results in the suppression of bone mineralization [20, 21] (Fig. 1).
1,25(OH)2D3-VDR signaling in positive and negative calcium balance. In case of sufficient calcium supply, 1,25(OH)2D3 s stimulates active intestinal calcium absorption which guarantee normal bone homeostasis. However, in case of low calcium supply, hyperparathyroidism stimulated 1,25(OH)2D3 production. High levels of PTH and 1,25(OH)2D3 preserve serum calcium by stimulating both RANKL-induced bone resorption and inhibiting bone mineralization
In contrast to the above data suggesting that 1,25(OH)2D3 suppresses bone mineralization, 1,25(OH)2D3 stimulates osteoblast differentiation from mesenchymal stromal cells in humans [22, 23]. Further, mice with VDR overexpression in osteoblasts showed increased bone mass [24]. To resolve the contradiction on the vitamin D-dependent mineralization, a stage-dependent variation of osteoblast function and vitamin D signaling should be considered important future research projects.
Vitamin K
The vitamin K family members share a same 2-methyl-1,4-naphthoquinone nucleus but differ in their isopropyl side chain. Vitamin K1 or phylloquinone (PK) is found in green, leafy vegetables, and vitamin K2 or menaquinones (MK-n) are present in animal products and fermented foods. Long-chain MK-n generated by bacteria in fermented foods are an important source of vitamin K. On the other hand, MK-4 is ubiquitously present in animal foods, including meat and eggs, but it contributes to small dietary intakes compared with PK and MK-7. Interestingly, MK-4 can be synthesized from PK in tissues and is present in much higher amounts compared with PK.
Vitamin K Regulates Bone Mineralization
Vitamin K is a cofactor of GGCX, an enzyme that converts specific glutamic acid residues in several substrate proteins to Gla residues. Gla residues form calcium-binding groups, which are essential for the protein’s biological activity. Gla-containing proteins are involved in blood coagulation, bone metabolism, vascular repair, prevention of vascular calcification, and regulation of cell proliferation and signal transduction [3, 25, 26]. Vitamin K undergoes a cyclic interconversion, known as the vitamin K cycle, comprising the reduction of the vitamin K quinone form into hydroquinone, oxidation to 2,3-epoxide (vitamin K epoxide), and reduction to quinone [27]. The formation of Gla from glutamate is coupled with the conversion of hydroquinone to vitamin K epoxide. Both these activities occur for a cofactor of GGCX. The warfarin-sensitive microsomal enzyme, vitamin K epoxide reductase (VKOR), recycles vitamin K epoxide back to hydroquinone, thus completing the vitamin K cycle (Fig. 2). From a nutritional perspective, the fact that cells possess the machinery for efficient recycling of vitamin K consumed during γ-carboxylation can be regarded as a local salvage pathway to preserve limited stores of vitamin K. This is clinically illustrated in infants born with a defective VKOR who often present with severe coagulopathy and/or skeletal defects.
After oral ingestion, vitamin K derivatives are misallied by bile acids and pancreatic juice in the gastrointestinal tract and then absorbed from the upper part of the small intestine. The micelles in the small intestine are transferred via chylomicrons, similar to other fat-soluble vitamins, into the bloodstream via lymphatic vessels. The transfer of PK and MK-4 to tissues has been studied in detail using mice and rats; PK and MK-4 are distributed in almost all tissues in a relatively short time [28]. In mice fed with normal feed (including PK and menadione (MD) but not MK-4), PK and MK-4 are detected in almost all body tissues, but the concentration of MK-4 is several hundred times higher than that of PK due to the conversion of PK into MK-4 in vivo. Okano et al. proved that PK is converted into MK-4 in vivo using deuterium-labeled PK [29]. Nakagawa et al. reported that the key enzyme responsible for the conversion of PK into MK-4 is UbiA prenyltransferase domain-containing protein 1 (UBIAD1) [30]. When PK is converted into MK-4, a side-chain cleavage reaction occurs and MD (without side chain) is detected in the intestinal lymph of PK orally administered rats [31]. These results indicate that the side-chain cleavage reaction of PK occurs in the small intestinal epithelium where PK becomes MD, which is then transferred to each tissue and converted by UBIAD1 into MK-4 to exert its various physiological activities. Recently, Takada et al. reported that Niemann–Pick C1-like 1 (NPC1L1) protein, a cholesterol transporter, plays a central role in intestinal PK uptake and modulates the anticoagulant effect of warfarin. They suggested that intestinal VK absorption is NPC1L1-dependent by in vitro studies using NPC1L1-overexpressing intestinal cells and in vivo studies using Npc1l1-knockout mice [32].
Vitamin K is a cofactor of GGCX, an enzyme that Gla-specific glutamic acid residues of vitamin K-dependent protein (VKDP) (Fig. 2). OC is a bone-specific VKDP and is synthesized by osteoblasts during the later phase of bone mineralization. OC is the most abundant noncollagenous protein in bone, and its concentration in blood is tightly connected to osteoblast function, differentiation, and vitamin K sufficiency. The OC molecule contains three Glu residues, which need vitamin K-dependent post-translational γ-carboxylation to Gla residues. The post-translational modification of OC is regulated by several factors. Administration of vitamin K derivatives enhances the carboxylation process. Both dietary intake of vitamin K and a pharmacological dose of MK-4 induce rapid alteration of γ-carboxylation of Glu residues in OC molecules. Therefore, undercarboxylated OC (ucOC) is used to diagnose the osteoporosis pathology accompanied by vitamin K deficiency and to assess any therapeutic effect [33]. Recently, many postmenopausal osteoporotic patients treated with bisphosphonates were reported to have vitamin K deficiency as indicated by low vitamin K intake and high serum ucOC concentrations, despite having similar reductions in bone turnover as women with normal vitamin K intake. Because high serum ucOC concentrations are thought to be an independent risk factor for incident fractures in osteoporotic patients treated with bisphosphonates, nutritional management of vitamin K appears to be essential to reduce the occurrence of fractures in postmenopausal osteoporotic women treated with bisphosphonates who have vitamin K deficiency [34]. It is speculated that bisphosphonate inhibits farnesyl pyrophosphate (FPP) synthase in the mevalonate pathway and may decrease the synthesized amount of GGPP which is a side-chain source of MK-4 biosynthesis from PK [35••]. Thus, both vitamin K intake and MK-4 biosynthesis are important for bone health.
In addition, MK-4 binds to the steroid and xenobiotic nuclear receptor (SXR) leading to an enhanced expression of several components of the bone matrix [36, 37]. Systemic SXR knockout mice displayed a marked reduction in bone mass through both bone formation decrease and bone resorption increase. These reports indicate that vitamin K is a transcriptional regulator of extracellular matrix-related genes, some of which are involved in collagen assembly.
Finally, MK-4 is considered as the most physiologically active form among vitamin K derivatives, and its biosynthesis by UBIAD1 is important in vivo. Vitamin K deficiency being a risk factor for fracture susceptibility, vitamin K should be ingested to maintain healthy bones as it will be converted by UBIAD1 into active MK-4 in the bone tissue.
GGCX Mutations Cause Ectopic Mineralization
In vitamin K-mediated mineralization, GGCX activity is a predominant factor in either bone mineralization or ectopic calcification. Specifically, GGCX activates Gla proteins by adding a carboxyl group to the gamma position of glutamine [3]. Gla proteins include vitamin K-dependent coagulation factors (factors II, VII, IX, and X), protein C, protein S, and protein Z [38], as well as bone component proteins, such as matrix Gla protein (MGP) [39], OC [40], periostin (POSTN) [41], and Gla-rich protein [42].
We reported a 55-year-old male with a novel homozygous deletion mutation, c.2,221delT, p.S741LfsX100, in the GGCX gene [43•]. In the study, histopathological examination revealed calcium deposits in elastic fibers and vessel walls (Fig. 3), and collagen accumulation in the mid-dermis. Calcification in vessel walls possibly leads to attenuation of vessel elasticity and microcirculation insufficiency, followed by collagen accumulation in response to hypoxic conditions [44]. Alternatively, the partial osteogenic properties of GGCX dermal fibroblasts, as evidenced in our previous study, might involve increased collagen synthesis. Furthermore, collagen accumulation could explain the difference in cutaneous phenotypes between GGCX syndrome (i.e., cutis laxa-like sagging skin) and PXE (e.g., yellow-white papules and plaques).
A biopsied specimen was stained with von Kossa. Massive calcium deposits as well as elastic fiber calcification were found (a), and in the deeper dermis, the internal elastic lamina and a part of arteriole walls were positive for calcification (b). The bar depicts 500 μm in (a) and 50 μm in (b). [43•]
Our studies of GGCX dermal fibroblasts demonstrated that mutated GGCX molecule was larger, but its expression level and intracellular distribution were indistinguishable from wild-type GGCX molecule [43•]. An immunostaining and ELISA assay showed increase in undercarboxylated matrix gamma-carboxyglutamic acid protein (ucMGP), a representative substrate of GGCX as well as a potent calcification inhibitor, indicated that mutated GGCX was enzymatically inactive. Under osteogenic conditions, calcium deposition was exclusively observed in GGCX dermal fibroblasts. Furthermore, GGCX dermal fibroblasts contained 23- and 7.7-fold more alkaline phosphatase (ALP)-positive cells than the control, without and with osteogenic induction, respectively. GGCX dermal fibroblasts produced more ALP, mRNA level and enzyme activity were higher than that in dermal fibroblasts from the controls (normal dermal fibroblasts) after osteogenic induction. Other osteogenic marker mRNA levels were also higher in GGCX dermal fibroblasts than in normal dermal fibroblasts, which include bone morphogenetic protein 6, runt-related transcription factor 2 (RUNX2), and POSTN without osteogenic induction; osterix (OSX), collagen type I alpha 2 (COL1A2), and POSTN with osteogenic induction. By a lack of GGCX activity in dermal fibroblasts, the upregulation osteogenic marker expressions accompanied, and calcification thereby progressed [43•]. These data suggests that dermal fibroblasts from GGCX syndrome may trans-differentiate into osteogenic mesenchymal cells, which could link to an ectopic calcification in the dermis.
Summary
Vitamin D and K were discovered as nutritional factors in food of plant origin and animal products. After absorbed from dietary supply, those activities are altered by various chemical reactions in the body. The biological function of 1,25(OH)2D3 depends on a strict control of activation process resulting in the expression of the hydroxylase enzymes involved in vitamin D metabolism. Needless to say, efficient absorption of calcium in the small intestine is a main target of vitamin D function to maintain calcium homeostasis. During mineralized tissue development, it has been postulated that vitamin D directly stimulates osteoblastic bone formation and mineralization; nevertheless, there was no definite evidence to support this concept. Therefore, it is considered paradoxical for the local action, but vitamin D suppresses bone mineralization due to osteoblasts function. Furthermore, the intermediate components of vitamin K cyclic conversion are essential for a function of the protein which is important to mineralization. In particularly, bone and soft tissue mineralization, vitamin K is classically known for its role as a cofactor of GGCX and activates proteins such as osteocalcin.
Thus, through these local actions of vitamin D and K, physiological and non-physiological mineralization is regulated by nutrient factor derived from dietary supply, and the systemic calcium metabolism and homeostasis are thereby affected. In the study on role of vitamins in mineralization process, setting of the optimums dietary requirement of these vitamins is important to maintain skeletal integrity. More importantly, concrete molecular mechanisms of these vitamins in the early stage of the development of the mineralization have begun to be established.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance, •• Of major importance
Steenbock H, Black A. Fat-soluble vitamins XXIII. The induction of growth-promoting and calcifying properties in fats and their unsaponifiable constituents by exposure to light. J Biol Chem. 1925;64:263–98.
Shearer MJ, Vitamin K. Vitamin K. Lancet. 1995;345:229–34.
Furie B, Bouchard BA, Furie BC. Vitamin K-dependent biosynthesis of gamma-carboxyglutamic acid. Blood. 1999;93(6):1798–808.
Zhu JG, et al. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc Natl Acad Sci U S A. 2013;10:15650–5.
DeLuca HF. History of the discovery of vitamin D and its active metabolites. Bonekey Rep. 2014;3:8–15.
Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–76.
•• Uekawa A, Yamanaka H, Lieben L, Kimira Y, Uehara M, Yamamoto Y, et al. Phosphate-dependent luminal ATP metabolism regulates transcellular calcium transport in intestinal epithelial cells. FASEB J. 2018. A recent review, which describes vitamin D-independent transcellular calcium absorption.;32:1903–15.
Masuyama R, Nakaya Y, Tanaka S, Tsurukami H, Nakamura T, Watanabe S, et al. Dietary phosphorus restriction reverses the impaired bone mineralization in vitamin D receptor knockout mice. Endocrinology. 2001;142:494–7.
Balsan S, et al. Long-term nocturnal calcium infusions can cure rickets and promote normal mineralization in hereditary resistance to 1,25-dihydroxyvitamin D. J Clin Invest. 1986;77:61–1667.
Xue Y, Fleet JC. Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice. Gastroenterology. 2009;136:1317–27. e1311-12
Takeda S, Yoshizawa T, Nagai Y, Yamato H, Fukumoto S, Sekline K, et al. Stimulation of osteoclast formation by 1,25-dihydroxyvitamin D requires its binding to vitamin D receptor (VDR) in osteoblastic cells: studies using VDR knockout mice. Endocrinology. 1999;140:1005–8.
Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, et al. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology. 1999;140:4982–7.
Masuyama R, Nakaya Y, Katsumata S, Kajita Y, Uehara M, Tanaka S, et al. Dietary calcium and phosphorus ratio regulates bone mineralization and turnover in vitamin D receptor knockout mice by affecting intestinal calcium and phosphorus absorption. J Bone Miner Res. 2003;18:1217–26.
Panda DK, Miao D, Bolivar I, Li J, Huo R, Hendy GN, et al. Inactivation of the 25-hydroxyvitamin D 1α-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem. 2004;279:16754–66.
Fraser WD. Hyperparathyroidism. Lancet. 2009;374:145–58.
Fu Q, Manolagas SC, O’Brien CA. Parathyroid hormone controls receptor activator of NF-κB ligand gene expression via a distant transcriptional enhancer. Mol Cell Biol. 2006;26:6453–68.
Kim S, Yamazaki M, Zella LA, Shevde NK, Pike JW. Activation of receptor activator of NF-κB ligand gene expression by 1,25-dihydroxyvitamin D3 is mediated through multiple long-range enhancers. Mol Cell Biol. 2006;26:6469–86.
Galli C, Zella LA, Fretz JA, Fu Q, Pike JW, Weinstein RS, et al. Targeted deletion of a distant transcriptional enhancer of the receptor activator of nuclear factor-kappaB ligand gene reduces bone remodeling and increases bone mass. Endocrinology. 2008;149:146–53.
Meyer MB, Goetsch PD, Pike JW. Genome-wide analysis of the VDR/RXR cistrome in osteoblast cells provides new mechanistic insight into the actions of the vitamin D hormone. J Steroid Biochem Mol Biol. 2010;121:136–41.
Lieben L, Masuyama R, Torrekens S, van Looveren R, Schrooten J, Baatsen P, et al. Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J Clin Invest. 2012;122:1803–15.
Lieben L, Carmeliet G. Vitamin D signaling in osteocytes: effects on bone and mineral homeostasis. Bone. 2013;54:237–43.
van Driel M, Koedam M, Buurman CJ, Roelse M, Weyts F, Chiba H, et al. Evidence that both 1alpha,25-dihydroxyvitamin D3 and 24-hydroxylated D3 enhance human osteoblast differentiation and mineralization. J Cell Biochem. 2006;99:922–35.
Zhou S, Glowacki J, Kim SW, Hahne J, Geng S, Mueller SM, et al. Clinical characteristics influence in vitro action of 1,25-dihydroxyvitamin D3 in human marrow stromal cells. J Bone Miner Res. 2012;27:1992–2000.
Gardiner EM, et al. Increased formation and decreased resorption of bone in mice with elevated vitamin D receptor in mature cells of the osteoblastic lineage. FASEB J. 2000;14:1908–16.
Vermeer C, Jie KS, Knapen MH. Role of vitamin K in bone metabolism. Annu Rev Nutr. 1995;15:1–22.
Morris DP, Stevens RD, Wright DJ, Stafford DW. Processive post-translational modification. Vitamin K-dependent carboxylation of a peptide substrate. J Biol Chem. 1995;270:30491–8.
Stafford DW. The vitamin K cycle. J Thromb Haemost. 2005;3(8):1873–8.
Shearer MJ, Newman P. Metabolism and cell biology of vitamin K. Thromb Haemost. 2008;100:530–47.
Okano T, Shimomura Y, Yamane M, Suhara Y, Kamao M, Sugiura M, et al. Conversion of phylloquinone (vitamin K1) into menaquinone-4 (vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem. 2008;283:11270–9.
Nakagawa K, Hirota Y, Sawada N, Yuge N, Watanabe M, Uchino Y, et al. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature. 2010;468:117–21.
Hirota Y, Tsugawa N, Nakagawa K, Suhara Y, Tanaka K, Uchino Y, et al. Menadione (vitamin K3) is a catabolic product of oral phylloquinone (vitamin K1) in the intestine and a circulating precursor of tissue menaquinone-4 (vitamin K2) in rats. J Biol Chem. 2013;288:33071–80.
Takada T, Yamanashi Y, Konishi K, Yamamoto T, Toyoda Y, Masuo Y, et al. NPC1L1 is a key regulator of intestinal vitamin K absorption and a modulator of warfarin therapy. Sci Transl Med. 2015;7(275):275ra23.
Tsugawa N, Shiraki M, Suhara Y, Kamao M, Ozaki R, Tanaka K, et al. Low plasma phylloquinone concentration is associated with high incidence of vertebral fracture in Japanese women. J Bone Miner Metab. 2008;26:79–85.
Iwamoto J, Takada T, Sato Y. Vitamin K nutritional status and undercarboxylated osteocalcin in postmenopausal osteoporotic women treated with bisphosphonates. Asia Pac J Clin Nutr. 2014;23(2):256–62.
•• Hirota Y, Nakagawa K, Sawada N, Okuda N, Suhara Y, Uchino Y, et al. Functional characterization of the vitamin K2 biosynthetic enzyme UBIAD1. PLoS One. 2015;10(4):e0125737. Authors demonstrated the enzymatic function of UBIAD1 family and clarified bioactive structure.
Tabb MM, Sun A, Zhou C, Grün F, Errandi J, Romero K, et al. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J Biol Chem. 2003;278:43919–27.
Ichikawa T, Horie-Inoue K, Ikeda K, Blumberg B, Inoue S. Steroid and xenobiotic receptor SXR mediates vitamin K2-activated transcription of extracellular matrix-related genes and collagen accumulation in osteoblastic cells. J Biol Chem. 2006;281:16927–34.
Rezaie AR, Bae JS, Manithody C, Qureshi SH, Yang L. Protein Z-dependent protease inhibitor binds to the C-terminal domain of protein Z. J Biol Chem. 2008;283(29):19922–6.
Price PA, Urist MR, Otawara Y. Matrix Gla protein, a new gamma-carboxyglutamic acid-containing protein which is associated with the organic matrix of bone. Biochem Biophys Res Commun. 1983;117(3):765–71.
Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69(3):990–1047.
Coutu DL, Wu JH, Monette A, Rivard GÉ, Blostein MD, Galipeau J. Periostin, a member of a novel family of vitamin K-dependent proteins, is expressed by mesenchymal stromal cells. J Biol Chem. 2008;283(26):17991–8001.
Viegas CS, et al. Gla-rich protein is a novel vitamin K-dependent protein present in serum that accumulates at sites of pathological calcifications. Am J Pathol. 2009;175(6):2288–98.
• Okubo Y, Masuyama R, Iwanaga A, Koike Y, Kuwatsuka Y, Ogi T, et al. Calcification in dermal fibroblasts from a patient with GGCX syndrome accompanied by upregulation of osteogenic molecules. PLoS One. 2017;12(5):e0177375. Authors demonstrated a role of GGCX in ectopic calcification in dermal fibroblasts.
Lokmic Z, et al. Hypoxia and hypoxia signaling in tissue repair and fibrosis. Int Rev Cell Mol Biol. 2012;296:139–85.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Oral Disease and Nutrition
Rights and permissions
About this article
Cite this article
Nakagawa, K., Okubo, Y. & Masuyama, R. Vitamin Status and Mineralized Tissue Development. Curr Oral Health Rep 5, 89–95 (2018). https://doi.org/10.1007/s40496-018-0174-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40496-018-0174-2