Abstract
Purpose of Review
Osteoblasts are responsible for bone matrix production during bone development and homeostasis. Much is known about the transcriptional regulation and signaling pathways governing osteoblast differentiation. However, less is known about how osteoblasts obtain or utilize nutrients to fulfill the energetic demands associated with osteoblast differentiation and bone matrix synthesis. The goal of this review is to highlight and discuss what is known about the role and regulation of bioenergetic metabolism in osteoblasts with a focus on more recent studies.
Recent Findings
Bioenergetic metabolism has emerged as an important regulatory node in osteoblasts. Recent studies have begun to identify the major nutrients and bioenergetic pathways favored by osteoblasts as well as their regulation during differentiation. Here, we highlight how osteoblasts obtain and metabolize glucose, amino acids, and fatty acids to provide energy and other metabolic intermediates. In addition, we highlight the signals that regulate nutrient uptake and metabolism and focus on how energetic metabolism promotes osteoblast differentiation.
Summary
Bioenergetic metabolism provides energy and other metabolites that are critical for osteoblast differentiation and activity. This knowledge contributes to a more comprehensive understanding of osteoblast biology and may inform novel strategies to modulate osteoblast differentiation and bone anabolism in patients with bone disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skeletal development and postnatal homeostasis depend upon the proper differentiation and activity of bone forming osteoblasts. Osteoblasts differentiate from multipotent mesenchymal progenitor cells. These progenitors are initially proliferative before undergoing terminal differentiation into postmitotic matrix synthesizing osteoblasts. The process of differentiation occurs in a well-defined temporal sequence that is regulated by the transcription factors RUNX2 and OSX. RUNX2 is essential for committing to the osteoblast lineage. OSX functions downstream of RUNX2 to regulate osteoblast differentiation and the induction of osteoblast genes like Ibsp and Bglap. Another transcription factor, ATF4, is important for terminal differentiation and to regulate amino acid consumption and bone matrix production [1, 2]. In a general sense, osteoblasts are secretory cells responsible for producing and secreting collagen type I (COL1A1) and other extracellular proteins that comprise bone matrix. Defects in osteoblast generation or activity leads to reduced bone formation, decreased bone mass, and increased fracture risk. Elucidating the mechanisms that regulate their differentiation and bone forming activity will be critical to design effective bone anabolic therapeutics. Cellular bioenergetics is an area that has been under intense scrutiny in the bone field. Recent studies have begun to elucidate the bioenergetic substrates and pathways utilized by osteoblasts and generate mechanistic insights into how bioenergetic metabolism influences osteoblast differentiation. In this review, we summarize what is known about bioenergetic metabolism and its role in osteoblast differentiation and bone formation with an emphasis on more recent studies.
General Overview of Bioenergetic Metabolism
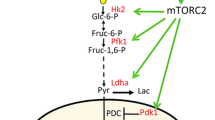
Osteoblast differentiation and bone formation are energetically demanding processes due in part to high rates of macromolecule (e.g., protein, DNA, and RNA) biosynthesis associated with proliferation and matrix production. Because of this, recent studies have focused on identifying the fuel sources and metabolic pathways utilized by osteoblasts to generate adenosine 5′triphosphate (ATP). The central hub of energetic metabolism in cells is the tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS) (Fig. 1). Osteoblasts metabolize glucose, fatty acids, and amino acids in a network of interconnected metabolic pathways including glycolysis, β-oxidation, and amino acid metabolism which produce intermediate metabolites that can enter the TCA cycle through a process known as anaplerosis. Individual TCA metabolites can also exit the TCA cycle via cataplerosis. Some intermediates, like malate and oxaloacetate (OAA), function to transfer electrons across the NADH impermeable mitochondrial membrane through the malate aspartate shuttle [3, 4•]. Other intermediates are important for fatty acid (e.g., citrate) and amino acid biosynthesis (e.g., α-ketoglutarate (αKG), OAA, fumarate, and succinate) while αKG also functions as an important cofactor for many different enzymes including prolyl hydroxylases, Jumonji domain containing histone demethylases and TET family of DNA demethylases [5,6,7]. Substrate oxidation can generate ATP either directly or indirectly by generating reducing equivalents in the form of nicotinamide adenine dinucleotide (NADH) and flavine adenine dinucleotide (FADH2). For example, one round of the TCA cycle produces one ATP, three NADH, and one FADH2. NADH and FADH2 subsequently transfer electrons to the electron transport chain (ETC) which consists of four enzyme complexes that function to transfer electrons to oxygen and create a proton gradient that ATPase uses for ATP synthesis (Fig. 1). In addition to energy production, the ETC has many other functions including regulating aspartate biosynthesis, proliferation, redox homeostasis, and generating reactive oxygen species (ROS) (Fig. 1) [8, 9, 10•, 11]. Thus, bioenergetic metabolism may impact osteoblast differentiation in more ways than by simply providing ATP.
Energetic metabolism in osteoblasts. Schematic depiction of the major metabolic pathways in osteoblasts including glycolysis (blue), TCA cycle (green), fatty acid metabolism (brown), and glutamine metabolism (purple). Major signaling pathways (black) that regulate metabolism in osteoblasts are highlighted. GLUT1, glucose transporter 1; HK, hexokinase; G6P, glucose-6-phosphate; 3PG, 3-phosphoglycerate; LDHA, lactate dehydrogenase A; MCT1, monocarboxylate transporter 1; SHMT, serine hydroxymethyltransferase; PDK, pyruvate dehydrogenase kinase 1; AMPK, AMP-activated protein kinase; Hif1a, hypoxia inducible factor 1a; PTH, parathyroid hormone; PTH1R, parathyroid hormone 1 receptor; IGF1, insulin-like growth factor 1; IGF1R, insulin-like growth factor 1 receptor; Lrp5/6, low-density lipoprotein receptor–related protein 5 or 6; Fzd, frizzled; mTORC1/2, mechanistic target of rapamycin complex 1/2; CPT1/2, carnitine palmitoyltransferase 1/2; TCA, tricarboxylic acid; α-KG, α-ketoglutarate; OAA, oxaloacetate; Asp, aspartate; MAS, malate aspartate shuttle; ETC, electron transport chain; Cyt c, cytochrome c; ROS, reactive oxygen species; AMP, adenosine monophosphate; ATP, adenosine triphosphate; NAD, nicotinamide adenine dinucleotide; FAD, flavin adenine dinucleotide
Osteoblast differentiation is associated with rapid shifts in bioenergetic metabolism. Differentiating osteoblasts are characterized by an abundance of high transmembrane potential mitochondria [12]. Consistent with this, mitochondrial oxygen consumption rapidly increases during osteoblast differentiation [10•, 13•, 14•]. Multiple studies have shown this is due in part to increased mitochondrial biogenesis. For example, one recent study described increased expression of all mitochondrial ETC complexes while another found complexes III, IV, and V waned over time [10•]. Consistent with increased mitochondrial biogenesis and activity, ATP levels increase during differentiation, although this has been attributed more to increased aerobic glycolysis than OXPHOS [10•, 12, 13•]. Regardless, mitochondrial activity is essential for osteoblast differentiation. Mice lacking the mtDNA polymerase gamma (POLγ) have accelerated bone loss due to reduced osteoblast differentiation and activity that is attributed to mitochondrial dysfunction [15•]. Pharmacological inhibition of mitochondrial biogenesis using tigecycline inhibited osteoblast differentiation without affecting viability in vitro [14•]. Similarly, reducing OXPHOS by targeting the nuclear receptor PPARδ inhibited osteoblast differentiation in vitro and bone formation in vivo [14•]. Multiple groups have inhibited mitochondrial complex I activity using Rotenone and shown this prevents osteoblast differentiation and matrix mineralization in vitro [10•, 14•]. Interestingly, the mitochondrial complex II inhibitor thenoyltrifluoroacetone had no effect on osteoblast differentiation suggesting complex II is dispensable for osteoblast differentiation [10•]. It is interesting to note complex II is thought to maintain coenzyme Q10 in a reduced state to protect mitochondrial lipids and proteins from oxidative damage as complex II does not pump protons across the inner mitochondrial membrane unlike complexes I and III [16]. In addition to energetics, mitochondria may influence epigenetic modifications and cell signaling through the production of cataplerotic citrate. Cytosolic citrate is converted by ATP citrate lyase (ACLY) into acetyl-CoA that can used to acetylate histones and other proteins [17]. In ST2 cells, WNT alters glucose flux away from the TCA cycle ultimately limiting citrate availability. This results in decreased histone acetylation and epigenetic inhibition of alternative cell fates [18]. In C3H10T1/2 cells, WNT stimulates mitochondrial biogenesis [19] which is associated with β-catenin acetylation and activity [20•]. Thus, mitochondria exert a multifaceted influence on osteoblast differentiation by regulating both energetics and the availability of substrates for protein and DNA modifications.
On the other hand, electron leakage from the mitochondrial ETC is the principal generator of reactive oxygen species (ROS) in mammalian cells [21, 22]. ROS include the superoxide anion (O2–), hydrogen peroxide (H2O2), and hydroxyl radicals (OH), all of which have inherent chemical properties to damage lipids, proteins, and DNA, a process termed as oxidative stress [23]. Generally, a leaking electron can be captured by O2, forming superoxide anion (O2−), which is rapidly converted to hydrogen peroxide (H2O2) by superoxide dismutase (SODs). If mitochondrial ROS exceeds a certain threshold, this will impair OXPHOS complexes and further stimulate ROS production [24]. Due to increased mitochondrial activity, both proliferating osteoblast progenitors and mature osteoblasts have increased ROS production [10•, 25]. ROS are detrimental to osteoblast differentiation and must be well controlled. SOD2 is essential to control mitochondrial ROS, promote mitochondrial biogenesis, mitochondrial function, and osteoblast differentiation [10•]. Importantly, mice lacking SOD2 specifically in osteocytes had increased cellular superoxide and accelerated age-dependent bone loss due to decreased osteoblast activity and increased osteoclastogenesis [26] (Table 1). Excessive ROS can result in mitochondrial dysfunction and activation of the mitochondrial permeability transition pore (MPTP), mitochondrial swelling and decreased ATP production. This is associated with age-related bone disease. Inhibiting MPTP opening through genetic deletion of cyclophilin D (CypD) protects mitochondrial function and promotes both osteoblast differentiation in vitro and bone anabolism in vivo [27, 28•] (Table 1). The precise regulation of osteoblast differentiation by ROS is muddled. ROS can oxidize the cysteine and methionine residues within proteins causing reversible and/or irreversible conformational changes [29, 30]. ROS has been shown to limit the DNA binding ability of RUNX1 (a close homologue to RUNX2) through cysteine and methionine oxidation [31]. Other studies found ROS inhibited osteoblast differentiation by reducing RUNX2 expression and activity either through transcriptional repression [32] or secondarily by affecting DNA binding and transactivation of target promoters downstream of Nrf2 [33]. Alternatively, H2O2 can activate AMPK directly through oxidative modifications (including Cys–SOH and S-glutathionylation) and subsequent conformational change of the AMPK-α subunit although this may be an indirect consequence of REDOX changes [34, 35]. Nevertheless, given that AMPK plays a critical role regulating Runx2 stability, it is likely that ROS can indirectly regulate Runx2 and osteoblast differentiation downstream of AMPK [36].
Glycolysis
Glucose is a major energy source for most mammalian cells including osteoblasts. Osteoblasts in vitro and bones in vivo are characterized by robust glucose consumption [36, 37, 38] which has been shown to rapidly increase in response to osteogenic stimuli like WNT, IGF, and PTH [4•, 39, 40]. Glucose is transported into the cell by the GLUT family of glucose transporters of which osteoblasts express Glut1, Glut3, and Glut4 [36, 41, 42]. GLUT1 is the major glucose transporter in osteoblasts as genetic studies in mice show GLUT1 is essential for osteoblast differentiation and required for the bone anabolic effects of WNT7b [36, 43•] (Table 1). Mechanistically, GLUT1 provides glucose to regulate RUNX2 protein expression by maintaining ATP levels to limit AMPK activation and preventing RUNX2 ubiquitination and degradation. RUNX2 potentiates further glucose uptake and bone matrix production by increasing GLUT1 expression [36]. This highlights the exquisite sensitivity of RUNX2 and the dependence of osteoblast differentiation on glucose uptake and ATP availability.
Glucose is metabolized in the cytoplasm by glycolysis [44]. Complete metabolism of glucose via glycolysis yields two pyruvate molecules, two ATP, and two reducing equivalents in the form of NADH. Pyruvate can be converted to either lactate, used for alanine biosynthesis or further oxidized in the TCA cycle. The reduction of pyruvate into lactate is catalyzed by the enzyme lactate dehydrogenase (LDH) dependent on the oxidation of NADH. It is thought the regeneration of oxidized NAD+ is essential for further glycolysis. Alternatively, the enzyme pyruvate dehydrogenase (PDH) decarboxylates pyruvate to form acetyl-CoA, which can subsequently enter the TCA cycle. Glycolysis occurs in two stages, the first of which consumes two ATP and ultimately generates fructose 1,6-bisphosphate (F1,6BP), which is cleaved to form two interconvertible three carbon molecules glyceraldehyde 3-phosphate (G3P) and dihydroxyacetone phosphate (DHAP). In the second stage of glycolysis, each G3P is eventually converted to pyruvate, producing two ATP in the process. It is important to note that individual glycolytic intermediates can feed into other metabolic pathways. For example, G6P is metabolized in the pentose phosphate pathway (PPP) to generate ribose 5-phosphate, the backbone of nucleic acids, and the reducing equivalent nicotinamide adenine dinucleotide phosphate (NADPH). NADPH is essential for anabolic reactions and to reduce oxidized glutathione (GSH) and thioredoxin (TRX) to neutralize ROS [45]. 3-phosphoglycerate (3PG) feeds into the serine biosynthetic pathway which produces NADPH and serine which can subsequently be converted to glycine, an important constituent of both collagen and GSH. The serine biosynthetic pathway is also important for one-carbon metabolism to generate methyl groups for the methylation of DNA and histones [46]. Thus, in addition to ATP production, glycolysis produces several intermediate metabolites that contribute to various biosynthetic pathways and regulate diverse cellular processes predicted to be important for osteoblast differentiation.
Osteoblasts primarily metabolize glucose into lactate both in vitro and in vivo [4•, 39, 40]. Recent metabolic tracing studies estimate that up to 80% of glucose is converted to lactate in mature osteoblasts even in the presence of abundant oxygen [40]. This phenomenon is also known as the Warburg effect or aerobic glycolysis [47]. Consistent with high lactate production, approximately 40% of ATP in immature osteoblasts and almost 80% of ATP in mature osteoblasts is estimated to be generated via glycolysis [13•, 48•, 49•]. Lactate itself can be converted to pyruvate by LDHB and undergoes oxidative metabolism to induces osteoblast differentiation in part by stabilizing HIF1a [50]. It is not entirely clear why lactate production is preferred compared to pyruvate oxidation in the TCA cycle. OXPHOS produces more than 15 times more ATP than glycolysis; however, glycolysis produces ATP at a rate almost 100 times faster that OXPHOS [51, 52]. One possibility is that increasing lactate production limits OXPHOS-dependent ROS generation and oxidative stress. Lactate production also regenerates NAD+ which facilitates glycolysis and rapid ATP production independent of oxygen availability. This in turn would allow osteoblasts to rapidly utilize available glucose and provide more glycolytic intermediates for biosynthetic reactions [40, 53, 54]. Along those lines, mitochondrial malic enzyme (ME), which converts pyruvate to malate and produces NAD+ via the malate aspartate shuttle, was shown to be essential for glycolysis in cultured osteoblasts [4•]. This suggests an important function of the mitochondria is to sustain glycolysis in osteoblasts.
Glycolysis is directly regulated by many osteogenic signals and is essential for osteoblast differentiation and bone anabolism. For example, genetic stabilization of HIF1α in osteoblasts stimulates glycolysis which is essential for increased bone formation in this model [55]. Conversely, the NOTCH pathway inhibits osteoblast differentiation in part by suppressing the expression of multiple glycolytic enzymes and thus limiting glycolysis (Table 1). Similarly, MiR-34a, a negative regulator of osteoblast differentiation, reduces glucose uptake and glycolysis by targeting GLUT1, HK2, and LDHA to prevent osteoblast differentiation in human MSC [56•]. Importantly, reintroduction of LDHA could overcome the inhibition of osteoblast differentiation by miR-34a [56•]. Conversely, the osteogenic signals WNT and PTH both increase HK2, PDK1, and LDHA expression to stimulate glycolysis during osteoblast differentiation albeit via distinct mechanisms [39, 40]. WNT activates mTORC2 directly whereas PTH acts indirectly through IGF and mTORC2 to induce glycolytic enzyme expression [39, 40]. More recent studies have identified other signals that regulate glycolysis including the myokine irisin and nitric oxide (NO) [57, 58•]. These studies underscore how glycolysis is an essential feature of differentiating osteoblasts and must be tightly regulated for osteoblast differentiation and bone formation. Highlighting the necessity of glycolytic upregulation, numerous studies have demonstrated that pharmacological inhibition of glycolysis using either the PDK inhibitor dichloroacetate (DCA) or the PFK3B inhibitor 3PO reduces bone anabolism in numerous and diverse genetic mouse models of high bone mass [39, 55, 59•] (Table 1). Conversely, treating mice with the LDHA inhibitor oxamate increased bone formation and improved bone strength with more pronounced effect in aged mice [60•]. It is not clear if this discrepancy is due to disparate requirements for glycolysis during physiological and pathological bone formation, aging effects, or off target inhibition of aspartate aminotransaminase [61]. It is likely due to mitochondrial compensation as oxamate stimulated OXPHOS in vitro [60•], whereas inhibiting glycolysis using DCA or 3PO should limit pyruvate production and its subsequent oxidation. It will be important to evaluate the malate aspartate shuttle and glycolysis directly in this model.
Amino Acid Metabolism
Amino acids are another potential energy source in osteoblasts. In addition to being critical for protein synthesis, amino acids can be metabolized to generate ATP either directly or indirectly. For example, proline oxidation occurs in the inner mitochondrial membrane to form pyrroline-5-carboxylate (P5C) by proline dehydrogenase (PRODH). Proline oxidation by PRODH generates FADH2 which donates electrons to complex II of the ETC coupling proline oxidation to ATP synthesis [62]. In comparison, other amino acids can be metabolized to produce anapleurotic TCA substrates. These amino acids are categorized as either ketogenic, glucogenic, or both. Ketogenic amino acids are metabolized to form acetyl-CoA, whereas glucogenic amino acids metabolized into either pyruvate, OAA, αKG, fumarate, or succinyl-CoA. Amino acid metabolism through the TCA cycle has recently been shown to be critical for various cellular processes including proliferation, differentiation, and protein synthesis [9, 63, 64, 65•, 66•, 67•, 68•].
Relatively little is known about the direct contribution of amino acids to osteoblast energetics. Most recent studies have focused on the amino acid glutamine. Multiple studies have shown that glutamine uptake and metabolism increase during osteoblast differentiation [65•, 66•, 67•, 68•]. Glutamine uptake in osteoblasts is mediated primarily by the transporter SLC1A5, which also transports asparagine [67•, 68•]. SLC1A5 expression increases during differentiation and is regulated by both WNT and PTH as well as the transcription factor ATF4 [66•, 67•, 69]. In addition to SLC1A5, WNT increases glutamine uptake through SLC7A7 in a β-catenin-dependent manner [67•]. Once inside the cell, glutamine metabolism is initiated by the enzyme glutaminase (GLS) which deaminates glutamine to form glutamate which can be further deaminated to produce αKG. Genetic ablation of GLS at different stages of osteoblast differentiation uniformly results in decreased bone mass and prevents PTH-induced bone formation due to decreased osteoblast differentiation and bone formation [65•, 66•, 70] (Table 1). Tracing studies performed in both bone marrow stromal cells (BMSC) and calvarial osteoblasts (cOB) found that glutamine carbon is enriched in αKG and other TCA cycle intermediates [65•, 66•, 68•]. Moreover, glutamine metabolism is essential to maintain αKG levels in osteoblasts and exogenous αKG can rescue proliferation in bone marrow stromal cells (BMSC) deprived of glutamine [65•] and proliferation and osteoblast differentiation in committed osteoblast precursors lacking GLS [66•]. One recent study found that αKG supplementation in drinking water attenuated age-associated bone loss and rejuvenates BMSCs [71]. This phenotype was attributed to epigenetic changes although bioenergetics were not evaluated in this model. Thus, glutamine metabolism likely contributes directly to OXPHOS in osteoblasts. Consistent with this, in ST2 cells, WNT increases both GLS expression and the flux of glutamine into the TCA cycle to fulfill the energetic demands caused by WNT induced protein synthesis [70]. However, one recent study found that GLS inhibition did not affect OXPHOS in calvarial osteoblasts indicating glutamine metabolism does not contribute substantially to osteoblast energetics [4•]. It is important to note that GLS inhibition did not affect osteoblast differentiation in this study. Further studies into the role of glutamine metabolism in osteoblast energetics are warranted.
Amino acid metabolism may regulate osteoblast energetics and differentiation secondarily. For example, arginine is catabolized by NOS to generate NO which activates glycolysis [58•]. αKG can be generated from glutamate by transamination in the malate aspartate shuttle. Our data indicates that transamination is a major route of αKG formation in osteoblasts ([65•], unpublished). Here, the transaminases GOT1 or GOT2 produce aspartate and αKG using oxaloacetate and glutamate [3]. Highlighting the importance of this reaction, inhibiting transaminase activity using AOA reduces proliferation in BMSC [65•, 68•]. Likewise, glutamine-derived aspartate synthesis is essential for chondrocyte proliferation [72•]. Aspartate biosynthesis occurs in the malate aspartate shuttle. Thus, limiting glutamine uptake and/or metabolism may impact osteoblast bioenergetics by limiting the malate aspartate shuttle. In this instance, limiting glutamine availability or metabolism would reduce glutamine-derived glutamate (and αKG) which would impede the malate aspartate shuttle and limit glycolysis. Consistent with this hypothesis, inhibiting transaminase activity reduces glucose uptake in osteoblasts [4•]. Alternatively, aspartate, glutamine, and glycine are all essential for the biosynthesis of nucleotides like adenine which is the precursor of ATP.
In addition to αKG and other TCA intermediates, tracing studies found that glutamine carbon is enriched in many downstream metabolites including GSH and NEAA ([65•, 66•] and unpublished). Glutamine-derived NEAA are essential for protein and matrix biosynthesis and osteoblast differentiation [66•]. In addition, NEAA may also contribute to energetics. For example, arginine biosynthesis is essential for osteoblast differentiation and was recently linked to glycolysis. Here, the enzyme argininosuccinate lyase (ASL) provides arginine which is then catabolized by NOS to provide NO and regulate glycolysis [58•]. Mechanistically, it is not clear how NO regulates glycolysis although it is likely through S-nitrosylation of glycolytic proteins [58•]. On the other hand, GSH is critical to neutralize ROS and regulate osteoblast viability [66•, 68•]. GSH levels increase during osteoblast differentiation in BMSC and were found to be significantly higher in committed osteoblast progenitors compared to committed adipocyte progenitors [49•, 73]. HIF1α regulates GLS expression in periosteal osteoblasts and enhances glutamine-derived GSH production [74]. This is essential for periosteal cell survival which is critical for bone healing in a critical size defect model [66•, 74] (Table 1). Mechanistically, glutamine-derived GSH may function to neutralize ROS generated by OXPHOS to prevent oxidative stress and promote mitochondrial activity.
β-oxidation of Lipids
Lipids are another energy-rich carbon source in osteoblasts estimated to provide between 40 and 80% of the energy provided by glucose [75]. Lipids can be metabolized to produce energy in a multistep process known as β-oxidation. β-oxidation occurs in the mitochondrial matrix and ultimately results in the formation of acetyl-CoA. Fatty acids are first transported into the mitochondria by the carnitine shuttle. Here, carnitine palmitoyltransferase 1 (CPT1) generates acyl-carnitine which is shuttled inside the mitochondria in exchange for carnitine. Acyl-carnitine is converted to acyl-CoA on the inner mitochondrial membrane by CPT2. Acyl-CoA then undergoes β-oxidation in the mitochondrial matrix where two carbons are sequentially cleaved off as acetyl-CoA. Even chain fatty acids can be completely oxidized into acetyl-CoA, whereas odd chain fatty acids ultimately form acetyl-CoA and succinyl-CoA. Complete oxidation of lipids by β-oxidation yields the most ATP per molecule compared to glucose or amino acids (Table 1).
Compared to glucose, investigation into fatty acid metabolism in osteoblasts has received less attention. A few elegant studies have demonstrated that bone is a major site of postprandial lipid uptake, and that fatty acid oxidation can contribute to ATP production [75, 76, 77•]. Consistent with this, inhibiting β-oxidation using etomoxir, an inhibitor of CPT1, reduces the oxygen consumption rate (OCR) in mouse BMSC and [14C]-oleate oxidation to 14CO2 in calvarial osteoblasts [78, 79]. Cpt1 mRNA expression increases during differentiation and may be directly regulated by PPARδ [14•]. Consistent with these mRNA changes, fatty acid uptake and β-oxidation increase during osteoblast differentiation although the dynamics of these changes are not entirely clear. Studies of calvarial osteoblasts found free fatty acid uptake increases transiently, whereas [14C]-oleate oxidation to 14CO2 increased nearly 300% during differentiation [4•, 79]. By comparison, bone marrow stromal cells (BMSC) have higher reliance on β-oxidation at earlier stages of differentiation as determined by OCR. This discrepancy likely reflects the different developmental stage and/or origin of BMSC and calvarial cells respectively as well as the disparate methods utilized.
Recent studies have highlighted the essential nature of fatty acids for osteoblast differentiation and bone formation. Fatty acid availability is crucial for osteoblast specification and differentiation. When fatty acids are replete mesenchymal progenitor cells differentiate into osteoblasts. Conversely when fatty acids are absent, mesenchymal progenitor cells increase SOX9 expression and undergo chondrocyte rather than osteoblast differentiation [80•]. This likely reflects the distinct metabolic state of chondrocytes which are highly glycolytic and are characterized by low expression of fatty acid oxidation genes compared to osteoblasts [6, 80•].
Osteoblasts also are characterized by the presence of lipid droplets both in vitro and in vivo following steroid treatment, excess alcohol consumption, and aging [78, 81,82,83,84]. The purpose of these lipid droplets in osteoblasts is unclear, however inhibiting lipid droplet synthesis using triacsin C prevented osteoblast differentiation in vitro [78]. Lipid droplets may function to store excess lipids for later metabolism to fuel differentiation and bone formation. Alternatively, lipid droplets may function to protect membranes from peroxidation reactions due to excessive ROS [85].
The importance of fatty acid oxidation for osteoblast differentiation is clear as etomoxir prevents osteoblast differentiation in vitro [79]. Similarly, knocking down Cpt1a in periosteal derived cells prevents osteoblast differentiation and bone healing in a fracture model [80•] (Table 1). Interestingly, genetic ablation of CPT2 in mature osteoblasts resulted in a sexually dimorphic bone phenotype with a transient decrease in bone volume in males compared to females which had decreased bone mass at all ages [77•] (Table 1). This appears to result from effects of estrogen as CPT2 mutant osteoblasts increased glucose consumption and glycolysis in the absence of exogenous estrogen [77•]. This demonstrates the plasticity of osteoblast nutrient selection is greater in male mice due to less hormonal regulation.
Like glycolysis, multiple osteogenic signaling pathways regulate fatty acid metabolism in the bone. Early studies in osteoblast cultures found 1,25(OH)2D3 and insulin either stimulated or reduced fatty acid oxidation while PTH had no effect [75]. However, PTH may influence fatty acid availability by inducing lipolysis in adipocytes which generates fatty acids that can be transferred to BMSC in vitro [86•]. Recent studies have focused on regulation by the WNT signaling pathway specifically through the coreceptor Lrp5 [79]. LRP5 activity is both necessary and sufficient to regulate the expression of β-oxidation genes and fatty acid oxidation in vitro [79]. Mechanistically, the canonical WNT/β-catenin pathway directly stimulates fatty acid oxidation in osteoblasts [79]. It is interesting that WNT rapidly stimulates both glycolysis and β-oxidation albeit through distinct pathways (mTOR and β-catenin respectively). This may reflect a need for an additional source of anapleurotic acetyl-CoA (from β-oxidation) to sustain the TCA cycle in osteoblasts.
Summary
In this review, we have highlighted the recent advances in our understanding of bioenergetic metabolism in osteoblasts. However, we remain a long way from a complete understanding of the molecular and cellular function of bioenergetics in osteoblasts during differentiation, bone formation and in disease. With this in mind, many outstanding questions remain. Is bioenergetic metabolism involved in bone and mineral pathologies (e.g., vascular calcification, aging, and diabetes related bone loss or ectopic ossification) and is this important for disease progression? Can metabolism be exploited to modulate osteoblast differentiation or bone anabolism? A wealth of knowledge about cellular metabolism has been gleaned from studies of cancer cells and diabetes which have resulted in novel metabolic therapies [87,88,89,90]. Perhaps these metabolic strategies can be repurposed to reduce excessive bone formation or pathological mineralization in humans. On the other hand, enhancing metabolism could stimulate osteoblast differentiation or activity and be used to promote bone formation in patients with osteopenia or osteoporosis. Indeed, the two primary bone anabolic agents, teriparatide, and romosozumab activate pathways that induce bone anabolism in part through metabolic regulation [39, 66•, 91]. Thus, pharmaceutical or dietary strategies to enhance osteoblast metabolism may be useful to stimulate bone anabolism in the elderly or in patients with low bone mass disorders.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry syndrome. Cell. 2004;117(3):387–98. https://doi.org/10.1016/s0092-8674(04)00344-7.
Elefteriou F, Benson MD, Sowa H, Starbuck M, Liu X, Ron D, et al. ATF4 mediation of NF1 functions in osteoblast reveals a nutritional basis for congenital skeletal dysplasiae. Cell Metab. 2006;4(6):441–51. https://doi.org/10.1016/j.cmet.2006.10.010.
Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nature Reviews Cancer. 2016;16(10):619–34. https://doi.org/10.1038/nrc.2016.71.
• Lee W-C, Ji X, Nissim I, Long F. Malic enzyme couples mitochondria with aerobic glycolysis in osteoblasts. Cell Reports. 2020;32(10):108108. https://doi.org/10.1016/j.celrep.2020.108108This study demonstrates that increased expression of malic enzyme ME2 promotes glycolytic flux into malate aspartate shuttle during osteoblast differentiation. Malate aspartate shuttle replenishes NAD+ to further facilitate glycolysis.
Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518(7539):413–6. https://doi.org/10.1038/nature13981.
Stegen S, Laperre K, Eelen G, Rinaldi G, Fraisl P, Torrekens S, et al. HIF-1α metabolically controls collagen synthesis and modification in chondrocytes. Nature. 2019;565(7740):511–5. https://doi.org/10.1038/s41586-019-0874-3.
D'Aniello C, Cermola F, Palamidessi A, Wanderlingh LG, Gagliardi M, Migliaccio A, et al. Collagen Prolyl Hydroxylation–Dependent Metabolic Perturbation Governs Epigenetic Remodeling and Mesenchymal Transition in Pluripotent and Cancer Cells. Cancer Research. 2019;79(13):3235–50. https://doi.org/10.1158/0008-5472.Can-18-2070.
Birsoy K, Wang T, Chen WW, Freinkman E, Abu-Remaileh M, Sabatini DM. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell. 2015;162(3):540–51. https://doi.org/10.1016/j.cell.2015.07.016.
Sullivan LB, Gui DY, Hosios AM, Bush LN, Freinkman E, Vander Heiden MG. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell. 2015;162(3):552–63. https://doi.org/10.1016/j.cell.2015.07.017.
• Gao J, Feng Z, Wang X, Zeng M, Liu J, Han S, et al. SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Differ. 2018;25(2):229–40. https://doi.org/10.1038/cdd.2017.144This study shows that mitochondrial activity increases along with elevated oxidative stress during osteoblast differentiation. In response, SIRT3 enhances SOD2 activity to mitigate the oxidative stress to support osteoblast differentiation.
Nagano T, Nakashima A, Onishi K, Kawai K, Awai Y, Kinugasa M, et al. Proline dehydrogenase promotes senescence through the generation of reactive oxygen species. J Cell Sci. 2017;130(8):1413–20. https://doi.org/10.1242/jcs.196469.
Komarova SV, Ataullakhanov FI, Globus RK. Bioenergetics and mitochondrial transmembrane potential during differentiation of cultured osteoblasts. Am J Physiol Cell Physiol. 2000;279(4):C1220–9. https://doi.org/10.1152/ajpcell.2000.279.4.C1220.
• Guntur AR, Gerencser AA, Le PT, DeMambro VE, Bornstein SA, Mookerjee SA, et al. Osteoblast-like MC3T3-E1 cells prefer glycolysis for ATP production but adipocyte-like 3T3-L1 cells prefer oxidative phosphorylation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2018;33(6):1052–65. https://doi.org/10.1002/jbmr.3390This study shows that differentiated osteoblasts preferentially rely on glycolysis for ATP production, while differentiated adipocytes mainly use oxidative phosphorylation.
• Müller DIH, Stoll C, Palumbo-Zerr K, Böhm C, Krishnacoumar B, Ipseiz N, et al. PPARδ-mediated mitochondrial rewiring of osteoblasts determines bone mass. Scientific Reports. 2020;10(1):8428. https://doi.org/10.1038/s41598-020-65305-5This study shows that PPARδ is required for the increased oxidative phosphorylation during osteoblast differentiation. Deletion of PPARδ impairs osteoblast differentiation and reduces bone mass.
• Dobson PF, Dennis EP, Hipps D, Reeve A, Laude A, Bradshaw C, et al. Mitochondrial dysfunction impairs osteogenesis, increases osteoclast activity, and accelerates age related bone loss. Scientific reports. 2020;10(1):11643. https://doi.org/10.1038/s41598-020-68566-2This article demonstrates that mitochondrial dysfunction caused by mutations in mitochondrial DNA polymerase results in bone loss due to reduced osteoblast differentiation and increased osteoclast activity.
Wojtovich AP, Smith CO, Haynes CM, Nehrke KW, Brookes PS. Physiological consequences of complex II inhibition for aging, disease, and the mKATP channel. Biochim Biophys Acta. 2013;1827(5):598–611. https://doi.org/10.1016/j.bbabio.2012.12.007.
Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324(5930):1076–80. https://doi.org/10.1126/science.1164097.
Karner CM, Esen E, Chen J, Hsu FF, Turk J, Long F. Wnt protein signaling reduces nuclear Acetyl-CoA levels to suppress gene expression during osteoblast differentiation. J Biol Chem. 2016;291(25):13028–39. https://doi.org/10.1074/jbc.M115.708578.
An JH, Yang JY, Ahn BY, Cho SW, Jung JY, Cho HY, et al. Enhanced mitochondrial biogenesis contributes to Wnt induced osteoblastic differentiation of C3H10T1/2 cells. Bone. 2010;47(1):140–50. https://doi.org/10.1016/j.bone.2010.04.593.
• Shares BH, Busch M, White N, Shum L, Eliseev RA. Active mitochondria support osteogenic differentiation by stimulating β-catenin acetylation. J Biol Chem. 2018;293(41):16019–27. https://doi.org/10.1074/jbc.RA118.004102This study shows that stimulating OXPHOS stabilizes β-catenin through acetylation and in turn promotes osteoblast differentiation. This paper highlights metabolic alterations can directly interact with osteogenic signaling pathways.
Shi X, Zhang Y, Zheng J, Pan J. Reactive oxygen species in cancer stem cells. Antioxid Redox Signal. 2012;16(11):1215–28. https://doi.org/10.1089/ars.2012.4529.
Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol. 2011;7(8):504–11. https://doi.org/10.1038/nchembio.607.
Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–62. https://doi.org/10.1016/j.cub.2014.03.034.
Galloway CA, Yoon Y. Perspectives on: SGP symposium on mitochondrial physiology and medicine: what comes first, misshape or dysfunction? The view from metabolic excess. J Gen Physiol. 2012;139(6):455–63. https://doi.org/10.1085/jgp.201210771.
Guntur AR, Le PT, Farber CR, Rosen CJ. Bioenergetics during calvarial osteoblast differentiation reflect strain differences in bone mass. Endocrinology. 2014;155(5):1589–95. https://doi.org/10.1210/en.2013-1974.
Kobayashi K, Nojiri H, Saita Y, Morikawa D, Ozawa Y, Watanabe K, et al. Mitochondrial superoxide in osteocytes perturbs canalicular networks in the setting of age-related osteoporosis. Scientific Reports. 2015;5(1):9148. https://doi.org/10.1038/srep09148.
Shum LC, Hollenberg AM, Baldwin AL, Kalicharan BH, Maqsoodi N, Rubery PT, et al. Role of oxidative metabolism in osseointegration during spinal fusion. PLOS ONE. 2020;15(11):e0241998. https://doi.org/10.1371/journal.pone.0241998.
• Shares BH, Smith CO, Sheu TJ, Sautchuk R Jr, Schilling K, Shum LC, et al. Inhibition of the mitochondrial permeability transition improves bone fracture repair. Bone. 2020;137:115391. https://doi.org/10.1016/j.bone.2020.115391This study demonstrates that protecting mitochondrial integrity through inhibition of the opening of mitochondrial permeability transition pores promotes fracture healing.
Finkel T. From sulfenylation to sulfhydration: what a thiolate needs to tolerate. Sci Signal. 2012;5(215):pe10. https://doi.org/10.1126/scisignal.2002943.
Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45(5):549–61. https://doi.org/10.1016/j.freeradbiomed.2008.05.004.
Mochin MT, Underwood KF, Cooper B, McLenithan JC, Pierce AD, Nalvarte C, et al. Hyperglycemia and redox status regulate RUNX2 DNA-binding and an angiogenic phenotype in endothelial cells. Microvasc Res. 2015;97:55–64. https://doi.org/10.1016/j.mvr.2014.09.008.
Arai M, Shibata Y, Pugdee K, Abiko Y, Ogata Y. Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life. 2007;59(1):27–33. https://doi.org/10.1080/15216540601156188.
Hinoi E, Fujimori S, Wang L, Hojo H, Uno K, Yoneda Y. Nrf2 negatively regulates osteoblast differentiation via interfering with Runx2-dependent transcriptional activation. J Biol Chem. 2006;281(26):18015–24. https://doi.org/10.1074/jbc.M600603200.
Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, Abraham E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J Biol Chem. 2010;285(43):33154–64. https://doi.org/10.1074/jbc.M110.143685.
Hinchy EC, Gruszczyk AV, Willows R, Navaratnam N, Hall AR, Bates G, et al. Mitochondria-derived ROS activate AMP-activated protein kinase (AMPK) indirectly. Journal of Biological Chemistry. 2018;293(44):17208–17. https://doi.org/10.1074/jbc.RA118.002579.
Wei J, Shimazu J, Makinistoglu MP, Maurizi A, Kajimura D, Zong H, et al. Glucose uptake and Runx2 synergize to orchestrate osteoblast differentiation and bone formation. Cell. 2015;161(7):1576–91. https://doi.org/10.1016/j.cell.2015.05.029.
Peck WA, Birge SJ, Fedak SA. Bone cells: biochemical and biological studies after enzymatic isolation. Science. 1964;146(3650):1476–7. https://doi.org/10.1126/science.146.3650.1476.
Zoch ML, Abou DS, Clemens TL, Thorek DL, Riddle RC. In vivo radiometric analysis of glucose uptake and distribution in mouse bone. Bone Res. 2016;4:16004. https://doi.org/10.1038/boneres.2016.4.
Esen E, Lee SY, Wice BM, Long F. PTH promotes bone anabolism by stimulating aerobic glycolysis via IGF signaling. J Bone Miner Res. 2015;30(11):1959–68. https://doi.org/10.1002/jbmr.2556.
Esen E, Chen J, Karner CM, Okunade AL, Patterson BW, Long F. WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell Metab. 2013;17(5):745–55. https://doi.org/10.1016/j.cmet.2013.03.017.
Zoidis E, Ghirlanda-Keller C, Schmid C. Stimulation of glucose transport in osteoblastic cells by parathyroid hormone and insulin-like growth factor I. Mol Cell Biochem. 2011;348(1-2):33–42. https://doi.org/10.1007/s11010-010-0634-z.
Li Z, Frey JL, Wong GW, Faugere MC, Wolfgang MJ, Kim JK, et al. Glucose transporter-4 facilitates insulin-stimulated glucose uptake in osteoblasts. Endocrinology. 2016;157(11):4094–103. https://doi.org/10.1210/en.2016-1583.
• Chen H, Ji X, Lee WC, Shi Y, Li B, Abel ED, et al. Increased glycolysis mediates Wnt7b-induced bone formation. Faseb J. 2019;33(7):7810–21. https://doi.org/10.1096/fj.201900201RRThis study shows that WNT-7b stimulates glucose consumption and glycolysis, which in turn increases bone mass. Deletion of GLUT1 reduces WNT-7b induced high bone mass phenotype.
Karner CM, Long F. Glucose metabolism in bone. Bone. 2018;115:2–7. https://doi.org/10.1016/j.bone.2017.08.008.
Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 2014;510(7504):298–302. https://doi.org/10.1038/nature13236.
Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. Nature Reviews Cancer. 2016;16(10):650–62. https://doi.org/10.1038/nrc.2016.81.
Warburg O. Uber den stoffwechsel der karzinomezellen. Biochem Z. 1924;152:309–44.
• Lee SY, Abel ED, Long F. Glucose metabolism induced by Bmp signaling is essential for murine skeletal development. Nat Commun. 2018;9(1):4831. https://doi.org/10.1038/s41467-018-07316-5This paper shows that GLUT1 is required for chondrocyte proliferation, matrix production and maturation during endochondral ossification. BMP signaling regulates GLUT1 expression via mTORC1-Hif1a cascade.
• Misra BB, Jayapalan S, Richards AK, Helderman RCM, Rendina-Ruedy E. Untargeted metabolomics in primary murine bone marrow stromal cells reveals distinct profile throughout osteoblast differentiation. Metabolomics. 2021;17(10):86. https://doi.org/10.1007/s11306-021-01829-9This study provides a complete metabolomic data set of bone marrow stromal cells during osteoblast differentiation.
Wu Y, Wang M, Feng H, Peng Y, Sun J, Qu X, et al. Lactate induces osteoblast differentiation by stabilization of HIF1α. Mol Cell Endocrinol. 2017;452:84–92. https://doi.org/10.1016/j.mce.2017.05.017.
Peek CB, Levine DC, Cedernaes J, Taguchi A, Kobayashi Y, Tsai SJ, et al. Circadian clock interaction with HIF1α mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab. 2017;25(1):86–92. https://doi.org/10.1016/j.cmet.2016.09.010.
Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11(5):325–37. https://doi.org/10.1038/nrc3038.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (New York, NY). 2009;324(5930):1029–33. https://doi.org/10.1126/science.1160809.
Dirckx N, Moorer MC, Clemens TL, Riddle RC. The role of osteoblasts in energy homeostasis. Nature Reviews Endocrinology. 2019;15(11):651–65. https://doi.org/10.1038/s41574-019-0246-y.
Regan JN, Lim J, Shi Y, Joeng KS, Arbeit JM, Shohet RV, et al. Up-regulation of glycolytic metabolism is required for HIF1α-driven bone formation. Proc Natl Acad Sci U S A. 2014;111(23):8673–8. https://doi.org/10.1073/pnas.1324290111.
• Hong M, Zhang XB, Xiang F, Fei X, Ouyang XL, Peng XC. MiR-34a suppresses osteoblast differentiation through glycolysis inhibition by targeting lactate dehydrogenase-A (LDHA). In Vitro Cell Dev Biol Anim. 2020;56(6):480–7. https://doi.org/10.1007/s11626-020-00467-0This study demonstrates that miR-34a inhibits the expression of glycolytic genes and suppresses osteoblast differentiation in part by directly targeting LDHA.
Zhang D, Bae C, Lee J, Lee J, Jin Z, Kang M, et al. The bone anabolic effects of irisin are through preferential stimulation of aerobic glycolysis. Bone. 2018;114:150–60. https://doi.org/10.1016/j.bone.2018.05.013.
• Jin Z, Kho J, Dawson B, Jiang M-M, Chen Y, Ali S, et al. Nitric oxide modulates bone anabolism through regulation of osteoblast glycolysis and differentiation. The Journal of Clinical Investigation. 2021;131(5):e138935. https://doi.org/10.1172/JCI138935This study shows that arginine metabolism provides nitric oxide that activates glycolysis to support osteoblast differentiation and bone formation. This study highlights the interaction between amino acid metabolism and glycolysis.
• Lee SY, Long F. Notch signaling suppresses glucose metabolism in mesenchymal progenitors to restrict osteoblast differentiation. J Clin Invest. 2018;128(12):5573–86. https://doi.org/10.1172/jci96221This study reveals that Notch signaling suppresses glycolysis via reduced phosphorylation of AMPK. Inhibition of glycolysis reverses the high bone mass phenotype of Notch2 mutant mice.
• Hollenberg AM, Smith CO, Shum LC, Awad H, Eliseev RA. Lactate dehydrogenase inhibition with oxamate exerts bone anabolic effect. Journal of Bone and Mineral Research. 2020;35(12):2432–43. https://doi.org/10.1002/jbmr.4142This study found that inhibition of lactate dehydrogenase using oxamate stimulates OXPHOS and promotes osteoblast differentiation and increases bone mass.
Thornburg JM, Nelson KK, Clem BF, Lane AN, Arumugam S, Simmons A, et al. Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res. 2008;10(5):R84. https://doi.org/10.1186/bcr2154.
Phang JM, Liu W, Hancock CN, Fischer JW. Proline metabolism and cancer: emerging links to glutamine and collagen. Curr Opin Clin Nutr Metab Care. 2015;18(1):71–7. https://doi.org/10.1097/MCO.0000000000000121.
Hosios AM, Hecht VC, Danai LV, Johnson MO, Rathmell JC, Steinhauser ML, et al. Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev Cell. 2016;36(5):540–9. https://doi.org/10.1016/j.devcel.2016.02.012.
Krall AS, Xu S, Graeber TG, Braas D, Christofk HR. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat Commun. 2016;7:11457. https://doi.org/10.1038/ncomms11457.
• Yu Y, Newman H, Shen L, Sharma D, Hu G, Mirando AJ, et al. Glutamine metabolism regulates proliferation and lineage allocation in skeletal stem cells. Cell Metab. 2019;29(4):966–78 e4. https://doi.org/10.1016/j.cmet.2019.01.016This paper demonstrates glutaminase-mediated glutamine metabolism is required for skeletal stem cell proliferation and both osteoblast specification and differentiation. Mechanistically, transaminase dependent α-ketoglutarate production is required for SSC proliferation.
• Stegen S, Devignes CS, Torrekens S, Van Looveren R, Carmeliet P, Carmeliet G. Glutamine Metabolism in osteoprogenitors is required for bone mass accrual and PTH-induced bone anabolism in male Mice. J Bone Miner Res. 2020. https://doi.org/10.1002/jbmr.4219This paper shows that PTH promotes glutamine consumption and catabolism in osteoblasts. Mechanistically, glutamine metabolism contributes to amino acid and nucleotide biosynthesis required for osteoblast differentiation, and glutathione production to promote osteoblast viability.
• Shen L, Sharma D, Yu Y, Long F, Karner CM. Biphasic regulation of glutamine consumption by WNT during osteoblast differentiation. Journal of Cell Science. 2021;134(1):jcs251645. https://doi.org/10.1242/jcs.251645This study shows that WNT regulates glutamine consumption through two amino acid transporters via distinct signaling pathways. WNT rapidly induces the expression Slc7a7 via β-catenin signaling pathway, while Slc1a5 is regulated via mTORC1-ATF4 cascade for sustained glutamine consumption.
• Sharma D, Yu Y, Shen L, Zhang G-F, Karner CM. SLC1A5 provides glutamine and asparagine necessary for bone development in mice. eLife. 2021;10:e71595. https://doi.org/10.7554/eLife.71595This study found the neutral amino acid transporter SLC1A5 provides glutamine and asparagine to regulate protein synthesis and osteoblast differentiation. Mechanistically, glutamine and asparagine are used to synthesize non-essential amino acids to support osteoblast differentiation.
Hu G, Yu Y, Tang YJ, Wu C, Long F, Karner CM. The amino acid sensor Eif2ak4/GCN2 is required for proliferation of osteoblast progenitors in mice. Journal of Bone and Mineral Research. 2020;35(10):2004–14. https://doi.org/10.1002/jbmr.4091.
Karner CM, Esen E, Okunade AL, Patterson BW, Long F. Increased glutamine catabolism mediates bone anabolism in response to WNT signaling. J Clin Invest. 2015;125(2):551–62. https://doi.org/10.1172/JCI78470.
Wang Y, Deng P, Liu Y, Wu Y, Chen Y, Guo Y, et al. Alpha-ketoglutarate ameliorates age-related osteoporosis via regulating histone methylations. Nature Communications. 2020;11(1):5596. https://doi.org/10.1038/s41467-020-19360-1.
• Stegen S, Rinaldi G, Loopmans S, Stockmans I, Moermans K, Thienpont B, et al. Glutamine metabolism controls chondrocyte identity and function. Dev Cell. 2020;53(5):530–44.e8. https://doi.org/10.1016/j.devcel.2020.05.001This study highlights the multi-functional role of glutamine in chondrocytes. Glutamine metabolism contributes to the epigenetic regulation of chondrogenic genes, aspartate synthesis for cell proliferation and matrix synthesis, and glutathione synthesis to offset ROS for cell survival.
Tencerova M, Figeac F, Ditzel N, Taipaleenmaki H, Nielsen TK, Kassem M. High-fat diet-induced obesity promotes expansion of bone marrow adipose tissue and impairs skeletal stem cell functions in mice. J Bone Miner Res. 2018;33(6):1154–65. https://doi.org/10.1002/jbmr.3408.
Stegen S, van Gastel N, Eelen G, Ghesquiere B, D'Anna F, Thienpont B, et al. HIF-1alpha promotes glutamine-mediated redox homeostasis and glycogen-dependent bioenergetics to support postimplantation bone cell survival. Cell Metab. 2016;23(2):265–79. https://doi.org/10.1016/j.cmet.2016.01.002.
Adamek G, Felix R, Guenther HL, Fleisch H. Fatty acid oxidation in bone tissue and bone cells in culture. Characterization and hormonal influences. The Biochemical journal. 1987;248(1):129–37. https://doi.org/10.1042/bj2480129.
Niemeier A, Niedzielska D, Secer R, Schilling A, Merkel M, Enrich C, et al. Uptake of postprandial lipoproteins into bone in vivo: impact on osteoblast function. Bone. 2008;43(2):230–7. https://doi.org/10.1016/j.bone.2008.03.022.
• Kim SP, Li Z, Zoch ML, Frey JL, Bowman CE, Kushwaha P, et al. Fatty acid oxidation by the osteoblast is required for normal bone acquisition in a sex- and diet-dependent manner. JCI Insight. 2017;2(16):e92704. https://doi.org/10.1172/jci.insight.92704This study demonstrates that fatty acid oxidation mediated by CPT2 is critical for bone formation in female mice.
Rendina-Ruedy E, Guntur AR, Rosen CJ. Intracellular lipid droplets support osteoblast function. Adipocyte. 2017;6(3):250–8. https://doi.org/10.1080/21623945.2017.1356505.
Frey JL, Li Z, Ellis JM, Zhang Q, Farber CR, Aja S, et al. Wnt-Lrp5 signaling regulates fatty acid metabolism in the osteoblast. Mol Cell Biol. 2015;35(11):1979–91. https://doi.org/10.1128/mcb.01343-14.
• van Gastel N, Stegen S, Eelen G, Schoors S, Carlier A, Daniëls VW, et al. Lipid availability determines fate of skeletal progenitor cells via SOX9. Nature. 2020;579(7797):111–7. https://doi.org/10.1038/s41586-020-2050-1This study reveals that lipid scarcity induces chondrogenesis of skeletal progenitors through activation of SOX9 expression via FOXO during bone healing. In turn, SOX9 further suppresses fatty acid oxidation, allowing cells to adapt to the avascular environment with nutrient restriction.
Wang Y, Li Y, Mao K, Li J, Cui Q, Wang GJ. Alcohol-induced adipogenesis in bone and marrow: a possible mechanism for osteonecrosis. Clin Orthop Relat Res. 2003;410:213–24. https://doi.org/10.1097/01.blo.0000063602.67412.83.
McGee-Lawrence ME, Carpio LR, Schulze RJ, Pierce JL, McNiven MA, Farr JN, et al. Hdac3 deficiency Increases marrow adiposity and induces lipid storage and glucocorticoid metabolism in osteochondroprogenitor cells. Journal of Bone and Mineral Research. 2016;31(1):116–28. https://doi.org/10.1002/jbmr.2602.
Maurel DB, Boisseau N, Benhamou CL, Jaffre C. Alcohol and bone: review of dose effects and mechanisms. Osteoporos Int. 2012;23(1):1–16. https://doi.org/10.1007/s00198-011-1787-7.
Enlow DH, Conklin JL, Bang S. Observations on the occurrence and the distribution of lipids in compact bone. Clin Orthop Relat Res. 1965;38:157–69. https://doi.org/10.1097/00003086-196500380-00022.
Bensaad K, Favaro E, Lewis CA, Peck B, Lord S, Collins JM, et al. Fatty acid uptake and lipid storage induced by HIF-1α contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep. 2014;9(1):349–65. https://doi.org/10.1016/j.celrep.2014.08.056.
• Maridas DE, Rendina-Ruedy E, Helderman RC, DeMambro VE, Brooks D, Guntur AR, et al. Progenitor recruitment and adipogenic lipolysis contribute to the anabolic actions of parathyroid hormone on the skeleton. Faseb j. 2019;33(2):2885–98. https://doi.org/10.1096/fj.201800948RRThis study shows that PTH promotes lipolysis in adipocytes to release fatty acids. Fatty acids are taken up by neighboring osteoblasts and promote their differentiation.
Krall AS, Mullen PJ, Surjono F, Momcilovic M, Schmid EW, Halbrook CJ, et al. Asparagine couples mitochondrial respiration to ATF4 activity and tumor growth. Cell Metabolism. 2021;33(5):1013–26.e6. https://doi.org/10.1016/j.cmet.2021.02.001.
Wu Q, ba-alawi W, Deblois G, Cruickshank J, Duan S, Lima-Fernandes E, et al. GLUT1 inhibition blocks growth of RB1-positive triple negative breast cancer. Nature Communications. 2020;11(1):4205. https://doi.org/10.1038/s41467-020-18020-8.
Gross MI, Demo SD, Dennison JB, Chen L, Chernov-Rogan T, Goyal B, et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther. 2014;13(4):890–901. https://doi.org/10.1158/1535-7163.Mct-13-0870.
Elgogary A, Xu Q, Poore B, Alt J, Zimmermann SC, Zhao L, et al. Combination therapy with BPTES nanoparticles and metformin targets the metabolic heterogeneity of pancreatic cancer. Proceedings of the National Academy of Sciences. 2016;113(36):E5328–E36. https://doi.org/10.1073/pnas.1611406113.
Fairfield H, Falank C, Harris E, Demambro V, McDonald M, Pettitt JA, et al. The skeletal cell-derived molecule sclerostin drives bone marrow adipogenesis. J Cell Physiol. 2018;233(2):1156–67. https://doi.org/10.1002/jcp.25976.
Li W, Deng Y, Feng B, Mak KK. Mst1/2 Kinases modulate glucose uptake for osteoblast differentiation and bone formation. J Bone Miner Res. 2018;33(6):1183–95. https://doi.org/10.1002/jbmr.3413.
• Dirckx N, Tower RJ, Mercken EM, Vangoitsenhoven R, Moreau-Triby C, Breugelmans T, et al. Vhl deletion in osteoblasts boosts cellular glycolysis and improves global glucose metabolism. J Clin Invest. 2018;128(3):1087–105. https://doi.org/10.1172/JCI97794This study demonstrates that stabilization of HIF1a promotes bone formation via increased glycolysis. The study also highlights the association between glucose metabolism in osteoblasts and whole body glucose homeostasis.
Yao Q, Khan MP, Merceron C, LaGory EL, Tata Z, Mangiavini L, et al. Suppressing mitochondrial respiration is critical for hypoxia tolerance in the fetal growth plate. Dev Cell. 2019;49(5):748–63.e7. https://doi.org/10.1016/j.devcel.2019.04.029.
Ambrogini E, Almeida M, Martin-Millan M, Paik JH, Depinho RA, Han L, et al. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab. 2010;11(2):136–46. https://doi.org/10.1016/j.cmet.2009.12.009.
Rached MT, Kode A, Xu L, Yoshikawa Y, Paik JH, Depinho RA, et al. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab. 2010;11(2):147–60. https://doi.org/10.1016/j.cmet.2010.01.001.
Kim J-H, Singhal V, Biswal S, Thimmulappa RK, DiGirolamo DJ. Nrf2 is required for normal postnatal bone acquisition in mice. Bone Research. 2014;2(1):14033. https://doi.org/10.1038/boneres.2014.33.
Funding
Work in the Karner lab is supported by National Institute of Health R01 grants (AR076325 and AR071967) to C.M.K.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Skeletal Biology and Regulation
Rights and permissions
About this article
Cite this article
Shen, L., Hu, G. & Karner, C.M. Bioenergetic Metabolism In Osteoblast Differentiation. Curr Osteoporos Rep 20, 53–64 (2022). https://doi.org/10.1007/s11914-022-00721-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-022-00721-2