Abstract
Of all the economically important plant parasitic nematodes, root-knot nematodes (Meloidogyne species) are amongst the most widespread, the best recognized and most widely studied. This is partly because infected roots develop galls where the nematodes feed, which with severe infection give roots a ‘knotted’ appearance. They have a remarkably wide host range, and are ubiquitous especially in tropical and sub-tropical regions of the world. Juveniles move through the soil rhizosphere and locate host roots, in which they migrate intercellularly to pro-vascular cells to form a feeding site. Here, in response to nematode secretions, they re-program the development of about 6 cells into ‘giant cells’, which provide them with the nourishment needed for them to complete their life cycle of about 4–5 weeks. Giant cells form by repeated mitosis without cytokinesis, and so become multi-nucleate. Wall ingrowths typical of transfer cells develop both where giant cell walls contact vascular elements and between neighbouring giant cells. These amplify the surface area of the cell membrane and enable solutes and water to enter the cells, and to move between giant cells in response to nematode feeding. The developing giant cells become filled with cytoplasm, prominent amoeboid nuclei, mitochondria and small vacuoles, and appear to be very active metabolically. Giant cell development is accompanied with a host of changes in gene expression, cytoskeleton changes, plant hormone changes and structural changes. The delicate interaction between the nematode and its associated giant cells is mediated via feeding tubes, derived from nematode gland secretions, which act as an ultrafilter and pressure regulator. As the infective worm-like J2 larvae develop via 3 moults to reach the adult stage, they swell and adult females become spherical, and lays eggs into a gelatinous matrix. Most root-knot nematodes reproduce by mitotic parthenogenesis, although facultative meiotic parthenogenesis also occurs (e.g. in M. hapla). The availability of new molecular technologies that enable detailed study of gene expression and regulation in giant cells, and new genomic technologies applied both to host and nematode pathogen, is leading to rapid advances in understanding host-pathogen interactions of this important plant pest species.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Root-knot nematodes (Meloidogyne species, Family Tylenchidae, genus Tylenchus), together with cyst nematodes (Heterodera and Gobodera species), are economically the most important nematode pests of broadscale and vegetable crops worldwide. Root knot nematodes have a very wide host range that includes almost all vascular plants, and although their infection is normally confined to host plant roots, tubers or tap roots in the soil, they can also reproduce in plant leaves if infiltrated into the leaf air spaces. Root-knot nematodes are classified as obligate biotrophic sedentary endoparasites, and they are possibly the easiest nematode pests to identify and study, because in most cases infection of roots or other organs is accompanied by the formation of a characteristic gall.
Although there are in the order of 60 species of root-knot nematodes, a smaller subset of these are very widespread—probably the best known species are M. incognita, M. javanica, M. arenaria and M. hapla. The nature and function of the feeding cells induced by all of these species are very similar. However differences do exist between the species in gall structure, number of lateral roots originating from galls and tolerance to cold. For example, M. hapla has the unfortunate common name ‘Northern root-knot nematode’ because it is more cold-tolerant, but this is inappropriate for the Southern hemisphere, where north equates to a hotter climate! An overview of the life cycle of root-knot nematodes is provided in Fig. 5.1 and described further below.
Schematic representation of the life cycle of root-knot nematodes. J2 root-knot nematodes (RKN) migrate to and enter a host root in the zone of elongation. An individual nematode then migrates to pro-vascular ‘target cells’ where it re-programs them by stimulating mitosis and cell expansion to form giant cells from which it feeds and becomes surrounded by a gall. After three moults within the root (not shown), the adult female becomes spherical and lays eggs in a gelatinous egg mass. The giant cells are multinucleate ‘transfer cells’ with wall ingrowths that amplify their plasma membranes to enhance the flow of solutes to the nematode from vascular tissues
Most root-knot nematode species reproduce by mitotic parthenogenesis, an asexual or clonal form of reproduction in which mitosis occurs and the oocytes keep the original diploid or polyploid chromosome number. Some species of root-knot nematodes, such as M. hapla, reproduce by facultative meiotic parthenogenesis. In this case, there is a first meiotic division in oocytes, and when males and sperm are present, usually under conditions of crowding or stress, sexual reproduction can occur to produce hybrid progeny. In the absence of males, diploidy is restored by fusion of sister haploid nuclei to generate diploid, inbred, progeny. As for cyst nematodes, after embryogenesis occurs in root-knot nematode adult females, the first stage juvenile (J1) develops within the protective eggshell and then moults to produce the second stage (J2) juvenile. Eggs are normally laid in a gelatinous egg mass, and in some temperate species (e.g. M. naasi), or under conditions of stress, there can be a period of diapause before the J2s hatch, but for most root-knot nematode species J2s hatch rapidly under favourable conditions (Fig. 5.2a).
a Freshly hatched M. hapla J2s labelled with fluorescein diacetate (FDA). b (left to right) J2 M. javanica, an individual J2 labelled with FDA, visible (arrow) in Arabidopsis roots in vitro 3 DPI, also outlined (right). (From Goto et al. 2010)
2 Root Invasion and Migration
Once J2 have hatched, they move through the soil in moisture films that surround soil particles towards roots of host plants. Growing plant roots influence the surrounding soil to generate a ‘rhizosphere’, as a result of secreting polysaccharide ‘slime’, which helps root tips penetrate the soil, and the sloughing off of root cap cells and degeneration of root hair cells. In some cases C4 acids are actively secreted by roots, and these C4 acids act as chelating agents, and aid release and uptake of mineral nutrients such as phosphorus. Mycorrihzal fungi may also be associated with roots and these also help extend the zone surrounding the root from which it can sequester nutrients. Without any clear gradients the movement of J2s is random or sometimes they are attracted to each other. However, normally the rhizosphere provides gradients of compounds that the nematodes can detect, and so enables them to move to and locate host plant roots. Although J2s are normally attracted to growing root tips, they are also attracted to emerging lateral roots and sites where other J2s have entered roots.
It also appears that signalling may occur in both directions before the J2 reaches a host root. Weerasinghe et al. (2005) provided evidence that root-knot nematode juveniles (M. incognita) may send out signals in advance that can influence root development and responsiveness to the nematode. Rhizobia bacteria, which form nitrogen fixing nodules with legume hosts, secrete lipochitooligosaccharide signalling molecules, generally known as ‘Nod’ (NF) factors that affect root hairs 2–4 h in advance of the arrival of the bacteria and root entry. Even in the absence of the bacteria, Nod factors alone can induce a series of changes in root hair cells that involve cytoskeleton, ion flux changes, root hair deformation and the formation of pseudonodules. Weerasinghe et al. (2005) demonstrated that water or culture medium that had been in contact with washed M. incognita J2s appeared to contain a signalling molecule(s) functionally equivalent to Nod factors, which they termed ‘NemF’. Lotus japonicus hairs from roots grown in solution are normally straight and smooth. At 1–4 h after treatment of such L. japonicus roots with water exposed to J2 M. incognita, root hairs became wavy and branched, with accompanying changes to the cytoskeleton and nuclear movements, similar to changes seen in root hairs treated with NF. This phenomenon also occurred when tomato roots, a good host for root-knot nematodes, were treated in the same way, indicating that the plant response is not confined just to legume hosts, but did not occur in treated Arabidopsis thaliana (Columbia), which is a poorer host for root-knot nematodes. The suggestion from these results, which also included studies on mutants of NF perception is that, at least in part, root responses to root-knot nematodes and rhizobial nodule formation may have some signalling pathways in common, and that root-knot nematodes may have conscripted some of those mechanisms from rhizobia, perhaps by horizontal gene transfer. However, flavonoid-deficient Medicago truncatula roots which did not support rhizobial nodulation did support root-knot nematode giant cell formation (Wasson et al. 2009), so clearly there are also major differences in pathways of host responses between root-knot nematodes and rhizobium nodules.
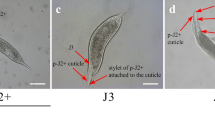
Once the J2 reaches a host root, it enters the root using a combination of mechanical penetration with its hollow mouth stylet, together with enzymatic secretions (Fig. 5.3) that can degrade polysaccharide components of plant cell walls. Unlike migration of J2 cyst-nematodes, root-knot nematode J2s migrate intercellularly, that is between cells rather then through the cells themselves. Migration of J2s can be followed using differential interference contrast optics and time-lapse photography. J2s can also be pre-stained with fluorescent dyes and monitored with fluorescence optics and confocal laser microscopy (Fig. 5.2b) (Goto et al. 2010). The effects of nematode migration can also be studied using plant lines in which specific cell types or genes have been tagged with green fluorescent protein (GFP) from Aequorea victoria or other similar fluorescent tags, and subsequent changes in gene expression with nematode migration and feeding site development can be monitored (see below).
Although A. thaliana is an atypical host, the small size and transparency of its roots nevertheless make it a good subject for studies using time time-lapse photography. Wyss and Grundler (1992) used video-enhanced contrast microscopy to show that J2s of M. incognita usually invade roots in the zone of elongation behind the tip. Local exploration of the root surface involves head rubbing and stylet movements at epidermal cells and entry is usually achieved by destroying the thin-walled epidermal and sub-epidermal cells, followed by intercellular migration. Surprisingly, on entry into the root behind the tip, their migration is first in the direction of the root-tip, where they turn round, and move away from the tip towards the differentiating vascular cylinder. This migration of root-knot J2s through cell walls also requires secretion of wall degrading enzymes, and it appears that a cocktail of such enzymes is secreted and enable separation of the middle lamella of cells just ahead of the migrating nematode—this aspect is described in more detail in Chap. 12. Despite this enzymatic breakdown of some cell wall components during migration, which is accompanied by mechanical pressure and the rupture of plasmodesmata that normally interconnect the symplasts of adjacent plant cells (Jones and Payne 1978a), in susceptible interactions the host plant is not known to respond to these disturbances, or to the oligosaccharides or other wall fragments generated, with any typical defence reaction. Either elicitors of host defence are not produced, or the nematode can prevent the activation of host defences (see Chap. 13 for more details on this aspect). It is not clear that the behaviour of J2 migration described for A. thaliana is typical of migration in other species, although similar behaviour has been observed in early stage infection of tomato roots (Goto and Jones unpublished). In other situations, when there are multiple secondary infections away from the growing root tips, infection and migration behaviour is likely to be different.
Once within the root, as the nematode migrates, it must respond to a completely new set of signals and gradients that enable it to detect its location and to migrate towards a site suitable for the induction of feeding cells, normally termed ‘giant cells’ where it will become sedentary.
3 Giant Cell Formation and Function
Once the responses from stylet thrusting by the J2 indicate that potentially responsive feeding cells, usually pro-vascular cells, have been reached, the nematode stops migrating and induces the formation of about six giant cells. This involves secretion of proteins and/or other components probably from the subventral oesophageal gland cells. The genes whose products enable the nematode to invade host plants and induce giant cells are usually referred to as ‘parasitism’ genes, and knowledge about them is described in detail in Chaps. 12–14 of this book. Root-knot nematodes have a characteristic pattern of head movement and feeding behaviour, which consists of head and stylet movements, interspersed with stylet-tip thrusting and pumping of the metacorpal bulb (Wyss and Grundler 1992). Pre-parasitic J2s store their food reserves mainly as lipids, but two to three days after root invasion, once they sense that the feeding cells are responding, they convert their lipid reserves to glycogen. Lipid reserves do not accumulate again until the adult stage is reached (Dropkin and Acedo 1974). One of the first external signs that a root-knot nematode J2 has become sedentary is the swelling of the root and the formation of a gall, which can be seen from about 24 h after infection.
3.1 Giant Cell Characteristics
The process of giant cell formation follows a typical developmental pathway, which differs of course from the cellular differentiation that would normally take place. The nematode can therefore re-program plant differentiation, and different endoparasitic nematodes re-program host cell development in different ways. Infection of the same host genotype of soybean by root-knot, cyst and reniform nematodes shows that each nematode induces their typical, distinctly different feeding sites (Jones and Dropkin 1975; Jones 1981). Early after a root-knot nematode has identified giant cell initials, such as in tissues examined 24 h after infection, there is no cell wall breakdown, but there is frequent evidence of recent mitotic events in cells immediately adjacent to the nematode or one or two cell layers away. The first sign of giant cell formation is stimulation of cell division and the formation of binucleate cells, in which the vesicles that would normally fuse to form the new cell plate between daughter nuclei after mitosis align apparently as in a normal cell plate, but subsequently fail to fuse and then disperse. This results in formation of a binucleate cell, but at this stage the cells are not obviously different in size from neighbouring cells. This nuclear division cycle—nuclear mitosis without cytokinesis—is stimulated and repeated to generate four, eight, sixteen, thirty-two or more nuclei in developing giant cells. Apart from the first nuclear division, in which spindle alignment appears normal, its alignment is abnormal in later mitotic cycles. In later cycles, metaphase plates with different number of chromosomes have been documented, such that polyploid nuclei may form, and it is probable that endo-reduplication without mitosis occurs during later stages. In some instances, partial cell plate vesicle fusion occurs, usually when nuclear division has occurred near the cell wall, and cell wall stubs can be found (Fig. 5.4c) (Jones and Payne 1978a). A similar alignment followed by dispersion of cell plate vesicles occurs when dividing plant cells are treated with caffeine (Jones and Payne 1978b). The process of giant cell development from the initial cells has been followed by electron microscopy over a period of 72 h, and composite tracings of electron micrographs of this process were provided by Jones and Payne (1978a). The J2 nematode retains the ability to move its head and insert its stylet into different giant cell initials during early and later stages of the interaction (Fig. 5.4d).
Scanning electron micrographs of roots of Impatiens balsamina infected with M. incognita, cut through feeding sites: A—cytoplasmic contents undigested, B-F cytoplasmic contents removed. a 10 DPI, the giant cell is filled with granular contents, wound xylem has differentiated outside the giant cell (x595). b A similar giant cell with cytoplasm removed to show wall ingrowths next to xylem elements. c 10 DPI giant cells and surrounding gall cells, note (arrow) partial cross walls in one giant cell, and apparent reversion of one developing giant cell to a xylem element with lignified thickenings (x85). d The space formerly occupied by and adult nematode (N) in relation to associated giant cells (GC), the arrow indicates the position of the head of the nematode (x115). e Extensive ingrowth development on an outer giant cell wall where it contacts xylem elements (x2,292). f Wall between two giant cells (from micrograph D). Note the pit fields, which contain numerous plasmodesmata, and wall ingrowths (x2,500). (From Jones and Dropkin, 1976)
3.2 The Cytoskeleton
Giant cell initials start out as vacuolated cells, but they expand rapidly over a period of about 2 weeks; central vacuoles are replaced with many smaller vacuoles, and ground cytoplasm (ribosomes, polysomes etc.) and organelles (Golgi bodies, mitochondria, endoplasmic reticulum, etc.) rapidly increase in number and density. Nuclei and nucleoli also become enlarged, with amoeboid profiles and strongly contrasting heterochromatic regions (Jones 1981). Given the stimulation of mitosis in giant cell development, coupled with the rapid expansion and other cytological features, it is not surprising that alterations of cytoskeletal components occur in developing giant cells (de Almeida Engler et al. 2004 and Chap. 18). As the giant cells elongate and expand, surrounding cells of the gall also divide and expand to accommodate giant cell expansion, and gall cells outside giant cells do not become crushed. When giant cells development starts near the zone of elongation they are usually longer than when formed in other tissues, and intrusive growth of giant cells (that is, the extension of giant cells by separation and growth between normal cells, particularly at either end of the complex), may also occur (Jones and Northcote 1972; Jones and Payne 1978a).
3.3 Giant Cells as Multinucleate Transfer Cells
About 3 days after induction, wall ingrowths typical of transfer cells start to form where giant cell initials contact vascular tissues—xylem or phloem sieve elements, (Fig. 5.4). Transfer cells are normally found in plants in a number of anatomical locations where a substantial flux of solutes is thought to cross from the apoplast (cell wall) compartment into the symplast (cytoplasmic compartment in which protoplasts are linked via plasmodesmata). These are typically in minor leaf veins, departing leaf traces, in root xylem parenchyma, at vascular discontinuities (e.g. the base of a developing cereal grain), in rhizobium nodules or the epidermis of water plants (Gunning and Pate 1974). The form of ingrowth is typical of each plant species, varying from branching finger-like structures to plates and flanges, and they are non-lignified secondary wall deposits. Wall ingrowths are typically polarised in the direction of solute flow. As giant cells enlarge, the extent of wall ingrowths, enveloped by the plasma membrane, increases dramatically opposite vascular cells (particularly xylem vessels), such that there is a 15–20 fold amplification in the surface area of the plasma membrane (Fig. 5.4b, e). In rare cases (e.g. Helianthemum) ingrowths of xylem and phloem transfer cells are morphologically different, and in root-knot nematode infected Helianthemum, when one giant cell abuts both xylem elements and phloem sieve tubes, the morphology of the wall ingrowths reflects the xylem type, not the phloem type, even next to sieve elements (Jones and Gunning 1976). When the walls between neighbouring giant cells are examined, very well developed wall ingrowths also occur in both giant cells, separated by thinner regions of walls where there are large pit fields that contain numerous plasmodesmata (Fig. 5.4f). This gives such walls a very irregular appearance in sections (sometimes mis-interpreted as wall breakdown) and the number of plasmodesmata suggests that secondary (de novo) formation of plasmodesmata may have occurred. This is in stark contrast to walls between giant cells and non-giant cells, where pit fields with plasmodesmata are not evident (Fig. 5.4b), and individual plasmodesmata, if present, are rare or perhaps non-functional (Jones and Dropkin 1976).
Because root-knot nematode giant cells form from pro-vascular cells, vascular continuity in the root is compromised, and adjacent cells often differentiate to form shorter ‘wound-type’ vascular elements with typical xylem thickenings (Fig. 5.4a, b, c)—in a symmetrical infection these wound xylem elements form an irregular cage-like structure around the giant cells. In some cases forming giant cells can revert to differentiate into tracheids with typical xylem wall thickenings, perhaps if the associated nematode stops feeding from it (Fig. 5.4c) (Jones and Dropkin 1976). Although not so obvious, phloem sieve elements also differentiate around giant cells to maintain phloem continuity.
3.4 Changes in Gene Expression in Giant Cells
Giant cells pass through a series of distinct stages during the life-cycle of the associated root knot nematode. These include the initial stages of induction, gall formation, stimulation of nuclear division, mitosis without cytokinesis, wall ingrowth formation, cell elongation and expansion, increase in cytoplasmic contents, DNA endoreduplication and maximum metabolic activity and eventually senescence of the cell contents as the nematode ceases to feed and completes its life cycle. Some changes in gene expression can be inferred, for example, by studying the responses of transgenic plants with different promoter-reporter gene constructs, or by using mutant lines of Arabidopsis, on infection with root-knot nematodes. Many of the results from these approaches have been reviewed comprehensively for example by Gheysen and Mitchum (2009), to which the reader is referred. Some reporter genes linked to specific promoters are strongly up-regulated in giant cells at different times of their development. Most research has concentrated on identifying genes that are up-regulated early in giant cell formation (e.g. see Gheysen and Mitchum 2009), but there are also promoter/reporter gene combinations in which expression only occurs three weeks or more after giant cell induction. Alcohol dehydrogenase (adh) and plant haemoglobin promoters linked to a gus reporter gene are such examples, and since both of these promoters normally express under conditions of low oxygen tension, this suggests that the oxygen tension in giant cells drops at the time of peak nutrient demand by the egg-laying female (Hutangura 1999). Similarly there are many genes that are down-regulated in giant cells.
Using a sterile culture system with glass inserts to observe nematode feeding sites during the entire nematode life cycle by confocal laser-scanning microscopy (Blinco and Jones unpublished), transgenic A. thaliana plants with an enhancer trap based on the yeast transcriptional activator GAL4 and an upstream activation sequence (UAS) linked to a modified GFP marker were used to monitor changes in gene expression in and around giant cells. For example, in a line where GFP was under the control of elements conferring specific expression in sieve elements, strong up-regulation of GFP was also observed within the giant cells (Fig. 5.5a, b). Strong down-regulation of endodermal cell specific GFP expression was observed around the giant cells in a second line (Fig. 5.5c, d), suggesting that a functional endodermal cell layer may not be present around giant cells.
Transgenic Arabidopsis thaliana plants with an enhancer trap (yeast transcriptional activator GAL4 and an upstream activation sequence UAS linked to a modified GFP marker) infected with M. javanica (line numbers refer to Nottingham Arabidopsis Stock Centre accessions). In line N9156, GFP expression is localised to phloem cells and giant cells. a The transmitted light image shows the giant cells in the central cylinder of the root at 6 DPI. b Confocal image shows strong up-regulation of GFP which co-localises with the giant cells seen in a. c and d GFP is expressed in the endodermis and root cap cells of line N9124. C Transmission and D confocal images of this line at 5 DPI. Panel D shows complete down-regulation of GFP in endodermis cells surrounding the giant cells (this pattern was retained to at least 20 DPI). Scale bars: 100 µm
3.5 Changes in the Cell Cycle in Giant Cells
As indicated above, there is no doubt that the mitotic cycle is stimulated in giant cells, although it is not followed by cytokinesis. In plant cells, there are five groups of cyclin genes: A, B, C, D and H, which encode the regulatory subunits of specific protein kinases. These genes are responsible for controlling the stages of cell cycle progression, particularly at the principal control points late in the G1 phase and at the G2/M boundary (Francis and Sorrell 2001). A- and B- type cyclins accumulate periodically through G2 and early M phases, with A-type cyclins also essential during the S phase (Joubès et al. 2001). D-type cyclins are important in regulating the progression of the G1 phase, and in the transition from G1 to S. Cell cycle re-entry is one of the first events to occur in GC initials. Expression of A- and B-type cyclins has previously been studied in root-knot nematode-induced GCs and cyst nematode induced syncytia in Arabidopsis (de Almeida Engler et al. 1999; Goverse et al. 2000). Using a GUS reporter gene, strong promoter activity of both the CycA2;1 and the CycB1;1 genes was observed in young GCs and neighbouring cells within the gall tissue (de Almeida Engler et al. 1999). This is evidence for activation of cell cycle progression through S to G2 and G2 to M phases. However, it is possible that the trigger for the initiation of GC formation occurs before the S phase. Using laser capture microdissection (LCM) to collect cytoplasmic contents from 4 day post-inoculation giant cells in tomato roots followed by RT-PCR two D-type cyclin genes, LeCycD3;2 and LeCycD3;3, were expressed at higher levels in giant cells compared to other cell-cycle-related cyclin genes, suggesting that the induction of the G1 phase of the cell cycle may be triggered in response to stimulation by the infecting nematode (Ramsay et al. 2004). A series of experiments aimed at understanding changes in cell cycle events in developing giant cells, using, for example, inhibitors of the cell cycle, cell cycle mutants and silencing specific cell cycle genes has been summarised by Gheysen and Mitchum (2009). The overall picture is quite complex, and the current information on this aspect is described in more detail in Chap. 17.
3.6 Plant Growth Regulators
A number of studies on relations of root-knot nematodes with host plants suggest that plant hormones, including auxins and cytokinins, are involved in giant cell and gall formation (e.g. Jones 1981; Hutangura et al. 1999; Karczmarek et al. 2004). Auxin responsive promoters are up-regulated early in giant cell formation (18–24 h post infection), but by 96–120 h post infection the response is much lower, suggesting that auxin accumulation is needed as a trigger for giant cell formation, but not for later enlargement. The local increase in auxin is probably a result of inhibition of local auxin transport, which correlates with gall and lateral root formation, and reduction of root tip growth, and may be preceded and regulated by changes in flavonoid metabolism (Hutangura et al. 1999). The involvement of cytokinins is less clear—similar experiments suggest that cytokinin levels are raised before giant cell development starts, but are not raised in giant cells (Lohar et al. 2004), nevertheless, cytokinin treatment can convert resistant plants to be susceptible to root-knot nematodes (Bird and Loveys 1980).
3.7 Giant Cells and Feeding Tubes
Although not present in the initial stages of giant cell formation, the presence of ‘feeding tubes’ is a typical feature of the root-knot nematode giant cell interaction. They were first noticed by light microscopy as straight rods in giant cells, and the first electron micrographs of their structure were described by Paulson and Webster (1970) and Jones and Northcote (1972). Feeding tubes in giant cells appear to form rapidly when feeding starts, within about 15 min of stylet insertion (Wyss and Zunke 1986) by self-assembly of nematode secretions, probably originating from the dorsal oesophageal gland cell. The stylet aperture is located just to one side of the tip of the mouth stylet, and the consensus view is that the stylet is only just inserted through the giant cell wall, such that at the stylet aperture the plasma membrane of the giant cell is breached but a seal is maintained. Nematode secretions are released that then self-assemble into the feeding tube (rather like the self assembly of rod-like virus coat proteins), which is sealed at the distal end and grows from the basal end, the diameter being relatively constant (about 1 µm) and the length varying up to 100 µm. The aperture of the nematode stylet is continuous within the lumen of the feeding tube, and the tube itself is semi-crystalline (Jones and Northcote 1972) and proteinaceous. When formed, the feeding tube interacts with host components, and is surrounded by a complex mass of host membranes. Because root-knot nematodes can feed from different giant cells, the stylet must be withdrawn after feeding from one giant cell and the associated feeding tube is released such that a number of feeding tubes can be found in the cytoplasm of one giant cell. The latter are no longer surrounded with the complex membrane system.
The role of the feeding tube is generally thought to be twofold—it acts as an ultrafilter and as a pressure regulator. The size exclusion value for root-knot feeding tubes has been studied by expression of fluorescent proteins in transgenic plants, and appears to be between 32–40 k Da (e.g. Urwin et al. 1997; McCarter et al. pers. comm): this is somewhat larger than the size exclusion for feeding tubes in cyst-nematode syncytia (see Chap. 4). Because of the raised solute levels in giant cells, their hydrostatic pressure appears to be very high, and so the feeding tube also serves to ensure that the giant cell does not burst or leak when a nematode feeds from it. The absence of a feeding tube as giant cells are initiated may be significant in that it may enable uptake of siRNAs by the nematode that triggers RNAi, such that early concerns that feeding tubes might prevent this mechanism of transgenic resistance appear not to be a problem (Bakhetia et al. 2005).
3.8 Electrophysiology of Giant Cells
The ability of giant cell plasma membranes to seal around inserted objects, such as an electrophysiological micropipette, is also remarkable. In a series of electrophysiological experiments by Jones et al. (1975), in contrast to other cells, they were able to make continuous recordings of transmembrane potentials from giant cells over many hours whilst carrying out electrophysiological experiments. These were the first results to indicate H+ ion co-transport was involved in uptake of sugars in higher plants (Jones et al. 1975), and a model for how this might occur at wall ingrowths is provided in Jones (1981). Subsequent research supports this proposal that H+ ATPase symporter proteins (e.g. for sucrose uptake and amino acid permeases) are co-localised at high density in the plasma membranes of transfer cell ingrowths (Offler et al. 2002). Jones et al. (1974) also recorded a series of action potentials from giant cells, that were regular in nature, reproducible, and not associated with nematode feeding. Similar trains of action potentials, apparently reflecting the same phenomenon have been recoded as rhythmic Ca2+ oscillations—termed ‘calcium spiking’. In plants, Ca2+ oscillations are involved in abscisic acid signaling in guard cells, in pollen tube elongation, and more significantly in the nodulation factor (NF) signal transduction pathway of legumes (Capoen et al. 2009). The underlying mechanism is a rhythmic sequestration and release of Ca2+ ions from the endomembrane system.
4 Giant Cell Induction—A Deliberate Controlled Event
With information on the molecular process involved in giant cell formation remaining elusive, it is possible to question whether they are simply induced by the plant itself as an endogenous response to nutrient withdrawal by the nematode, rather than by input of specific nematode molecules. In terms of the general processes involved in development and function of endoparasitic nematode feeding sites—giant cells, syncytia or nurse cells—there are two general phases, initial establishment of the feeding cells followed by formation of the mature transfer cell-like structures. The answer to the above question can be found by comparison of the first phase of initial establishment. Although different species of endoparasitic nematodes always induce a similar final form of feeding structure, clear differences exist in the nature of cell expansion, wall degradation, and stimulation of mitosis (Jones and Dropkin 1975; Jones 1981), indicating that initial feeding cell establishment is a deliberate process differentially controlled by secretions from the different nematode species.
Once feeding cells have been established, the second phase could in a large part relate to the nematode acting as a nutrient sink, such that withdrawal of solutes induces a programmed response to the source- sink situation in which the plant cells attempt to maintain homeostasis. This process includes increased transcriptional, metabolic and transport properties common to different feeding structures after their establishment (Jones 1981). Development of transfer cells in plants can be thought of as forming in this manner, with the typical formation of wall ingrowths, increase in cytoplasm and mitochondria being induced by the source-to-sink flow of solutes. If this is correct, it remains to be determined for how long initial nematode secretions are required, or if their nature changes during the nematode-host cell interaction in a way that reflects differences in patterns of gene expression in different feeding structures.
4.1 Nematode Secretions
Induction of feeding cells involves a delicate interaction between host and parasite with secretion of specific components, mainly from gland cells, into the giant cell initials. The subventral gland cells appear to be more active during the J2 migration phase, whereas the dorsal gland cell is more active during the prolonged sedentary phase (Hussey 1989). Some of the components, encoded by nematode parasitism genes have been identified as proteins and peptides, and have been termed collectively the ‘parasitome’—that is, those genes/products that are required by the nematode for successful parasitism. Various approaches have been used to identify parasitism genes and new knowledge has been generated rapidly as molecular techniques have advanced. A major advance was to isolate mRNA from glands of dissected parasitic J2s and to identify genes expressed in gland cells with the potential to be secreted into host cells (Huang et al. 2003). Proteomics approaches have also been used to identify proteins present in secretions of parasitic J2s that may be involved in modifying host cells (Jaubert et al. 2002; Bellafiore et al. 2008). Of the 50 or more parasitism genes expressed in root-knot and cyst nematode gland cells, few of the secreted products are common between these species, and more than 70% are pioneer genes with no significant homology in databases (Davis et al. 2009). The availability of full genome sequences of two species of root-knot nematodes (M. hapla and M. incognita) undoubtedly with more to come, application of comparative genomics, and new approaches for functional analysis of parasitism genes, such as over-expression in plant cells or silencing by RNA interference in the nematode, will provide new information that will lead to a much better understanding of how giant cells and other nematode feeding cells are induced and maintained. These aspects are described in more detail in Parts II and III of this volume.
5 Host Resistance to Root-Knot Nematodes
Although the focus of this chapter is on the susceptible plant response, and the formation of giant cells by root-knot nematodes, there are plants with natural resistance to root-knot nematodes, either as a result of being a non-host or through deployment of specific resistance genes. A series of genes that confer specific resistance to nematode infection have now been identified and characterised. These are typically of the ‘R’ gene type in which an avirulence (Avr) component from the nematode is recognised by the R gene, followed by a signalling cascade in plant cells in which usually a hypersensitive resistance response prevents successful nematode root colonisation. Probably the best characterised of such resistance genes is the Mi-1.2 gene, which was introduced into tomato in a cross with Solanum peruvianum. This gene encodes a typical nucleotide binding leucine rich repeat (NB/LRR) cytoplasmic protein, which confers resistance to M. incognita, M. javanica and M. arenaria, and is remarkable in that it also confers resistance to the potato aphid (Rossi et al. 1998) and the white fly Bemesia tabaci (Nombela et al. 2003). Natural resistance genes may be transferred by conventional plant breeding or by introduction into transgenic plants, although compatibility in the signalling cascade is required for them to function in different host plants. Transgenic resistance may also be achieved using RNA interference (RNAi) targeted to nematode genes (Yadav et al. 2006), or by prevention of feeding cell formation (Jones et al. unpublished).
6 Concluding Remarks
Root-knot nematodes are ubiquitous, polyphagous and highly successful plant pathogens, which have probably evolved from free-living nematodes by a process that probably included horizontal gene transfer of bacterial genes and they have subsequently evolved the capacity to control the development of giant cells at their feeding sites. They live in a benign environment in the root protected by surrounding gall cells, with a ready made food source available to them while they complete their life cycle. From being a relatively neglected group of pathogens, plant parasitic nematodes, and particularly root-knot nematodes, are now at the forefront of the latest research. Some of this new emphasis can be attributed to the availability of significant new resources derived from the choice of C. elegans as the model nematode. Certainly, the pace of advancements in understanding this host-pathogen interaction is accelerating, although often each new piece of information requires further testing and raises new questions. However, even without the C. elegans model, the host of advances in technology would still have been applied to study of nematode-plant interactions. In particular, advances in micro-techniques have enabled changes in gene expression and metabolism in giant cells to be greatly expanded. Combined with new DNA and transcriptome sequencing technologies for both host plant and nematode pathogens, improvements in bioinformatics and comparative genomics that enable large new datasets to be analysed and compared, and an ability in functional genomics (e.g. RNAi and over-expression) to study gene function, we are now entering a new era in which we can expect to develop fundamental understanding of how a root-knot nematode finds the correct cells and re-programs them to form giant cells for their benefit. Much new information is provided in later chapters of this book, and we can predict confidently that this will result in new effective methods to control plant-parasitic nematodes.
Abbreviations
- DPI:
-
Days Post Infection
- GFP:
-
Green Fluorescent Protein
- RKN:
-
Root-Knot Nematode
- J1:
-
first stage Juvenile
- J2:
-
second stage Juvenile
- NF:
-
Nodulation Factor; other abbreviations defined when first used.
References
Bakhetia M, Charlton WL, Urwin PE, McPherson MJ, Atkinson HJ (2005) RNA interference and plant parasitic Nematodes. Trends Plant Sci 10:632–637
Bellafiore S, Shen Z, Rosso MN, Abad P, Shih P, Briggs S (2008) Direct identification of the Meloidogyne incognita secretome reveals proteins with host cell reprogramming potential. PLoS Pathog 4:e1000192
Bird AF, Loveys BR (1980) The involvement of cytokinins in a host–parasite relationship between the tomato (Lycopersicon esculentum) and a nematode (Meloidogyne javanica). Parasitology 80:497–505
Capoen W, Sofie Goormachtig S, Holsters M (2009) Water-tolerant legume nodulation. J Exp Bot 61:1251–1255
Davis EL, Hussey RS, Baum T (2009) Parasitism genes: what they reveal about parasitism. In: Berg RH, Taylor CG (eds) Cell biology of plant nematode parasitism. Springer, Heidelberg, pp 15–44
de Almeida Engler J, De Vleesschauwer V, Burssens S, Celenza JL, Inzé D, Van Montagu M, Engler G, Gheysen G (1999) Molecular markers and cell cycle inhibitors show the importance of cell cycle progression in nematode-induced galls and syncytia. Plant Cell 11:793–807
de Almeida Engler JJ, Van Poucke K, Karimi M, De Groodt R, Gheysen G, Engler G (2004) Dynamic cytoskeleton rearrangements in giant cells and syncytia of nematode-infected roots. Plant J 38:12–26
Dropkin VH, Acedo J (1974) An electron microscopic study of glycogen and lipid in female Meloidogyne incognita (root-knot nematode). J Parasitol 63:1013–1021
Francis D, Sorrell DA (2001) The interface between the cell cycle and plant growth regulators. Plant Growth Regul 33:1–12
Gheysen G, Mitchum MG (2009) Molecular insights in the susceptible plant response to nematode infection. In: Berg RH, Taylor CG (eds) Cell biology of plant nematode parasitism. Plant cell monographs. Springer-Verlag, Berlin
Goto DB, Fosu-Nyarko J, Sakuma F, Sadler G, Flottman-Reid M, Uehara T, Kondo N, Yamaguchi J, Jones MGK (2010) In planta observation of live fluorescent plant endoparasitic nematodes during early stages of infection. Nematology Res 40:15–19
Goverse A, de Almeida Engler J, Verhees J, van der Krol S, Helder J, Gheysen G (2000) Cell cycle activation by plant parasitic nematodes. Plant Mol Biol 43:747–761
Gunning BES, Pate JS (1974) Transfer cells. In: Robards AW (ed) Dynamic aspects of plant ultrastructure. McGraw-Hill, Maidenhead
Huang G, Gao B, Maier T, Allen R, Davis EL et al (2003) A profile of putative parasitism genes expressed in the esophageal gland cells of the root-knot nematode Meloidogyne incognita. Mol Plant Microbe Interact 16:376–381
Hussey RS (1989) Disease-inducing secretions of plant-parasitic nematodes. Annu Rev Phytopathol 27:123–141
Hutangura P, Mathesius U, Jones MGK, Rolfe BG (1999) Auxin induction is a trigger for root gall formation caused by root-knot nematodes in white clover and is associated with the activation of the flavonoid pathway. Aust J Plant Physiol 26:221–231
Jaubert S, Ledger TN, Laffaire JB, Piotte C, Abad P, Rosso MN (2002) Direct identification of stylet secreted proteins from root-knot nematodes by a proteomic approach. Mol Biochem Parasitol 121:205–211
Jones MGK (1981) Host cell responses to endoparasitic nematodes. Ann Appl Biol 97:353–372
Jones MGK, Dropkin VH (1975) Cellular alterations induced in soybean by three endoparasitic nematodes. Physiol Plant Pathol 5:119–124
Jones MGK, Dropkin VH (1976) Scanning electron microscopy of nematode induced giant transfer cells. Cytobios 15:159–161
Jones MGK, Gunning BES (1976) Transfer cells and nematode-induced giant cells in Helianthemum. Protoplasma 87:273–279
Jones MGK, Northcote DH (1972) Multinucleate transfer cells induced in coleus roots by the root-knot nematode Meloidogyne incognita. Protoplasma 75:381–395
Jones MGK, Payne HL (1978a) The early stages of nematode-induced giant cell formation in roots of Impatiens balsamina. J Nematol 10:70–84
Jones MGK, Payne HL (1978b) Cytokinesis in Impatiens balsamina and the effect of caffeine. Cytobios 20:79–91
Jones MGK, Novacky A, Dropkin VH (1974) ‘Action potentials’ in nematode induced plant transfer cells. Protoplasma 80:401–405
Jones MGK, Novacky A, Dropkin VH (1975) Transmembrane potentials of parenchyma cells and nematode-induced transfer cells. Protoplasma 85:15–37
Joubès J, Lemaire-Chamley M, Delmas F, Walter J, Hernould M, Mouras A, Raymond P, Chevalier C (2001) A new C-type cyclin-dependant kinase from tomato expressed in dividing tissues does not interact with mitotic and G1 cyclins. Plant Physiol 126:1403–1415
Karczmarek A, Overmars H, Helder J, Goverse A (2004) Feeding cell development by cyst and root-knot nematodes involves a similar early, local and transient activation of a specific auxin-inducible promoter element. Mol Plant Pathol 5:324–346
Lohar DP, Schaff JE, Laskey JG, Kieber JJ, Bilyeu KD, Bird DMcK (2004) Cytokinins play opposite roles in lateral root formation, and nematode and rhizobial symbioses. Plant J 38:203–214
Nombela G, Williamson VM, Muñiz M (2003) The root-knot nematode resistance gene Mi-1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci. Mol Plant Microbe Interact 16:645–649
Offler CE, McCurdy DW, Patrick JW, Talbot MJ (2002) Transfer cells: cells specialized for a special purpose. Annu Rev Plant Biol 54:431–454
Paulson RE, Webster JM (1970) Giant cell formation in tomato roots caused by Meloidogyne incognita and M. hapla (Nematode) infection: A light and electron microscope study. Can J Bot 48:271–276
Ramsay K, Wang ZH, Jones MGK (2004) Using laser capture microdissection to study gene expression in early stages of giant cells induced by root-knot nematodes. Mol Plant Pathol 5:587–592
Rossi M, Goggin FL, Milligan SB, Kaloshian I, Ullman DE, Williamson VM (1998) The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc Natl Acad Sci U S A 95:9750–9754
Urwin PE, McPherson MJ, Atkinson HJ (1997) Enhanced transgenic plant resistance to nematodes by dual proteinase inhibitor constructs. Planta 204:472–479
Wasson AP, Ramsay K, Jones MGK, Mathesius U (2009) Differing requirements for flavonoids during the formation of lateral roots, nodules and root knot nematode galls in Medicago truncatula. New Phytol 183:167–179
Weerasinghe RR, David McK, Bird D, Allen NS (2005) Root-knot nematodes and bacterial Nod factors elicit common signal transduction events in Lotus japonicas. Proc Natl Acad Sci U S A 102:3147–3152
Wyss U, Grundler FMW (1992) Feeding behaviour of sedentary plant parasitic nematodes. Eur J Plant Pathol 98:165–173
Wyss U, Zunke U (1986) Observations on the behaviour of second-stage juveniles of Heterodera schachtii inside host roots. Revue Nématol 9:153–165
Yadav BC, Veluthambi K, Subramaniam K (2006) Host-generated double-stranded RNA induces RNAi in plant-parasitic nematodes and protects the host from infection. Mol Biochem Parasitol 148:219–222
Acknowledgements
MJ thanks the EU COST Action 872 program for support to participate in its meetings. Research in the authors’ laboratories is supported by the Australian Research Council (MJ) and the Japanese Ministry of Education, Culture, Sports, Science and Technology (DG).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Jones, M.G., Goto, D.B. (2011). Root-knot Nematodes and Giant Cells. In: Jones, J., Gheysen, G., Fenoll, C. (eds) Genomics and Molecular Genetics of Plant-Nematode Interactions. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0434-3_5
Download citation
DOI: https://doi.org/10.1007/978-94-007-0434-3_5
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-0433-6
Online ISBN: 978-94-007-0434-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)