Abstract
The pentose phosphate pathway plays a pivotal role in cellular physiology. It synthesizes the nucleotide precursor ribose-5-phosphate and NADPH, required for redox homeostasis maintenance and lipogenesis. Cancer cells undergo metabolic reprogramming required to sustain proliferation and fully achieve malignant capabilities. Such metabolic reprogramming involves multiple metabolic pathways, and the pentose phosphate pathway becomes essential in tumour metabolism and biology. According to that, many changes occur in this metabolic pathway over the tumorigenic process, and the enzymes in this pathway become directly involved in the metabolism of cancer cells as well as in other important features of tumours. Also, a greater reliance on this pathway has been detected in some types of tumours. In this chapter, a detailed view of the role of the pentose phosphate pathway and its enzymes in tumour metabolism is provided. In addition, the potentiality of this pathway as therapeutic target in cancer treatment is discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pentose Phosphate Pathway
- Metabolic Reprogram
- Reactive Oxygen Species Detoxification
- TKTL1 Protein
- Diphenyl Urea Derivative
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 The Pentose Phosphate Pathway in Cell Metabolism
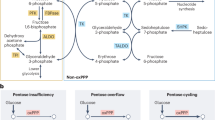
The pentose phosphate pathway (PPP) was first described in 1926 and constitutes an essential metabolic pathway involved in the synthesis of nucleic acid precursors and in the generation of reducing power, both indispensable to the maintenance of cell integrity. It is classically divided into two parts, known as the oxidative (ox-PPP) and the nonoxidative (nonox-PPP) branches (see Fig. 7.1). The ox-PPP catalyses the irreversible transformation of glucose-6-phosphate into ribulose-5-phosphate with the subsequent production of important amounts of NADPH and CO2. The nonox-PPP branch is a reversible pathway that interconverts pentose phosphate and other sugar phosphate, contributing to the synthesis of ribose-5-phosphate as well as to the redirection of the excess of pentose phosphate towards glycolysis. It has been estimated that the percentage of glucose metabolized through PPP ranges from 5 to 30 % depending on the tissue, with higher percentages in lipid-synthesizing tissues (such as the liver, white adipose tissue, lactating mammary glands, adrenal glands, and gonads) and in red blood cells (Luzzatto and Notaro 2001; Riganti et al. 2012). Throughout the next pages, a detailed view of the PPP and the function that this pathway plays in cancer will be provided, with particular emphasis in the role of the main enzymes of PPP in cancer cell biology.
1.1 The Oxidative Branch of the Pentose Phosphate Pathway
The oxidative branch of the pentose phosphate pathway is a major source of metabolic precursors for biosynthetic processes (i.e. for nucleic acid synthesis) and reducing power (i.e. for lipid synthesis, maintenance of reduced pool of glutathione, etc). It operates as an irreversible pathway which produces ribulose-5-phosphate, NADPH, and CO2 by consuming glucose-6-phosphate and NADP+. This pathway consists of three metabolic reactions which are considered to operate as depicted in Fig. 7.1.
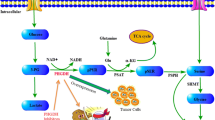
The first enzyme of the ox-PPP is glucose-6-phosphate dehydrogenase (G6PD; EC 1.1.1.49). It catalyses the oxidation of glucose-6-phosphate to 6-phosphoglucono-δ-lactone, a cyclic and unstable lactone ester of phosphogluconic acid. This irreversible reaction produces NADPH from NADP+ and is highly regulated, among others, by NADPH and palmitoyl-CoA both negatively modulating G6PD enzymatic activity (Fig. 7.2). G6PD can be active in human cells as a dimer or tetramer (formed by the association of two dimers), each inactive monomer being composed of 515 amino acids (Au et al. 2000; Riganti et al. 2012). The amount of NADP+ is critical for the activity of this enzyme, since it is necessary for stabilizing the dimer (Au et al. 2000). On the contrary, NADPH lacks stabilizing effects (Kotaka et al. 2005) and its binding to G6PD instead of NADP+ leads to a reduction of G6PD activity. Therefore, G6PD activity is directly modulated by the NADP+/NADPH ratio. Here, it is worth noting that G6PD usually works at 1–2 % of its maximal potential in healthy subjects, because of the high concentration of NADPH in resting conditions. Upon NADPH oxidation, NADP+/NADPH ratio increases and G6PD shifts to the most active state, increasing ox-PPP flux (Eggleston and Krebs 1974). Consistent with the role of G6PD in the synthesis of NADPH for lipogenesis, a negative regulation of this enzyme by the lipid intermediate palmitoyl-CoA has also been described (Asensio et al. 2007; Kawaguchi and Bloch 1974; Taketa and Pogell 1966). Accordingly, G6PD deficiency, which is the most common enzyme deficiency in the world (Cappellini and Fiorelli 2008; Luzzatto and Notaro 2001), yields a diminution of NADPH and a subsequent decrease in cholesterol synthesis (Rawat et al. 2012) and protection under oxidative stress (Rajasekaran et al. 2007) due to a lower production of reduced glutathione from NADPH. Besides regulation by direct binding of cofactors, G6PD is also modulated by signalling pathways. Thus, G6PD activity is upregulated by the action of EGF and PDGF (Tian et al. 1994; Stanton et al. 1991), whereas G6PD mRNA levels are increased through the action of insulin via PI3K activation (Talukdar et al. 2005; Wagle et al. 1998).
Main regulatory mechanisms of G6PD. G6PD is positively and negatively modulated by different mechanisms. The figure shows simply the main positive and negative regulators of G6PD as well as the transitions between the monomeric-dimeric-tetrameric states of the enzyme. Dotted lines indicate indirect effects on the enzyme by the involvement of additional effectors. G6P glucose-6-phosphate, 6PG 6-phosphogluconate, R5P ribose-5-phosphate, F6P fructose-6-phosphate, G3P glyceraldehyde-3-phosphate, DHEA dehydroepiandrosterone, EGF epidermal growth factor, PDGF platelet-derived growth factor
The second enzyme of the ox-PPP is 6-phosphogluconolactonase (6PGL; EC 3.1.1.31), which accelerates the spontaneous ring-opening hydrolysis of 6-phosphoglucono-δ-lactone to produce 6-phosphogluconate.
Finally, 6-phosphogluconate dehydrogenase (6PGD; EC 1.1.1.44) catalyses the oxidative decarboxylation of 6-phosphogluconate and yields ribulose-5-phosphate, CO2, and NADPH. The resulting ribulose-5-phosphate can be then converted into ribose-5-phosphate by a reversible reaction of the nonox-PPP (see next section) and used for the synthesis of nucleotides.
1.2 The Nonoxidative Branch of the Pentose Phosphate Pathway
The nonoxidative branch of the pentose phosphate pathway is a reversible pathway that interconverts different carbon length sugar phosphate by two- and three-carbon unit exchange. This pathway includes four reversible enzymatic reactions.
Ribose-5-phosphate isomerase (RPI, EC 5.3.1.6.) interconverts ribulose-5-phosphate and ribose-5-phosphate via production of an enediol intermediate. Ribose-5-phosphate produced in this reaction is used in the biosynthesis of coenzymes (including NADH, NADPH, FAD, and B12) and nucleic acids (DNA and RNA).
Ribulose-5-phosphate-3-epimerase (RPE, EC 5.1.3.1.) interconverts ribulose-5-phosphate to another ketose, xylulose-5-phosphate. This reaction, as the previously described one, proceeds also by an enediol intermediate.
Transketolase (TKT; EC 2.2.1.1.) is a thiamine pyrophosphate-dependent enzyme that acts in two steps of the nonox-PPP transferring in both steps two-carbon units from one sugar phosphate (donor) to another (acceptor). Its mechanism of action involves the binding of a ketose phosphate substrate (xylulose-5-phosphate), expulsion of the glyceraldehyde-3-phosphate product, and transfer of the two-carbon unit to an aldose phosphate (ribose-5-phosphate), yielding a molecule of sedoheptulose-7-phosphate. Transketolase can process a variety of 2-keto sugar phosphates in a similar manner.
Transaldolase (TA, EC 2.2.1.2) reaction is similar to the glycolytic aldolase reaction of glycolysis and catalyses the transference of three-carbon units. Its mechanism of action involves the binding of a ketose phosphate substrate (sedoheptulose-7-phosphate), expulsion of the erythrose-4-phosphate product, and transfer of the three-carbon unit to an aldose phosphate (glyceraldehyde-3-phosphate) to yield the product fructose-6-phosphate.
Despite the PPP was described almost a century ago, the precise reaction scheme of the nonox-PPP remains controversial and incompletely understood. 14C-isotope labelling experiments demonstrated that the degree of 14C-isotope incorporation in carbon atoms of fructose-6-phosphate and its distribution differs from what is predicted by the proposed scheme of individual reactions (Horecker et al. 1954). Indeed, Horecker, who originally discovered the reactions of the nonox-PPP, wrote: “From the results with labelled pentose phosphate, it is apparent that the transketolase-transaldolase sequence of reactions is by itself insufficient to account for the hexose monophosphate formation, since the distribution of isotope in the product differs from that predicted by these reactions”. The discrepancies between the widely accepted reaction scheme of the PPP and the observed experimental results suggest that this pathway has not been properly characterized yet.
Transketolase enzyme reaction is the rate-limiting step of the nonox-PPP (Comin-Anduix et al. 2001; Sabate et al. 1995). The characterization of the reaction modus and substrate specificity of TKT is of utmost interest to resolve the discrepancy between experimental results and PPP reaction schemes presented in textbooks. One possible explanation might lie on the existence of additional nonox-PPP enzymes not yet considered in the reaction schemes, such as TKT isoforms, which may carry out non-standard transketolase reactions. Throughout evolution of higher vertebrates, genome duplication led to duplication of the TKT gene giving rise to the transketolase-like 1 (TKTL1) precursor gene. This duplication was followed by an integration of the TKTL1 precursor mRNA into the genome leading to the intronless transketolase-like 2 gene (TKTL2). After this, the TKTL1 precursor gene mutated creating the recent TKTL1 gene (Coy et al. 2005). In comparison to known transketolase proteins, the TKTL1 gene encodes for TKTL1 protein isoform harbouring a 38-amino acid deletion due to deletion of original TKT gene exon 3. This deletion results in the generation of a transketolase protein similar to His103 yeast mutant, which has been reported to be capable of catalysing one-substrate reaction as well as to display a reduced affinity to thiamine (Selivanov et al. 2004). This reaction transforms a five-carbon molecule into three- and two-carbon molecules (Coy et al. 2005). Therefore, it is speculated on the possibility that TKTL1 enzyme catalyses also this reaction, although this hypothesis requires further research. Thus, the existence of different TKT isoforms provides a greater complexity in the operating modus of the nonox-PPP.
Several studies examined the effect of TKTL1 protein suppression on the total transketolase enzyme activity of different cell lines and tissues (Hu et al. 2007; Zhang et al. 2007; Yuan et al. 2010; Xu et al. 2009). In these studies, expression of human TKTL1 protein was inhibited by RNA interference in different cell models and a significant inhibition of transketolase reaction and/or associated glucose metabolism was found. Therefore, these findings demonstrate that TKTL1 is an active enzyme that clearly contributes to total transketolase activity in mammalian cells. Moreover, several in vivo studies using RNA inhibitory experiments and TKTL1 knockout mice clearly demonstrated at the highest level of evidence that TKTL1 has an important function in colon mucosal repair (Bentz et al. 2011). Thus, knockout of TKTL1 in mice led to suppression of mucosal repair and aggravates murine experimental colitis (Bentz et al. 2011). Also, it has been reported that TKTL1 plays an important and protective role against ROS allowing the maintenance of redox homeostasis in vitro (Xu et al. 2009; Wanka et al. 2012) and in vivo (Bentz et al. 2011). Recently, it has been demonstrated that downregulation of the Werner syndrome protein (WRN) induced an increase in oxidative stress accompanied by the downregulation of TKTL1, as well as of G6PD, and isocitrate dehydrogenase 1 (IDH1), further supporting the role of TKTL1 in redox homeostasis (Baomin et al. 2014; Li et al. 2009). This data is also in line with the strong expression of TKTL1 in germ cells (Rolland et al. 2013), the protective role of TKTL1 in the brain (Wanka et al. 2012; Coy et al. 2005), and the evolution of cognitive functions during transition from Neanderthals to Homo sapiens (Green et al. 2010; Prüfer et al. 2014; Pääbo 2014).
Depending on the cellular needs, the PPP can operate in different modus, and its two branches (ox-PPP and nonox-PPP) can be partially decoupled. When both ribose-5-phosphate and NADPH are needed, the main flux of carbons is driven through the ox-PPP. However, when the need of ribose-5-phosphate is greater than the need of NADPH, the carbon flux can be driven in a greater extent through the nonox-PPP. On the contrary, when there is a high demand of NADPH but not that of ribose-5-phosphate, the excess of this metabolite is directed towards glycolysis through the nonox-PPP. In the same line, when both NADPH and ATP are needed, but ribose-5-phosphate is not, glycolytic carbons are shunted into the ox-PPP and subsequently into the nonox-PPP and glycolysis.
2 The Role of the Pentose Phosphate Pathway in Cancer Metabolism
The widely recognized as the main characteristic of tumour cells is its accelerated and uncontrolled proliferation. Accordingly, the requirements of nutrients are particularly high in proliferating and tumour cells, since they need not only to preserve their integrity and perform their physiological functions but also to generate a new daughter cell. Then, as cancer is a tissue-proliferation disorder, it is expected that cancer cells rewire metabolism at the service of proliferation to provide themselves with energy and precursors of macromolecules (Ward and Thompson 2012; Schulze and Harris 2012). Furthermore, development of malignancy involves a metabolic reprogramming closely related to the acquisition of the well-known cancer hallmarks, extending thus the role of metabolism beyond growth and proliferation (Kroemer and Pouyssegur 2008; Hanahan and Weinberg 2011).
The metabolic feature associated to cell malignant transformation that has been known for the longest time is the enhanced aerobic glycolysis, consisting in an increased metabolism of glucose to lactate even in the presence of oxygen. This phenomenon is commonly referred to as the “Warburg effect” due to Otto Warburg, who first described it in the 1920s (Warburg et al. 1924, 1927). Although this adaptation accounts for one of the essential metabolic requirements of cancer cells, the high production of energy, two additional metabolic requirements must be fullfilled by cancer cells to survive and proliferate: biosynthesis of macromolecules and maintenance of redox homeostasis (Cantor and Sabatini 2012), two metabolic processes in which PPP importantly participates in. In brief, cell division requires high amounts of nucleic acids for DNA replication and significant synthesis of lipids for membrane duplication. The former are produced from the pentose phosphate generated through the PPP, and the latter are synthesized from acetyl-CoA and NADPH partly produced by the ox-PPP. Thus, the PPP promotes nucleotide and lipids synthesis but also allows carbon recirculation through nonox-PPP to glycolysis in order to preserve the formation of other molecules with a significant role in tumour physiology such as ATP, amino acids, or lactate. Moreover, the capacity of ox-PPP of producing NADPH allows the maintenance of the redox balance, which is widely accepted to be altered in cancer cells and requires additional mechanisms to be maintained (Trachootham et al. 2009; Sosa et al. 2013).
2.1 Oxidative Branch of the Pentose Phosphate Pathway in Cancer
As described earlier, the ox-PPP is an irreversible metabolic pathway driven by GPGD, 6PGL, and 6PGD. Given that this pathway is involved in two cellular essential processes related to anabolism and redox homeostasis (synthesis of ribose and NADPH), the role of the ox-PPP and its constituent enzymes in tumour biology has been mainly studied in the context of cell proliferation, transformation, and maintenance of redox state of cancer cells.
2.1.1 Glucose-6-Phosphate Dehydrogenase
G6PD usually works at a low basal rate in non-transformed cells (Riganti et al. 2012). Nevertheless, it can exert a strong proliferative role when it becomes deregulated. The key role of G6PD in tumorigenesis is supported by the fact that G6PD gene overexpression transforms NIH3T3 cells and induces tumours in nude mice (Kuo et al. 2000). In accordance, it has also been described that cells overexpressing G6PD proliferate more than wild-type cells, suggesting that G6PD levels correlates with cell proliferation rate (Tian et al. 1999; Leopold et al. 2003).
As described earlier, the ox-PPP branch is one of the main metabolic pathways involved in the production of NADPH, which is essential to the maintenance of the reduced antioxidant pool, such as reduced glutathione. In this sense, the essential role of the enzyme G6PD in protection against oxidative stress is sturdily documented (Gao et al. 2009; Ho et al. 2007; Cheng et al. 2004). Cells lacking G6PD show increased propensity for oxidant-induced senescence and increased sensitivity to diamide-induced oxidative damage. In fact, in an attempt to separately evaluate the role of G6PD in ribose synthesis and redox homeostasis, it has been concluded that G6PD is dispensable for pentose synthesis but essential to defence against oxidative stress (Pandolfi et al. 1995). In this regard, it is widely accepted that most tumours deal with increased levels of reactive oxygen species (ROS), leading to conditions of high oxidative stress. Compared to normal cells, malignant cells display higher levels of endogenous oxidative stress in vitro and in vivo (Szatrowski and Nathan 1991; Kawanishi et al. 2006). Breast tumours are a paradigmatic example, since they are characterized by persistent ROS generation (Brown and Bicknell 2001; Kang 2002) and reliance on ox-PPP branch to modulate oxidative stress. In these tumours, markers of constitutive oxidative stress have been detected in samples from in vivo breast carcinomas (Toyokuni et al. 1995; Portakal et al. 2000) as well as elevated levels of 8-hydroxy-2′-deoxyguanosine, one of the major oxidatively modified DNA base products, compared with normal control samples from the same patient (Toyokuni et al. 1995). Consequently, breast tumours also display greater reliance on ROS detoxification systems, which increases gradually as tumour progresses. Advanced breast tumours display an increased need to detoxify ROS as demonstrated by the higher expression of the ox-PPP enzymes detected in a genome-scale study based on the gene expression analysis of a large cohort of clinical samples (Jerby et al. 2012). Also, metastases of breast cancer display an increased expression of enzymes of the PPP such as G6PD and 6PGL (Chen et al. 2007), and breast cancer cells MCF7 (derived from metastatic pleural effusion) have an increased expression of G6PD compared with the near-normal breast cancer cells MCF10 (Drabovich et al. 2012).
Given the important role of G6PD in healthy and cancer cell physiology, it is not surprising that G6PD expression and activity is regulated by some of the most important oncogenes and tumour suppressor genes. G6PD upregulation has been reported in NIH3T3 fibroblast transfected with a mutated copy of K-RAS gene (de Atauri et al. 2011; Vizan et al. 2005), indicating that K-RAS regulates G6PD expression by a yet not described mechanism. Also, as mentioned above, G6PD is positively regulated by PI3K, one of the most frequently activated oncogenes in various types of cancer (Samuels et al. 2004; Luo et al. 2003). On the contrary, G6PD activity is negatively regulated by tumour suppressor gene P53, which impairs dimer formation by direct binding to the enzyme, therefore decreasing G6PD activity (Jiang et al. 2011). However, tumour-associated P53 mutants lack the G6PD-inhibitory activity, enhancing ox-PPP flux. Therefore, enhanced PPP glucose flux due to P53 inactivation increases glucose consumption and direct glucose towards biosynthesis in tumour cells. According to the role of G6PD in oxidative stress, G6PD is also regulated by transcription factors involved in response to cellular stress, such as NRF2, which has been recently described to play a key role in tumorigenesis (DeNicola et al. 2011; Mitsuishi et al. 2012). This transcription factor is frequently upregulated in various types of human cancers, resulting in an overactivation of its target genes and providing cells with additional capabilities of malignance (Singh et al. 2006; Solis et al. 2010; Tsai et al. 2008). A significant portion of the NRF2 target genes are metabolic genes involved in PPP and NADPH production, such as G6PD, PGD (phosphoglycerate dehydrogenase), TKT, TALDO1 (transaldolase), ME1 (malic enzyme 1), and IDH1, all of them containing antioxidant response element (ARE) sequences in their promoters. This provides additional evidence of the relevant function that PPP and NADPH production have in tumorigenesis.
2.1.2 6-Phosphogluconate Dehydrogenase
The role of 6PGD in cancer was initially related to the detection and prognosis of tumours. High 6GPD activity in primary breast tumours was associated to poor relapse-free survival times when compared with those with low 6GPD activity (Brocklehurst et al. 1986; Kolstad et al. 1967). More recently, several reports have described a functional role of this enzyme in cancer pathogenesis. 6PGD inhibition in lung cancer cell lines resulted in tumour growth inhibition by senescence induction both in vitro and in vivo, what may be partly due to accumulation of growth-inhibitory metabolic intermediates (Sukhatme and Chan 2012). Furthermore, it has been also described that 6PGD inhibition downregulate c-Met receptor activation by inhibiting the phosphorylation of activating tyrosine residues. This downregulation of c-Met receptor subsequently inhibited cell migration in vitro, providing a functional role of 6PGD in cancer cell migration and c-Met signalling (Chan et al. 2013).
2.2 Nonoxidative Branch of the Pentose Phosphate Pathway in Cancer
As described above, the nonox-PPP is a metabolic pathway that consists of two reversible enzymatic reactions: transketolase (TKT) and transaldolase (TA). It has been reported that the TKT family includes genes encoding two other TKT-like proteins (TKTL1 and TKTL2) in addition of TKT. Among them, TKTL1 has been reported to be overexpressed in several cancer cell lines and tissues such as the colon, lung bladder, thyroid, breast, liver larynx and brain (Langbein et al. 2006; Zerilli et al. 2008; Zhang et al. 2007; Foldi et al. 2007; Völker et al. 2007) and its expression correlates with poor prognosis of patients (Langbein et al. 2006; Schwaab et al. 2011; Lange et al. 2012; Kayser et al. 2011; Völker et al. 2007; Grimm et al. 2013) and resistance to radio- and chemotherapy (Schwaab et al. 2011). The reversibility of this branch confers great versatility to the pathway, allowing the cell to activate ribose-5-phosphate synthesis or glycolytic recirculation of the PPP intermediates depending on its metabolic requirements. Thereby, if ox-PPP is active and nucleotide precursors are synthesized efficiently, nonox-PPP is able to reincorporate the excess of pentose phosphate into the glycolytic pathway, guaranteeing energy obtaining and the supply of many metabolic precursors (glycerol, amino acids, acetyl-CoA, …) essential to cell proliferation. On the other hand, if the ox-PPP is not active, the nonox-PPP can produce the required amount of ribose-5-phosphate. However, if the activation of the ox-PPP is not accompanied by the activation of the nonox-PPP, pentose phosphate may accumulate, and tumour requirements would not be fulfilled. Therefore, nonox-PPP, apart from playing its own role, is important to enable the activation of the ox-PPP during tumorigenesis.
Given the reversibility of the reactions involved in the nonox-PPP, the Warburg effect can enhance the use of this branch (Pelicano et al. 2006). In experiments in vitro using pancreatic adenocarcinoma cells, around 85 % of the ribose has been reported to be synthesized through the nonox-PPP (Boros et al. 1997). Accordingly, in experiments in vivo using pancreatic ductal adenocarcinoma mouse model, ribose biogenesis is mainly carried out through the nonox-PPP, being this pathway essential to tumour progression in vivo (Ying et al. 2012). Furthermore, the function of the nonox-PPP in cancer is also demonstrated by additional studies reporting the involvement of this pathway in oncogenic transformation (Smith et al. 2009; Xu et al. 2009; Sun et al. 2010) and several processes accompanying it, such as metabolic reprogramming, uncontrolled tumour cell proliferation invasiveness and metastasis (Frederiks et al. 2008; Kohrenhagen et al. 2008; Krockenberger et al. 2007; Langbein et al. 2006, 2008; Schwaab et al. 2011; Kayser et al. 2011; Zerilli et al. 2008). The specific role of each enzyme is described below.
2.2.1 Transaldolase
The role of TA in cancer cells remains unclear, since available data supports both pro- and anti-tumorigenic role of this enzyme. Higher rates of TA expression have been reported in specific groups of head and neck squamous cell carcinoma (HNSCC) (Chung et al. 2004) and in lung epithelium of smokers in comparison with non-smokers (Hackett et al. 2003). A role of TA, but not necessarily its overexpression, has also been described in many other tumours (Samland and Sprenger 2009). Furthermore, it has been reported that in vivo, TA forms a complex with two enzymes of the ox-PPP, G6PD and 6PGD, being this complex upregulated in cancer (Huang et al. 2005). This finding led some researchers to propose the overexpression of TA as a biomarker for cancer development (Riganti et al. 2012). However, the overexpression of TA in the above-mentioned tumours is frequently related to better prognosis and recurrence-free survival rate (Chung et al. 2004). Thus, despite the enzymatic complex in which TA has been identified might be involved in carcinogenesis, the presence of G6PD in the complex might largely explain the role of this complex in carcinogenesis, while TA might be a mere bystander. In accordance with this reasoning, TA overexpression led to contrary metabolic effects than those induced by G6PD overexpression, i.e. it accelerated the turnover of NADPH, decreased the amount of reduced glutathione, and increased the cell sensitivity to ROS-induced apoptosis (Banki et al. 1996). According to this data, a tumour suppression role of TA is more likely than an oncogenic role. In fact, TALDO1 is located in chromosome 11 (11p15.5–p15.4) (Banki et al. 1997), a region containing tumour suppressor genes that is frequently deleted in cancers, such as oesophageal cancer (Lam et al. 2002), and its expression has been reported in differentiating and maturating processes that are totally opposed to malignant transformation (Grossman et al. 2004). In conclusion, there are indications that may lead to suggest a possible role of TA as tumour suppressor gene; however, further studies are required in order to validate this hypothesis.
2.2.2 Transketolases
The role of transketolases in cancer development has been more clearly described than TA’s. TKT activity has been reported as the PPP enzymatic activity with the highest control coefficient of tumour growth in mice with Ehrlich’s ascites tumour (Comin-Anduix et al. 2001) as well as to be increased in (pre)neoplastic lesions in rat liver (Frederiks et al. 2008). Also, inhibition of TKT by oxythiamine led to a decrease of 90 % in final tumour mass in mice hosting Ehrlich’s ascites tumour (Boros et al. 1997). These findings clearly prove the essential role that TKT plays in tumorigenesis.
From the two active transketolases described in tumours, TKT and TKTL1, the latter has been suggested as novel candidate oncogene (Smith et al. 2009) and a putative drug target for those tumours in which it is overexpressed (Riganti et al. 2012; Sun et al. 2010). Moreover, increased TKTL1 is activated by hypomethylation (Sun et al. 2010), and its levels correlate with activated proliferation and tumour progression (Diaz-Moralli et al. 2011; Krockenberger et al. 2010; Zerilli et al. 2008; Langbein et al. 2008), whereas TKTL1 silencing leads to inhibition of glucose metabolism, cell proliferation, and tumour growth (Hu et al. 2007; Zhang et al. 2007; Xu et al. 2009; Sun et al. 2010). Also a protective role for TKTL1 under starvation conditions has been reported. Recently, Wanka et al. demonstrated that Tp53-induced glycolysis and apoptosis regulator (TIGAR) protect glioma cells from starvation-induced cell death only when TKTL1 protein is present (Wanka et al. 2012).
The role of transketolases in cancer cells has been related to the hypoxia-inducible factor 1α (HIF-1α), the master regulator of the response to hypoxia that is deeply involved in tumour physiology (Sun et al. 2010; Zhao et al. 2010). It has been reported that TKT participates in a component of HIF-1α-dependent imatinib (Gleevec) resistance in chronic myeloid leukaemia cells (Zhao et al. 2010). The inhibition of TKT expression by shRNA in imatinib-resistant cells led to their resensitization, whereas the same treatment in cells overexpressing TKTL1 was ineffective. These results showed that both TKT and TKTL1 play a similar role in conferring resistance to imatinib (Zhao et al. 2010). Moreover, it has been also reported a collaborative role of HIF-1α and TKTL1 in the metabolic reprogramming of TKTL1-mediated head and neck squamous cell carcinoma tumorigenesis, where TKTL1 contributes to carcinogenesis through increased aerobic glycolysis and HIF-1α stabilization (Sun et al. 2010), reinforcing the idea of a physiological connection between transketolases and HIF-1α in cancer. Also, hypoxia (Bentz et al. 2013) as well as chemotherapy (Wanka et al. 2012) induce the expression of TKTL1.
3 The Pentose Phosphate Pathway as Potential Cancer Therapeutic Target
The significance of cancer and its worldwide impact have revealed the need of developing effective therapies to fight against this fatal disease. As mentioned above, one of the first and most obvious cancer features is the accelerated and uncontrolled proliferation of tumour cells. Given that cell division requires genome replication, researchers have developed pharmacologic strategies targeted to hinder the synthesis of nucleotides in order to impair tumour growth (Farber and Diamond 1948; Heidelberger et al. 1957). Molecules such as aminopterin, methotrexate, and 5-fluorouracil have been long and widely used in cancer therapy due to the capability of these molecules of inhibiting either nucleotide synthesis or DNA replication, thus impairing primarily cancer cell proliferation (Farber and Diamond 1948; Heidelberger et al. 1957). Their effectiveness opened the door to study other potential therapeutic targets in the pathway of synthesis of the sugar component of nucleotides, as is the PPP. This pathway has been considered a rational therapeutic target because it meets two essential requirements of cancer cells: ribose synthesis for nucleotides production and NADPH synthesis for redox homeostasis maintenance and lipogenesis (Butler et al. 2013; Vander Heiden 2011). Accordingly, the main enzymes of the PPP, G6PD, and TKT have been proposed as potential therapeutic targets in cancer (Boros et al. 1997; de Atauri et al. 2011; Rais et al. 1999; Ramos-Montoya et al. 2006), and inhibitors of the two enzymes have been designed and assessed. Therefore, the inhibitor of G6PD, dehydroepiandrosterone (DHEA), and the inhibitor of TKT, oxythiamine (OT), have been described as potential antitumoral agents (Rais et al. 1999; Ramos-Montoya et al. 2006; Cascante et al. 2002).
Deficiency in G6PD is found in approximately 400 million people worldwide, with patients suffering mild anaemia but no other serious health issues, which opens a potential therapeutic window for inhibition of this enzyme in cancer treatment. The reduction of G6PD levels seems to exert different effects on cell proliferation. In cancer cells, inhibition of G6PD leads to a clear decrease in proliferation (Li et al. 2009). Also, G6PD inhibition in human foreskin fibroblast reduces cell growth and induces cellular senescence (Ho et al. 2007). Nevertheless, the complete absence of the enzyme in G6PD-deleted embryonic stem cells does not reduce proliferation, but makes cells more sensitive to strong antioxidants (Fico et al. 2004). In accordance to that, the inhibition of the ox-PPP is especially attractive since it not only targets the production of nucleotide precursors but also the ROS protection system of cancer cells. Targeting cancer cells with ROS-mediated mechanisms has been proposed as an interesting therapeutic approach. As mentioned earlier, cancer cells usually work with increased levels of ROS and acquire protective and compensating mechanisms by activation of ROS detoxification mechanisms. Moderate levels of ROS can promote many aspects of tumour biology (Cairns et al. 2011). However, a delicate balance exists between ROS-producing and ROS-removing reactions. Since this equilibrium is forced in cancer cells, they are more sensitive to further external insults affecting this balance, promoting ROS formation, inhibiting ROS removal reactions, or both actions simultaneously (Trachootham et al. 2009). Thus, given the role of G6PD in ROS detoxification, inhibition of this enzyme is likely to break this equilibrium, dismantling the ROS adaptive response and causing cell death. This hypothesis is fully supported by the fact that G6PD-deficient cells show enhanced oxidative stress and increased sensitivity to oxidative damage, indicating that G6PD plays a fundamental role in controlling ROS levels, and that it might be a potential target in a ROS-based cancer therapy approach (Cheng et al. 2004; Gao et al. 2009; Ho et al. 2007).
The above-mentioned crucial roles of G6PD in cell physiology have led researchers to assess the efficacy of the G6PD inhibitor DHEA against breast cancer by performing several clinical trials (search for clinical trial identifier NCT00972023 and NCT02000375 at www.clinicaltrials.gov/ct2/search).
Also, TKT has been explored as potential therapeutic target. It has been observed that inhibition of the nonox-PPP provokes a greater decrease in tumour proliferation than the inhibition of the ox-PPP. In vivo testing of OT and DHEA in C57BL/6 mice hosting Ehrlich’s ascites tumour cells revealed a 90.4 and a 46 % decrease in the final tumour mass, respectively, after 3 days of treatment (Boros et al. 1997). Likewise, the administration of OT and DHEA resulted in cell cycle arrest in Ehrlich’s ascites tumour in vivo, and the combined administration of both drugs displayed a synergic effect (Rais et al. 1999).
However, several limitations complicate the interpretation of OT-mediated TKT inhibition experiments as well as hinder the efficacy of OT in cancer treatment: first, the lack of specificity caused by the fact that OT is an antimetabolite of thiamine that potentially affects all thiamine-dependent enzymatic activities, such as pyruvate dehydrogenase and α-ketoglutarate dehydrogenase; second, the versatility of the nonox-PPP and the bidirectional activity catalysed by TKT, which means that the inhibition of the enzyme does not necessarily lead to an inhibition of the synthesis of pentose phosphates; and third, the above-mentioned evidence that the transketolase mainly overexpressed in cancer cells might be TKTL1, whose activity is not clearly reported to be thiamine dependent and, therefore, might be insensitive to OT-mediated inhibition. To overcome the lack of specificity of OT, recently, diphenyl urea derivatives have also been reported as transketolase inhibitors by likely interfering with the enzyme dimerization. This new family of inhibitors represent a new avenue for the design of more selective inhibitors of TKT/TKTL1 with a novel binding mode, which is not based on mimicking the thiamine pyrophosphate cofactor binding (Obiol-Pardo et al. 2012).
On the other hand, clinical and experimental data support the requirement of thiamine to sustain enhanced nonox-PPP flux in tumours, being this fact further supported by the signs of thiamine deficiency in cancer patients. Thus, it has been hypothesized that thiamine supplementation in cancer patients may promote tumour growth (Boros et al. 1998). Certainly, evidence of significant stimulatory effect on tumour proliferation by thiamine supplementation has been provided using an Ehrlich’s ascites tumour mouse model (Comin-Anduix et al. 2001). Interestingly, it has also been reported in this study that overdoses of thiamine (2,500 times the recommended dietary intake (RDI)) slightly decreases tumour cell proliferation, suggesting that cancer patients requiring thiamine to treat thiamine deficiency should receive overdoses of thiamine to avoid the range of thiamine concentrations that supports proliferation. Other studies in breast cancer also have shown that tumour latency was significatively longer in animals fed with a low-thiamine diet compared with animals with normal-thiamine diet (Daily et al. 2012). The importance of the thiamine availability in cancer and its relation with tumour growth through promoting TKT and other thiamine-dependent pathways is an open area of research that might bring new opportunities for therapeutic intervention and dietary modification to reduce disease progression in cancer patients (Zastre et al. 2013).
Conclusion
In summary, an evolving body of evidence indicates that PPP plays a fundamental role both in healthy and cancer cells physiology. However, given the particular metabolic requirements and biochemical architecture of cancer cells, ox- and nonox-PPP are likely to play a critical role in some types of tumours. A better knowledge of the biochemistry and regulation of PPP in cancer cells as well as the identification of those tumours largely reliant on PPP will surely culminate in novel and interesting findings with clinical relevance. Also, further research on the promising role of TKTL1 in cancer biology will likely provide new and interesting knowledge of the mechanisms underlying tumour metabolic reprogramming.
References
Asensio C, Levoin N, Guillaume C, Guerquin MJ, Rouguieg K, Chretien F, Chapleur Y, Netter P, Minn A, Lapicque F (2007) Irreversible inhibition of glucose-6-phosphate dehydrogenase by the coenzyme A conjugate of ketoprofen: a key to oxidative stress induced by non-steroidal anti-inflammatory drugs? Biochem Pharmacol 73(3):405–416
Au SW, Gover S, Lam VM, Adams MJ (2000) Human glucose-6-phosphate dehydrogenase: the crystal structure reveals a structural NADP(+) molecule and provides insights into enzyme deficiency. Structure 8(3):293–303
Banki K, Eddy RL, Shows TB, Halladay DL, Bullrich F, Croce CM, Jurecic V, Baldini A, Perl A (1997) The human transaldolase gene (TALDO1) is located on chromosome 11 at p 15.4–p15.5. Genomics 45(1):233–238
Banki K, Hutter E, Colombo E, Gonchoroff NJ, Perl A (1996) Glutathione levels and sensitivity to apoptosis are regulated by changes in transaldolase expression. J Biol Chem 271(51):32994–33001
Baomin L, Iglesias-Pedraz J, Leng-Ying C, Fei Y, Cadenas E, Sita R, Comai L (2014) Downregulation of the Werner syndrome protein induces a metabolic shift that compromises redox homeostasis and limits proliferation of cancer cells. Aging Cell 13:367–378
Bentz S, Pesch T, Wolfram L, de Valliere C, Leucht K, Fried M, Coy JF, Hausmann M, Rogler G (2011) Lack of transketolase-like (TKTL) 1 aggravates murine experimental colitis. Am J Physiol Gastrointest Liver Physiol 300(4):G598–G607
Bentz S, Cee A, Endlicher E, Wojtal KA, Naami A, Pesch T, Lang S, Schubert P, Fried M, Weber A, Coy JF, Goelder S, Knüchel R, Hausmann M, Rogler G (2013) Hypoxia induces the expression of transketolase-like 1 in human colorectal cancer. Digestion 88(3):182–192. doi:10.1159/000355015, Epub 2013 Oct 26
Boros LG, Brandes JL, Lee WN, Cascante M, Puigjaner J, Revesz E, Bray TM, Schirmer WJ, Melvin WS (1998) Thiamine supplementation to cancer patients: a double edged sword. Anticancer Res 18(1B):595–602
Boros LG, Puigjaner J, Cascante M, Lee WN, Brandes JL, Bassilian S, Yusuf FI, Williams RD, Muscarella P, Melvin WS, Schirmer WJ (1997) Oxythiamine and dehydroepiandrosterone inhibit the nonoxidative synthesis of ribose and tumor cell proliferation. Cancer Res 57(19):4242–4248
Brocklehurst D, Champion AE, Cheek TR, Dewhurst DG (1986) The value of 6-phosphogluconate dehydrogenase (6-PGDH) activity as a marker of tumour cellularity and prognostic indicator in primary breast cancer. Tumour Biol 7(2–3):99–104
Brown NS, Bicknell R (2001) Hypoxia and oxidative stress in breast cancer. Oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res 3(5):323–327
Butler EB, Zhao Y, Munoz-Pinedo C, Lu J, Tan M (2013) Stalling the engine of resistance: targeting cancer metabolism to overcome therapeutic resistance. Cancer Res 73(9):2709–2717
Cairns RA, Harris IS, Mak TW (2011) Regulation of cancer cell metabolism. Nat Rev Cancer 11(2):85–95. doi:10.1038/nrc2981
Cantor JR, Sabatini DM (2012) Cancer cell metabolism: one hallmark, many faces. Cancer Discov 2(10):881–898
Cappellini MD, Fiorelli G (2008) Glucose-6-phosphate dehydrogenase deficiency. Lancet 371(9606):64–74
Cascante M, Boros LG, Comin-Anduix B, de Atauri P, Centelles JJ, Lee PW (2002) Metabolic control analysis in drug discovery and disease. Nat Biotechnol 20(3):243–249
Comin-Anduix B, Boren J, Martinez S, Moro C, Centelles JJ, Trebukhina R, Petushok N, Lee WN, Boros LG, Cascante M (2001) The effect of thiamine supplementation on tumour proliferation. A metabolic control analysis study. Eur J Biochem 268(15):4177–4182
Coy JF, Dressler D, Wilde J, Schubert P (2005) Mutations in the transketolase-like gene TKTL1: clinical implications for neurodegenerative diseases, diabetes and cancer. Clin Lab 51(5–6):257–273
Chan B, VanderLaan PA, Sukhatme VP (2013) 6-Phosphogluconate dehydrogenase regulates tumor cell migration in vitro by regulating receptor tyrosine kinase c-Met. Biochem Biophys Res Commun 439(2):247–251
Chen EI, Hewel J, Krueger JS, Tiraby C, Weber MR, Kralli A, Becker K, Yates JR 3rd, Felding-Habermann B (2007) Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res 67(4):1472–1486
Cheng ML, Ho HY, Wu YH, Chiu DT (2004) Glucose-6-phosphate dehydrogenase-deficient cells show an increased propensity for oxidant-induced senescence. Free Radic Biol Med 36(5):580–591
Chung CH, Parker JS, Karaca G, Wu J, Funkhouser WK, Moore D, Butterfoss D, Xiang D, Zanation A, Yin X, Shockley WW, Weissler MC, Dressler LG, Shores CG, Yarbrough WG, Perou CM (2004) Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell 5(5):489–500
Daily A, Liu S, Bhatnagar S, Karabakhtsian RG, Moscow JA (2012) Low-thiamine diet increases mammary tumor latency in FVB/N-Tg(MMTVneu) mice. Int J Vitam Nutr Res 82(4):298–302
de Atauri P, Benito A, Vizan P, Zanuy M, Mangues R, Marin S, Cascante M (2011) Carbon metabolism and the sign of control coefficients in metabolic adaptations underlying K-ras transformation. Biochim Biophys Acta 1807(6):746–754
DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, Scrimieri F, Winter JM, Hruban RH, Iacobuzio-Donahue C, Kern SE, Blair IA, Tuveson DA (2011) Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475(7354):106–109
Diaz-Moralli S, Tarrado-Castellarnau M, Alenda C, Castells A, Cascante M (2011) Transketolase-like 1 expression is modulated during colorectal cancer progression and metastasis formation. PLoS One 6(9):e25323
Drabovich AP, Pavlou MP, Dimitromanolakis A, Diamandis EP (2012) Quantitative analysis of energy metabolic pathways in MCF-7 breast cancer cells by selected reaction monitoring assay. Mol Cell Proteomics 11:422–434
Eggleston LV, Krebs HA (1974) Regulation of the pentose phosphate cycle. Biochem J 138(3):425–435
Farber S, Diamond LK (1948) Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med 238(23):787–793
Fico A, Paglialunga F, Cigliano L, Abrescia P, Verde P, Martini G, Iaccarino I, Filosa S (2004) Glucose-6-phosphate dehydrogenase plays a crucial role in protection from redox-stress-induced apoptosis. Cell Death Differ 11(8):823–831
Foldi M, Stickeler E, Bau L, Kretz O, Watermann D, Gitsch G, Kayser G, Zur Hausen A, Coy JF (2007) Transketolase protein TKTL1 overexpression: a potential biomarker and therapeutic target in breast cancer. Oncol Rep 17(4):841–845
Frederiks WM, Vizan P, Bosch KS, Vreeling-Sindelarova H, Boren J, Cascante M (2008) Elevated activity of the oxidative and non-oxidative pentose phosphate pathway in (pre)neoplastic lesions in rat liver. Int J Exp Pathol 89(4):232–240
Gao LP, Cheng ML, Chou HJ, Yang YH, Ho HY, Chiu DT (2009) Ineffective GSH regeneration enhances G6PD-knockdown Hep G2 cell sensitivity to diamide-induced oxidative damage. Free Radic Biol Med 47(5):529–535
Green RE, Krause J, Briggs AW, Maricic T, Stenzel U, Kircher M, Patterson N, Li H, Zhai W, Fritz MH, Hansen NF, Durand EY, Malaspinas AS, Jensen JD, Marques-Bonet T, Alkan C, Prufer K, Meyer M, Burbano HA, Good JM, Schultz R, Aximu-Petri A, Butthof A, Hober B, Hoffner B, Siegemund M, Weihmann A, Nusbaum C, Lander ES, Russ C, Novod N, Affourtit J, Egholm M, Verna C, Rudan P, Brajkovic D, Kucan Z, Gusic I, Doronichev VB, Golovanova LV, Lalueza-Fox C, de la Rasilla M, Fortea J, Rosas A, Schmitz RW, Johnson PL, Eichler EE, Falush D, Birney E, Mullikin JC, Slatkin M, Nielsen R, Kelso J, Lachmann M, Reich D, Paabo S (2010) A draft sequence of the Neandertal genome. Science 328(5979):710–722
Grimm M, Schmitt S, Teriete P, Biegner T, Stenzl A, Hennenlotter J, Muhs HJ, Munz A, Nadtotschi T, Konig K, Sanger J, Feyen O, Hofmann H, Reinert S, Coy JF (2013) A biomarker based detection and characterization of carcinomas exploiting two fundamental biophysical mechanisms in mammalian cells. BMC Cancer 13:569
Grossman CE, Qian Y, Banki K, Perl A (2004) ZNF143 mediates basal and tissue-specific expression of human transaldolase. J Biol Chem 279(13):12190–12205. doi:10.1074/jbc.M307039200
Hackett NR, Heguy A, Harvey BG, O’Connor TP, Luettich K, Flieder DB, Kaplan R, Crystal RG (2003) Variability of antioxidant-related gene expression in the airway epithelium of cigarette smokers. Am J Respir Cell Mol Biol 29(3 Pt 1):331–343
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Heidelberger C, Chaudhuri NK, Danneberg P, Mooren D, Griesbach L, Duschinsky R, Schnitzer RJ, Pleven E, Scheiner J (1957) Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature 179(4561):663–666
Ho HY, Cheng ML, Chiu DT (2007) Glucose-6-phosphate dehydrogenase—from oxidative stress to cellular functions and degenerative diseases. Redox Rep 12(3):109–118
Horecker BL, Gibbs M, Klenow H, Smyrniotis PZ (1954) The mechanism of pentose phosphate conversion to hexose monophosphate. I. With a liver enzyme preparation. J Biol Chem 207(1):393–403
Hu LH, Yang JH, Zhang DT, Zhang S, Wang L, Cai PC, Zheng JF, Huang JS (2007) The TKTL1 gene influences total transketolase activity and cell proliferation in human colon cancer LoVo cells. Anti-Cancer Drugs 18(4):427–433
Huang JB, Espinoza J, Romero R, Petty HR (2005) Transaldolase is part of a supramolecular complex containing glucose-6-phosphate dehydrogenase in human neutrophils that undergoes retrograde trafficking during pregnancy. Metabolism 54(8):1027–1033
Jerby L, Wolf L, Denkert C, Stein GY, Hilvo M, Oresic M, Geiger T, Ruppin E (2012) Metabolic associations of reduced proliferation and oxidative stress in advanced breast cancer. Cancer Res 72(22):5712–5720
Jiang P, Du W, Wang X, Mancuso A, Gao X, Wu M, Yang X (2011) p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol 13(3):310–316. doi:10.1038/ncb2172
Kang DH (2002) Oxidative stress, DNA damage, and breast cancer. AACN Clin Issues 13(4):540–549
Kawaguchi A, Bloch K (1974) Inhibition of glucose 6-phosphate dehydrogenase by palmitoyl coenzyme A. J Biol Chem 249(18):5793–5800
Kawanishi S, Hiraku Y, Pinlaor S, Ma N (2006) Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol Chem 387(4):365–372
Kayser G, Sienel W, Kubitz B, Mattern D, Stickeler E, Passlick B, Werner M, Zur Hausen A (2011) Poor outcome in primary non-small cell lung cancers is predicted by transketolase TKTL1 expression. Pathology 43(7):719–724
Kohrenhagen N, Voelker HU, Schmidt M, Kapp M, Krockenberger M, Frambach T, Dietl J, Kammerer U (2008) Expression of transketolase-like 1 (TKTL1) and p-Akt correlates with the progression of cervical neoplasia. J Obstet Gynaecol Res 34(3):293–300
Kolstad P, Bergsjo P, Koller O, Pihl A, Sanner T (1967) Detection of preinvasive and early invasive cancer by 6-phosphogluconate dehydrogenase determinations. Am J Obstet Gynecol 98(6):804–807
Kotaka M, Gover S, Vandeputte-Rutten L, Au SW, Lam VM, Adams MJ (2005) Structural studies of glucose-6-phosphate and NADP+ binding to human glucose-6-phosphate dehydrogenase. Acta Crystallogr D Biol Crystallogr 61(Pt 5):495–504
Krockenberger M, Honig A, Rieger L, Coy JF, Sutterlin M, Kapp M, Horn E, Dietl J, Kammerer U (2007) Transketolase-like 1 expression correlates with subtypes of ovarian cancer and the presence of distant metastases. Int J Gynecol Cancer 17(1):101–106
Krockenberger M, Engel JB, Schmidt M, Kohrenhagen N, Hausler SF, Dombrowski Y, Kapp M, Dietl J, Honig A (2010) Expression of transketolase-like 1 protein (TKTL1) in human endometrial cancer. Anticancer Res 30(5):1653–1659
Kroemer G, Pouyssegur J (2008) Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 13(6):472–482
Kuo W, Lin J, Tang TK (2000) Human glucose-6-phosphate dehydrogenase (G6PD) gene transforms NIH 3T3 cells and induces tumors in nude mice. Int J Cancer 85(6):857–864
Lam CT, Tang CM, Lau KW, Lung ML (2002) Loss of heterozygosity on chromosome 11 in esophageal squamous cell carcinomas. Cancer Lett 178(1):75–81
Langbein S, Frederiks WM, Zur Hausen A, Popa J, Lehmann J, Weiss C, Alken P, Coy JF (2008) Metastasis is promoted by a bioenergetic switch: new targets for progressive renal cell cancer. Int J Cancer 122(11):2422–2428. doi:10.1002/ijc.23403
Langbein S, Zerilli M, Zur Hausen A, Staiger W, Rensch-Boschert K, Lukan N, Popa J, Ternullo MP, Steidler A, Weiss C, Grobholz R, Willeke F, Alken P, Stassi G, Schubert P, Coy JF (2006) Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br J Cancer 94(4):578–585
Lange CA, Tisch-Rottensteiner J, Böhringer D, Martin G, Schwartzkopff J, Auw-Haedrich C (2012) Enhanced TKTL1 expression in malignant tumors of the ocular adnexa predicts clinical outcome. Ophthalmology 119(9):1924–1929. doi:10.1016/j.ophtha.2012.03.037, Epub 2012 Jun 1
Leopold JA, Walker J, Scribner AW, Voetsch B, Zhang YY, Loscalzo AJ, Stanton RC, Loscalzo J (2003) Glucose-6-phosphate dehydrogenase modulates vascular endothelial growth factor-mediated angiogenesis. J Biol Chem 278(34):32100–32106
Li D, Zhu Y, Tang Q, Lu H, Li H, Yang Y, Li Z, Tong S (2009) A new G6PD knockdown tumor-cell line with reduced proliferation and increased susceptibility to oxidative stress. Cancer Biother Radiopharm 24(1):81–90
Luo J, Manning BD, Cantley LC (2003) Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell 4(4):257–262
Luzzatto L, Notaro R (2001) Malaria. Protecting against bad air. Science 293(5529):442–443
Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, Yamamoto M, Motohashi H (2012) Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 22(1):66–79
Obiol-Pardo C, Alcarraz-Vizan G, Cascante M, Rubio-Martinez J (2012) Diphenyl urea derivatives as inhibitors of transketolase: a structure-based virtual screening. PLoS One 7(3):e32276
Paabo S (2014) The human condition—a molecular approach. Cell 157(1):216–226
Pandolfi PP, Sonati F, Rivi R, Mason P, Grosveld F, Luzzatto L (1995) Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J 14(21):5209–5215
Pelicano H, Martin DS, Xu RH, Huang P (2006) Glycolysis inhibition for anticancer treatment. Oncogene 25(34):4633–4646
Portakal O, Ozkaya O, Erden Inal M, Bozan B, Kosan M, Sayek I (2000) Coenzyme Q10 concentrations and antioxidant status in tissues of breast cancer patients. Clin Biochem 33(4):279–284
Prufer K, Racimo F, Patterson N, Jay F, Sankararaman S, Sawyer S, Heinze A, Renaud G, Sudmant PH, de Filippo C, Li H, Mallick S, Dannemann M, Fu Q, Kircher M, Kuhlwilm M, Lachmann M, Meyer M, Ongyerth M, Siebauer M, Theunert C, Tandon A, Moorjani P, Pickrell J, Mullikin JC, Vohr SH, Green RE, Hellmann I, Johnson PL, Blanche H, Cann H, Kitzman JO, Shendure J, Eichler EE, Lein ES, Bakken TE, Golovanova LV, Doronichev VB, Shunkov MV, Derevianko AP, Viola B, Slatkin M, Reich D, Kelso J, Paabo S (2014) The complete genome sequence of a Neanderthal from the Altai Mountains. Nature 505(7481):43–49
Rais B, Comin B, Puigjaner J, Brandes JL, Creppy E, Saboureau D, Ennamany R, Lee WN, Boros LG, Cascante M (1999) Oxythiamine and dehydroepiandrosterone induce a G1 phase cycle arrest in Ehrlich’s tumor cells through inhibition of the pentose cycle. FEBS Lett 456(1):113–118
Rajasekaran NS, Connell P, Christians ES, Yan LJ, Taylor RP, Orosz A, Zhang XQ, Stevenson TJ, Peshock RM, Leopold JA, Barry WH, Loscalzo J, Odelberg SJ, Benjamin IJ (2007) Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell 130(3):427–439
Ramos-Montoya A, Lee WN, Bassilian S, Lim S, Trebukhina RV, Kazhyna MV, Ciudad CJ, Noe V, Centelles JJ, Cascante M (2006) Pentose phosphate cycle oxidative and nonoxidative balance: a new vulnerable target for overcoming drug resistance in cancer. Int J Cancer 119(12):2733–2741
Rawat DK, Hecker P, Watanabe M, Chettimada S, Levy RJ, Okada T, Edwards JG, Gupte SA (2012) Glucose-6-phosphate dehydrogenase and NADPH redox regulates cardiac myocyte L-type calcium channel activity and myocardial contractile function. PLoS One 7(10):e45365
Riganti C, Gazzano E, Polimeni M, Aldieri E, Ghigo D (2012) The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic Biol Med 53(3):421–436
Rolland AD, Lavigne R, Dauly C, Calvel P, Kervarrec C, Freour T, Evrard B, Rioux-Leclercq N, Auger J, Pineau C (2013) Identification of genital tract markers in the human seminal plasma using an integrative genomics approach. Hum Reprod 28(1):199–209
Sabate L, Franco R, Canela EI, Centelles JJ, Cascante M (1995) A model of the pentose phosphate pathway in rat liver cells. Mol Cell Biochem 142(1):9–17
Samland AK, Sprenger GA (2009) Transaldolase: from biochemistry to human disease. Int J Biochem Cell Biol 41(7):1482–1494
Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE (2004) High frequency of mutations of the PIK3CA gene in human cancers. Science 304(5670):554
Schulze A, Harris AL (2012) How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature 491(7424):364–373
Schwaab J, Horisberger K, Strobel P, Bohn B, Gencer D, Kahler G, Kienle P, Post S, Wenz F, Hofmann WK, Hofheinz RD, Erben P (2011) Expression of transketolase like gene 1 (TKTL1) predicts disease-free survival in patients with locally advanced rectal cancer receiving neoadjuvant chemoradiotherapy. BMC Cancer 11(1):363
Selivanov VA, Kovina MV, Kochevova NV, Meshalkina LE, Kochetov GA (2004) Kinetic study of the H103A mutant yeast transketolase. FEBS Lett 567(2–3):270–274
Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, Brock MV, Biswal S (2006) Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med 3(10):e420
Smith IM, Glazer CA, Mithani SK, Ochs MF, Sun W, Bhan S, Vostrov A, Abdullaev Z, Lobanenkov V, Gray A, Liu C, Chang SS, Ostrow KL, Westra WH, Begum S, Dhara M, Califano J (2009) Coordinated activation of candidate proto-oncogenes and cancer testes antigens via promoter demethylation in head and neck cancer and lung cancer. PLoS One 4(3):e4961. doi:10.1371/journal.pone.0004961, Epub 2009 Mar 23
Solis LM, Behrens C, Dong W, Suraokar M, Ozburn NC, Moran CA, Corvalan AH, Biswal S, Swisher SG, Bekele BN, Minna JD, Stewart DJ, Wistuba II (2010) Nrf2 and Keap1 abnormalities in non-small cell lung carcinoma and association with clinicopathologic features. Clin Cancer Res 16(14):3743–3753
Sosa V, Moline T, Somoza R, Paciucci R, Kondoh H, LLeonart ME (2013) Oxidative stress and cancer: an overview. Ageing Res Rev 12(1):376–390
Stanton RC, Seifter JL, Boxer DC, Zimmerman E, Cantley LC (1991) Rapid release of bound glucose-6-phosphate dehydrogenase by growth factors. Correlation with increased enzymatic activity. J Biol Chem 266(19):12442–12448
Sukhatme VP, Chan B (2012) Glycolytic cancer cells lacking 6-phosphogluconate dehydrogenase metabolize glucose to induce senescence. FEBS Lett 586(16):2389–2395
Sun W, Liu Y, Glazer CA, Shao C, Bhan S, Demokan S, Zhao M, Rudek MA, Ha PK, Califano JA (2010) TKTL1 is activated by promoter hypomethylation and contributes to head and neck squamous cell carcinoma carcinogenesis through increased aerobic glycolysis and HIF1alpha stabilization. Clin Cancer Res 16(3):857–866. doi:10.1158/1078-0432.CCR-09-2604, Epub 2010 Jan 26
Szatrowski TP, Nathan CF (1991) Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res 51(3):794–798
Taketa K, Pogell BM (1966) The effect of palmitoyl coenzyme A on glucose 6-phosphate dehydrogenase and other enzymes. J Biol Chem 241(3):720–726
Talukdar I, Szeszel-Fedorowicz W, Salati LM (2005) Arachidonic acid inhibits the insulin induction of glucose-6-phosphate dehydrogenase via p38 MAP kinase. J Biol Chem 280(49):40660–40667
Tian WN, Braunstein LD, Apse K, Pang J, Rose M, Tian X, Stanton RC (1999) Importance of glucose-6-phosphate dehydrogenase activity in cell death. Am J Physiol 276(5 Pt 1):C1121–C1131
Tian WN, Pignatare JN, Stanton RC (1994) Signal transduction proteins that associate with the platelet-derived growth factor (PDGF) receptor mediate the PDGF-induced release of glucose-6-phosphate dehydrogenase from permeabilized cells. J Biol Chem 269(20):14798–14805
Toyokuni S, Okamoto K, Yodoi J, Hiai H (1995) Persistent oxidative stress in cancer. FEBS Lett 358(1):1–3
Trachootham D, Alexandre J, Huang P (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8(7):579–591
Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, Bremer R, Gillette S, Kong J, Haass NK, Sproesser K, Li L, Smalley KS, Fong D, Zhu YL, Marimuthu A, Nguyen H, Lam B, Liu J, Cheung I, Rice J, Suzuki Y, Luu C, Settachatgul C, Shellooe R, Cantwell J, Kim SH, Schlessinger J, Zhang KY, West BL, Powell B, Habets G, Zhang C, Ibrahim PN, Hirth P, Artis DR, Herlyn M, Bollag G (2008) Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA 105(8):3041–3046
Vander Heiden MG (2011) Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov 10(9):671–684
Vizan P, Boros LG, Figueras A, Capella G, Mangues R, Bassilian S, Lim S, Lee WN, Cascante M (2005) K-ras codon-specific mutations produce distinctive metabolic phenotypes in NIH3T3 mice [corrected] fibroblasts. Cancer Res 65(13):5512–5515
Völker HU, Scheich M, Schmausser B, Kämmerer U, Eck M (2007) Overexpression of transketolase TKTL1 is associated with shorter survival in laryngeal squamous cell carcinomas. Eur Arch Otorhinolaryngol 264(12):1431–1436, Epub 2007 Jul 18
Wagle A, Jivraj S, Garlock GL, Stapleton SR (1998) Insulin regulation of glucose-6-phosphate dehydrogenase gene expression is rapamycin-sensitive and requires phosphatidylinositol 3-kinase. J Biol Chem 273(24):14968–14974
Wanka C, Steinbach JP, Rieger J (2012) Tp53-induced glycolysis and apoptosis regulator (TIGAR) protects glioma cells from starvation-induced cell death by up-regulating respiration and improving cellular redox homeostasis. J Biol Chem 287(40):33436–33446
Warburg O, Posener K, Negelein E (1924) Über den Stoffwechsel der Carcinomzelle. Biochem Z 152:309–344
Warburg O, Wind F, Negelein E (1927) The metabolism of tumors in the body. J Gen Physiol 8(6):519–530
Ward PS, Thompson CB (2012) Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 21(3):297–308
Xu X, Zur Hausen A, Coy JF, Löchelt M (2009) Transketolase-like protein 1 (TKTL1) is required for rapid cell growth and full viability of human tumor cells. Int J Cancer 124(6):1330–1337. doi:10.1002/ijc.24078
Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL, Yan H, Wang W, Chen S, Viale A, Zheng H, Paik JH, Lim C, Guimaraes AR, Martin ES, Chang J, Hezel AF, Perry SR, Hu J, Gan B, Xiao Y, Asara JM, Weissleder R, Wang YA, Chin L, Cantley LC, Depinho RA (2012) Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149(3):656–670
Yuan W, Wu S, Guo J, Chen Z, Ge J, Yang P, Hu B (2010) Silencing of TKTL1 by siRNA inhibits proliferation of human gastric cancer cells in vitro and in vivo. Cancer Biol Ther 9(9):710–716
Zastre JA, Sweet RL, Hanberry BS, Ye S (2013) Linking vitamin B1 with cancer cell metabolism. Cancer Metab 1(1):16
Zerilli M, Amato MC, Martorana A, Cabibi D, Coy JF, Cappello F, Pompei G, Russo A, Giordano C, Rodolico V (2008) Increased expression of transketolase-like-1 in papillary thyroid carcinomas smaller than 1.5 cm in diameter is associated with lymph-node metastases. Cancer 113(5):936–944. doi:10.1002/cncr.23683
Zhang S, Yang JH, Guo CK, Cai PC (2007) Gene silencing of TKTL1 by RNAi inhibits cell proliferation in human hepatoma cells. Cancer Lett 253(1):108–114
Zhao F, Mancuso A, Bui TV, Tong X, Gruber JJ, Swider CR, Sanchez PV, Lum JJ, Sayed N, Melo JV, Perl AE, Carroll M, Tuttle SW, Thompson CB (2010) Imatinib resistance associated with BCR-ABL upregulation is dependent on HIF-1alpha-induced metabolic reprogramming. Oncogene 29(20):2962–2972
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Wien
About this chapter
Cite this chapter
Benito, A., Diaz-Moralli, S., Coy, J.F., Centelles, J.J., Cascante, M. (2015). Role of the Pentose Phosphate Pathway in Tumour Metabolism. In: Mazurek, S., Shoshan, M. (eds) Tumor Cell Metabolism. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1824-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1824-5_7
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1823-8
Online ISBN: 978-3-7091-1824-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)