Abstract
Management of haemostasis during surgery has many components that start with good surgical technique, good preoperative planning and anaesthetic support [1]. Spine surgery implies in some specific aspects: bone surface exposure can be a very significant source of bleeding [2]; some spine tumours (e.g. renal cancer metastasis) are well known from its potential bleeding. Revision surgical cases, platelet dysfunction and coagulopathies are also factors that have been considered. Retroperitoneal spine surgery can create significant source of bleeding during exposure or accidental vascular lesion of tumour, deformity and degenerative and trauma causes. The choice and the proper positioning of the patient in the surgical table will also prevent additional risk of bleeding. In posterior spinal surgery, there are two specific requirements: adequate position of the spine and an unrestricted abdomen with reduction of bleeding from epidural venous system.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Management of haemostasis during surgery has many components that start with good surgical technique, good preoperative planning and anaesthetic support [1]. Spine surgery implies in some specific aspects: bone surface exposure can be a very significant source of bleeding [2]; some spine tumours (e.g. renal cancer metastasis) are well known from its potential bleeding. Revision surgical cases, platelet dysfunction and coagulopathies are also factors that have been considered. Retroperitoneal spine surgery can create significant source of bleeding during exposure or accidental vascular lesion of tumour, deformity and degenerative and trauma causes. The choice and the proper positioning of the patient in the surgical table will also prevent additional risk of bleeding. In posterior spinal surgery, there are two specific requirements: adequate position of the spine and an unrestricted abdomen with reduction of bleeding from epidural venous system.
1 Definition and Pathophysiology
Several factors can contribute to the occurrence of intraoperative bleeding related to the surgical procedure itself (Table 17.1). Spine surgery implies in some specific aspects: bone surface exposure can be a very significant source of bleeding [2]; some spine tumours (e.g. renal cancer metastasis) are well known from its potential bleeding. Revision surgical cases, platelet dysfunction and coagulopathies are also factors that have been considered. Retroperitoneal spine surgery can create significant source of bleeding during exposure or accidental vascular lesion of tumour, deformity and degenerative and trauma causes. The choice and the proper positioning of the patient in the surgical table will also prevent additional risk of bleeding. In posterior spinal surgery, there are two specific requirements: adequate position of the spine and an unrestricted abdomen with reduction of bleeding from epidural venous system. Intraoperative blood loss is a common problem that can be encountered especially in multilevel spine fusion procedures. Currently, in the literature, there is no clear definition for significant haemorrhage in spine surgery, and there are no exact reports on consequences associated with major blood loss under these circumstances [4]. Major blood loss may lead to blood, platelet and factor transfusions. Although blood screening has improved the safety considerably over the years, there are still known risks of transfusion, including potential transfusion reactions and alloimmunization as well as infectious risks, such as hepatitis, human immunodeficiency virus, cytomegalovirus and transfusion-associated bacterial sepsis. Furthermore, there is emerging data suggesting that blood transfusion may be associated with an increased risk of post-operative infections. Additionally, the costs of blood replacement must be considered.
Spinal surgery can include now a great number of different scenarios with patient- and procedure-related aspects (Table 17.2) [5]. Spine surgeon must adopt effective surgical techniques that reduce the amount of the exposed, bleeding tissue during surgery to decrease blood loss and avoid the risks and costs associated with transfusion.
2 Haemostasis in Adult Patients
Adult patients can have thin periosteum bones with wider vascular channels. Epidural venous bleeding can be very significant in obese patients. The spine in adult cases can be stiffer than adolescents; facet joints can have degenerative deformation that may require extensive bone resection. Osteotomies are a source of bleeding irrespective of the surgeon’s choice especially in cases that required sagittal balance correction. Adult patients with medical comorbidities cannot tolerate hypotension or controlled hypotension because of risk damage caused by decreased perfusion to critical organs. Adult patients have frequent use of different types of medications and herbal supplements that can increase bleeding. Patients sometimes forget or miss to tell medical staff about these habits. Some values of estimated blood loss (EBL) are listed from current literature, but good rule is always control blood losses during the surgery and made the correct replacement therapy.
3 Haemostasis in Paediatric Patients
Paediatric patients can comprise a very heterogeneous group of patients. Paediatric spine pathology can be flexible or rigid ones as seen in some congenital malformations. Comorbidities present in the paediatric group can pose other specific management. In the paediatric group, all preoperative estimates cannot be exact; therefore in all cases, volume of blood suctioned from the operative field, blood collected on sponges (determined from weighing by operating room nurses), drapes, gowns and sometimes on the floor can be of utmost importance on this matter.
Paediatric patients can tolerate controlled hypotension better than adult patients. The length of time of surgery and extra loss with harvesting autogenous iliac crest bone are other factors increasing blood loss in these patients. Some studies about EBL in specific surgical treatment of some paediatric spine pathologies are listed in Table 17.2 for reference. Effective haemostasis in surgery can offer various advantages to the patient, surgeon and health-care facility. As a result of intraoperative blood loss, the need for allogenic or autologous blood transfusions and the risks associated with blood transfusions are increased [6, 7]. Reduced length of stay in the intensive care unit (ICU) and overall length of hospital stay have been related to reductions in the amount of blood transfused. Excessive intraoperative blood loss also has been shown to significantly increase the risk of major perioperative complications [8, 9].
4 Techniques for Maintaining Haemostasis in Surgery
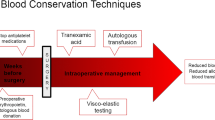
Surgeons have an array of options to control bleeding, including mechanical and thermal techniques and devices as well as pharmacotherapies and topical agents which are listed in Table 17.3.
4.1 Mechanical Techniques
Application of direct pressure or compression at a bleeding site is often the surgeon’s first choice to assist in the control of bleeding. Other mechanical methods, including sutures, staples and ligating clips, are useful if the source of bleeding is easily identifiable and able to be sealed. Compression or other mechanical methods, however, may not be appropriate during all surgical procedures, for example, if the source of bleeding is diffuse or hard to identify or the patient has an inherent or surgery-induced coagulopathy resulting from the type of surgical procedure (e.g. hemodilution, hypothermia) or prior administration of antiplatelet or anticoagulant medications [10]. One of the earliest topical haemostatic agents was cotton, in the form of gauze sponges. Although such materials concentrate blood and coagulation products via physical adsorption, they are not absorbed by the body, and upon removal, the clot may be dislodged, leading to further bleeding. Autologous blood donation (AUT) has emerged as one of the principal means to avoid or reduce allogeneic blood transfusion. These techniques involve collection and reinfusion of the patient’s own blood, preoperative acute normovolemic hemodilution, intraoperative salvage of blood from surgical field and post-operative blood salvage (collected and reinfused within first 6–8 post-operative hours) [11]. AUT has some other advantages, for example, in patients with rare blood groups, with multiple alloantibodies. It can be used safely and effectively in adult but also in adolescents. García-Erce et al. [12] showed in their study that preoperative blood autologous donation needs to be associated with other blood-saving methods (haemostatic drugs, for example, EPO and perioperative blood salvage) in some specific scoliosis patients for better results. In addition, blood retrieval is not recommended in patients with haemoglobin (Hb) levels lower than 11 g/dL [13]. By maintaining adequate haemoglobin concentrations during repeated blood collection, it is possible to reduce the interval between donations and retrieve a larger number of autologous blood units, thereby covering the predicted requirements. A meta-analysis study published by Henry et al. concluded that preoperative donation of autologous blood reduces exposure to allogeneic blood transfusion by 68% [14]. However, for those patients who donated autologous blood, the risk of receiving any transfusion (allogeneic and/or autologous) was increased by 24%. The increased rate of exposure to any transfusion may be attributed to two factors: (1) patients who donate autologous blood in general have lower preoperative haemoglobin levels than those patients who do not predonate autologous blood and therefore have an increased probability of requiring an intraoperative and/or post-operative blood transfusion; (2) the availability of predonated autologous blood engenders a more liberal transfusion policy. An analysis we performed of 35 non-randomized studies of AUT showed that the overall transfusion rate (allogeneic and/or autologous) was 67% in patients allocated to AUT [15]. This result is similar to what was seen in this meta-analysis of randomized controlled trials, which showed an overall transfusion rate (allogeneic and/or autologous) of 78% in those patients randomized to AUT. On the basis of the current evidence, AUT appears effective in reducing exposure to allogeneic blood. However, preoperative autologous donation exposes patients to other potential risks associated with blood donation and blood transfusion. As reported, the incidence of reactions occurring at the time of donation is similar for allogeneic and autologous donors (between 2% and 5%), with most reactions being mild and of a vasovagal origin [16]. Autologous blood can become contaminated with bacteria and can cause circulatory overload, particularly in elderly patients if used in a liberal fashion without a transfusion protocol. As with any transfusion, there is the ever-present risk of transfusing the wrong blood due to clerical, laboratory or ward error [17]. The overall benefits of AUT probably outweigh the harms for some groups, for instance, those who have been alloimmunized through repeated transfusion and are contemplating elective surgery. However, a full assessment of the balance of benefit and harm requires a better understanding of the clinical value of legitimate indications for red cell transfusion. Intraoperative cell salvage (ICS) and post-operative autologous transfusion (PAT) seem to avoid some of the problems of blood storage. During surgery, the intraoperatively salvaged blood can be processed to obtain a red cell concentrate ready for transfusion [18]. This procedure has few complications, the most normal being dilution coagulopathy when a large volume of processed blood is being transfused. However, in spine surgery, the effectiveness of ICS (inhaled corticosteroid) is controversial, and its selective use for operations with high intraoperative blood loss is recommended [19]. Finally, in a retrospective study by Reitman et al. [20], the USB (unwashed filtered shed blood) group required fewer post-operative transfusions (1 U to 36% of patients in the US group versus 1 U to 50% of patients in the control group). However, the authors concluded that the difference was less than expected and that the use of USB was not cost-effective during most elective lumbar procedures. PAT consists of recuperation and reinfusion of shed blood from post-operative draining, total knee arthroplasty being the operation where it has been used the most. There are now in the market a number of devices for collecting post-operative shed blood, the principal differentiating characteristic being the existence or not of a washing process for the salvaged blood. When the ICS is not used, PAT is normally performed by using devices that recuperate and retransfuse shed blood to the patient as unwashed filtered shed blood (USB). USB contains certain activated coagulation factors as well as degrading products of the fibrinogen so that its reinfusion could lead to a coagulopathy. When analysing the evolution of the levels of these proteins in samples obtained from the patients at 1 and 24 h after reinfusion, a trend to normalization was seen, and no alterations were detected in standard coagulation times [21]. In 13 studies, nearly 700 patients undergoing surgery who received a reinfusion of an average of 560 mL of USB did not experience clinically significant coagulopathy or increase in post-operative bleeding [22]. Bone wax is a well-known topical haemostatic agent composed of beeswax and baseline. It allows clot formation by stopping blood flow from damaged vessels into the bone [23]. Bone wax is known to inhibit osteogenesis and bone healing in some animal studies [24, 25]. It should never be left in fusion sites and within the spinal canal. It must never be used also in contaminated fields [26]. Preoperative embolization of spinal lesions of great bleeding potential seems to be a rational surgical strategy when it is available and the lesion is reachable by endovascular selective catheterization [27]. Vertebral metastases are responsible for 30–70% of spinal tumours [28]. The most highly vascular metastases are from thyroid and renal cell carcinoma [29]. Some publications in the literature clearly showed that preoperative endovascular embolization reduced intraoperative blood loss [30, 31]. This procedure has been described as beneficial in cases of vertebral aneurysmal bone cysts [32], vertebral haemangiomas [33], osteoblastoma, chondroma, chondrosarcoma [34] and many types of vertebral metastases [35]. The embolization procedure is more frequently performed in lumbar and thoracic spine tumours than in cervical spine lesions. The reason is that in cervical spine lesions, one can see frequent anastomoses between carotid, vertebral and subclavian arteries. The risk of cerebral or spinal cord embolization in these cases is increased [36]. The protocol must include the correct vascular anatomy of the region of interest, the identification of blush pattern of the lesion and the selection of the specific material for embolization (e.g. coils, polyvinyl alcohol (PVA) particles). All endovascular procedures should precede in 20 days at least the surgical treatment of the lesion. Partial embolization cases seem to not reduce the amount of bleeding during the surgery, so it must be informed to the surgical team for proper or adjusted measures at the time of surgery.
4.2 Thermal Techniques
Thermal techniques, such as cryotherapy, harmonic scalpels, lasers and ultrasonic osteotome, also have become viable surgical options to reduce bleeding. In spinal tumour surgery, preoperative embolization procedure sometimes cannot be enough to reduce the blood flow or cannot be accomplished for anatomical limitations. Cryocoagulation can be performed intraoperatively after adequate exposure of the tumour. The system uses liquid nitrogen as the circulating agent with which freezing is induced. Probe temperatures can reach a nadir of −180°C. Probe sizes used on this purpose are in 3 and 5 mm diameter. Straight- and flat-head probes can be used on this technique. These can be inserted eccentrically in the tumour and gradually moved towards its centre and towards the spinal cord and canal. Ultrasonography is used to monitor the ice ball of the cryotherapy, as well as to ensure that the spinal cord or spinal nerves are not affected. Cryotherapy treatment times varies from 5 to 10 min. Somatosensory-evoked potentials must be monitored during the procedure. The spinal cord and spinal nerves should be protected at all times from the probe. The extent of cryocoagulation is controlled using intraoperative ultrasonography (with a 12-mHz transducer) or by establishing physical separation of the spinal cord from the tumour. The echogenicity of the frozen tissue differs distinctly from that of the unfrozen tissue such that the extent of freezing is visible on the ultrasound and controlled accordingly. Following freezing of the tumour, the probe can be removed, and resection of the tumour is then conducted. The other advantages of this method besides the reduction of intraoperative bleeding are that it allows a more radical tumour excision, prevents intraoperative spillage from tumour content and therefore permits a better spinal reconstruction [37]. Harmonic scalpel (HS) is an ultrasonically activated coagulator which generates less heat and minimal smoke during surgery compared to electrocauterization (EC). The lower degree of heat generation causes less thermal injury to the tissue than regular EC. The outstanding quality of the HS is its ability to coagulate and cut vessels. Cakir et al. [38] made a cost-effective study with two matched blinded posterior spine surgery groups. The author concluded that the use of HS resulted in statistically significantly less intraoperative and post-operative less blood loss and less operating times than EC. Although the HS is an expensive device, the personnel costs for autologous blood predonation were not taken into consideration. This device was considered cost neutral in cases with major anticipated blood loss. The ultrasonic BoneScalpel™ (Bone Scalpel, Misonix, USA) is a tissue-specific device that allows to the surgeon to make precise osteotomies while protecting collateral or adjacent soft tissue structures. The device is comprised of a blunt ultrasonic blade that oscillates at over 22,500 cycles with an imperceptible microscopic amplitude. The recurring impacts pulverize the non-compliant crystalline structure resulting in a precise cut. The more compliant adjacent soft tissue is not affected by the ultrasonic oscillation. One recent paper reported an experience with 128 consecutive spine surgeries with the use of the ultrasonic scalpel [39]. The majority of the patients had previous spine surgeries and/or spinal deformity. In all cases, the ultrasonic scalpel was successfully used to create the needed osteotomies with high precision to facilitate the surgical procedure without percussion on the spinal column or injury to the underlying nerves. The major advantage (although difficult to objectively quantify) of this ultrasonic device is the reduction of bleeding which helps to create and maintain visibility in the surgical field. The authors have noticed that by virtue of the precision and ease of control (oscillation versus rotation), the efficiency of the surgery has improved. As a result, those often technically challenging osteotomy procedures can now be performed in less time with the ultrasonic scalpel. The ultrasonic scalpel uses a narrow blade with a self-irrigating system that provides lubrication and cooling into the cutting cavity and limits the risk of mechanical and thermal injury [40]. However, they reported one incident of dural tear from the overheating of the local tissue by the scalpel blade sitting in one position. It is imperative that the surgeon continues to move the device and not let it bind in one position. A total of 11 dural injuries (8.6%) occurred in their case series. Since majority of the patients had previous spine surgery and/or spinal deformity, this dural injury rate is comparable with previous reports [41].
4.3 Chemical Techniques
Depending on the procedure and location of the bleeding tissue, it may be impractical or impossible to effectively stop blood loss via mechanical or thermal haemostatic techniques. For example, in bony surfaces, parenchymal tissues, inflamed or friable vessels or tissues containing multiple and diffused capillaries, it is extremely difficult to maintain haemostasis with these methods. The use of effective pharmacological methods during surgery can be a useful option or an adjunct to other methods in these situations. The pharmacological methods seek to augment surgical haemostasis by enhancing the natural coagulative mechanisms or in reduction of bleeding by indirect effects as in case of specific anaesthesiology techniques. Neuraxial blockade is the term for central blocks involving the spinal, epidural and caudal spaces. While it is now an invaluable adjunct and even occasionally an alternative to general anaesthesia, its use is not a new phenomenon [42]. Regardless of the class of local anaesthetic, these drugs can be divided into ones that are short, intermediate or long acting. Lidocaine has traditionally been the agent of choice or slightly longer surgical procedures that require an intermediate-acting local anaesthetic. Some centres have also adopted the use of mepivacaine for its longer length of action with a similar onset profile. Of note is the potential for an increased incidence of hypotension due to venous pooling from the beta effects of epinephrine-containing solutions. This phenomenon seems to be especially true to patients receiving lumbar epidural anaesthesia. Hypotension can also occur which is attributed to the reduction of sympathetic outflow via opioid receptors in the sympathetic ganglia. Longer-acting local anaesthetics used for epidural anaesthesia typically consist of either bupivacaine or ropivacaine in varying concentrations.
Another class of analgesic adjuvants includes alpha-adrenergic agonists. Clonidine is the main drug used in this class due to its production as a preservative-free preparation. The effects of epidurally administered clonidine are seen as early as 20 min after injection, with peak effects occurring in 1 h. The analgesic potency has been described as being comparable to epidurally administered morphine [43]. Adding clonidine to opioids in the epidural space has an additive effect, which results in a lower dose of narcotic necessary for optimal pain control. This as a consequence diminishes the incidence of respiratory depression that potentially occurs with neuraxial opioids. Clonidine is lipophilic and as a result is quickly redistributed systemically despite neuraxial injection. It therefore has both central and peripheral effects. At lower doses, the central effects cause sympatholysis leading to hypotension, while the peripheral effects at higher doses cause vasoconstriction. Clonidine administered in the low thoracic or lumbar region typically produces blood pressure effects similar to that seen with intravenous administration [44]. When given in the mid or upper thoracic regions, epidurally administered clonidine causes an even greater decrease in blood pressure [45]. This more substantial drop in blood pressure is attributed to blocking thoracic dermatomes that contribute to sympathetic fibres innervating the heart. In addition to the hypotensive potential of clonidine, bradycardia and nausea with or without vomiting are also potential side effects. Controlled hypotensive anaesthesia (CHA) has been used for many years as a means of reducing intraoperative blood loss and facilitating surgical exposure. Reduced intraoperative blood pressure leads to a direct reduction in bleeding from surgically injured arteries and arterioles. Venous dilation, in turn, decreases venous bleeding, especially from cancellous bony sinuses that do not collapse when transected [46]. There have been a number of prospective trials demonstrating the efficacy of controlled hypotension, alone or in combination with other techniques, at reducing the blood loss and transfusion requirement of major spinal surgery [47, 48]. However, other studies showed that CHA does not decrease transfusion requirements compared with normotensive anaesthesia in scoliosis surgery [49]. Anaesthetic agents used to induce deliberate hypotension in current practice include the volatile gases (isoflurane, desflurane and sevoflurane) and intravenous sedative medications (thiopental and propofol). Interference with the ability to measure and compare somatosensory- or motor-evoked potentials is the principal limitation to using any anaesthetic agents to produce hypotension. Concerns about hypotension are addressed during spine surgeries. Hypotension makes the patient more susceptible to cardiac arrest if a sudden surgical catastrophe or if a massive haemorrhage or tension pneumothorax occurs. In adult or critically ill patients, the ischaemic threshold of individual organs is impossible to estimate, and because monitoring of perfusion is indirect at best, there have been occasional case reports of complications noted following uneventful anaesthetics [50]. New onset or worsening of neurologic deficit below the level of surgery may result from direct injury or overdistraction of the spinal cord, hypoperfusion or a combination of the two. Continuous electrophysiologic monitoring of either the anterior spinal cord (motor-evoked potentials) or posterior cord (somatosensory-evoked potentials) is the standard of care for most complex spinal surgeries. Electrical evidence of decreased spinal cord function should lead the provider to abandon the hypotensive technique, accepting the potential for increased haemorrhage in exchange for maximizing perfusion. Myocardial ischaemia or infarct is rare following hypotensive anaesthesia and is usually the result of unrecognized hypovolaemia and vasoconstriction, anaemia, occult coronary disease or a combination. Sudden desaturation has been described during controlled hypotension, which may put vulnerable patients at risk [51]. Risk factors for decreased myocardial reserve, such as advanced age, diabetes, atherosclerosis or resting hypertension, are all relative contraindications to controlled hypotension. These patients may already have flow-limited myocardial perfusion, as well as altered autoregulatory thresholds in other organ systems. Another possible side effect of hypotension is the development of perioperative ischaemic optic neuropathy (POION) during surgery. Although this complication is very rare in spinal surgery, with previous studies citing incidence rates between 0% and 0.12%, the condition is debilitating and is still a cause for concern [52]. These patients can complain loss of vision in one eye—characterized by loss of colour vision, visual field defect and relative afferent pupillary defect. Although the cause of POION is unclear, it is thought to be related to compromised blood flow to the optic nerve. The Johns Hopkins study reported that the four affected patients by POION experienced anaemia, hypotension or both, during surgery. Other possible risk factors were prone position, long procedure times and significant intraoperative hydration. It is generally accepted that surgeons and anaesthesiologists should aim for mean arterial blood pressure of 50–60 mmHg to provide safe and adequate hypotension during spinal surgery in healthy patients [53]. In 1999, in a randomized trial performed on 235 elderly patients, the mean intraoperative arterial blood pressure reduced to as low as 45–55 mmHg was equally as safe as the less hypotensive group’s mean pressure of 55–70 mmHg with respect to short- and long-term risks [54]. Local vasoconstrictors are used by infiltration of paraspinal muscles with epinephrine and ornipressin to reduce bleeding in many spine procedures [55]. However, no relation between intraoperative blood loss and the amount of injected epinephrine was observed [56]. The literature in this matter is scarce. Some reports of epinephrine usage in hypotensive epidural anaesthesia result in a reduced intraoperative bleeding in orthopaedic procedures [57]. Aprotinin, tranexamic acid (TXA) and epsilon aminocaproic acid (EACA) are drugs widely used in many types of surgeries as cardiac surgery, orthopaedic surgery and vascular surgery. Aprotinin is a non-specific, serine protease inhibitor, derived from the bovine lung, with antifibrinolytic properties. It acts as an inhibitor of several serine proteases, including trypsin, plasmin, plasma kallikrein and tissue kallikrein. Aprotinin also inhibits the contact phase activation of coagulation that both initiates coagulation and promotes fibrinolysis [58]. Drug regimen will not be given (see text below). TXA and EACA are synthetic lysine analogues (synthetic derivatives of the amino acid lysine) that act as effective inhibitors of fibrinolysis. TXA and EACA act principally by blocking the lysine binding sites on plasminogen molecules, inhibiting the formation of plasmin and therefore inhibiting fibrinolysis. Tranexamic acid is about 10 times more potent than aminocaproic acid and binds much more strongly to both the strong and weak sites of the plasminogen molecule than EACA [17]. TXA drug regimen usually given in patients with congenital bleeding disorders is 10 mg/kg and is suggested also for cardiac and major orthopaedic surgery. For complex procedures taking many hours, this could be followed by a maintenance infusion of 1 mg/kg/h. TXA is contraindicated in patients with renal and urethral pathologies because of the risk of clot formation and hydronephrosis [59]. EACA drug regimen usually given is 75–150 mg/kg followed by 12.5–30 mg/kg/h. EACA has been associated with hypotension, cardiac arrhythmias, myopathy and rhabdomyolysis but is the only available antifibrinolytic agent in some places. In 2013, the Cochrane Database of Systematic Reviews published a specific and a very comprehensive meta-analysis study with specific objective to assess all randomized controlled trials (RCTs) studying these drugs in respect of blood loss during surgery in adult patients, who need for red blood cell (RBC) transfusion, and adverse events, particularly vascular occlusion, renal dysfunction and death [60]. This review summarizes data from 252 RCTs that recruited over 25,000 participants. Of the 252 included trials, 173 were conducted in cardiac surgery, 53 trials were in orthopaedic surgery, 14 involved liver surgery, 5 were conducted in vascular surgery, 4 involved thoracic surgery, 1 involved gynaecological surgery, 1 involved neurosurgery and 1 trial was in orthognathic surgery.
Twenty trials of TXA versus control involving orthopaedic surgery reported total blood loss data (intraoperative and post-operative blood loss combined). These trials included a total of 1201 patients, of whom 605 were randomized to TXA and 596 were randomized to a control group. The use of TXA in orthopaedic surgery significantly reduced the total amount of blood lost during the perioperative period (MD −446.19 mls, 95% CI −554.61 to −337.78 mls). Heterogeneity between these trials was statistically significant (Chi2 = 85.30, df = 19, P < 0.00001; I2 = 78%). The use of TXA was not associated with an increased risk of myocardial infarction stroke, DVT renal failure or renal dysfunction. Of the 65 trials of TXA that provided data on the number of patients exposed to allogeneic blood transfusion, 28 were assessed as having adequate allocation concealment of treatment schedule. For these 28 trials, the use of TXA reduced the rate of allogeneic blood transfusion by a relative 41% (RR 0.59, 95% CI 0.51–0.69). The use of EACA in orthopaedic surgery reduced blood loss during the perioperative period by around 300 mls per patient (MD −299.69 mls, 95% CI −522.54 to −76.84 mls). Heterogeneity between these trials was not statistically significant (Chi2 = 0.73, df = 1, P = 0.39; I2 = 0%). The use of EACA was not associated with an increased risk of myocardial infarction, stroke, DVT, pulmonary embolism or renal failure/dysfunction. Of the 16 trials that provided data on the number of patients exposed to allogeneic blood transfusion, 5 were assessed as having adequate allocation concealment of treatment schedule. For these trials, the use of EACA did not statistically significantly reduce the rate of allogeneic blood transfusion (RR 0.82, 95% CI 0.58–1.16). Data from the head-to-head trials suggest an advantage of aprotinin over the lysine analogues TXA and EACA in terms of reducing perioperative blood loss, but the differences were small. In 2008, a large pharmacoepidemiological study by Schneeweiss et al. [61] confirmed the increased risk of death with aprotinin. After adjustment, the estimated risk of death was 64% higher in the aprotinin group than in the aminocaproic acid group (relative risk, 1.64; 95% confidence interval [CI], 1.50–1.78). This difference remained statistically significant after a range of analytical procedures including a propensity score-matched analysis and an instrumental variable analysis. Consequently, the balance of benefit and harm favours the use of the lysine analogues over aprotinin and justifies the regulatory action that resulted in the withdrawal of aprotinin from international markets in 2008 [62]. This study concluded that tranexamic acid and epsilon aminocaproic acid provide worthwhile reductions in blood loss and the need for allogeneic red cell transfusion in adult surgical patients. Based on the results of randomized trials, their efficacy does not appear to be offset by serious adverse effects. The evidence is stronger for tranexamic acid than for epsilon aminocaproic acid. Another study published on the Cochrane Database of Systematic Reviews assess the efficacy and safety of aprotinin, tranexamic acid and aminocaproic acid in reducing blood loss and transfusion requirements in children undergoing scoliosis surgery including children and patients under 18 years of age [63]. The total number of participants in the included studies was 254, of whom 127 received placebo and 127 received antifibrinolytic drugs. Antifibrinolytic drugs decreased the amount of blood transfused by 327 mL and the amount of blood loss by 427 mL. However, the actual need of transfusion was not significantly decreased. Although no deaths or adverse events were noted with the use of these antifibrinolytic drugs, the number of children evaluated was too small and the duration of follow-up too short to draw any conclusion on their safety. The risk of being transfused was 13% lower in patients receiving antifibrinolytic drugs; however, the difference was not statistically significant (95% confidence interval (CI), 0.67–1.12). The authors concluded that antifibrinolytic drugs decrease blood loss in children undergoing scoliosis surgery and, therefore, could be added to the armamentarium of available techniques to reduce bleeding. Although the decrease in blood loss can be considered clinically important, whether antifibrinolytic drugs reduce the need for transfusions remains unclear. Further studies will be needed to clarify the efficacy of this class of drugs on this purpose. Erythropoietin (EPO) is a hormone that regulates erythropoiesis, acting on erythroid colony-forming units by stimulating progenitor cell differentiation in the bone marrow. EPO accelerates maturation of proerythroblasts to reticulocytes, stimulates the synthesis of haemoglobin (Hb) and promotes the release of reticulocytes to the circulation and their differentiation into mature red blood cells [64]. Recombinant human erythropoietin (rHuEPO) is a biosynthetic form of the natural hormone having the same biochemical structure and biological effect [65]. There are two types of rHuEPO: the alpha type and the beta type, presented in lyophilized form and reconstituted with saline solution [66]. Recombinant human erythropoietin can be administered intravenously or subcutaneously. It reaches peak plasma concentration faster by the intravenous route, although the half-life is shorter (5–10 h) [67]. Availability is lower with subcutaneous administration, but the half-life is longer (12–18 h), and this route is usually recommended for the management of perioperative anaemia [68]. Initially, the recommended dose was 300 IU/kg/day during 15 days [15, 69]. However, Goldberg demonstrated that a weekly dose of 600 IU/kg starting 3 weeks before the procedure achieved a higher increase in haemoglobin (1.44 g/dL versus 0.73 g/dL) with comparable safety and efficacy and lower cost [70]. Current recommended dosage is 600 IU/kg of subcutaneous rHuEPO weekly, given over 3 weeks (days 21, 14 and 7 before the surgical procedure) and on the day of surgery. In practice, administration of a 40,000 IU vial weekly is recommended in adults with a mean weight of around 65 kg. The 40,000 IU dose is authorized exclusively for preoperative rHuEPO treatment. When haemoglobin levels reach 15 g/dL at any of the preoperative analyses, rHuEPO administration is interrupted indefinitely [71]. Blood management is most difficult in adult patients with complex deformities requiring very aggressive surgery with circumferential approaches and long spinal instrumentation. Colomina et al. made a specific study in spine surgery and divided their patients in three groups, one who did not received EPO (degenerative and deformity cases) and other two groups who received EPO (degenerative (>2 levels) and adult deformity cases, respectively). Two groups received rHuEPO based on their protocol: the adult deformity patients, 98% complied with AUT estimations and only 14.6% required allogenic blood transfusion. In patients with degenerative cases (>2 levels) of surgery and a predicted blood loss of around 50% of total volume, nearly 97% did not require allogenic transfusions when rHuEPO treatment was associated with the AUT programme [72]. The authors conclude that surgery involving blood losses under 30% of the patient’s total volume can be accomplished without the need for allogenic transfusion, if the baseline haemoglobin level is greater than 13 g/dL. Recombinant human erythropoietin administration is very effective for attaining this level and can be used as the only blood-sparing technique in these patients. When the expected blood loss is around 50% of the total volume, AUT is the standard technique applied. Addition of rHuEPO to AUT in these patients improves haemoglobin levels and facilitates retrieval of the autologous blood units required. This combination can avoid allogenic transfusion in 95% of the patients. When surgery is associated with a predicted blood loss close to the patient’s total blood volume, the combined blood-sparing technique should always be used. Despite the complexity and aggressiveness of the procedure, with the combination of AUT and rHuEPO, more than 80% of the patients operated will not require allogenic transfusions. The disadvantages of rHuEPO therapy include side effects and cost. Side effects may include hypertension, myocardial infarction, angina and deep venous thrombosis, though previous literature showed that these effects were not increased compared with control groups [73]. The cost of the epoetin alfa dose regimen, a specific type of rHuEPO, is approximately US$400 per injection, or a total of US$1600 in the USA [74]. Desmopressin: Deamino-8-d-arginin vasopressin (DDVAP or desmopressin) is an analogue of the natural hormone vasopressin or antidiuretic hormone (ADH). It increases the release of factor VIIc and von Willebrand factor (vWF) from endothelial cells, along with a paradoxical increase of plasminogen activator and prostaglandins. DDAVP, originally developed and licensed for the treatment of inherited defects of haemostasis, given by slow intravenous infusion at a dose of 0.3 μg/kg, acts by releasing ultralarge von Willebrand factor multimers from endothelial cells, leading to an enhancement of primary haemostasis [75]. Despite the successful use of DDAVP in patients with von Willebrand disease, congenital platelet disorders, renal failure, cirrhosis and long-term salicylate treatment, its effectiveness during spinal surgery has been controversial, as it is based on a small number of experimental studies [76]. Carless et al. published a systematic review about desmopressin use for minimizing allogenic blood transfusion in patients without congenital bleeding disorders failing to show any real and effective efficacy on this matter [77]. However, in patients with spinal disorders with von Willebrand disease requiring surgical treatment, a very specific and detailed preoperative surgical strategy will be needed using this drug for successful treatment [78]. Absorbable topical haemostatic agents have since been developed and provide useful adjunctive therapy when conventional methods of haemostasis are ineffective or impractical. Topical haemostatic agents can be applied directly to the bleeding site and may prevent continuous unrelenting bleeding throughout the entire procedure and into the post-operative recovery period. They are in the market in multiple types and options.
Collagen-based products: The efficacy of collagen-derived haemostatic agents has been established in standardized animal studies and clinically in man [79, 80]. They lead to thrombocyte adhesion and activation of coagulation factor XII (Hageman factor). Like gelatin products, collagen-based haemostatics can be of bovine, porcine or equine origin and are available in different forms, such as sheets, powder or aggregates. As they can cause adhesion formation and immune reactions, they should not be left in the spinal canal. They can interfere with bone healing and cause allergic reactions and infection. This should be applied dry with clean and dry instruments, and pressure with gloved fingers should never be placed, as the collagen-based products would adhere to the glove more than on the haemorrhage site. Post-operative adhesion of the haemostatic agent to neural structures is possible. Therefore, it is recommended to tease way the excess of product after 5–10 min. Swelling occurs, although less so than with other products, and surgeons should be aware of this when these products are left in place in rigid compartments [23]. Oxidized cellulose presents multiple mechanisms of action, including physical and mechanical actions in tamponade, blood absorption, swelling and gel formation and then surface interactions with proteins, platelets and intrinsic and extrinsic pathway activation. One major advantage of oxidized cellulose is its definite and potent action against a wide variety of pathogenic organisms, both in vivo and in vitro. This beneficial effect is immediate and is exerted by a low pH effect. The current theory is that this chemical haemostatic reduces the effective initial inoculum with an acid hostile ambient, allowing the host’s natural defences to overcome the organism [81]. Activation of the initial coagulation phase is caused by their surface effect. They also induce a moderate acceleration of fibrinogen polymerization and seem to act as a caustic haemostat by decreasing pH [82]. Bacteriostatic properties of this product should be preferred in particularly contaminated fields. Obviously, should this event occur, the use of no agent at all is always better. Wadding or packing in rigid cavities (neural foramina) should be avoided, due to risks related to swelling phenomena. Gelatin-based: Absorbable gelatin sponges are available in multiple formulations (sheds, powder, foam). Except for thrombin-soaked formulations, their haemostatic effect appears to be a physical effect rather than a “surface effect”. When compared with collagen-based products, gelatin sponges have been reported to form a better quality clot [83]. During spine procedures, attention should be paid to remove the excess, and the surgeon should be aware that gelatin can interfere with bone healing. In infected spaces, its use is contraindicated, because it may enhance the infectious process. It could be suggested placing it dry with moderate pressure on the bleeding site. This agent can double in volume by swelling and can also cause compressive complications. If soaked in thrombin, GF has an increased haemostatic action. Thrombin: The active topical haemostatic agent thrombin has been widely used in surgery for years. The agent has had a long history of clinical efficacy and safety in many surgical procedures [84]. Unlike passive agents, which rely on the presence of normal clotting processes, however, the active agent thrombin acts at the end of the clotting cascade, rendering its action less susceptible to coagulopathies caused by clotting factor deficiencies or platelet malfunction [85]. Thrombin can provide a logical and useful choice in patients receiving antiplatelet and/or anticoagulation agents, which is occurring in an increasing proportion of surgical cases [10]. Thrombin relies on the presence of fibrinogen in the patient’s blood; however, it is ineffective in patients who have afibrinogenaemia, a rare condition reported to affect one in one million people. Additionally, thrombin itself does not need to be removed from the site of bleeding before wound closure, unlike many of the passive topical haemostatic agents, and degeneration and reabsorption of the resulting fibrin clot are achieved during normal wound healing [86]. The sprayable formulation of thrombin offers potential advantages in the surgical setting because wide surfaces can be treated instantaneously without the need for tamponade. Awareness of the potential of bovine thrombin products to induce antibodies has been raised; however, the clinical implications are still largely unclear [87]. Although many patients will demonstrate no clinical or laboratory abnormalities after the development of antibodies to thrombin preparations, abnormalities in blood coagulation tests are occasionally reported and have rarely been fatal [88]. Fibrin sealants are sterile, virally inactivated preparation of purified human fibrinogen and thrombin. Fibrin glue bypasses the clotting cascade by catalysing the conversion of fibrinogen into fibrin to immediately produce activated clotting factors [89]. Stimulating the formation of a fibrin clot, fibrin “glues” initiate the final stage of the clotting cascade. Two major families can be distinguished: the combination of a fibrinogen component together with a thrombin/Ca solution and, more recently, a collagen/thrombin component that uses the endogenous fibrinogen of the bleeding source. There are several concerning issues regarding the use of fibrin glue in the setting of lumbar spine surgery. From a practical standpoint, the approximate 20-min preparation time can be relatively significant when approaching the end of an operation. Once applied, the coagulum needs 3–5 min to attain optimal adherence and 2 h to reach its ultimate strength. The feasibility of providing appropriate setting conditions during bleeding, irrigation, building intradural pressure and movement remains questionable. There is also concern regarding the effect of fibrin glue on bone formation. Although some studies have shown fibrin glue to be a successful scaffold for bone growth when combined with osteoinductive or osteoconductive substances, multiple animal studies have raised concern that fibrin glue may inhibit bony fusion, even when using recombinant bone morphogenetic proteins [90, 91]. A Cochrane review on the use of fibrin sealants in surgery suggests efficacy, but this conclusion is hampered by the small number of trials and limitations in the methodology. Fibrin sealants have been reported to be effective in spine surgery and to diminish scar formation [92, 93].
Haemostatic matrices: The bicomponent medium of the second family of fibrin glues contains thrombin and a gelatin matrix. A tamponade effect of the swelling collagen granules restricts bleeding, while the gelatin matrix provides the structural integrity required to remain in situ. Its main advantage is that it can easily be used on wet and bleeding tissues, and favourable results have been reported in spinal procedures [10].
5 Assessing the Risk of Blood Transfusions in Spine Surgery
Many researches on literature discuss and try to define predictors that can be used to estimate the risk or probability of blood transfusion in elective spine procedures. Nuttall et al. made a retrospective analysis of 244 patients who have submitted to spine procedures and after statistical linear multiple regression modelling concluded that low preoperative haemoglobin concentration, tumour surgery, increased number of posterior levels surgically fused, history of pulmonary disease, less amount of autologous blood available and no use of the Jackson table were significant determinants of the number of allogeneic RBC units transfused on the day of surgery. In the current study, idiopathic scoliosis was not found to be a predictor of increased RBC or coagulation product transfusions. A benefit from the use of hypotensive anaesthesia technique was not found in their study [94]. In another retrospective study, Lenoir et al. analysed 230 patients who were submitted to spine procedures [95]. Patients’ data were analysed in a retrospective study trying to identify an individual probability of allogeneic transfusion in adult patients undergoing elective thoracolumbar spine surgery. These patients were submitted in a specific anaesthesia and transfusion protocols, and all patients received tranexamic acid in a continuous intravenous infusion until the skin closed. Different factors collected were tested in statistic techniques. The authors created a specific score including age, preoperative haemoglobin level, number of spine fusion levels and osteotomy as follows (PMTSS (predictive model of transfusion in spine surgery) score calculation, see Table 17.4).
Predictive model of transfusion in spine surgery (PMTSS) is calculated as the arithmetic sum of points assigned to each item, except in case of osteotomy, where the maximum number of points [4] is allocated in any case. When age <50 years, fusion level <2, haemoglobin (Hb) >14 or no osteotomy is planned, 0 points are, respectively, allocated for each item. The score is then comprised between 0 and 4 and defined five distinct levels of allogeneic transfusion risk.
Patients with score total above two points should be screened to preoperative blood-sparing techniques to reduce allogeneic blood transfusion. However, this study has some specific limitations recognized by the own authors; perhaps, this scale nowadays cannot be so precise because some new recent minimal invasive techniques used in spine surgery today that can be an effective treat to many spinal pathologies without no such bleeding were used before. Studied relationship between preoperative levels of fibrinogen, bleeding and transfusion requirements in 82 consecutive adolescent idiopathic scoliosis patients. They analysed specific factors, all patients were submitted again a specific anaesthetic protocol and all cases received tranexamic acid intravenously at the initiation of all procedures. Intraoperative and post-operative blood volumes were recorded. In their study, when the predictive values of a fibrinogen concentration in the lower quartile (<2.8 g/L) on extensive bleeding and transfusions were calculated, high negative predictive values were obtained (94% and 88%, respectively), while the positive predictive values were low (47% and 30%, respectively). This means that for an individual patient with a fibrinogen value above 2.8 g/L, it is unlikely that the patient will bleed extensively during or after the procedure. Lower fibrinogen concentrations on the other hand do not necessarily result in a high bleeding volume or transfusion rate but provide information that these patients have an increased risk for these events. One may speculate if patients with high risk for bleeding complications and preoperative fibrinogen levels in the lower normal range may benefit from prophylactic fibrinogen administration. Fibrinogen concentrations obtained from human plasma are commercially available and currently used to treat inherited and acquired fibrinogen deficiency [96]. In a recent pilot study in cardiac surgery, patients who received prophylactic treatment with 2 g fibrinogen had a reduction in post-operative bleeding with 32% [3]. As seen in other studies, the authors also suggest that other studies will be needed to answer the significance of their results. As we can see, there are a growing number of tools and different strategies that can be used to avoid or lower the amount of allogeneic blood transfusion. These strategies must be organized and fashioned in specific situations to accomplish this objective. In a near future, other drugs and solutions will be part of our everyday routine.
References
Lawson JH. The clinical use and immunologic impact of thrombin in surgery. Semin Thromb Hemost. 2006;32(Suppl 1):98–110.
Samudrala S. Topical hemostatic agents is surgery: a Surgeon’s perspective. AORN J. 2008;88:S1–11.
Karlsson M, Ternstrom L, Hyllner M, et al. Prophylactic fibrinogen infusion reduces bleeding after coronary artery bypass surgery. A prospective randomised pilot study. Thromb Haemost. 2009;102:137–44.
Elgafy H, Bransford RJ, McGuire R, Dettori J, Fischer D. Are there effective measures to decrease massive hemorrhage in major spine fusion surgery? Spine. 2010;35(95):S47–56.
Hu SS. Blood loss in adult spinal surgery. Eur Spine J. 2004;13(Suppl 1):S3–5.
Block JE. Severe blood loss during spinal reconstructive procedures: the potential usefulness of topical hemostatic agents. Med Hypotheses. 2005;65(3):617–21.
Bochicchio G, Dunne J, Bochicchio K, Scalea T. The combination of platelet-enriched autologous plasma with bovine collagen and thrombin decreases the need for multiple blood transfusions in trauma patients with retroperitoneal bleeding. J Trauma. 2004;56(1):76–9.
Carreon LY, Puno RM, Dimar JR 2nd, Glassman SD, Johnson JR. Perioperative complications of posterior lumbar decompression and arthrodesis in older adults. J Bone Joint Surg Am. 2003;85(11):2089–92.
McDonnell MF, Glassman SD, Dimar JR 2nd, Puno RM, Johnson JR. Perioperative complications of anterior procedures on the spine. J Bone Joint Surg Am. 1996;78(6):839–47.
Renkens KL Jr, Payner TD, Leipzig TJ, et al. A multicenter, prospective, randomized trial evaluating a new hemostatic agent for spinal surgery. Spine. 2001;26(15):1645–50.
Ridgeway S, Tai C, Alton P, Barnardo P, Harrison DJ. Pre-donated autologous blood transfusion in scoliosis surgery. J Bone Joint Surg Br. 2003;85:1032–6.
García-Erce J, Muñoz M, Bisbe E, Sáez M, Solano VM, Beltrán S, Ruiz A, Cuenca J, Vicente-Thomas J. Predeposit autologous donation in spinal surgery: a multicenter study. Eur Spine J. 2004;13(Suppl 1):S34–9.
Keating EM, Meding JB. Perioperative blood management practices in elective orthopaedic surgery. J Am Acad Orthop Surg. 2002;10:393–400.
Henry D A, Carless P A, Moxey A J, O'Connell D, Ker K, Fergusson DA. Pre-operative autologous donation for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2001;(5):CD003602. https://doi.org/10.1002/14651858.CD003602.pub2.
Carless P, Moxey AJ, O'Connell D, Henry D. Autologous transfusion techniques: a systematic review of their efficacy. Transfus Med. 2004;14:123–44.
McVay PA, Andrews A, Hoag MS, Polan D, Skettino S, Stehling LC. Moderate and severe reactions during autologous blood donations are no more frequent than during homologous blood donations. Vox Sang. 1990;59:70–2.
Faught C, Wells P, Fergusson D, Laupacis A. Adverse effects of methods for minimizing perioperative allogeneic transfusion: a critical review of the literature. Transfus Med Rev. 1998;12:206–25.
Muñoz M, García-Vallejo JJ, Ruiz MD, Romero R, Olalla E, Sebastían C. Transfusion of post-operative shed blood: laboratory characteristics and clinical utility. Eur Spine J. 2004;13(Suppl 1):S107–13.
Chanda A, Smith DR, Nanda A. Autotransfusion by cell saver technique in surgery of lumbar and thoracic spinal fusion with instrumentation. J Neurosurg. 2002;96(Suppl 3):298–303.
Reitman CA, Watters WC 3rd, Sassard WR. The CS in adult lumbar fusion surgery: a cost-benefit outcomes study. Spine. 2004;29:1580–1583. discussion on p. 1584.
Sebastián C, Romero R, Olalla E, Ferrer C, García-Vallejo JJ, Muñoz M. Postoperative blood salvage and reinfusion in spinal surgery. Blood quality, effectiveness and impact on patient blood parameters. Eur Spine J. 2000;9:458–65.
Muñoz M, Sánchez-Arrieta Y, García-Vallejo JJ, Mérida FJ, Ruiz MD, Maldonado J. Autotransfusión pre y posoperatoria. Estudio comparativo de la hematología, bioquímica y metabolism eritrocitário en sangre predonada y sangre de drenaje postoperatorio. Sangre (Bar). 1999;44:433–50.
Schonauer C, Tessitore E, Barbagallo G, Albanese V, Moraci A. The use of local agents: bone wax, gelatin, collagen, oxidized cellulose. Eur Spine J. 2004;13(Suppl 1):S89–96.
Howard TC, Kelley RR. The effect of bone wax on the healing of experimental rat tibial lesions. Clin Orthop. 1969;63:226–32.
Geary JR, Frantz VK. New absorbable hemostatic bone wax. Experimental and clinical studies. Ann Surg. 1950;132:1128–37.
Johnson P, Fromm D. Effects of bone wax on bacterial clearance. Surgery. 1981;89(2):206–9.
Guzman R, Dubach-Schwizer S, Heini P, Lovblad K, Kalbermatten D, Schroth G, Remonda L. Preoperative transarterial embolization of vertebral metastases. Eur Spine J. 2005;14:263–8.
Boland PJ, Lene JM, Sundersen N. Metastatic disease of spine. Clin Orthop. 1982;1169:95–102.
Shi HB, Suh DC, Lee HK, Lim SM, Kim DH, Choi CG, Lee CS, Rhim SC. Preoperative transarterial embolization of spinal tumors: embolization techniques and results. AJNR Am J Neuradiol. 1999;20:2009–15.
Görich J, Solymosi L, Hasan I, Sittek H, Majdali R, Reiser M. Embolisation von Knochenmetastasen. Radiologie. 1995;35:55–9.
Manke C, Bretschneider T, Lenhart M, Strotzer M, Neumann C, Gmeinwieser J, Feuerbach S. Spinal metastases from renal cell carcinoma: effect of preopertative embolization on intraoperative blood loss. AJNR Am J Neuroradiol. 2001;22:997–1003.
Cory DA, Fritsch SA, Cohen MD, et al. Aneurysmal bone cysts: imaging findings and embolotherapy. Am J Roentgenol. 1989;153:369–73.
Esparza J, Castro S, Portillo JM, et al. Vertebral hemangiomas: spinal angiography and preoperative embolization. Surg Neurol. 1978;10:171–3.
Chiras J, Gaston A, Gaveau T et al. [Preoperative embolization in spinal pathology. Apropos of 21 cases]. J Radiol. 1983;64:397-403 (Fr).
Gellad FE, Sadato N, Numaguchi Y, et al. Vascular metastatic lesions of the spine: preoperative embolization. Radiology. 1990;176:683–6.
Vetter SC, Strecker EP, Ackermann LW, Harms J. Preoperative embolization of cervical spine tumors. Cardiovasc Intervent Radiol. 1997;20:343–7.
Nader R, Brent T, Nauta HJW, Crow W, vanSonnenberg E, Hadjipavlou A. Preoperative embolization and intraoperative cryocoagulation as adjuncts in resection of hypervascular lesions of the thoracolumbar spine. J Neurosurg Spine. 2002;97:294–300.
Cakir B, Ulmar B, Schmidt R, Kelsch G, Geiger P, Mehrkens H, Puhl W, Richter M. Efficacy and cost effectiveness of harmonic scalpel compared with electrocautery in posterior instrumentation of the spine. Eur Spine J. 2006;15:48–54.
Hu X, Ohmiess D, Lieberman I. Use of an ultrasonic osteotome device in spine surgery: experience from the first 128 patients. Eur Spine J. 2013;22(12):2845–9. https://doi.org/10.1007/s00586-013-2780-y.
Sanborn MR, Balzer J, Gerszten PC, Karausky P, Cheng BC, Welch WC. Safety and efficacy of a novel ultrasonic osteotome device in a ovine model. J Clin Neurosci. 2011;18:1528–33.
McMahon P, Dididze M, Levi AD. Incidental durotomy after spinal surgery: a prospective study in an academic institution. J Neurosurg. 2012;17:30–6.
Corning J. Spinal anesthesia and local medications of the cord. N Y J Med. 1885;42:483–5.
Tamsen A, Gordh T. Epidural clonidine produces analgesia. Lancet. 1984;2(8396):231–2.
De Kock M, Crochet B, Morimont C, Scholtes JL. Intravenous or epidural clonidine for intra- and postoperative analgesia. Anesthesiology. 1993;79(3):525–31.
De Kock M. Site of hemodynamic effects of alpha sub 2-adrenergic agonists. Anesthesiology. 1991;75:715–6.
Dutton RP. Controlled hypotension for spinal surgery. Eur Spine J. 2004;13(Suppl 1):S66–71.
Malcolm-Smith NA, McMaster MJ. The use of induced hypotension to control bleeding during posterior fusion for scoliosis. J Bone Joint Surg Br. 1983;65:255–8.
Mandel RJ, Brown MD, McCullough NC, et al. Hypotensive anestesia and autotransfusion in spinal surgery. Clin Orthop. 1981;154:27.
Lennon RL, Hosking MP, Gray JR, et al. The effects of intraoperative blood salvage and induced hypotension on transfusion requirements during spinal surgical procedures. Mayo Clin Proc. 1987;62:1090–4.
Murphy MA. Bilateral posterior ischemic optic neuropathy after lumbar spine surgery. Ophthalmology. 2003;110:1454–7.
Bernard JM, Le Penven-Henninger C, Passuti N. Sudden decreases in mixed venous oxygen saturation during posterior spinal fusion. Anesth Analg. 1995;80:1038–41.
Chang SH, Miller NR. The incidence of vision loss due to perioperative ischemic optic neuropathy associated with spine surgery: the Johns Hopkins Hospital experience. Spine. 2005;30(11):1299–302.
Urmey WF. Combined regional and general anesthesia for orthopedic spine fusion surgery. Tech Reg Anesth Pain Manag. 2000;4(2):101–5.
Williams-Russo P, Sharrock NE, Mattis S, et al. Randomized trial of hypotensive epidural anesthesia in older adults. Anesthesiology. 1999;91(4):926–35.
Szpalski M, Gunzburg R. Management of haemostasis in spine surgery (touch breefings). Eur Musc Rev. 2008:53–7.
Guay J, Haig M, Lortie L, et al. Predicting blood loss in surgery for idiopathic scoliosis. Can J Anaesth. 1994;41:775–81.
Kiss H, Raffl M, Neumann D, et al. Epinephrine-augmented hypotensive epidural anesthesia replaces tourniquet use in total knee replacement. Clin Orthop Relat Res. 2005:184–9.
Fritz H, Wunderer G. Biochemistry and applications of aprotinin, the kallikrein inhibitor from bovine organs. Arzneimittelforschung. 1983;33:479–94.
Schulman S. Pharmacologic tools to reduce bleeding in surgery. Hematology. 2012;2012:517–21.
Henry DA, Carless PA, Moxey AJ, O'Connell D, Stokes BJ, Fergusson DA, Ker K. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011;(5):CD001886. DOI: https://doi.org/10.1002/14651858.CD001886.pub1.
Schneeweiss S, Seeger JD, Landon J, Walker AM. Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med. 2008;358:771–83.
Manufacturer removes remaining stocks of Trasylol [press release]. Silver Spring, MD: US Food and Drug Administration; 2008. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116895.htm
A T, Cepeda MS, Schumann R, Carr DB. Antifibrinolytic agents for reducing blood loss in scoliosis surgery in children. Cochrane Database Syst Rev. 2008;(5):CD006883. https://doi.org/10.1002/14651858.CD006883.pub3.
Browne JK, Cohen AM, Egrie JC, Lai PH, Lin FK, Strickland T, Watson E, Stebbing N. Erythropoietin: gene cloning, protein structure, and biological properties. Cold Spring Harb Symp Quant Biol. 1986;51(1):693–702.
Cheung W, Minton N, Gunawardena K. Pharmacokinetics and pharmacodynamics of epoetin alfa once weekly and three times weekly. Eur J Clin Pharmacol. 2001;57:411–8.
Rosencher N, Ozier Y. Peri-operative use of EPO. Transfus Clin Biol. 2003;10:159–64.
Rosencher N, Woimant G, Ozier Y, Conseiller C. Preoperative strategy for homologous blood salvage and peri-operative erythropoietin. Transfus Clin Biol. 1999;6:370–9.
Goodnough LT, Monk TG, Andriole GL. Erythropoietin therapy. N Engl J Med. 1997;336:933–8.
de Andrade JR, Jove M, Landon G, Frei D, Guilfoyle M, Young DC. Baseline hemoglobin as a predictor of risk of transfusion and response to epoetin alfa in orthopedic surgery patients. Am J Orthop. 1996;25:533–42.
Goldberg MA. Perioperative epoetin alfa increases red blood cell mass and reduces exposure to transfusions: results of randomized clinical trials. Semin Hematol. 1997;34:41–7.
Stowell CP, Chandler H, Jove M, Guilfoyle M, Wacholtz MC. An open-label, randomized study to compare the safety and efficacy of perioperative epoetin alfa with preoperative autologous blood donation in total joint arthroplasty. Orthopedics. 1999;22:s105–12.
Colomina M, Bagó J, Pellsé F, Godet C, Villanueva C. Preoperative erythropoietin in spine surgery. Eur Spine J. 2004;13(Suppl 1):S40–9.
de Andrade JR, Frei D, Guilfoyle M. Integrated analysis of thrombotic/vascular event occurrence in epoetin alfa–treated patients undergoing major, elective orthopedic surgery. Orthopedics. 1999;22(1 Suppl):S113–8.
Shapiro GS, Boachi-Adjei O, Dhawlikar SH, Maier LS. The use of epoetin alfa in complex spine deformity surgery. Spine. 2002;27(18):2067–71.
Ruggeri ZM, Mannucci PM, Lombardi R, Federici AB, Zimmerman TS. Multimeric composition of factor VIII/von Willebrand factor following administration of DDAVP: implications for pathophysiology and therapy of von Willebrand’s disease subtypes. Blood. 1982;59:1272–8.
Kovesi T, Royston D. Pharmacological approaches to reducing allogeneic blood exposure. Vox Sang. 2003;84:2–10.
Carless P A, Stokes B J, Moxey A J, Henry DA. Desmopressin use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2004;(5):CD001884. https://doi.org/10.1002/14651858.CD001884.pub2.
Bolan C, Rick M, Polly D Jr. Transfusion medicine management for reconstructive spinal repair in a patient with von Willebrand’s disease and a history of heavy surgical bleeding. Spine. 2001;26(23):E552–6.
Orgill DP, Ehret FW, Regan JF, et al. Polyethylene glycol/microfibrillar collagen composite as a new resorbable hemostatic bone wax. J Biomed Mater Res. 1998;39:358–63.
Kruger J. Blutstillung bei neurochirurgischen Operationen. Eine Vergleichsstudie zwischen einem Kollagenvlies (LyostyptR) und einem Gelatine-Schwammchen (MarbagelanR). Zentralbl Neurochir. 1992;53:33–6.
Wagner WR, Pachence JM, Ristich J, Johnson PC. Comparative in vitro analysis of topical hemostatic agents. J Surg Res. 1996;66:100–8.
Levy ML, Day DJ, Fukushima T. Surgicel fibrillar absorbable oxidized regenerated cellulose. Neurosurgery. 1997;41:701–2.
Dehen M, Niederdellmann H, Lachner J. Mechanical properties of collagen or gelatine-stabilized blood clots. Dtsch Zahnarztl Z. 1990;45:553–6.
Lundblad RL, Bradshaw RA, Gabriel D, Ortel TL, Lawson J, Mann KG. A review of the therapeutic uses of thrombin. Thromb Haemost. 2004;91(5):851–60.
Oz MC, Rondinone JF, Shargill NS. FloSeal Matrix: new generation topical hemostatic sealant. J Card Surg. 2003;18(6):486–93.
Morikawa T. Tissue sealing. Am J Surg. 2001;182(2 Suppl):29S–35S.
Ortel TL, Charles LA, Keller FG, et al. Topical thrombin and acquired coagulation factor inhibitors: clinical spectrum and laboratory diagnosis. Am J Hematol. 1994;45(2):128–35.
Winterbottom N, Kuo JM, Nguyen K, et al. Antigenic responses to bovine thrombin exposure during surgery: a prospective study of 309 patients. J Appl Res. 2002;2(1) http://jrnlappliedresearch.com/articles/Vol2Iss1/winterbottom.htm
Weinstein JN, Tosteson TD, Lurie JD, Tosteson AN, Hanscom B, Skinner JS, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA. 2006;296:2441–50.
Turgut M, Erkus M, Tavus N. The effect of fibrin adhesive (Tisseel) on interbody allograft fusion: an experimental study with cats. Acta Neurochir. 1999;141:273–8.
Zarate-Kalfopulos B, Estrada-Villasenor E, Lecona-Buitron H, Arenas-Sordo Mde L, Garza-Hernandez AC, Reyes-Sanches A. Use of fibrin glue in combination with autologous bone graft as bone enhancer in posterolateral spinal fusion. An experimental study in New Zealand rabbits. Cir Cir. 2007;75:201–5.
Tredwell SJ, Sawatzky B. The use of fibrin sealant to reduce blood loss during Cotrel-Dubousset instrumentation for idiopathic scoliosis. Spine. 1990;15:913–5.
Carless PA, Anthony DM, Henry DA. Systematic review of the use of fibrin sealant to minimize perioperative allogeneic blood transfusion. Br J Surg. 2002;89:695–703.
Nuttall G, Horlocker T, Santrach P, Oliver W, Dekutoski M, Bryant S. Predictors of blood transfusions in spinal instrumentation and fusion surgery. Spine. 2000;25(5):596–601.
Lenoir B, Merckx P, Paugam-Burtz C, Dauzac C, Agostini M, Guigui P, Mantz J. Individual probability of allogeneic erythrocyte transfusion in elective spine surgery. Anesthesiology. 2009;110:105–1060.
Fenger-Eriksen C, Lindberg-Larsen M, Christensen AQ, et al. Fibrinogen concentrate substitution therapy in patients with massive haemorrhage and low plasma fibrinogen concentrations. Br J Anaesth. 2008;101:769–73.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer-Verlag GmbH Germany
About this chapter
Cite this chapter
Dos Santos, F. (2023). Haemostasis in Spinal Surgery: An Overview. In: Vieweg, U., Grochulla, F. (eds) Manual of Spine Surgery. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-64062-3_17
Download citation
DOI: https://doi.org/10.1007/978-3-662-64062-3_17
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-64060-9
Online ISBN: 978-3-662-64062-3
eBook Packages: MedicineMedicine (R0)