Abstract
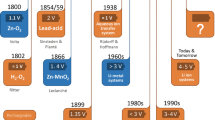
Rechargeable lithium-ion batteries have been continually developed since their introduction by Sony in 1991. Energy density is one of the key parameters for lithium-ion batteries. It was steadily increased by optimizing battery components such as electrode materials or electrolyte as well as by improving the cell construction technologies. The cell level progress during recent years is shown in Fig. 16.1. Both gravimetric (specific) and volumetric energy density were more than doubled.
The original version of this chapter was revised. The updated online version can be found at https://doi.org/10.1007/978-3-662-53071-9_33
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
Rechargeable lithium-ion batteries have been continually developed since their introduction by Sony in 1991. Energy density is one of the key parameters for lithium-ion batteries. It was steadily increased by optimizing battery components such as electrode materials or electrolyte as well as by improving the cell construction technologies. The cell level progress during recent years is shown in Fig. 16.1. The gravimetric energy density of standard cells (18650) increased from below 100 Wh/kg to about 250 Wh/kg today. Similarly, the volumetric energy density increased from about 200 Wh/L to around 700 Wh/L, see [1].
(a) Chronological development of lithium-ion cells’ mean practical energy densities (high energy design). Diagram after ref [1]. (b) Theoretical and (forecast) practical energy densities for different rechargeable cell systems (secondary elements). Practical energy density values are reference values only and strongly vary with the respective cell design (size, geometry, high energy, high power): Pb-acid battery (car battery, 12 V), NiMH – nickel metal hydride (cell level, AA), lithium-ion – mean value across different types (cell level), HT-Na/S – high temperature sodium/sulfur (cell level) Li/S – data from by Sion Power (cell level, pouch), Li/O2 – data from by Polyplus (cell level, primary element)) (c) Comparison between the different cell concepts of conventional lithium-ion cells, metal/sulfur cells and metal/oxygen cells.
It is obvious however, that a similar increase is not to be expected in the coming years since technology reaches its natural limits. Eventually, the electrode materials used will be the limiting parameters. Currently, carbon materials (mainly graphite) are used in anodes, while cathodes contain oxidic transition metal compounds. These are for example lithium cobalt oxide (LiCoO2), Li-NCM (Li(Ni1−x−yMnxCoy)O2), lithium iron phosphate (LiFePO4), or lithium manganese oxide (LiMn2O4). The theoretical gravimetric energy densityFootnote 1 for these cell types typically ranges from 350 to 400 Wh/kg.
If further cell components such as electrolyte, separator, current collector, additives, and housing are taken into account, energy density typically decreases by more than 50 percent. The transition from single cell to batteryFootnote 2 causes additional losses, resulting in values of 110–140 Wh/kg in electric vehicles. With today’s commercial cells’ values in mind, it can be predicted that a considerable energy density increase is not achievable with the conventional approaches (Fig. 16.1a and Table 16.1). Currently, important approaches to maximize energy and power density by improving electrode materials are in particular (a) partially or completely substituting lithium alloys containing tin and silicon for carbon in the negative electrode and (b) increasing the positive electrode’s nickel content.
Lithium-sulfur and lithium-air systems are much discussed next generation technologies and use cell chemistries considerably different from those of standard lithium-ion batteries. Developing these battery types to marketable systems in regards to gravimetric energy density would mean a great step forward when compared with the current lithium-ion technology.
Theoretical and provisional practical energy densities of both technologies are compared with conventional systems in Fig. 16.1b and Table 16.2. A lithium-air cell has a theoretical energy density of several thousand Wh/kg. This explains the fascination sparked by this cell system.
The most important reasons for the high energy density of both cell concepts are
-
(1)
the substitution of the light elements sulfur and oxygen for the cathode’s comparatively heavy transition metal compounds.
-
(2)
the storing of more lithium per formula unit. Conventional cathode materials change the transition metal’s oxidation state during the lithium’s intercalation and deintercalation. Hence, maximally one lithium ion per formula unit can be stored with the redox pairs Co4+/Co3+, Fe3+/Fe2+ and Mn4+/Mn3+. Effectively, only 0.8 lithium ions (LiMn2O4) and 0.5 lithium ions (LiCoO2) respectively can be utilized per formula unit. In contrast to that, sulfur and oxygen can incorporate 2 lithium ions each per formula unit during a complete reaction.
-
(3)
the replacement of the anode’s graphite with metallic lithium in the future. However, using a pure metal anode poses challenges that have yet to be resolved, most notably the dangerous dendrite formation. The continuous electrolyte consumption induced by the reaction with the metal anode during charging and discharging is a decisive factor for cycling stability.
Moreover, the use of sulfur or oxygen as active components in batteries is also attractive considering their elemental abundance.
This Chapter takes an in-depth look at the cell chemistries of next generation technologies using Li/S and Li/O2. It also discusses advantages and disadvantages as well as solutions for the current problems. “All-solid state batteries” become more and more interesting and are reviewed at the end of this Chapter.
2 The lithium-sulfur battery
2.1 Basic principle
The lithium-sulfur cell chemistry has been examined for several decades [2–4]. The reasons for this are the high energy density, the almost unlimited availability of sulfur, and its low toxicity. There have been great improvements during the last 10 or 15 years. But the technology still faces great challenges, even now. On first glance, a lithium-sulfur cell is based on reversible transformation of lithium by means of sulfur according to the following principle:
The theoretical cell voltage is calculated as E° = 2.24 V from the Gibbs energy of this reaction (ΔrG°(25 °C) = − 432.57 kJ/mol [Li2S]). Combined with the theoretical capacity of 1,167 mAh/g (Li2S), the resulting theoretical energy density is 2,613 Wh/kg (Li2S). This value is several times higher than that of conventional batteries. Literature often describes the cathode reaction by itself, thus the capacity mentioned there is usually referring to sulfur. It is 1,672 mAh/g (S).
Fig. 16.2a shows a schematic diagram of a cell. A suitable cathode structure must be generated since both sulfur and the discharge product Li2S are not electrically conducting. Usually, porous carbon particles with large surfaces are used as carrier material. They provide electronic contact and also ensure sufficient electrolyte accessibility. The considerable volume changes are characteristic for cell reactions. Li2S (ρ = 1.66 g/cm3, V m = 28.0 ml/mol) has a lower density than sulfur (ρ = 2.07 g/cm3, V m = 15.5 ml/mol). Therefore, the cathode must provide enough space to compensate for a volume increase of around 80 %. In general, a sulfur cathode contains between 50 and 70 % weight percent sulfur. The remaining weight is divided among the carbon carry material and the binding agent (small amount). Carbon also needs to be added to standard cathode materials as a conducting additive, but with lower percentages. The energy densities of a lithium-sulfur cell attainable in practice are much lower than the theoretical energy densities. This is mainly due to the high carbon content and the required high porosity.
(a) Schematic diagram of a lithium-sulfur cell. Metallic lithium is the anode material. The cathode consists of a mixture of sulfur and carbon particles, which is mechanically stabilized by means of a binding agent. (b) Typical voltage profile (charging and discharging cycle) of a lithium-sulfur cell.
The electrolyte is made of a mixture of organic solvents and the applicable conducting salt. As opposed to standard carbonate-based solvents such as ethylene carbonate (EC)/dimethyl carbonate(DMC) with the conducting salt LiPF6, the Li/S8 cell in general features a mixture of dimethoxyethane (DME, C4H10O2), 1,3-dioxolane (DOL, C3H6O2), and lithium bis(trifluoromethylsulfonyl)imide (LiN(SO2CF3)2, LiTFSI), because, at this stage, this mixture seems to be best compatible with the metallic lithium anodes.
This cell reaction seems quite simple at first glance, however if one takes a closer look it becomes very complex. Several intermediate steps for the reduction of sulfur to the sulfide ion (S2-) are the reason:

Most polysulfides dissolve very well in the electrolyte, hence the reaction mechanism is considerably different to that of conventional lithium-ion batteries where the reactions are pure solid state reactions. Fig. 16.3 shows polysulfide solubility in the electrolyte with the help of a demonstration. The electrolyte takes on color immediately after the beginning of the discharge reaction because of the dissolved species.
First discharging of a lithium-sulfur cell in a glass cell. In the beginning of the reaction, soluble polysulfides are formed at the cathode from sulfur and lithium. They diffuse to the lithium anode (Section 16.2.2 – Shuttle mechanism)
It is still unsure which species emerge, how quickly the individual partial reactions take place, and how high the respective concentrations are at a specific point in time. The influence of polysulfide formation on the cell reaction can be directly seen from the discharge voltage (Fig. 16.2b). The discharging process can be divided into three areas.
In the beginning (area 1), the discharge reaction starts with the reduction of elementary sulfur S8. Higher polysulfides such as Li2S8 and Li2S6 are formed. They dissolve in the electrolyte, which causes the discharge voltage to continually decrease. A minimum characterizes the transition to area 2. The incipient formation of solid Li2S (Li2S2) phase is the reason. The required nucleation enthalpy causes an additional overvoltage and subsequently a discharging cycle minimum. Sulfur reduction continues in area 2. Aside from the solid phases, low-order soluble polysulfides (Li2S4, Li2S3) exist. Ideally, a full transition from Li2S2 to Li2S takes place at the end of discharging. However, this is usually not achieved in cells. In general, 2/3 to 3/4 of theoretical capacity are reached. Reasons for this could be: insufficiently fast solid-state diffusion during the transition from Li2S2 to Li2S, low electrical conductivity of Li2S2 and Li2S, blocking of the porous electrode by the growing Li2S particles, or the shuttle mechanism (Section 16.2.2). Overall, the complex cell reaction leads to two plateaus which is why the measured cell voltage slightly deviates from the thermodynamic value of 2.24 V for the direct transition from lithium and sulfur to Li2S.
There is less knowledge on what happens when reversing the reaction. Charging features a continually increasing potential and appears to be simpler, namely by means of multiple electron transfer. Peled et al. [5] used cyclic voltammetry during the discharging process to show an incremental reduction, i.e., the voltammogramm displays several maximums. However, there was only a single maximum for the charging process (oxidation). This correlates with formation of S8 2-.
Oxidation to S8 at the end of the charging process apparently is incomplete [6, 7]. It is still unclear how much of the solid phases (S8, Li2S2, Li2S) eventually form during cell cycling and how high the share of the respective polysulfides is. Only gradually, elaborate in situ experiments shed light on cell chemistry [8, 9].
2.2 Shuttle mechanism
Polysulfides dissolve very well in electrolytes. This causes another lithium-sulfur cell characteristic, the so-called shuttle mechanism (Fig. 16.4). The polysulfides Sn 2− that form and dissolve at the cathode, diffuse to the lithium anode and are reduced to Li2S2 and Li2S.Footnote 3 Subsequent high-order polysulfide species react with these compounds and form low-order polysulfides S(n−x) 2−. This means that the desired electrochemical reaction of sulfur at the cathode partly also takes place at the anode in uncontrolled fashion (chemical or electrochemical reactions both are conceivable), which negatively influences cell characteristics.
The low-order polysulfides formed at the anode diffuse back to the cathode. When the cell is discharged, these diffused species are further reduced to Li2S2 or Li2S. Simply put, the cathode reaction partly takes place at the anode during the discharging process or, rather, the cell self-discharges. Both are undesirable effects decreasing capacity. In contrast to that, the diffusion to the cathode during the charging process is followed by a re-oxidation of the polysulfide species from low order to higher order. These polysulfides then diffuse to the anode again. This cycle is generally known as the shuttle mechanism. For a very strong shuttle, charging continues infinitely as the cell is “chemically short-circuited”. Kumaresan et al. [10] gave a mathematical description of the discharging and charging reaction and the shuttle mechanism.
Overall, the shuttle mechanism causes a loss of sulfur active mass in the cathode leading to poor cycle life.
2.3 Long-term stabilization concepts
Currently, several concepts are tested to improve Li/S cell cycling characteristics. These concepts concern the cathode (carbon/sulfur), the electrolyte, and the anode (lithium metal). The goals especially are to limit the active mass loss, to suppress the shuttle mechanism, and to keep the cathode structures mechanically stable over a longer period of time. Hence, special carbon materials with a defined porosity are used for the cathode. They ensure sufficient electrical sulfur contact and also immobilize the polysulfide species to limit their loss in the electrolyte. A great many number of scientific papers treating these issues have been published during recent years. Nazar et al. [11] for example melt-infiltrated a special carbon (CMK-3) with a defined pore size of d = 3 nm with sulfur at 155 °C in their highly regarded paper (Fig. 16.5). The material showed a considerably less pronounced shuttle mechanism and a comparably stable cycling behavior at high capacities when compared to not-infiltrated samples. This is due to the material’s special nanostructure.
(a) Production of a nano-structured cathode from mesoporous carbon (CMK3) and sulfur. (I) Carbon melt infiltration with liquid sulfur. (II) Sulfur cooling and crystallization (30 wt% CMK-3, 70 wt% S). (III) The volume increase that results from Li2S formation is compensated by the carbon that provides enough free space. (b) Nanocomposite (CMK-3/S) or PEG-modified material cycling stability (Material reproduced and modified by permission of Macmillan Publishers Ltd: Nature Materials [11], Copyright 2009)
Modifying the carbon surface with polyethylene glycol (PEG) resulted in additional improvement. Other researched carbon materials include activated carbon, carbon black, graphene, and carbon nanotubes.
It is important to note that, next to the carbon material, also the electrode preparation has a major influence on the cathode performance. Electrode sulfur surface load, layer thickness, binder content, and binder type are among significant parameters in this respect [12, 13]. Decrease in electrode layer thickness or decrease in sulfur surface load also are simple measures to improve cycling behavior. Research is also looking into polysulfide loss reduction by adding cathode additives such as Al2O3 or SiO2 (polysulfide trap). Currently, a high surplus of electrolyte and lithium is still required to guarantee sufficient cycling stability. These measures however defeat the purpose of creating a high energy density battery. Therefore, there must be a balanced approach between improving individual parameters and decreasing energy density [14 – 18].
Another approach is to prevent the diffusion of polysulfide species to the anode. To that end, solid or gel polymer electrolytes are used as barriers [19]. Compared to liquid electrolytes, however, this material has a decreased conductivity and contacting capability that cause higher overvoltages and thus lower cell energy efficiency. Aurbach et al. [20] researched lithium anode passivation by means of the electrolyte additive LiNO3. This additive forms a protective layer which effectively mitigates the parasitic polysulfide reduction a the lithium electrode. LiNO3 has established itself as standard additive in lithium-sulfur battery research. However, LiNO3 is continually broken down in the cell and therefore does not prevent aging, it only slows it down [21].
In addition to all of the above-mentioned cell concepts, additional approaches to create an electrochemical storage device based on lithium and sulfur compounds are currently being researched or rediscovered [22]. It seems as if sulfur can be reversibly implemented in Li+-conducting compounds such as lithium polysulfidophosphates [23]. A different approach substitutes a liquid catholyte for the solid C/S cathode, a concept similar to that of redox-flow batteries [24]. Other approaches are: cells based on dissolved polysulfides (an approach that had been looked into several decades ago [25] and that currently enjoys a renaissance [26–28]) and the opposite, namely all-solid state batteries [29–30] in which a solid electrolyte replaces the liquid electrolyte. It remains to be proven, whether these approaches are finally more promising than the conventional cell design, however.
2.4 Status quo
Up to now and in spite of many different approaches, Li/S cells could not be sufficiently improved to ensure satisfying behavior with respect to all important application parameters. For example, cycle life under practical conditions is often insufficient and volumetric energy densities are lower than expected. Also, compared to lithium-ion batteries, cell reaction speed (kinetics) is still insufficient due to the usage of nonconducting species such as S8, Li2S2, and Li2S. Increased research and development efforts are therefore required to develop a mass-marketable lithium-sulfur cell. There exist however novel applications relying on high gravimetric energy densities (e.g., aerial drones). The intensified research efforts produce a high publication rate of 5 to 10 per week. In addition to the lithium-sulfur battery discussed here, a sodium-sulfur battery that operates at ambient temperatures is also conceivable [31]. So far, however, the performance of these cells still lacks behind the lithium-sulfur cells.
Oxis Energy Limited (Oxfordshire, UK) and formerly also Sion Power Corp. (Tucson, USA) developed Li/S cells for a variety of applications. Energy densities of 400 Wh/kg on the cell level (500 Wh/kg targeted for 2019) are reported yet they are not available on the free market.
3 The lithium-air battery
3.1 Basic principle
Abraham et al. in 1996 were the first to describe the function of lithium-air batteries (more precisely lithium-O2 batteries) using non-aqueous electrolytes [32]. The main difference to standard batteries is that this cell type is an open system because − as with fuel cells − atmospheric oxygen is changed over at the cathode (Fig. 16.6). One might assume that the discharge product of an Li/O2 cell is Li2O, similar to fuel cells where H2O is formed from hydrogen and oxygen. However, it is lithium peroxide (Li2O2) that is generally formed as a discharge product for thermodynamic reasons. Research has shown that potentially formed Li2O is difficult to oxidize again. Metastable lithium superoxide probably develops as interstage product.
\({\rm{Anode\,reaction}}:2\,{\rm{Li}} \to 2\,{\rm{L}}{{\rm{i}}^ + } + 2\,{{\rm{e}}^ - }\) | \({\rm{Anode\,reaction}}:2\,{\rm{Li}} \to 2\,{\rm{L}}{{\rm{i}}^ + } + 2\,{{\rm{e}}^ - }\) |
\({\text{Cathode reaction}}:{\text{0}}.{\text{5}}\,{{\text{O}}_{\text{2}}} + {\text{2}}{{\text{e}}^ - } \to {{\text{O}}^{{\text{2}} - }}\) | \({\text{Cathode reaction}}:{{\text{O}}_{\text{2}}}{\text{ + 2}}\,{{\text{e}}^ - } \to {{\text{O}}_{\text{2}}}^{{\text{2}} - }\) |
\({\text{Overall reaction}}:2{\text{Li}}+{\text{0}}.{\text{5}}\,{{\text{O}}_{\text{2}}} \to {\text{Li}}_2{\text{O}}\) (E° = 2.91 V) | \({\text{Overall reaction}}:2{\text{Li}}+{{\text{O}}_{\text{2}}} \to {\text{Li}}_2{\text{O}}_2\) (E° = 2.96 V) |
(a) Schematic diagram of a lithium-air cell and electrode reaction for Li2O2 as the discharge product (oxygen reduction reaction, ORR). (b) Typical charging and discharging cycle. The displayed capacity relates to the mass of the carbon material used. The thermodynamically calculated voltage is E° = 2.96 V
Battery mass increases with an increasing discharge state because oxygen is introduced from the outside during discharging. This is why the described theoretical energy density of this system varies considerably. If the oxygen mass is included in the calculation, the free reaction enthalpy ΔrG°(25 °C) = – 439.08 kJ/mol (Li2O) combined with a capacity of 1,793 mAh/g (Li2O) results in a theoretical energy density of 5,220 Wh/kg (Li2O) (or 1,168 mAh/g and 3,458 Wh/kg for Li2O2 as discharge product). Without the oxygen mass, the energy density for both reaction products is above 11,000 Wh/kg. This is similar to theoretical energy densities of standard fuels where oxygen mass is also not included in energy density calculations.
Fig. 16.6 shows that a suitable cathode structure (conductive carbon matrix covered with catalytic particles, if necessary) is required to enable the cell reaction and to provide enough free space for the resulting solid reaction products. The reaction products have a very low conductivity. This is why large-surface carbon materials (> 50 m2/g) are generally used to ensure a homogeneous distribution of product particles that are as small as possible. Since it is difficult to specify the theoretical cathode capacity, literature specifies measured capacity as absolute (mAh) or sets it in reference to the carbon carrier material’s weight (mAh/g[C]).
For operating a lithium-oxygen cell with air, several undesired side reactions with air components must be prevented by using suitable membranes. Examples are: unwanted introduction of N2 (nitride formation, Li3N), H2O (hydroxide formation, LiOH), and CO2 (carbonate formation, Li2CO3). Currently, no simple solutions have been found in this respect. Hence, cell chemistry research is not conducted with air, but with oxygen. Also, cell drying-out, i.e., electrolyte evaporation from the cathode that is in contact with the atmosphere, must be prevented during operation. At the same time, lithium anode corrosion by dissolved oxygen in the electrolyte must be inhibited. In practice, this also calls for an additional protective layer. Because of the additionally required components, the attainable energy density will be much lower in practice than the theoretical values.
3.2 Electrolyte stability, efficiency, and reversibility
Fig. 16.6b shows a typical charging and discharging cycle of a Li/O2 cell. The big difference between the measured discharge voltage (~2.6 V) and the charging voltage (> 3.5 V) is representative for this cell type and results in pronounced hysteresis. This is caused by high overvoltages during cell reactions, especially during the charging process (oxygen oxidation). The extent of the hysteresis scales inversely with the energy efficiency of the cell reaction which is only around 60 to 70 % for many current aprotic Li/O2 cells. This observation led to increased catalyst usage (MnO2, Pt, Au, etc.) and better cathode materials (activated carbon, carbon black, graphene, nanotubes, etc.) with the hope to reduce overpotentials and to improve rechargeability.
Early research results indicated complex cell reactions [33–35]. However, it was recently proven that carbonate-based electrolytes (i.e., 1 M LiPF6 in propylene carbonate) that had been used until then irreversibly degrade during cell reaction [36, 37]. Instead of the desired product (Li2O2), a multitude of degradation products (Li2CO3, CO2, H2O, C3H6[OCO2Li]2, …) were accounted for. Partially, these degradation products are further degraded during charging. This is due to the reactivity of the superoxide radical (O2·−) which forms as an intermediate during oxygen reduction. Thus, capacity values achieved in experiments were based not only on the reversible formation and degradation of Li2O2, they also were a result of irreversible electrolyte degradation. Even worse, many of the suggested catalysts accelerated this degradation process even more [38].
Research focus has changed due to these results and currently is directed toward developing electrolytes with a sufficient stability. It was not possible to identify a suitable system for practical applications till now. However, cell reactions, which still are accompanied by undesired secondary reactions, can at least be better examined in glyme or DMSO based electrolytes. For example, the discharge product Li2O2 generally occurs as nanoscopic particles shaped as a torus (Fig. 16.7). The use of gold instead of carbon as electrode may also ease scientific examinations of the electrode reaction [39].
3.3 Status quo
The current Li/O2 battery status quo without a doubt still requires more fundamental research. At present, there is no cell concept for aprotic electrolytes that is able to prove reversible formation of Li2O2 (or Li2O) across several cycles without simultaneous electrolyte degradation in practice.
Recent research results make it obvious that currently the greatest challenge for commercialization is the development of chemically stable electrolytes and electrode materials. In terms of the electrolyte, additional requirements need to be fulfilled aside from chemical stability. These are: lithium-ion conductivity, oxygen solubility and diffusivity as well as suitable electrode wetting. To better determine and understand the many various secondary reactions, current research uses a great variety of analysis methods. Gas analysis or pressure monitoring during cell cycling are methods that can provide clear evidence for side reactions, for example.
A more recent approach to reducing charging overvoltages uses redox mediators such as tetrathiafulvalene (TTF), lithium iodide, or tetramethylpiperidinoxyl (TEMPO) [40–46]. Oxidation is attained through dissolved compounds in this case. During charging, these compounds themselves are electrochemically oxidized at first, then they are reduced by Li2O2, which then decomposes under release of O2. Another important aspect is the understanding of water impurities on the cell chemistry [47–51].
Surprisingly, it could be proven that a substitution of sodium for lithium makes the cell reaction much more reversible. The discharge products in this case are not nanoscopic Na2O2 particles however, but large sodium superoxide (NaO2) crystallites (Fig. 16.7) [52]. A catalyst may not be required because overvoltages are very low. Compared to an Li/O2 cell, energy density is lower (2,643 Wh/kg[Na] and 1,105 Wh/kg[NaO2], respectively).
Li/O2 cells not only are developed by academic institutions, but also by companies [37, 53]. Li/O2 batteries with an aqueous electrolyte are an alternative to the non-aqueous Li/O2 batteries discussed here [54]. In such a system, oxygen from the ambient air reacts with lithium and water to lithium hydroxide (LiOH). Very high energy densities are to be expected with this cell concept as well. It is a prerequisite however, to effectively protect the lithium anode against the aqueous electrolyte.
4 Challenges of using lithium metal as anode
The use of lithium metal as anode is most appealing for achieving highest energy densities.
Reversible dissolution and re-plating of lithium that are required for rechargeable batteries faces a range of difficulties and related safety concerns so far largely prevent its use in application. This is due especially to the chemical reactivity of lithium with the electrolyte as well as to the formation of dendrites during charging.
In general, lithium reacts with all known electrolytes for thermodynamic reasons. A key criteria for using lithium metal in an battery therfore is that the electrolyte forms a passivating layer that prevents further parasitic reactions. The concept of such an solid electrolyte interphase (SEI) has been introduced by Peled in 1979 [55]. The layer must be conductive to lithium ions and block electrons to achieve this. It should be mentioned that all lithium-ion batteries with graphite anode are also operated outside of the electrolyte’s stability range. Caused by a reaction with the carbonate-based electrolyte (i.e., LiPF6 in EC/DMC and additives), a very stable SEI is formed that has a thickness of only a few nanometers. Contrary to that, ether-based solvents such as dioxolane or glyme are preferable for metallic lithium anodes [56]. This in spite of the fact that they are irreversibly degraded over time. Creeping corrosion of the lithium electrode requires that lithium anode cell systems have to be operated with excess lithium and liquid electrolyte. This negatively impacts the energy density of the cell. It should be pointed out that especially when lithium-sulfur cells are researched in a laboratory environment, this is often done with a great excess of liquid electrolyte and lithium. This excess must be considerably reduced to achieve a marketable cell concept. Therefore, there is great need to develop suitable approaches to improve the behavior of lithium electrodes with electrolytes in general.
Next to the thermodynamic instability of lithium in contact with electrolytes, the phenomenon of dendrite formation is a key challenge. Dendrites are well known to form during metal plating and hence during charging of a battery with lithium electrode. If the dendrites grow all the way to the cathode, they would cause a short circuit in the cell resulting in thermal runaway. It is possible that the dendrites fully become detached from the anode during cycling. Then they would not be available for the electrode reaction (“dead lithium”). Cell capacity would continually decline. An excess of lithium could balance out this loss. The amount of added lithium strongly depends on the system researched and the degree of cell optimization. Earlier research with lithium anodes (MoS2 cathode) could give some pointers in this respect. A threefold lithium excess resulted in a service life of 300 cycles [57].
The following requirements must be fulfilled to use lithium in rechargeable cells: parasitic reactions must be kept as low as possible and lithium plating should be as planar as possible, i.e., dendrite formation needs to be prevented. It might be possible to achieve these goals if a suitable electrolyte composition is developed that promotes formation of a pertinent SEI in the contact with lithium. An alternative could be coatings or membranes. These could be applied directly to the anode and may also function as separator. Another approach to prevent dendrite formation subjects the cell to mechanical pressure above the lithium yield point [58]. There is also research looking into different additives to improve lithium electrode reversibility [59 – 63].
If lithium cannot be used, conventional anode materials such as graphite may be used instead. This would however considerably reduce the theoretically achievable energy density (Table 16.2) to more unattractive values. This is why another approach favors silicon as anode material in Li/S cells [64 – 65]. Assuming formation of Li4. 4Si, the theoretical cell voltage and energy density would amount to 2.04 V and 1,863 Wh/kg (3,299 Wh/l), respectively. Another alternative is tin (E° = 1.72 V, 922 Wh/kg and 2,628 Wh/l, respectively) [19]. On the other hand, the use of metals such as Si or Sn as substitute is not straightforward either as the large volume expansion during lithium intercalation leads to particle cracking and poor SEI stability.
5 All-solid state batteries
A very recent trend is the development of rechargeable solid state batteries (SSB), often also noted as “all-solid state batteries” (ASSB) or “solid state lithium batteries” (SSLB) in order to highlight the fact that this type of battery is constructed without the typical liquid electrolyte [66 – 69]. The current rate of development is fast, and only some of the most important topics related to the development of SSB shall be discussed here briefly (see [67 – 68] for a discussion of the essential challenges).
Two major types of SSLB have to be distinguished on the basis of the cell geometry and cell charge capacity: Thin film SSLB (tf-SSLB) have a relatively low area capacity in the order of 0.1 mAh/cm2 and are typically prepared by gas phase deposition. Thin film SSB are already commercialized, but the corresponding market is yet small. Commercial tf-SSLB are usually based on single cells of the type Li/”LiPON”/LCO, i.e. contain a thin film cathode (a few µm thick) of LiCoO2, a thin film solid electrolyte separator made of “LiPON” (an amorphous Li3PO4 film containing a few percent of nitrogen) and a lithium metal anode. Once well-functioning, tf-SSB can be run reversibly for thousands of cycles without significant degradation. Production of large scale batteries on the basis of multi-layer tf-SSB will not be cost-efficient, and therefore, the further development and use will be restricted to low-energy mobile applications (smart cards, flexible batteries for textiles, etc.).
Thick film or large scale SSB are still in the state of research [67 – 71]. Here, two general trends can be identified: In “solidified” lithium ion batteries (SE-LIB; Solid Electrolyte LIB) the liquid electrolyte is substituted by either a solid polymer or a glass-ceramic electrolyte, or a combination of both. As solid electrolytes have usually a higher density than liquid (organic) electrolytes, SE-LIB will only gain improved energy density if the use of high capacity electrodes or of high voltage cathodes are enabled. One particular development in the direction of high energy SSB would then be LiM-SSB (Lithium Metal SSB) in which a thick lithium metal anode is utilized. Due to the high specific capacity of the lithium metal anode, LiM-SSB could offer a significant jump in energy density (up to +70%), but both severe mechanical effects during metal dissolution and deposition, as well as the risk the abovementioned dendrite growth through the solid electrolyte separator have to be overcome.
Power density is considered as “Achilles heel” of SSB, as solid electrolytes are considered as comparably poor ionic conductors. This is not correct, and today already quite a number of inorganic solid electrolytes with lithium ion conductivity higher than that of liquid electrolytes have been reported, see Fig. 16.8 for comparison [72 – 73]. In the case of SE-LIB, authors of a recent report even suggest higher rate capability than in the case of conventional LIB [70]. This can be of great relevance for fast charging and will surely drive further research.
It is generally accepted that interface kinetics is key to SSB with sufficient performance [67 – 68]. Once the lithium metal anode is employed, SEI formation with the solid electrolyte will take place, as the best solid electrolytes are all thermodynamically unstable against reduction [74 – 75]. If the forming SEI is highly resistive, the cell impedance will increase and will reduce the energy efficiency. If the forming SEI is highly conductive it may stabilize the cell, as in the case of SEI in conventional LIB. At the cathode/solid electrolyte interface also degrading interfacial reaction may take place and may also increase the cell impedance.
In summary, solid state batteries may open an attractive avenue toward high energy and high power lithium batteries. It is too early to judge the potential commercial success, as still some critical issues have to be solved.
6 Outlook
Lithium-sulfur and lithium-air batteries are two of the few systems with which it seems feasible to achieve a gravimetric energy density increase compared to lithium-ion batteries. Increase in volumetric energy density is less significant however. When sulfur or oxygen are used instead of transition metal compounds, there is also hope for producing low-cost batteries provided that the need for special carbons (or alternative electrode materials) or catalysts does not increase costs.
Both systems still pose great challenges from a technological point of view. This is especially true for lithium-air systems, because no suitable aprotic electrolyte could be found up till now. Both systems require protection of the lithium metal anode from parasitic secondary reactions. Special electrolytes or protective layers are required to prevent the shuttle mechanism in Li/S systems and the anode's oxygen corrosion in Li/O2 cells. Furthermore, dendrite formation must be averted. Also, solid, insulating reaction products develop in both cell types during cycling (S, Li2S2, Li2S and Li2O2, Li2O). They may block the electrode and considerably impair the cell reaction's kinetics. Therefore, it is probably necessary to use cathode structures with a suitable porosity to promote a fine (nanometer range) reaction product distribution. A lithium-air cell also requires an effective and low-cost membrane, which specifically enables oxygen transport into and from the cell. This calls for innovations on both material and cell level.
It is hard to forecast whether and, if possible, when the next generation technologies for secondary elements described here can actually be put into practice. This is due to the current development status and the very diverse requirements for different application areas. A study conducted by the Fraunhofer Institute for Systems and Innovation Research expects rechargeable Li/S8 cells (400 Wh/kg, 100 cycles) between 2020 and 2030 and Li/O2 cells (>300 Wh/kg, > 500 cycles) later than 2030. This shows that there are still fundamental problems to be solved. In terms of primary elements, lithium-air and similar systems may be on the market earlier than that.
Change history
03 June 2019
No Header
Notes
- 1.
The theoretical (gravimetric) energy density is the stored chemical energy based on the pure electrode materials’ mass.
- 2.
Initially, the terms “cell” and “battery” had strictly different definitions. An electrochemical cell is the smallest battery unit and consists of anode, cathode, electrolyte, separator, current collector, and housing. As opposed to that, a battery consists of at least two cells connected in series or in parallel. A 12-V lead battery for instance is made of six 2-V cells. Nowadays however, a cell is often called battery also. The electrochemical processes do not differ from cell to battery and this is why the present Chapter does not differentiate between those two terms. Specifying the practical energy densities however calls for a differentiation. All practical energy density values (with the exception of lead batteries) in this Chapter refer to cells.
- 3.
The polysulfide species Sn 2– that form at the cathode during discharging dissolve in the electrolyte there. A concentration gradient versus the anode develops, which causes the polysulfides to diffuse toward the anode. Step by step, the polysulfides are distributed in the electrolyte.
Bibliography
Gesamt-Roadmap Energiespeicher für die Elektromobilität 2030, Fraunhofer-Institut für System und Innovationsforschung ISI, Karlsruhe, Dezember 2015
Herbert D, Ulam J (1962) Inventors; electric dry cells and storage batteries
Nole DA, Moss V, Cordova R (1970) Inventors; battery employing lithium-sulphur electrodes with nonaqueous electrolyte
Abraham KM (1981) Status of rechargeable positive electrodes for ambient-temperature lithium batteries. J Power Sources 7(1):1 − 43
Yamin H, Penciner J, Gorenshtain A, Elam M, Peled E (1985) The electrochemical-behavior of polysulfides in tetrahydrofuran. J Power Sources 14(1−3):129 − 134
Akridge JR, Mikhaylik YV, White N (2004) Li/S fundamental chemistry and application to hig-performance rechargeable batteries. Solid State Ionics 175(1 – 4):243 – 245
Mikhaylik YV, Akridge JR (2004) Polysulfide shuttle study in the Li/S battery system. J Electrochem Soc 151(11):A76 − A1969
Nelson J, Misra S, Yang Y, Jackson A, Liu Y, Wang H et al (2012) In operando x-ray diffraction and transmission x-ray microscopy of lithium sulfur batteries. J Am Chem Soc 134(14):6337 – 6343
Dominko R, Demir-Cakan R, Morcrette M, Tarascon J-M (2011) Analytical detection of soluble polysulphides in a modified Swagelok cell. Electrochem Commun 13(2):117 – 120
Kumaresan K, Mikhaylik Y, White RE (2008) A mathematical model for a lithium-sulfur cell. J Electrochem Soc 155(8):A576 − A582
Ji X, Lee KT, Nazar LF (2009) A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat Mater 8(6):500 – 506
Schneider H, Garsuch A, Panchenko A, Gronwald O, Janssen N, Novak P (2012) Influence of different electrode compositions and binder materials on the performance of lithium-sulfur batteries. J Power Sources 205:420 – 425
Cheon SE, Ko KS, Cho JH, Kim SW, Chin EY, Kim HT (2003) Rechargeable lithium sulfur battery – II. Rate capability and cycle characteristics. J Electrochem Soc 150(6):A800 – A805
Kang SH, Zhao X, Manuel J, Ahn HJ, Kim KW, Cho KK, Ahn JH (2014) Effect of sulfur loading on energy density of lithium sulfur batteries. PSSA 211(8):1895–1899
Hagen M, Fanz P, Tübke J (2014) Cell energy density and electrolyte/sulfur ratio in Li-S cells. J Power Sources 264:30–34
Brückner J, Thieme S, Grossmann HT, Dörfler S, Althues H, Kaskel S (2014) Lithium-sulfur batteries: influence of C-rate, amount of electrolyte and sulfur loading on cycle performance. J Power Sources 268:82–87
Cleaver T, Kovacik P, Marinescu M, Zhang T, Offer G (2018) Perspective—commercializing lithium sulfur batteries: are we doing the right research? J Electrochem Soc 165(1):A6029–A6033
Adelhelm P, Hartmann P, Bender CL, Busche M, Eufinger C, Janek J, Beilstein J (2015) From lithium to sodium: cell chemistry of room temperature sodium–air and sodium–sulfur batteries. J Nanotechnol 6:1016–1055
Hassoun J, Scrosati B (2010) A high-performance polymer tin sulfur lithium ion battery. Angewandte Chemie Int Edition 49(13):2371 – 2374
Aurbach D, Pollak E, Elazari R, Salitra G, Kelley CS, Affinito J (2009) On the surface chemical aspects of very high energy density, rechargeable li–sulfur batteries. J Electrochem Soc 156(8):A694 – A702
Jozwiuk A, Sommer H, Janek J, Brezesinski T (2015) Fair performance comparison of different carbon blacks in lithium-sulfur batteries with practical mass loadings – simple design competes with complex cathode architecture. J Power Sources 296:454–461
Medenbach L, Adelhelm P (2017) Cell concepts of metal-sulfur batteries (Metal = Li, Na, K, Mg): strategies for using sulfur in energy storage applications. Top Curr Chem 375(5):81
Lin Z, Liu Z, Fu W, Dudney NJ, Liang C (2013) Lithium Polysulfidophosphates: A Family of Lithium-Conducting Sulfur-Rich Compounds for Lithium-Sulfur Batteries. Angewandte Chemie. 125(29):7608 – 11
Yang Y, Zheng G, Cui Y (2013) A membrane-free lithium/polysulfide semi-liquid battery for large-scale energy storage. Energy & Environmental Science 6(5):1552 – 8
Rauh RD, Abraham KM, Pearson GF, Surprenant JK, Brummer SB (1979) A lithium/dissolved sulfur battery with an organic electrolyte. J Electrochem Soc 126(4):523–527
Zhang SS, Read JA (2012) A new direction for the performance improvement of rechargeable lithium/sulfur batteries. J Power Sources 200:77–82
Zheng G, Cui Y (2013) A membrane-free lithium/polysulfide semi-liquid battery for large-scale energy storage. Energy Environ Sci 6:1552–1558
Fu Y, Su YS, Manthiram A (2013) Highly reversible lithium/dissolved polysulfide batteries with carbon nanotube electrodes. Angew Chem Int Edit 52(27):6930–6935
Hassoun J, Scrosati B (2010) Moving to a solid‐state configuration: a valid approach to making lithium‐sulfur batteries viable for practical applications. Adv Mater 22(45):5198–5201
Nagata H, Chikusa Y (2014) A lithium sulfur battery with high power density. J Power Sources 264:206–210
Adelhelm P, Hartmann P, Bender CL, Busche M, Eufinger C, Janek J (2015) From lithium to sodium: cell chemistry of room temperature sodium–air and sodium–sulfur batteries. Beilstein J Nanotechnol 6:1016–1055
Abraham KM, Jiang Z (1996) A polymer electrolyte-based rechargeable lithium/oxygen battery. J Electrochem Soc 143(1):1 – 5
Read J (2002) Characterization of the lithium/oxygen organic electrolyte battery. J Electrochem Soc 149(9):A1190 – A1195
Sawyer DT, Valentine JS (1981) How super is superoxide. Acc Chem Res 14(12):393 − 400
Aurbach D, Daroux M, Faguy P, Yeager E (1991) The electrochemistry of noble-metal electrodes in aprotic organic-solvents containing lithium-salts. J Electroanal Chem 297(1):225 – 244
Mizuno F, Nakanishi S, Kotani Y, Yokoishi S, Iba H (2010) Rechargeable Li-air batteries with carbonate-based liquid electrolytes. Electrochem 78(5):403 – 405
Freunberger SA, Chen Y, Peng Z, Griffin JM, Hardwick LJ, Barde F et al (2011) Reactions in the rechargeable Li-O2 battery with alkyl carbonate electrolytes. J Am Chem Soc 133(20):8040 – 8047
McCloskey BD, Scheffler R, Speidel A, Bethune DS, Shelby RM, Luntz AC (2011) On the efficacy of electrocatalysis in nonaqueous Li-O2 batteries. J Am Chem Soc 133(45):18038 – 18041
Peng ZQ, Freunberger SA, Chen YH, Bruce PG (2012) A Reversible and Higher-Rate Li-O2 Battery. Science. 337(6094):563 – 6.
Chase GV, Zecevic S, Walker W, Uddin J, Sasaki KA, Giordani V, Bryantsev V, Blanco M, Addison D (2011) US Patent Application No 20120028137 A1 2011
Hase Y, Shiga T, Nakano M, Takechi K, Setoyama N (2009) US Patent Application No US 2009/0239113 A1 2009
Chen Y, Freunberger SA, Peng Z, Fontaine O, Bruce PG (2013) Charging a Li–O2 battery using a redox mediator. Nat Chem 5:489–494
Lim HD, Song H, Kim J, Gwon H, Bae Y, Park KY, Hong J, Kim H, Kim T, Kim YH, Lepró X, Ovalle-Robles R, Baughman R, Kang K (2014) Superior rechargeability and efficiency of lithium–oxygen batteries: hierarchical air electrode architecture combined with a soluble catalyst. Angew Chem Int Ed Engl 53(15):3926–3931
Bergner BJ, Schürmann A, Peppler K, Garsuch A, Janek J (2014) TEMPO: a mobile catalyst for rechargeable Li-O2 batteries. J Am Chem Soc 136(42):15054–15064
Feng N, Mu X, Zhang X, He P, Zhou H (2017) Intensive study on the catalytical behavior of N-methylphenothiazine as a coluble mediator to oxidize the Li2O2 cathode of the Li–O2 battery. ACS Appl Mater Interfaces 9(4):3733–3739
Liang Z, Lu YC (2016) Critical role of redox mediator in suppressing charging instabilities of lithium–oxygen batteries. J Am Chem Soc 138(24):7574–7583
Aetukuri NB, McCloskey BD, Garcia JM, Krupp LE, Viswanathan V, Luntz AC (2015) Solvating additives drive solution-mediated electrochemistry and enhance toroid growth in non-aqueous Li–O2 batteries. Nat Chem 7:50–56
Meini S, Piana M, Tsiouvaras N, Garsuch A, Gasteiger HA (2012) The effect of water on the discharge capacity of a non-catalyzed carbon cathode for Li-O2 batteries. Electrochem Solid-State Lett 15(4):A45–A48
Schwenke KU, Metzger M, Restle T, Piana M, Gasteiger HA (2015) The influence of water and protons on Li2O2 crystal growth in aprotic Li-O2 cells. J Electrochem Soc 162(4):A573–A584
Li F, Wu S, Li D, Zhang T, He P, Yamada A, Zhou H (2015) The water catalysis at oxygen cathodes of lithium–oxygen cells. Nat Commun 6:7843
Xia C, Black R, Fernandes R, Adams B, Nazar LF (2015) The critical role of phase-transfer catalysis in aprotic sodium oxygen batteries. Nat Chem 7:496–501
Hartmann P, Bender CL, Vracar M, Dürr AK, Garsuch A, Janek J, Adelhelm P (2013) A rechargeable room-temperature sodium superoxide (NaO2) battery. Nat Mater 12:228 – 232
http://www.ibm.com/smarterplanet/us/en/smart_grid/article/battery500.html
de Jonghe LC et al (2007) inventors; protected active metal electrode and battery cell structures with non-aqueous interlayer architecture
Peled E, Menkin S (2017) Review—SEI: past, present and future. J Electrochem Soc 164(7):A1703–A1719
Aurbach D et al (2009) On the surface chemical aspects of very high energy density, rechargeable Li-sulfur batteries. J Electrochem Soc 156(8):A694 − A702
Brandt K (1994) Historical development of secondary lithium batteries. Solid State Ionics.69(3 – 4):173 – 183
Monroe C, Newman J (2005) The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J Electrochem Soc 152(2):A396 – A404
Li W, Yao H, Yan K, Zheng G, Liang Z, Chiang Y-M, Cui Y (2015) The synergetic effect of lithium polysulfide and lithium nitrate to prevent lithium dendrite growth. Nat Commun 6:7436.
Ding F, Xu W, Graff GL, Zhang J, Sushko ML, Chen X, Shao Y, Engelhard MH, Nie Z, Xiao J, Liu X, Sushko PV, Liu J, Zhang J-G (2013) Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J Am Chem Soc 135(11):4450–4456.
Suo L, Hu Y-S, Li H, Armand M, Chen L (2013) A new class of solvent-in-salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat Commun 4.
Qian J, Henderson WA, Xu W, Bhattacharya P, Engelhard M, Borodin O, Zhang J-G (2015) High rate and stable cycling of lithium metal anode. Nat Commun 6.
Khurana R, Schaefer JL, Archer LA, Coates GW (2014) Suppression of lithium dendrite growth using cross-linked polyethylene/poly(ethylene oxide) electrolytes: a new approach for practical lithium-metal polymer batteries. J Am Chem Soc 136(20):7395–7402.
Yang Y, McDowell MT, Jackson A, Cha JJ, Hong SS, Cui Y (2010) New nanostructured Li2S/Silicon rechargeable battery with high specific energy. Nano Lett 10(4):1486 – 1491
Elazari R, Salitra G, Gershinsky G, Garsuch A, Panchenko A, Aurbach D (2012) Rechargeable lithiated silicon–sulfur (SLS) battery prototypes. Electrochem Commun 14(1):21 – 24
Handbook of Solid State Batteries, 2nd ed., Dudney N J, West W C, Nanda J (Eds.), World Scientific 2015
Janek J, Zeier W (2016) A solid future for battery development. Nat Energy 1(9):16141
Luntz A C, Voss J, Reuter K (2015) Interfacial challenges in solid-state Li ion batteries. J Phys Chem Lett 6:4599–4604
Robinson A L, Janek J (2014) Solid-state batteries enter EV fray. MRS Bulletin 39:1046
Kato, Y. et al. (2016) High-power all-solid-state batteries using sulfide superionic conductors. Nat Energy 1:16030
Oh G, Hirayama M, Kwon O, Suzuki K, Kanno R (2016) Bulk-type all solid-state batteries with 5 V class LiNi0.5Mn1.5O4 cathode and Li10GeP2S12 solid electrolyte. Chem Mater 28:2634–2640
Bachman JC, Muy S, Grimaud A, Chang HH, Pour N, Lux SF, Paschos O, Maglia F, Lupart S, Lamp P, Giordano L, Shao-Horn Y (2016) Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chem Rev 116(1):140–162
Minami T, Hayashi A, Tatsumisago M (2006) Recent progress of glass and glass-ceramics as solid electrolytes for lithium secondary batteries. Solid State Ionics 177:2715–2720
Wenzel S, Weber D, Leichtweiss T, Sann J, Janek J (2016) Interphase formation and degradation of charge transfer kinetics between a lithium metal anode and highly crystalline Li7P3S11 solid electrolyte. Solid State Ionics 286:24–33
Zhu Y, He X, Mo Y (2015) Origin of outstanding stability in the lithium solid electrolyte materials: insights from thermodynamic analyses based on first-principles calculations. ACS Appl Mater Interface 7:23685–23693
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer-Verlag GmbH Germany, part of Springer Nature
About this chapter
Cite this chapter
Janek, J., Adelhelm, P. (2018). Next generation technologies. In: Korthauer, R. (eds) Lithium-Ion Batteries: Basics and Applications. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-53071-9_16
Download citation
DOI: https://doi.org/10.1007/978-3-662-53071-9_16
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-53069-6
Online ISBN: 978-3-662-53071-9
eBook Packages: EnergyEnergy (R0)