Abstract
The search for new superconductors continued even after the discovery of the cuprate superconductors. We will briefly describe the most important developments. In 2001, Jun Akimitsu and colleagues from Tokyo reported the discovery of superconductivity in the compound magnesium diboride (MgB2) with the critical temperature TC = 39 K.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
The search for new superconductors continued even after the discovery of the cuprate superconductors. We will briefly describe the most important developments. In 2001, Jun Akimitsu and colleagues from Tokyo reported the discovery of superconductivity in the compound magnesium diboride (MgB2) with the critical temperature TC = 39 K. This was very surprising, since the two elements magnesium and boron are not superconducting themselves and the compound had been well known for a long time. The hexagonal crystal structure of MgB2 shows a layered structure of alternating planes of magnesium and boron atoms. Again, the formation of Cooper pairs due to the electron–phonon interaction is the basis of superconductivity. MgB2, however, is a case of so-called two-band superconductivity, in which charge carriers from two energy bands contribute differently to superconductivity. The wave function of the Cooper pairs shows no clear directional dependence.
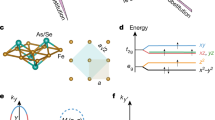
The discovery of the iron- and arsenic-containing pnictides in 2008 by Hideo Hosono and colleagues in Japan was another important step. It began with the compound LaOFeAs consisting of lanthanum (La), oxygen (O), iron (Fe) and arsenic (As), which was still doped with fluorine (F). In the compound LaO1−xFxFeAs, the critical temperature TC = 26 K was observed for x = 0.07. Also other elements of the light rare earths (Rare Earths, Re) such as praseodymium (Pr), neodymium (Nd) or samarium (Sm) instead of lanthanum resulted in superconducting compounds within the family ReO1−xFxFeAs. Critical temperature values up to the record value TC = 56 K in Sr0.5Sm0.5FeAsF were observed. In their electronic transport properties, the pnictides do not show pronounced anisotropy. The important structural elements are plane layers of iron atoms surrounded by tetrahedrally arranged As- or Se-anions, which play the role of CuO planes in the cuprates (Fig. 10.1). The layers are stacked on top of each other and separated from each other by blocking layers of alkali atoms, elements of the alkaline earths or rare earths and oxygen atoms. For doping, oxygen is partially replaced by fluorine.
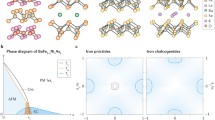
Similar to the case of the cuprates 22 years earlier, research on iron pnictides developed explosively worldwide. Similar to the cuprates, the iron pnictides are magnetically ordered in the undoped state. Unlike the cuprates, however, they are electrically conducting. They are an antiferromagnetic semimetal. It seems that in this case, superconductivity and magnetism are related. In Fig. 10.2, we show an overview of the different iron pnictides discovered until 2015. The different families are identified by the names 11, 111, 122, 1111, etc. More than 50 iron pnictides have been found by 2010. The pairing mechanism is still unclear. However, there is much evidence to suggest magnetic spin fluctuations as the basis for the formation of Cooper pairs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2021 Springer Fachmedien Wiesbaden GmbH, part of Springer Nature
About this chapter
Cite this chapter
Huebener, R.P. (2021). MgB2, Iron Pnictides. In: History and Theory of Superconductors. essentials(). Springer, Wiesbaden. https://doi.org/10.1007/978-3-658-32380-6_10
Download citation
DOI: https://doi.org/10.1007/978-3-658-32380-6_10
Published:
Publisher Name: Springer, Wiesbaden
Print ISBN: 978-3-658-32379-0
Online ISBN: 978-3-658-32380-6
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)