Abstract
Percutaneous tumor ablation has become one of the most prevalent treatment options for small renal cell carcinomas given continued favorable outcome and is already a treatment of choice for selected patients. Currently, the most common indications are elderly patients with small incidental tumors of unclear metastatic potential and high-risk surgical patients since this procedure has been shown to be safe in patients with multiple comorbidities. This chapter describes the indications, applicators, procedure, outcomes, and complications of percutaneous tumor ablation of renal cell carcinoma. Importantly, the technologies are continuously improving, and it is expected that patient selection and satisfaction will continue to expand and outcomes will continue to improve.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Renal Cell Carcinoma
- Partial Nephrectomy
- Tumor Ablation
- Poor Surgical Candidate
- Percutaneous Cryoablation
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Radio-frequency ablation and cryoablation are treatments for focal malignancy where a needle applicator is advanced through the skin into the center of a tumor (guided by ultrasound, computed tomography, or magnetic resonance imaging) and then induces hot or cold cytotoxic temperatures that rapidly result in tumor death (Goldberg et al. 2000). Once reserved for nonsurgical patients, these minimally invasive treatment options have now expanded to include a larger spectrum of patients, fueled by continued favorable outcome studies, scarce complications, lower immediate morbidity and mortality than surgery, lower cost, outpatient capacity, and, importantly, patient satisfaction (McAchran et al. 2005; Onishi et al. 2007). These benefits must be balanced with the acknowledgement of the relative newness of these procedures which is accompanied by lack of long-term data and that there are challenges including the inability to treat large, centrally located tumors near the renal hilum and difficulties monitoring ablation. But even as initial outcome data is published, rapid technological advancements and improvements in technique evoke even greater promise for success going forward.
1 Indications
According to the American Cancer Association, there are approximately 50,000 new cases of renal cell carcinoma diagnosed each year in the United States, an incidence that has more than doubled since 1950 (Jemal et al. 2008). Most of these are incidentally detected on high-resolution diagnostic imaging for unrelated indications. Many of these incidentally noted RCCs are T1a tumors (less 4 cm in diameter limited to the kidney) and found in elderly patients who are generally poorer surgical candidates as they were imaged for other comorbid conditions (Jayson and Sanders 1998). In addition, the benefit of a total or partial nephrectomy (currently the most common treatment option) is unclear for these small, nonaggressive RCCs whose natural course may not affect patient longevity (Hollingsworth et al. 2006). Indeed, prior to the development of these minimally invasive procedures, conservative watchful waiting was often advocated, despite substantial patient anxiety (Chow et al. 1999). For these patients, percutaneous tumor ablation is an attractive option since it spares the significant morbidity of surgery while offering effective and potentially curative treatment option (Onishi et al. 2007). Lastly, from a public health perspective, tumor ablation is likely preferred over surgery for small RCC treatment at a societal willingness-to-pay threshold (Pandharipande et al. 2008).
Patients needing nephron-sparing treatment such as those with a single kidney, with bilateral RCCs, or with genetic predisposition to multiple tumors are good candidates even if initially they are also candidates for partial nephrectomy (Goldberg and Dupuy 2001). For example, patients with von Hippel-Lindau syndrome are excellent candidates, as alternative treatments to multiple partial nephrectomies for recurring RCCs are important to save nephrons and prolong the time to dialysis. Patients already on dialysis are also at increased risk for renal cell carcinoma and, given their renal failure, are often poor surgical candidates as well. Other risk factors for renal cell carcinoma which may lead to poor surgical candidacy include smoking and obesity (Cohen and McGovern 2005).
Other uses of ablation in the treatment of RCC have included local tumor recurrence after nephrectomy (McLaughlin et al. 2003), intractable tumor-related hematuria (Wood et al. 2001), palliation for lung and symptomatic bone metastases (Zagoria et al. 2001; Dupuy et al. 1998), and tumor debulking in conjunction with immunotherapy in patients with stage IV disease (Goldberg and Dupuy 2001). Again, each case should be reviewed on a case by case basis.
2 Patient Selection
Patients are usually jointly evaluated by a urologist and interventional radiologist, but practices may vary amongst different centers. Regardless, each practitioner must weigh best interests of the patient. Since one-quarter of patients with RCC will have metastatic disease at diagnosis which precludes local treatment and indicates a poor 5-year survival rate, the extent of disease should be well established with sufficient abdominal and nonabdominal imaging to verify the extent of local tumor and metastatic involvement (Cohen and McGovern 2005). In addition, pretreatment imaging is important for treatment planning which can be performed via ultrasound, magnetic resonance, or CT guidance (Goldberg et al. 2000). Important laboratory values include prothrombin time, partial prothrombin time, complete blood cell count, creatinine, and screening for intravenous sedation or anesthesia. A biopsy prior to the procedure is not imperative but should be strongly considered, because imaging does not always accurately differentiate benign from malignant disease (Silverman et al. 2006).
Tumor size and location are the two most important factors that govern whether RCCs can be successfully treated (Gervais et al. 2005a). Since heat exponentially decreases from the radio frequency or cryo source, large tumors (>5 cm) pose a significant challenge, especially since a 0.5–1.0-cm “ablation margin” surrounding the tumor is also preferred (Goldberg and Dupuy 2001). In general, RCC tumors that are 4 cm in diameter or less are ideal for ablation, with highly favorable success rates (>90 %) when performed by well-trained clinicians (Levinson et al. 2008). Most tumors smaller than 3 cm can also be successfully treated in a single session (Zagoria et al. 2004). Tumors between 3.0 and 4.0 cm in diameter can also be successfully treated with confidence, but multiple ablations and sessions may be required (Gervais et al. 2005b). Indeed, as implied above when describing therapy for VHL RCC, one of the benefits cited for RF ablation is the ability to perform minimally invasive repeated treatments.
The location of the tumor also influences ablation results. The easiest tumors to treat are exophytic as they are surrounded by heat-insulating perirenal fat (Liu et al. 2006; Ahmed et al. 2004). As a result, even large exophytic tumors are almost always successfully treated, with 70 % or more requiring only a single RF session (Hui et al. 2008). Parenchymal tumors may be more difficult to treat, but centrally located tumors represent a larger obstacle for successful ablation given with the surrounding vascular tissue which draws heat away from the tumor (i.e., the heat-sink effect) (Lu et al. 2002). As a result, central tumors larger than 3 cm have an increased risk of treatment failure (Ogan et al. 2002). Additional factors affecting ablation are the electrical and thermal conductivities of the tumor and surrounding tissues which influence the capacity for energy deposition and heat accumulation, respectively (Ahmed et al. 2008; Solazzo et al. 2005).
Contraindications may include a poor life expectancy of less than 1 year, multiple metastases, or difficulty for successful treatment due to size or location of tumor (Ahrar et al. 2005). In general, large tumors (>5 cm) or tumors in the hilum or central collecting system are not typically recommended for percutaneous tumor ablation (Atwell et al. 2007; Gervais et al. 2005b). In addition, tumors located so that thermal injury may occur to the proximal ureter, resulting in urine extravasation and urinoma production, are usually deferred until an intraureteral stent has been placed by a urologist (Johnson et al. 2003). However, the only absolute contraindications include irreversible coagulopathies or severe medical instability such as sepsis.
3 Procedure
The aim of percutaneous tumor ablation is to kill all viable malignant cells within a designated area including a 5–10-mm “ablative” margin of surrounding tissue, if possible, with the minimal damage of the adjacent tissues (Goldberg et al. 2000). The most well-studied techniques and those that received most attention are radio-frequency ablation and cryoablation, but some emerging energy sources such as microwave and high-intensity focused ultrasound show some promise but are only available in controlled experimental situations.
3.1 Radio-frequency Ablation
RF delivers a high-frequency (460–500 kHz) alternating current into the tumor by means of an RF applicator, a single thin needle (usually 21–14 gauge) that is electrically insulated along all but the distal 1–3 cm of the shaft, or an array of multiple such tines extending from a central cannula. The current produces resistive friction in the tissue that is converted into heat, analogous to heat production from an electrical resister in a circuit. Heat, in turn, induces cellular destruction and protein denaturation. Cell death occurs at temperatures higher than 50 °C with complete tumor necrosis being achieved at 60–100 °C (Goldberg et al. 2000). Most RF systems used for the kidney are monopolar, and the current is completed via grounding pads placed on the patient’s thighs. Efforts to increase tumor ablation have led to the development of various RF applicators such as multitined applicators, cluster applicators, pulsed energy delivery, and others. Currently, there are three RF devices with 510-K Food and Drug Administration approval for soft tumor ablation (Valleylab Cool-tip™, Boulder, Colorado; Boston Scientific LeVeen®, Natick, Massachusetts; AngioDynamics StarBurst™, Queensbury, New York). No study has yet demonstrated a clear advantage of any one device, and new devices are sure to become available given increasing market demand.
3.2 Cryoablation
Cryoablation uses cooled cryoprobes to freeze and destroy tumor. Traditionally, given the large applicator size (up to 8-mm diameter, or 0.5 gauge) (Finley et al. 2008), it was almost always administered via laparoscope, but smaller applicators now enable percutaneous image-guided application (17 gauge) (Finley et al. 2008). Liquid nitrogen or argon is introduced into the probe in a controlled fashion, resulting in freezing of the surrounding tissues. The formation of ice crystals and subsequent thawing within a cell disrupts the cell membrane and other intracellular activities leading to cell death. Additional cells which are not directly killed may undergo apoptosis. A typical cryoablation session involves freezing, thawing, and refreezing which is particularly effective at mediating cellular disruption (Hoffmann and Bischof 2002; Rupp et al. 2002).
One cited benefit of cryoablation is the ability to visualize the ice ball in real time via CT or MR which allows the extent of cell death to be more reliably predicted (Edmunds et al. 2000). It is thought that complete cell death occurs 3 mm inside the edge of the ice ball with most operators extending the ice ball at least 5 mm beyond the tumor margin (Warlick et al. 2006). However, for RF ablation performed under CT guidance, a postprocedural scan with contrast will also often enable gross visualization of enhancing residual tumor which can then be re-treated (Goldberg et al. 2000). According to the most recent studies, cryoablation provides a safe and oncologically effective alternative to extirpative surgery for renal masses in patients with significant medical comorbidities (Kim et al. 2013). In patients with solitary kidneys, renal cryoablation is associated with superior perioperative outcomes compared to partial nephrectomy. Specifically, partial nephrectomy is not associated with greater loss of renal function than renal cryoablation regardless of the extent of tumor complexity (Panumatrassamee et al. 2013).
3.3 Emerging Technologies
Microwave energy is emerging as a potential source of heat for use in thermal ablation although the technology is still in its infancy with only small experimental series in patients (Liang et al. 2008; Clark et al. 2007). In contrast to radio frequency, within the tip of the inserted microwave applicator is an antenna for externally applied energy at 1,000–2,450 mHz. The deposited microwave energy results in rotation of polar molecules that is opposed by frictional forces which are then converted to heat (Carrafiello et al. 2008). One potential advantage of microwave over RF is greater energy deposition and higher temperatures, especially since microwave is not impeded by peri-applicator tissue charring like RF. The higher energy may also be more resilient to the heat-sink effect which can be an important obstacle for RF (Brace et al. 2007). As such, the technology is deserving of further consideration but requires further investigation as the efficacy and safety relative to more proven technologies are still to be determined (Liang et al. 2008).
High-intensity focused ultrasound is another emerging minimally invasive ablation tool with most research focused on fibroid treatment (Lenard et al. 2008). There has not yet been any well-documented investigation for the possible application for renal tumors. One theoretically attractive option is that cytotoxic heat accumulation is created via multiple high-intensity ultrasound waves delivered by a transducer on the skin without any physical penetration of the skin surface (Klingler et al. 2008).
3.4 Difficulties Comparing Modalities and Approaches
There is currently no conventional acceptance as to which modality is superior given insufficient data for even the two most widely used procedures: radio-frequency ablation vs. cryoablation. Cryoablation is almost always performed with laparoscopy owing to the large size of most applicators, and there is insufficient follow-up data for the more recently developed percutaneous cryoablation to be useful for comparison. On the other hand, it has been used longer so there is potentially more experience with that technique. Recognizing these differences, meta-analyses indicate that a second treatment session is necessary more often for percutaneous radio-frequency ablation than for laparoscopic cryoablation (primary efficacy rates of 87.1 and 94.8 %, respectively) (Kunkle and Uzzo 2008; Hui et al. 2008). However, the secondary efficacy rates after retreatment are similar (92 and 95 %, respectively, p > 0.05) (Hui et al. 2008). There is also no significant difference between metastatic progression (2.5 and 1.0 %, respectively, p > 0.05) (Kunkle and Uzzo 2008). However, meta-analysis does suggest that major complications may be lower in percutaneous radio-frequency ablation than laparoscopic cryoablation (3 % vs. 7 %, respectively) (Kunkle and Uzzo 2008). Additionally, other initial comparisons between percutaneous and laparoscopic cryoablation suggest that there may be higher overall complications, transfusion rates, analgesic use, and hospital stays with a laparoscopic approach compared to percutaneous treatment (40 %, 28 %, 17.8 mg, and 3.1 days vs. 22 %, 11 %, 5.1 mg, and 1.3 days, respectively, all p < 0.05) with similar failure rates at 1-year follow-up (4.2 and 5.3 %, respectively) (Finley et al. 2008). Further validation with randomized prospective data is necessary as these studies are small retrospective studies that cannot control for factors that may have influenced the choice of approach. An analysis of 5- and 10-year follow-up data will be helpful when available.
Regardless, we adhere to the notion that less invasive approaches should be preferred over more invasive in the setting of similar outcomes.
In conclusion, in the setting of RCC, percutaneous ablation procedures can be considered over laparoscopic ones, saving partial nephrectomy for cases when the first two approaches are not advised. However, comparing the individual percutaneous modalities (i.e., radio-frequency ablation and cryoablation) for definite advantages over each other is challenging, as both are being presently refined. Thus, the most important factors will continue to be proper patient selection and meticulous technique by experienced clinicians.
3.5 Adjuvant Therapy
Recent studies have demonstrated that the modification of tumor vessel density using antiangiogenic agents such as sorafenib (Nexavar®, Bayer HealthCare, Leverkusen, Germany) and sunitinib (SUTENT®, Pfizer Labs, New York, New York) increases the efficacy of RF coagulation. In one study, the administration of sorafenib prior to RF ablation markedly decreased microvascular density and led to significantly larger zones of RF-induced coagulation necrosis (Hakime et al. 2007). There is also the potential for combining treatment with radiation as has been examined in animal studies and in patients with lung cancer (Horkan et al. 2005; Dupuy et al. 2006). Although these methods are still in their investigational phase, there is promise for their rapid acceptance and adoption in standard clinical practice.
3.6 Biopsy Controversy
There is currently some debate about whether biopsy should be performed before ablation. For most institutions, pathologic confirmation of malignancy is a tacit or explicit requirement before any type of treatment is initiated, including thermal ablation, but some institutions are beginning to question whether a biopsy is always necessary (Beland et al. 2007). Additionally, ablation of the tumor will make pathologic examination of the tissue much more challenging if this is ever needed for future therapeutic considerations. The argument to not perform a biopsy is the need for two percutaneous procedures which are associated with increased costs and small but genuine procedural risks and that many tumors demonstrate imaging features that are highly suggestive of renal cell carcinoma perhaps making biopsy redundant (Herts and Baker 1995). In addition, even small amounts of hemorrhage associated with biopsy can potentially obscure the margins of the tumor which can increase the difficulty of the ablative procedure. Unlike laparoscopic surgery where port-site seeding is estimated to be 0.1 %, percutaneous tumor tract seeding has only been reported in a single case report (Castillo and Vitagliano 2008; Bush et al. 1977; Tanaka et al. 2008). To minimize the risks and costs, a biopsy can be easily performed immediately prior to ablation, but pathologic analysis cannot be rendered before tumor ablation commences.
3.7 Day of the Procedure
Regardless of the energy source, the percutaneous procedure is usually an outpatient procedure unless comorbid conditions require hospitalization or closer observation. Only conscious sedation is typically necessary for anesthesia, although some physicians and patients prefer general anesthesia. The patient is placed prone, and after local anesthesia is applied, the applicator is percutaneously advanced into the center of the tumor under image guidance (CT, ultrasound, or MR). Heat or cold is then applied for approximately 10–20 min as dictated per manufacturer recommendations. Depending upon the size and location of the tumor, the applicator may need to be readjusted and additional treatments administered. After the procedure, the patient is monitored for several hours and discharged home with oral analgesics for postprocedural pain with patients usually resuming to full activity in a couple of days. A laparoscopic approach is similar to other surgical laparoscopic procedures requiring general anesthesia. Patients are hospitalized at least overnight and may have slightly higher postprocedural pain (Finley et al. 2008).
4 Outcomes and Complications
4.1 Imaging Follow-Up
The success of percutaneous tumor ablation is assessed by postprocedural imaging, typically by computed tomography or MR starting 1 month after treatment. Imaging immediately following the procedure can be difficult to interpret, because peripheral inflammation may mimic the appearance of viable tumor. On computed tomography, viable tumor is usually nodular and maintains its enhancement (>10-HU postcontrast injection), whereas successfully ablated tumor loses its attenuation, consistent with coagulation necrosis (Gervais et al. 2005a, b). It has also been noted that tumors usually decrease in size immediately after ablation by about 20 %, while many continue to involute over time (Ganguli et al. 2008). Very rarely does the zone of ablation enlarge because of liquefactive necrosis (Merkle et al. 2005). The area of nonenhancement itself may be larger than the original tumor as the zone of ablation is expected to be larger than the tumor to allow for an “ablative margin.” Recurrent tumor will usually manifest as peripheral nodular or peripheral crescent enhancement (Gervais et al. 2005a, b). Subsequent follow-up imaging is usually performed at 3–6, 12, 18, and 24 months after ablation, and yearly thereafter.
Many patients who undergo percutaneous tumor ablation have renal insufficiency which is often a large contributing factor in deciding to undergo tumor ablation since it limits destruction to vital normal renal parenchyma. For these patients, follow-up imaging is often performed with MR to limit the toxicity of iodinated contrast administered with CT. Yet, unenhanced imaging is almost never sufficient for evaluation of recurrent tumor.
Current thinking is that even if the eGFR is estimated to be very low which may place the patient at risk for nephrogenic systemic fibrosis (Wertman et al. 2008), the risk-benefit ratio still usually favors proceeding with MR imaging with gadolinium rather than CT with iodine-based contrast agents (Geoffrey et al. 2007). Again, residual or recurrent tumor usually manifests as abnormal nodular or crescent enhancement with gadolinium. Immediate postablation MR imaging may demonstrate smooth rim peripheral enhancement secondary to surrounding hyperemia. Unenhanced T1 and T2 signal may be variable and complex due to hemorrhage, coagulated protein, and liquefactive necrosis (Merkle et al. 2005).
4.2 Imaging Pitfalls
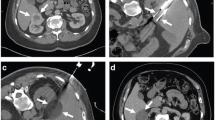
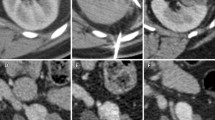
During ablation, gas is formed without and about the tumor as a by-product of tissue coagulation (Fig. 1). This may be visualized on immediate postprocedural imaging and should not be mistaken for bowel injury. In addition, hydrodissection is occasionally employed prior to ablation to protect the adjacent organs from heat injury. This involves infusing sterile water into the surrounding tissue to create a barrier between the adjacent organs and the ablative zone (Fig. 2). This should not be mistaken for hemorrhage or bowel injury.
Successful radio-frequency ablation of renal cell carcinoma. (a) Noncontrast CT demonstrates exophytic 2.5-cm tumor (arrow); (b) positioning of RF applicator with tip at distal end of tumor (arrow); (c) gas (arrows) is produced during ablation secondary to high-temperature coagulation; (d) immediate contrast-enhanced CT demonstrates nonenhancement of the tumor with a small surrounding “margin” of nonenhancement of the adjacent kidney (arrows) and small clinically insignificant perinephric hemorrhage (arrowhead); (e) 2 years after ablation, contrast-enhanced CT follow-up demonstrates continued nonenhancement of the tumor with a characteristic fat “halo” in the perirenal fat (arrowheads), suggesting complete treatment
The use of hydrodissection in thermal ablation to protect adjacent structures in a patient with multiple RCCs. (a) The colon (arrowheads) is closely approximated to the 1.3-cm tumor in the right kidney (black arrow), risking thermal injury; (b) 5 % dextrose in water (arrowheads) is injected into the perirenal fat which successfully separates the colon from the kidney (double-headed arrow); (c) applicator needle (arrow) is positioned into the tumor with the aid of a guiding needle (arrowhead); (d) immediate postablation CT demonstrates the ablative zone about the tumor (arrows); (e) 6-month follow-up MR with gadolinium demonstrates no recurrent tumor enhancement (arrows). A second 1.5-cm RCC in the right lower pole was also successfully treated during the same session (not shown)
Follow-up imaging also commonly demonstrates inflammatory stranding within the surrounding perirenal fat which should not be confused with residual tumor. Over time, a thin soft tissue halo may also appear within the surrounding fat due to encapsulation of fat necrosis which should not be interpreted as recurrent tumor (Fig. 1) (Gervais et al. 2003). More recently, it has also been observed that enhancing inflammatory nodules do rarely appear (<2 % of the time) after percutaneous ablation which may mimic tumor seeding of the applicator tract (Lokken et al. 2007). Real tumor tract seeding is exceeding rare and has only ever been reported once after radio-frequency ablation of RCC (Mayo-Smith et al. 2003). Instead, a new enhancing nodule within or adjacent to the applicator track is more likely to represent chronic inflammation containing histiocytes, granulation tissue, and fibrosis. These will usually appear as either a ring-enhancing nodule or ill-defined tram-tracking enhancement appearing 3–52 months after ablation (Lokken et al. 2007). Nodular enhancement along the ablated tumor margin, however, should be treated with suspicion for recurrence.
4.3 Outcomes
Results vary depending upon the modality (cryoablation vs. radio frequency) and applicator type (single vs. multitine), but meta-analyses across all percutaneous approaches yield a secondary effectiveness rate (i.e., no evidence of recurrence after multiple treatments is necessary) greater than 90 % for tumors smaller than 4 cm which is not significantly different than surgical treatment at 1 year (Hui et al. 2008).
4.3.1 Outcomes for Radio-frequency Ablation
Some midterm data is becoming available demonstrating recurrence-free survival rate of approximately 90 % at 5 years for tumors smaller than 4 cm (Levinson et al. 2008). Additionally, as technology and the learning curve have markedly progressed since treatment was performed for these initial survival data, future studies are expected to be as good or better. Again, treatment success is dependent upon size and location (exophytic vs. central) with near 100 % recurrence-free disease possible with selected tumor sizes (<4 cm) with larger tumors associated with increased risk of recurrence.
4.3.2 Outcomes for Cryoablation
Data is very limited for percutaneous cryoablation (Fig. 3), but short-term success (1 year) also appears to be excellent with success rates consistently above 95 % (Atwell et al. 2008). In addition, technical success was achieved with tumors ranging up to 7 cm, though the same principle of selecting tumors less than 4 cm still applies for optimal results (Stein and Kaouk 2007). Again, 5- and 10-year follow-up outcome data will be helpful with the caveat that current treatments will likely be superior given continuously technological improvements.
When comparing the outcomes of laparoscopic to percutaneous cryoablation at the same center, the procedural outcomes are demonstrably superior for percutaneous cryoablation including lower complications and transfusions (22 % vs. 40 %, respectively), shorter hospital stays (1.3 vs. 3.1 days, respectively), and lower narcotic use (5.1 vs. 17.8 mg, respectively). It should be noted that the complication rates reported in this series were higher than the accepted complication rate for percutaneous cryoablation (around 3 %) (Hui et al. 2008). Regardless, in short-term follow-up (13 months), cancer-specific survival is similar for percutaneous and laparoscopic cryoablation (100 and 100 %, respectively), and initial treatment failure was also not significantly different at 5.3 % (1/19) and 4.3 % (1/24), respectively.
4.4 Complications
The average complication rate is less than 5 % for both radio frequency and cryoablation, but almost none result in long-term morbidity. Radio-frequency ablation and cryoablation are both effective in the treatment of renal masses measuring 3 cm or smaller (Atwell et al. 2013). Major complications with either procedure are infrequent. Meta-analyses demonstrate a major complication rate of 3 % for percutaneous treatment vs. 7 % in the surgical treatment group (7 %; p < 0.05) which is the accepted clinical understanding (Hui et al. 2008; Johnson et al. 2004). The most common complications include perinephric hematoma, pneumothorax, nerve injury, and pain. Central tumors and tumors within the lower pole also run the risk of ureteral or ureteropelvic injury. A few case reports have documented nephrectomies that were necessary after ureteral injury or obstruction, but again, this is compared to oncologic treatment that could have included nephrectomy itself.
It is also important to note that the very low complication rate associated with RF ablation is reported in patients who were already deemed too high risk for surgical intervention because of advanced age or medical comorbidities. Thus, even in high-risk patients, percutaneous tumor ablation is associated with a very low complication rate.
5 Conclusion
Minimally invasive treatments for renal cell carcinoma such as percutaneous tumor ablation will undoubtedly become more prevalent as outcomes continue to be favorable and should be considered a viable treatment option for selected patients. Currently, the most common indications are elderly patients with small incidental tumors of unclear lethal potential and all high-risk surgical patients as this procedure has been shown to be safe in patients with multiple comorbidities. Multiple modalities are available, but the most common are RF and cryoablation which are likely similar in efficacy but still lack sufficient long-term data. These technologies are continuously improving, and it is expected that, as a result, patient selection and satisfaction will continue to expand.
References
Ahmed M, Liu Z, Afzal KS et al (2004) Radiofrequency ablation: effect of surrounding tissue composition on coagulation necrosis in a canine tumor model. Radiology 230(3):761–767
Ahmed M, Liu Z, Humphries S et al (2008) Computer modeling of the combined effects of perfusion, electrical conductivity, and thermal conductivity on tissue heating patterns in radiofrequency tumor ablation. Int J Hyperthermia 24(7):577–588
Ahrar K, Matin S, Wood CG et al (2005) Percutaneous radiofrequency ablation of renal tumors: technique, complications, and outcomes. J Vasc Interv Radiol 16:679–688
Atwell TD, Farrell MA, Callstrom MR et al (2007) Percutaneous cryoablation of large renal masses: technical feasibility and short-term outcome. AJR Am J Roentgenol 188(5):1195–1200
Atwell TD, Farrell MA, Leibovich BC et al (2008) Percutaneous renal cryoablation: experience treating 115 tumors. J Urol 179(6):2136–2140
Atwell TD, Schmit GD, Boorjian SA et al (2013) Percutaneous ablation of renal masses measuring 3.0cm and smaller: comparative local control and complications after radiofrequency ablation and cryoablation. AJR Am J Roentgenol 200(2):461–466
Beland MD, Mayo-Smith WW, Dupuy DE et al (2007) Diagnostic yield of 58 consecutive imaging-guided biopsies of solid renal masses: should we biopsy all that are indeterminate? AJR Am J Roentgenol 188(3):792–797
Brace CL, Laeseke PF, Sampson LA et al (2007) Microwave ablation with multiple simultaneously powered small-gauge triaxial antennas: results from an in vivo swine liver model. Radiology 244(1):151–156
Bush WH Jr, Burnett LL, Gibbons RP (1977) Needle tract seeding of renal cell carcinoma. AJR Am J Roentgenol 129(4):725–727
Carrafiello G, Laganà D, Mangini M et al (2008) Microwave tumors ablation: principles, clinical applications and review of preliminary experiences. Int J Surg 6(Suppl 1):S65–S69
Castillo OA, Vitagliano G (2008) Port site metastasis and tumor seeding in oncologic laparoscopic urology. Urology 71(3):373–378
Chow WH, Devesa SS, Warren JL et al (1999) Rising incidence of renal cell cancer in the United States. JAMA 281:1628–1631
Clark PE, Woodruff RD, Zagoria RJ et al (2007) Microwave ablation of renal parenchymal tumors before nephrectomy: phase I study. AJR Am J Roentgenol 188(5):1212–1214
Cohen HT, McGovern FJ (2005) Renal-cell carcinoma. N Engl J Med 353:2477–2490
Dupuy DE, Safran H, Mayo-Smith WW et al (1998) Radiofrequency ablation of painful osseous metastases. Radiology 209(P):389
Dupuy DE et al (2006) Radiofrequency ablation followed by conventional radiotherapy for medically inoperable stage I non-small cell lung cancer. Chest 129(3):738–745
Edmunds TB, Schulsinger DA, Durand DB et al (2000) Acute histologic changes in human renal tumors after cryoablation. J Endourol 14(2):139–143
Finley DS, Beck S, Box G et al (2008) Percutaneous and laparoscopic cryoablation of small renal masses. J Urol 180(2):492–498
Ganguli S, Brennan DD, Faintuch S et al (2008) Immediate renal tumor involution after radiofrequency thermal ablation. J Vasc Interv Radiol 19(3):412–418
Geoffrey EW, Leyendecker JR, Krehbiel KA et al (2007) CT and MR imaging after imaging-guided thermal ablation of renal neoplasms. Radiographics 27:325–339
Gervais DA, McGovern FJ, Arellano RS et al (2003) Renal cell carcinoma: clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology 226:417–424
Gervais DA, Arellano RS, McGovern FJ et al (2005a) Radiofrequency ablation of renal cell carcinoma. II. Lessons learned with ablation of 100 tumors. AJR Am J Roentgenol 185:72–80
Gervais DA, McGovern FJ, Arellano RS et al (2005b) Radiofrequency ablation of renal cell carcinoma. I. Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol 185:64–71
Goldberg SN, Dupuy DE (2001) Image-guided radiofrequency tumor ablation: challenges and opportunities – part I. J Vasc Interv Radiol 12:1021–1032
Goldberg SN, Gazelle GS, Mueller PR (2000) Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. Am J Radiol 174:323–331
Hakime A, Hines-Peralta AU, Peddy H et al (2007) Combination of radiofrequency ablation with antiangiogenic therapy for tumor ablation efficacy: study in mice. Radiology 244(2):464–470
Herts BR, Baker ME (1995) The current role of percutaneous biopsy in the evaluation of renal masses. Semin Urol Oncol 13(4):254–261
Hoffmann NE, Bischof JC (2002) The cryobiology of cryosurgical injury. Urology 60:40–49
Hollingsworth JM, Miller DC, Daignault S et al (2006) Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst 98:1331–1334
Horkan C et al (2005) Reduced tumor growth with combined radiofrequency ablation and radiation therapy in a rat breast tumor model. Radiology 235(1):81–88
Hui GC, Tuncali K, Tatli S et al (2008) Comparison of percutaneous and surgical approaches to renal tumor ablation: metaanalysis of effectiveness and complication rates. J Vasc Interv Radiol 19(9):1311–1320
Jayson M, Sanders H (1998) Increased incidence of serendipitously discovered renal cell carcinoma. Urology 51:203–205
Jemal A, Siegel R, Ward E et al (2008) Cancer statistics, 2008. CA Cancer J Clin 58:71–96
Johnson DB, Saboorian MH, Duchene DA et al (2003) Nephrectomy after radiofrequency ablation-induced ureteropelvic junction obstruction: potential complication and long-term assessment of ablation adequacy. Urology 62(2):351–352
Johnson DB, Solomon SB, Su LM et al (2004) Defining the complications of cryoablation and radio frequency ablation of small renal tumors: a multi-institutional review. J Urol 172:874–877
Kim EH, Tanagho YS, Bhayani SB et al (2013) Percutaneous cryoablation of renal masses: Washington University experience of treating 129 tumours. BJU 111(6):872–879
Klingler HC, Susani M, Seip R et al (2008) A novel approach to energy ablative therapy of small renal tumours: laparoscopic high-intensity focused ultrasound. Eur Urol 53(4):810–816
Kunkle DA, Uzzo RG (2008) Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer 113(10):2671–2680
Lenard ZM, McDannold NJ, Fennessy FM et al (2008) Uterine leiomyomas: MR imaging-guided focused ultrasound surgery – imaging predictors of success. Radiology 249(1):187–194
Levinson AW, Su LM, Agarwal D et al (2008) Long-term oncological and overall outcomes of percutaneous radio frequency ablation in high risk surgical patients with a solitary small renal mass. J Urol 180(2):499–504
Liang P, Wang Y, Zhang D et al (2008) Ultrasound guided percutaneous microwave ablation for small renal cancer: initial experience. J Urol 180(3):844–848
Liu Z, Ahmed M, Weinstein Y et al (2006) Characterization of the RF ablation-induced “oven effect”: the importance of background tissue thermal conductivity on tissue heating. Int J Hyperthermia 22(4):327–342
Lokken RP, Gervais DA, Arellano RS et al (2007) Inflammatory nodules mimic applicator track seeding after percutaneous ablation of renal tumors. AJR Am J Roentgenol 189:845–848
Lu DS, Raman SS, Vodopich DJ et al (2002) Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the “heat sink” effect. AJR Am J Roentgenol 178:47–51
Mayo-Smith WW, Dupuy DE, Parikh PM et al (2003) Imaging-guided percutaneous radiofrequency ablation of solid renal masses; techniques and outcomes of 38 treatment sessions in 32 consecutive patients. AJR Am J Roentgenol 180:1503–1508
McAchran SE, Lesani OA, Resnick MI (2005) Radiofrequency ablation of renal tumors: past, present, and future. Urology 66:15–22
McLaughlin CA, Chen MY, Torti FM et al (2003) Radiofrequency ablation of isolated local recurrence of renal cell carcinoma after radical nephrectomy. AJR Am J Roentgenol 181:93–94
Merkle EM, Nour SG, Lewin JS (2005) MR imaging follow-up after percutaneous radiofrequency ablation of renal cell carcinoma: findings in 18 patients during first 6 months. Radiology 235:1065–1071
Ogan K, Jacomides L, Dolmatch BL et al (2002) Percutaneous radiofrequency ablation of renal tumors: technique, limitations, and morbidity. Urology 60(6):954–958
Onishi T, Nishikawa K, Hasegawa Y et al (2007) Assessment of health-related quality of life after radiofrequency ablation or laparoscopic surgery for small renal cell carcinoma: a prospective study with medical outcomes Study 36-Item Health Survey (SF-36). Jpn J Clin Oncol 37:750–754
Pandharipande PV, Gervais DA, Mueller PR et al (2008) Radiofrequency ablation versus nephron-sparing surgery for small unilateral renal cell carcinoma: cost-effectiveness analysis. Radiology 248(1):169–178
Panumatrassamee K, Kaouk JH, Autorino R et al (2013) Cryoablation versus minimally invasive partial nephrectomy for small renal masses in the solitary kidney: impact of approach on functional outcomes. J Urol 189(3):818–822
Rupp CC, Hoffmann NE, Schmidlin FR et al (2002) Cryosurgical changes in the porcine kidney: histologic analysis with thermal history correlation. Cryobiology 45:167–182
Silverman SG, Gan YU, Mortele JK et al (2006) Renal masses in the adult patient: the role of percutaneous biopsy. Radiology 240(1):6–22
Solazzo SA, Liu Z, Lobo SM et al (2005) Radiofrequency ablation: importance of background tissue electrical conductivity – an agar phantom and computer modeling study. Radiology 236(2):495–502
Stein RJ, Kaouk JH (2007) Renal cryotherapy: a detailed review including a 5-year follow-up. BJU Int 99:1265–1270
Tanaka K, Hara I, Takenaka A et al (2008) Incidence of local and port site recurrence of urologic cancer after laparoscopic surgery. Urology 71(4):728–734
Warlick CA, Lima GC, Allaf ME et al (2006) Clinical sequelae of radiographic iceball involvement of collecting system during computed tomography-guided percutaneous renal tumor cryoablation. Urology 67(5):918–922
Wertman R, Altun E, Martin DR et al (2008) Risk of nephrogenic systemic fibrosis: evaluation of gadolinium chelate contrast agents at four American universities. Radiology 248(3):799–806
Wood BJ, Grippo J, Pavlovich CP (2001) Percutaneous radio frequency ablation for hematuria. J Urol 166:2303–2304
Zagoria RJ, Chen MY, Kavanagh PV et al (2001) Radio frequency ablation of lung metastases from renal cell carcinoma. J Urol 166(5):1827–1828
Zagoria RJ, Hawkins AD, Clark PE et al (2004) Percutaneous CT-guided radiofrequency ablation of renal neoplasms: factors influencing success. AJR Am J Roentgenol 183:201–207
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Hines-Peralta, A., Goldberg, S.N., Quaia, E., Stacul, F. (2014). Radio-frequency Ablation and Cryoablation for Renal Cell Carcinoma. In: Quaia, E. (eds) Radiological Imaging of the Kidney. Medical Radiology(). Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-54047-9_23
Download citation
DOI: https://doi.org/10.1007/978-3-642-54047-9_23
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-54046-2
Online ISBN: 978-3-642-54047-9
eBook Packages: MedicineMedicine (R0)