Abstract
Hyperphenylalaninemia (HPA), a disorder of phenylalanine catabolism, is caused primarily by a deficiency of the hepatic phenylalanine-4-hydroxylase (PAH) or by one of the enzymes involved in its cofactor tetrahydrobiopterin (BH4) biosynthesis (GTP cyclohydrolase I (GTPCH) and 6-pyruvoyl-tetrahydropterin synthase (PTPS)) or regeneration (dihydropteridine reductase (DHPR) and pterin carbinolamine-4a-dehydratase (PCD)) (Blau et al. 2001). BH4 is known to be the natural cofactor for PAH, all three isoforms of nitric oxide synthase (NOS), tyrosine-3-hydroxylase, as well as tryptophan-5-hydroxylase (Werner et al. 2011), the latter two being the key enzymes in the biosynthesis of the neurotransmitters dopamine and serotonin. Thus, with two exceptions (see below) any cofactor defect will result in a deficiency of biogenic amines accompanied by HPA. Because phenylalanine is a competitive inhibitor of the uptake of tyrosine and tryptophan and other LNAA across the blood-brain barrier and of the hydroxylases of tyrosine and tryptophan, depletion of catecholamines and serotonin occurs in untreated patients with PAH deficiency. Both groups of HPA (PAH and BH4 deficiency) are heterogeneous disorders varying from severe to mild and benign forms. Because of the different clinical and biochemical severities in this group of diseases, the terms “severe” or “mild” will be used based upon the type of treatment and involvement of the CNS. For the BH4 defects, symptoms may manifest during the first weeks of life, but usually are noted within the first 6 months of life. Birth is generally uneventful, except for an increased incidence of prematurity and lower birth weights in severe PTPS deficiency (Opladen et al. 2012).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Phenylalanine Level
- Dihydropteridine Reductase
- Sapropterin Dihydrochloride
- Sepiapterin Reductase
- Blood Phenylalanine Level
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Hyperphenylalaninemia (HPA), a disorder of phenylalanine catabolism, is caused primarily by a deficiency of the hepatic phenylalanine-4-hydroxylase (PAH) or by one of the enzymes involved in its cofactor tetrahydrobiopterin (BH4) biosynthesis (GTP cyclohydrolase I (GTPCH) and 6-pyruvoyl-tetrahydropterin synthase (PTPS)) or regeneration (dihydropteridine reductase (DHPR) and pterin-4a-carbinolamine dehydratase (PCD)) (Blau et al. 2001). BH4 is known to be the natural cofactor for PAH, tyrosine-3-hydroxylase, and tryptophan-5-hydroxylase as well as all three isoforms of nitric oxide synthase (NOS) (Werner et al. 2011), the latter two being the key enzymes in the biosynthesis of the neurotransmitters dopamine and serotonin. Thus, with two exceptions (see below) any cofactor defect will result in a deficiency of biogenic amines accompanied by HPA. Because phenylalanine is a competitive inhibitor of the uptake of tyrosine and tryptophan across the blood-brain barrier and of the hydroxylases of tyrosine and tryptophan, depletion of catecholamines and serotonin occurs in untreated patients with PAH deficiency. Both groups of HPA (PAH and BH4 deficiency) are heterogeneous disorders varying from severe, e.g., classical phenylketonuria (PKU), to mild and benign forms (see Sect. 1.4). Because of the different clinical and biochemical severities in this group of diseases, the terms “severe” or “mild” will be used based upon the type of treatment and involvement of the CNS. For the BH4 defects, symptoms may manifest during the first weeks of life but usually are noted within the first half year of life. Birth is generally uneventful, except for an increased incidence of prematurity and lower birth weights in severe PTPS deficiency (Opladen et al. 2012).
Two disorders of BH4 metabolism may present without HPA. These are dopa-responsive dystonia (DRD; Segawa disease) (Segawa 2011) and sepiapterin reductase (SR) deficiency (Friedman et al. 2012). While DRD is caused by mutations in the GTPCH gene and is inherited in an autosomal dominant manner, SR deficiency is an autosomal recessive trait. Both diseases evidence severe biogenic amine deficiencies. DRD usually presents with a dystonic gait and diurnal variation, while many patients with SR deficiency have initial diagnosis of cerebral palsy. At least two reports describe heteroallelic patients with DRD suggesting a wide spectrum of GTPCH variants.
A diagnosis of HPA is usually based upon the confirmation of an elevated blood phenylalanine level obtained on a normal diet, following a positive newborn screening test. Normal breast milk or formula feeding for only 24 h is sufficient to raise the baby’s blood phenylalanine sufficiently to trigger a positive test level (>120 μmol/l). In general, an infant will be found to have a positive screening test 12 h postnatal. The tandem mass spectrometry (TMS) is today the method of choice for newborns screening. A detection as early as possible is essential in order to introduce appropriate treatment to prevent effects on mental development.
In PAH and BH4 deficiencies, factors like a relatively high phenylalanine intake or catabolic situations may be responsible for high phenylalanine concentrations in blood. Once HPA has been detected, a sequence of quantitative tests (see Sect. 1.8) enables the differentiation between variants, i.e., BH4-non-responsive PKU (usually the patients with the most severe PAH deficiency), BH4-responsive PKU (Heintz et al. 2013), and BH4 deficiencies. Because the BH4 deficiencies are actually a group of diseases which may be detected because of HPA, but not simply and routinely identified by neonatal mass screening, selective screening for a BH4 deficiency is essential in every newborn with even slightly elevated phenylalanine levels. Differential testing for BH4 deficiencies should be done in all newborns with plasma phenylalanine levels greater than 120 μmol/l (2 mg/dl), as well as in older infants and children with neurological signs and symptoms.
BH4 deficiencies presenting without HPA are detectable only by investigations for neurotransmitter metabolites and pterins in CSF or by clinical signs and symptoms. In DRD, a phenylalanine loading test, a trial with l-dopa, and enzyme activity measurement in cytokine-stimulated fibroblasts and molecular testing are confirmatory for the diagnosis. SR deficiency can be definitely diagnosed by an enzyme assay of cultured fibroblasts or DNA testing, but phenylalanine loading test is also positive.
The goals of treatment are to control HPA in PAH and BH4 deficiencies and to restore CNS neurotransmitter homeostasis in BH4 deficiencies (Blau et al. 2010). To that aim, dietary restriction in phenylalanine intake, supplementation with BH4, and oral administration of dopamine and serotonin precursors (l-dopa/carbidopa and 5-hydroxytryptophan, respectively), as well as some other drugs are available (Opladen et al. 2012). In this respect it should be taken into account that some patients with PAH deficiency, historically only treated by diet, can be treated with BH4 (sapropterin dihydrochloride). At the same time, in patients with DPHR deficiency, in whom historically the HPA was not treated with BH4, the diet restricting phenylalanine intake is the treatment of choice. Only about 20 % of DHPR-deficient patients are on BH4 treatment (Opladen et al. 2012).
Late detection of PAH or BH4 deficiencies and late introduction of treatment lead to irreversible brain damage. In contrast to early and continuously treated patients with PAH deficiency, some patients with BH4 deficiencies show progressive neurological deterioration despite treatment. Patients with PCD deficiency are at risk for developing early-onset diabetes in puberty.
2 Nomenclature
No. | Disorder | Alternative Name | Abbreviation | Gene Symbol | Chromosomal Localization | Affected Protein | OMIM No. | Sub Type |
|---|---|---|---|---|---|---|---|---|
1.1 | Phenylalanine hydroxylase deficiency | Classic phenylketonuria | Classic PKU | PAH | 12q22-24.1 | Phenylalanine hydroxylase | 261600 | Mild to severe |
1.2 | GTP cyclohydrolase deficiency | arGTPCH | GCH1 | 14q22.1-22.2 | GTP cyclohydrolase I | 233910 | Autosomal recessive | |
1.3 | 6-Pyruvoyl-tetrahydropterin synthase deficiency | PTPS | PTS | 11q22.3-23.3 | 6-Pyruvoyl-tetrahydropterin synthase | 261640 | ||
1.4 | Dihydropteridine reductase deficiency | DHPR | QDPR | 4p15.3 | Dihydropteridine reductase | 261630 | Moderate and severe | |
1.5 | Pterin-4a-carbinolamine dehydratase deficiency | Primapterinuria | PCD | PCBD1 | 10q22 | Pterin-4a-carbinolamine dehydratase | 264070 | Benign HPA Early-onset diabetes |
1.6 | Dopa-responsive dystonia | Segawa disease | adGTPCH, DRD | GCH1 | 14q22.1-22.2 | GTP cyclohydrolase I | 600225 | |
1.7 | Sepiapterin reductase deficiency | SR | SPR | 2p14-p12 | Sepiapterin reductase | 182125 |
3 Metabolic Pathway
Biosynthesis and regeneration of tetrahydrobiopterin (BH4) including possible metabolic defects in hyperphenylalaninemia (HPA) and catabolism of phenylalanine. 1.1 phenylalanine-4-hydroxylase (PAH), 1.2/1.6 GTP cyclohydrolase I, 1.3 6-pyruvoyl-tetrahydropterin synthase (PTPS), 1.4 dihydropteridine reductase (DHPR), 1.5 pterin-4a-carbinolamine dehydratase (PCD), 1.7 sepiapterin reductase, carbonyl reductase (CR), aldose reductase (AR), dihydrofolate reductase (DHFR), aromatic amino acid decarboxylase (AADC), tyrosine hydroxylase (TH), and tryptophan hydroxylase (TPH). 7,8-BH2: 7,8-dihydrobiopterin; PTP: 6-pyruvoyl-tetrahydropterin; HVA: homovanillic acid; 5HIAA: 5-hydroxyindoleacetic acid. Pathological metabolites used as specific markers in the differential diagnosis are marked in squares
4 Signs and Symptoms
5 Reference Values
6 Pathological Values/Differential Diagnosis
7 Loading Tests
Loading test with BH4
Proposed algorithms for the BH4 (sapropterin dihydrochloride; Kuvan) challenge, screening, and initiating treatment in BH4-responsive PKU patients. (a) Initial screening test with blood Phe monitoring on the first day and BH4 (sapropterin dihydrochloride) administration (20 mg/kg) on two following days. (b) Efficiency testing in BH4-reponsive patients over several weeks with BH4 doses adjusted individually according to Phe tolerance and therapeutic blood Phe levels. Combined Phe (100 mg/kg) and BH4 (20 mg/kg) loading test is sometimes difficult to interpret and is therefore not recommended (Blau et al. 2011)
Loading tests with Phe
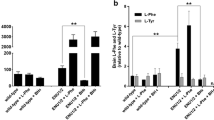
Oral phenylalanine loading with 100 mg Phe/kg body weight is performed, as previously described by Hyland et al. (1997). Samples were collected at time T-0, T-1h, T-2h, T-4h, and T-6h. Plasma was frozen immediately and kept in the dark; DBS were kept in dry conditions at 4 °C. The clearest differentiation measured by the standardized mean difference between the patients and controls was seen 2 h after Phe administration (Opladen et al. 2010). See also the flowchart with cut-off values. (a) Means and standard deviations (SDs) of phenylalanine/tyrosine (Phe/Tyr) ratios in pediatric patients after Phe loading [open circles dopa-responsive dystonia (DRD) patients; black squares controls; * p < 0.05; **p < 0.01] (b) Means and standard deviations (SDs) of biopterin concentration in pediatric patients after phenylalanine loading [open circles dopa-responsive dystonia (DRD) patients; black squares controls; **p < 0.01] (Opladen et al. 2010)
8 Diagnostic Flowcharts
Differential diagnosis of HPAs
Diagnostic flowchart for the laboratory diagnosis of PKU and BH4 deficiencies (Blau et al. 2011). Dried blood spots (DBS) or random urine (U) can be used for the differential diagnosis and depending on the profile of neopterin (Neo), biopterin (Bio), and primapterin (Pri) and dihydropteridine reductase (DHPR) activity in DBS, diagnosis of following BH4 deficiencies can be established: GTP cyclohydrolase I (GTPCH) deficiency (low or no detectable neopterin and biopterin), 6-pyruvoyl-tetrahydropterin synthase (PTPS) deficiency (high neopterin and low or no detectable biopterin), dihydropteridine reductase (DHPR) deficiency (normal neopterin and normal or elevated biopterin and no DHPR activity), and pterins-4acarbinolamine dehydratase (PCD) deficiency (elevated neopterin, low-normal biopterin, and elevated primapterin). N normal (Blau et al. 2011)
Screening for a BH4 deficiency should be done in all newborns and children with even slight HPA (plasma Phe >120 μmol/l) as well as in older children without HPA but with neurological symptoms suggestive of a neurotransmitter deficiency. The following protocol is suggested:
-
1.
Analysis of pterins in urine
-
2.
Measurement of DHPR activity in blood from a Guthrie card
-
3.
Analysis of phenylalanine and tyrosine in serum or plasma before and after a BH4 challenge
-
4.
DNA testing
The first two tests are essential and will allow the differentiation between all variants with BH4 deficiencies. With some limitations (DHPR def.), the BH4 loading test is an additional useful diagnostic tool for the rapid discrimination between classical PKU and biopterin variants. This test is also useful for identifying the recently described BH4-responsive PAH deficiency. For the interpretation and determination of the various disorders based upon loading tests, see Sect. 1.6.
Biochemical and loading test data are summarized in Fig. 1.5 (Blau et al. 2010):
Flow-chart for the differential diagnosis of BH4 responsive PKU and BH4 deficiencies. Phe phenylalanine, DBS dried blood spots, DHPR dihydropteridine reductase, Neo neopterin, Bio biopterin, Pri primapterin, GTPCH GTP-cyclohydrase I, PTPS 6-pyruvoyl-tetrahydropterin synthase, PCD pterin-4a-carbinolamine dehydratase, BH4 tetrahydrobiopterin. (Blau et al. 2010)
Interpretation of the Phe loading test
Phenylalanine (Phe) loading in children: revised shortened test procedure and test interpretation (Bio biopterin, Phe/Tyr Phe/tyrosine ratio, DBS dried blood spot, P plasma, BW body weight, dd diagnostic decision) (Opladen et al. 2010)
9 Diagnostic Flowchart in the Differential Diagnosis of Non-HPA Variants
Diagnostic algorithm for patients with a possible disorder of neurotransmitter metabolism. Sepiapterin reductase (SR) deficiency and other disorders of neurotransmitter metabolism should be considered in patients with (1) developmental delay with hypotonia, (2) suspect but unexplained cerebral palsy (CP) or CP with atypical features, and (3) uncharacterized l-dopa-responsive motor disorders. (1) In a patient with developmental delay and hypotonia, if oculogyric crises, diurnal fluctuation, sleep disturbance, or extrapyramidal or autonomic signs exist, a disorder of neurotransmitter biosynthesis is likely and cerebrospinal fluid (CSF) analysis should be done. If no other signs are present, CSF analysis should be considered if standard workup for hypotonia is unrevealing. If CSF analysis is abnormal, then mutational screening and/or measurement of enzymatic activity can be targeted to confirm the specific disorder suggested by the pattern of CSF abnormalities. If CSF evaluation is impractical, alternative evaluation may include l-dopa trial and/or phenylalanine load. If negative, CSF analysis must still be done to exclude a disorder of neurotransmitter metabolism. If l-dopa trial and/or phenylalanine loading are positive, CSF analysis will allow targeted mutational screening; however, one should keep in mind that phenylalanine (Phe) load can be positive in heterozygote carriers for phenylketonuria. Alternatively, CSF analysis may be skipped and broad mutational screening undertaken. Mutational and gene dosage screening may be time-consuming and costly, and false negatives may still occur. Therefore, this alternative evaluation route should be reserved for cases in which CSF analysis is not available or is declined, or in which other clinical features lead to suspicion of a specific diagnosis. (2) In a patient with unexplained CP or CP with atypical features, a disorder of neurotransmitter metabolism should be considered and diagnostic algorithm, as outlined above, should be followed. Atypical or unexplained features suggesting need for further metabolic investigation in a child with possible CP include lack of adequate antecedent, nondiagnostic magnetic resonance imaging, progressive symptoms, familial occurrence, episodic encephalopathy, and features not expected in the CPs such as diurnal variation, sleep disturbance, autonomic symptoms, or oculogyric crises. (3) All patients with an l-dopa-responsive motor disorder should be evaluated for a disorder of neurotransmitter metabolism. CSF analysis (after discontinuation of l-dopa therapy for at least 10 days) is the recommended first step. If l-dopa withdrawal is impractical, the results of CSF analyses may still be informative if either pterins or 5-hydroxyindoleacetic acid levels are abnormal. Alternatively, molecular investigations can be done, guided either by results of phenylalanine loading test or clinical symptoms with caveats as noted above.1 CSF analysis should consist of homovanillic acid, 5-hydroxyindoleacetic acid (5HIAA), pterins (neopterin, biopterin, and sepiapterin), and 5-methyltetrahydrofolate (Blau et al. 2010)
10 Specimen Collection
Test | Preconditions | Material | Handling | Pitfalls |
|---|---|---|---|---|
Phe | Free diet | Guthrie card, serum/plasma, CSF | Keep cool (−20 °C) for serum or plasma | |
Guthrie card at RT | ||||
Neo | Free diet, Phe in plasma high enough | Random urine, spotted urine | Keep cool, dark (−20 °C) | Infections (Neo ↑) |
Bio | Oxidized sample, dark, RT | |||
Serum/plasma | Keep cool, dark (−20 °C) | |||
CSF | EDTA tube (−20 °C) | |||
BH4, BH2 | CSF | DTE/DETAPAC tube (−80 °C) | ||
HVA | 1 h before medication, withdraw first 0.5 ml | CSF | (−80 °C) | |
5HIAA | ||||
5-CH3-THF | ||||
DHPR | Erythrocytes from heparinized blood | Frozen (−20 °C) | ||
Guthrie card | RT | |||
Fibroblasts | RT | |||
Min. 50 mg | Chorionic villi | Frozen (−80 °C) | ||
PTPS | Before medication, no BH4 | Erythrocytes from heparinized blood | frozen (−20 °C) | |
Min. 50 mg | Chorionic villi | Frozen (−80 °C) | ||
GTPCH | Fibroblasts | RT | ||
SR | Fibroblasts | RT |
11 Prenatal Diagnosis
Disorder | Material | Timing, Trimester |
|---|---|---|
1.1 | Fetal DNA | I |
1.2 | Amniotic fluid, liver, DNA | II |
1.3 | Amniotic fluid, liver, erythrocytes, amniocytes, DNA | II |
CV | I | |
1.4 | Amniotic fluid, liver, erythrocytes, amniocytes, DNA | II |
CV | I |
12 DNA Analysis
DNA analysis is possible for PAH and all BH4 deficiencies
Disorder | Material | Method |
|---|---|---|
1.1 | Genomic DNA | PCR/RFLP/SSCP/ sequencing |
1.2 | Genomic DNA/FB | PCR/DGGE/sequencing |
1.3 | Genomic DNA/FB | PCR/DGGE/sequencing |
1.4 | Genomic DNA/FB | PCR/RFLP/DGGE/sequencing |
1.5 | Genomic DNA | PCR/sequencing |
1.6 | Genomic DNA/FB | PCR/DGGE/sequencing |
1.7 | Genomic DNA/FB | PCR/sequencing |
13 Treatment
PAH or BH4 deficiency present either with or without HPA. In those presenting with HPA (1.1–1.5; see below), the main goal of treatment is to reduce or normalize blood phenylalanine levels without causing deficiencies of other amino acids and other nutrients that are usually received by intake of natural intake (Belanger-Quintana et al. 2011). This can be done by introduction of the low-phenylalanine (or low-natural protein) diet; in some patients administration of the synthetic cofactor BH4 can relax or even replace the diet (Keil et al. 2013). In such cases it is important to be sure that patients use normal amounts of natural protein and not causing deficiencies of amino acids.
In PAH deficiency, one of the areas that need consensus is whether to start treatment at blood phenylalanine levels above 360–600 μmol/l (van Spronsen et al. 2011). Treatment targets have not reached consensus yet, but for the first decade, more or less every center will try to keep phenylalanine below 360 μmol/l or even lower (Blau et al. 2010). Very recent data even show that outcome is even better when phenylalanine is kept below 240 μmol/l (Jahja R et al., J Pediatr, in press). The next issue to decide on is the search for BH4-responsive patients. To this end we refer to paragraph 1.7. All three existing methods (i.e., DNA, 7–24 days BH4 loading test (Levy 2007), and the 48-h BH4 loading test (Blau et al. 2011; Anjema et al. 2013)) have their own considerations. For all tests, long-term data are necessary to proof the predictive correctness of the method. Other issues that need attention are the amount of total protein (natural protein plus amino acids), giving extra tyrosine in case of low tyrosine concentrations (especially in case of pregnancy), and the question how strict treatment should be during adulthood (especially in males and in females after reproductive age).
One should be aware that there are individuals with “severe” PKU mutations that have escaped severe mental retardation despite high blood phenylalanine levels and very poor dietary control. One explanation for this phenomenon is that they have near normal brain phenylalanine levels, despite high blood phenylalanine levels. A number of studies have now demonstrated considerably variability in blood versus brain phenylalanine levels in PKU patients. Outcome in PKU appears to be related to both blood and brain phenylalanine levels. This, in all probability, will assume greater importance in making decisions about the strictness and duration of dietary control in the future.
In BH4 deficiencies, the mode of treatment depends on the type of disease, may differ with the patient’s age, and the policies in various countries and centers (Opladen et al. 2012). In addition, patients with HPA due to a cofactor defect need more strict blood phenylalanine control and additional supplementations with neurotransmitter precursors l-dopa and 5-hydroxytryptophan in a combination with the peripheral decarboxylase inhibitor carbidopa. Patients with dihydropteridine reductase deficiency (DHPR, 1.4) need additional folinic acid substitution. In patients revealing levodopa-induced peak-dose dyskinesia, slow-release forms of drugs can be used, and reaching the upper therapeutic limits of l-dopa may be an indication for the use of MAO and/or COMT inhibitors.
Patients with dopa-responsive dystonia (DRD, dominant GTPCH cyclohydrolase I deficiency, 1.6) and sepiapterin reductase deficiency (SR, 1.7) respond to low dosage l-dopa/carbidopa therapy and patients with SR deficiency need additional supplementation with 5-hydroxytryptophan and probably also BH4 (Friedman et al. 2012).
Prognosis and outcome strongly depend on the age when the diagnosis is made and treatment introduced but also on the type of mutation (Jäggi et al. 2008).
Recommendations for treatment and monitoring are not completely uniform worldwide. Therefore, where possible and necessary, recommendations have been combined and ranges of values indicating lower and upper limits are reported.
1.1 Phenylalanine hydroxylase deficiency (PKU)
Age | Protein requirement (g/kg BW/day)a | Phe tolerance (mg/day) | Target blood Phe (μmol/l) | Phe-free AAM | ||||
|---|---|---|---|---|---|---|---|---|
Germany | Netherlands | UK | USA | Type | g/dayb | |||
0–3 months | 2.1–2.7 | ~130–400 | 40–240 | 120–360 | 120–360 | 120–360 | 1 | 3–10 |
4–12 months | 2.1–2.0 | ~130–400 | 40–240 | 120–360 | 120–360 | 120–360 | 1 | 3–10 |
1–2 years | 1.7 | ~130–400 | 40–240 | 120–360 | 120–360 | 120–360 | 2 | 20–50 |
2–3 years | 1.7 | ~200–400 | 40–240 | 120–360 | 120–360 | 120–360 | 2 | 20–50 |
4–6 years | 1.6 | ~200–400 | 40–240 | 120–360 | 120–360 | 120–360 | 2 | 20–50 |
7–9 years | 1.4 | ~200–400 | 40–240 | 120–360 | 120–480 | 120–360 | 2 | 20–50 |
10–12 years | 1.1 | ~350–800 | 40–900 | 120–360 | 120–480 | 120–360 | 2 | 50–90 |
13–15 years | 1.0 | ~350–800 | 40–900 | 120–600 | 120–700 | 120–600 | 2 | 50–90 |
>16 years | 0.9 | ~450–1,000 | 40–1,200 | 120–600 | 120–700 | 120–900 | 3 | 60–150 |
Non-PKU Hyperphenylalaninemia
Treatment only necessary for pregnant women with blood Phe levels >250–360 μmol/l (see below). Clinical monitoring is advised for all patients with Phe >360 μmol/l
Tetrahydrobiopterin (BH4)-Responsive PKU/HPA
There are no specific recommendations for the treatment of this group of HPA. The following table summarizes the current knowledge based on several experimental trials.
Age | Protein requirement g/kg BW/day | Phe tolerance mg/day | Target blood Phe μmol/l | mg BH4/kg BWa |
|---|---|---|---|---|
All ages | See 1.1 | Near normal | See 1.1 | 5–20 |
Maternal PKU/HPA
Trimester | Protein requirement g/kg BW/day | Phe tolerance mg/day | Target blood Phe μmol/l | Phe-free AAM | |
|---|---|---|---|---|---|
Type | g/daya | ||||
1 | 1.1 | ~180–1,600 | 120–360 | 3 | 60–150 |
2–3 | 1.3–1.5 | ~180–1,600 | 120–360 | 3 | 60–150 |
1.2 GTP cyclohydrolase I deficiency and 1.3 6-Pyruvoyl-tetrahydropterin synthase deficiency (severe form)
No. | Symbol | Age | Medication/diet | Dosage (mg/kg/day) | Dosages per day |
|---|---|---|---|---|---|
1.2 | GTPCH | Newborn | l-Dopa | 1–3 | 3–6 |
1.3 | PTPS (severe) | Carbidopa | 10–25 %a | 3–6 | |
5-Hydroxytryptophan | 1–2 | 3–6 | |||
Tetrahydrobiopterin (BH4) | 5–10 | 3 | |||
<1–2 years | l-Dopa | 4–7 | 3–6 | ||
Carbidopa | 10–25 %a | 3–6 | |||
5-Hydroxytryptophan | 3–5 | 3–6 | |||
Tetrahydrobiopterin (BH4) | 5–10 | 2 | |||
>1–2 years | l-Dopa | 8–15 | 3–6 | ||
Carbidopa | 10–25 %a | 3–6 | |||
5-Hydroxytryptophan | 6–9 | 3–6 | |||
Tetrahydrobiopterin (BH4) | 5–10 | 2 |
Beware/Pitfalls
-
1.
Patients are on an unrestricted (i.e., protein-rich) diet.
-
2.
BH4 may significantly reduce plasma and CSF tyrosine levels. Consider nutrition and tyrosine supplementation.
-
3.
l-Dopa/carbidopa/5-hydroxytryptophan therapy should be introduced slowly but continuously increased according to the clinical picture in steps of 1 mg/kg/day per week. 5-Hydroxytryptophan may not be tolerated due to gastrointestinal side effect. In these cases monotherapy with l-dopa/carbidopa may be sufficient.
-
4.
l-Dopa/carbidopa/5-hydroxytryptophan therapy may reduce CSF folates (CH3-group trapping by l-dopa to 3-O-methyl-dopa). Determine 5-methyltetrahydrofolate in CSF. Consider folinic acid (5-formyltetrahydrofolate, leucovorin) substitution (10–20 mg/day).
-
5.
Drugs like trimethoprim-sulfamethoxazoles or methotrexate may induce hyperphenylalaninemia by inhibiting DHPR.
1.3 6-Pyruvoyl-tetrahydropterin synthase deficiency (mild form)
No. | Symbol | Age | Medication/diet | Dosage (mg/kg/day) | Dosages per day |
|---|---|---|---|---|---|
1.3 | PTPS (mild) | All ages | Tetrahydrobiopterin (BH4)a | 5–10 | 2 |
1.4 Dihydropteridine reductase deficiency
No. | Symbol | Age | Medication/diet | Dosage (mg/kg/day) | Dosages per day |
|---|---|---|---|---|---|
1.4 | DHPR | Newborn | l-Dopa | 1–3 | 3–6 |
Carbidopa | 10–25 %a | 3–6 | |||
5-Hydroxytryptophan | 1–2 | 3–6 | |||
Folinic acid | 15–20 mg/day | 1–2 | |||
Diet (see 1.1 PKU) | |||||
<1–2 years | l-Dopa | 4–7 | 3–6 | ||
Carbidopa | 10–25 %a | 3–6 | |||
5-Hydroxytryptophan | 3–5 | 3–6 | |||
Folinic acid | 15–20 mg/day | 1–2 | |||
Diet (see 1.1 PKU) | |||||
>1–2 years | l-Dopa | 8–15 | 3–6 | ||
Carbidopa | 10–25 %a | 3–6 | |||
5-Hydroxytryptophan | 6–9 | 3–6 | |||
Folinic acid | 15–20 mg/day | 1–2 | |||
Diet (see 1.1 PKU) |
Beware/Pitfalls
-
1.
Patients are on low-Phe diet (see Table 1.1); however, blood Phe levels should be close to normal. These patients are more sensitive to high Phe levels than other PKU.
-
2.
l-Dopa/carbidopa/5-hydroxytryptophan therapy should be introduced slowly but continuously increased according to the clinical picture in steps of 1 mg/kg/day per week. 5-Hydroxytryptophan may not be tolerated due to gastrointestinal side effect. In these cases monotherapy with l-dopa/carbidopa may be sufficient.
-
3.
Drugs like trimethoprim-sulfamethoxazoles or methotrexate may induce hyperphenylalaninemia by inhibiting DHPR.
1.5 Pterin-4a-carbinolamine dehydratase deficiency
No. | Symbol | Age | Medication/diet | Dosage (mg/kg/day) | Dosages per day |
|---|---|---|---|---|---|
1.5 | PCD | Newborn | Tetrahydrobiopterin (BH4)a | 5–10 | 2 |
>1 years | No treatmentb |
Beware/Pitfalls
-
1.
Patients are on an unrestricted (i.e., protein-rich) diet.
-
2.
BH4 may significantly reduce plasma and CSF tyrosine levels. Consider tyrosine supplementation.
-
3.
Drugs like trimethoprim-sulfamethoxazoles or methotrexate may induce hyperphenylalaninemia by inhibiting DHPR.
1.6 Dopa-responsive dystonia/autosomal dominant GTPCH deficiency
No. | Symbol | Age | Medication/diet | Dosage (mg/kg/day) | Dosages per day |
|---|---|---|---|---|---|
1.6 | DRD | Newborn | l-Dopa | 1–3 | 3–4 |
Carbidopa | 10–25 %a | 3–4 | |||
>1 years | l-Dopa | 4–12 | 3–4 | ||
Carbidopa | 10–25 %a | 3–4 |
Beware/Pitfalls
-
1.
l-Dopa/carbidopa therapy should be introduced slowly but continuously increased according to the clinical picture in steps of 1 mg/kg/day per week.
1.7 Sepiapterin reductase deficiency
No. | Symbol | Age | Medication/diet | Dosage (mg/kg/day) | Dosages per day |
|---|---|---|---|---|---|
1.7 | SR | Newborn | l-Dopa | 1–3 | 3–4 |
Carbidopa | 10–25 %a | 3–4 | |||
5-Hydroxytryptophan | 1–2 | 3–4 | |||
>1 years | l-Dopa | 4–10 | 3–4 | ||
Carbidopa | 10–25 %a | 3–4 | |||
5-Hydroxytryptophan | 3–9 | 3–4 |
Beware/Pitfalls
-
1.
l-Dopa/carbidopa/5-hydroxytryptophan therapy should be introduced slowly and increased in steps of not more than 1 mg/kg over days/weeks.
Alternative therapies/experimental trials
No. | Deficiency symbol | Age | Medication | Dosage (mg/kg/day) | Dosages per day |
|---|---|---|---|---|---|
1.1 | BH4-responsive PKU | >4 years | BH4a | 10–20 | 1–2 |
1.3–1.5 | GTPCH, PTPS, DHPR | All ages | Deprenylb | 0.1–0.3 | 3–4 |
Entacaponec | ~30 | 1–2 | |||
1.7 | SR | All ages | Deprenylb | 0.07–0.14 | 3–4 |
Sertralined | 0.71–2.14 | 2–3 | |||
Melatonine | 0.01–0.03 | 1–2 | |||
Bromocriptine | Not reported | Not reported |
Beware/Pitfalls
-
1.
Administration of MAO-B or COMT inhibitors allows a 30 % reduction of the daily dosage of neurotransmitter precursors.
14 Follow-Up/Monitoring
1.1. PAH deficiency
Age | Biochemical monitoring Phe and Tyr | Clinical monitoringa | Intellectual and personality development |
|---|---|---|---|
0–3 months | Weekly | 1–3 monthly | |
4–12 months | Weekly | 1–3 monthly | X |
1–2 years | Weekly | 2–6 monthly | |
2–3 years | Weekly | 2–6 monthly | X |
4–6 years | Fortnightly | 3–6 monthly | X |
7–9 years | Fortnightly | 6 monthly | |
10–12 years | Monthly | 6 monthly | X |
13–15 years | Monthly | 6 monthly | X |
Adolescents/adults | Monthly | 6–12 monthly | X |
Maternal PKU | Weeklyb | Bimonthlyc |
Standard protocol for intercurrent illness
Aim the best possible intake of fluid, energy, and Phe-free AAM, with special attention for higher need of energy, while taking AAM in these periods may be a real.
1.2–1.7. BH 4 deficiencies
Plasma Phe and Tyr are monitored in all forms of HPA, CSF investigations only in disorders affecting BH4 metabolism with and without HPA (1.2–1.7).
Test | Age | Frequency | Comments |
|---|---|---|---|
Phe and Tyr (blood) | 1–3 years | Weekly to fortnightly | Phe levels: 40–240 (360) μmol/la |
4–10 years | Fortnightly to monthly | ||
11–16 years | Monthly | Phe levels: 40–900 μmol/la | |
>16 years | Every 2–3 months | Phe levels: 40–1,200 μmol/la | |
Neopterin | <1 month | Fortnightly | Close to normal range |
Biopterin | |||
5HIAA | |||
HVA | |||
Folates (CSF)b | |||
1 month–1 years | Every 4–8 weeks | Close to normal range | |
<1 years | Monthly to yearly | Close to normal range | |
Glucose (P) | >5 years | Yearly | Only in PCD-deficient patients |
References
Anjema K, van Rijn M, Hofstede FC, et al (2013) Tetrahydrobiopterin responsiveness in phenylketonuria: prediction with the 48-hour loading test and genotype. Orphanet J Rare Dis 10:103
Belanger-Quintana A, Burlina A, Harding CO, Muntau AC (2011) Up to date knowledge on different treatment strategies for phenylketonuria. Mol Genet Metab 104(Suppl):S19–25
Blau N, Thöny B, Cotton RGH, Hyland K (2001) Disorders of tetrahydrobiopterin and related biogenic amines. In: Scriver CR, Beaudet al, Sly WS, Valle D, Childs B, Vogelstein B (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 1725–1776
Blau N, Van Spronsen FJ, Levy HL (2010) Phenylketonuria. Lancet 376:1417–1427
Blau N, Hennermann JB, Langenbeck U, Lichter-Konecki U (2011) Diagnosis, classification and genetics of phenylketonuria and tetrahydrobiopterin (BH4) deficiencies. Mol Genet Metab 104:S2–9
Friedman J, Roze E, Abdenur JE et al (2012) Sepiapterin reductase deficiency: a treatable mimic of cerebral palsy. Ann Neurol 71:520–530
Heintz C, Cotton RG, Blau N (2013) Tetrahydrobiopterin, its mode of action on phenylalanine hydroxylase and importance of genotypes for pharmacological therapy of phenylketonuria. Hum Mutat 34:927–936
Hyland K, Fryburg JS, Wilson WG et al (1997) Oral phenylalanine loading in dopa-responsive dystonia – a possible diagnostic test. Neurology 48:1290–1297
Jäggi L, Zurflüh MR, Schuler A et al (2008) Outcome and long-term follow-up of 36 patients with tetrahydrobiopterin deficiency. Mol Genet Metab 93:295–305
Keil S, Anjema K, van Spronsen FJ et al (2013) Long-term follow-up and outcome of phenylketonuria patients on sapropterin: a retrospective study. Pediatrics 131:e1881–e1888
Levy HB, Burton S, Cederbaum C, Scriver (2007) “Recommendations for evaluation of responsiveness to tetrahydrobiopterin (BH(4)) in phenylketonuria and its use in treatment.” Mol Genet Metab 92(4): 287–291
Opladen T, Okun JG, Burgard P, Blau N, Hoffmann GF (2010) Phenylalanine loading in pediatric patients with dopa-responsive dystonia: revised test protocol and pediatric cut-off values. J Inerit Metab Dis 33:697–703
Opladen T, Hoffmann FG, Blau N (2012) An international survey of patients with tetrahydrobiopterin deficiencies presenting with hyperphenylalaninaemia. J Inerit Metab Dis 35:963–73
Segawa M (2011) Hereditary progressive dystonia with marked diurnal fluctuation. Brain Dev 33:195–201
van Spronsen FJ, Huijbregts SC, Bosch AM, Leuzzi V (2011) Cognitive, neurophysiological, neurological and psychosocial outcomes in early-treated PKU-patients: a start toward standardized outcome measurement across development. Mol Genet Metab 104(Suppl):S45–51
Werner ER, Blau N, Thöny B (2011) Tetrahydrobiopterin: biochemistry and pathophysiology. Biochem J 438:397–414
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Blau, N., van Spronsen, F.J. (2014). Disorders of Phenylalanine and Tetrahydrobiopterin Metabolism. In: Blau, N., Duran, M., Gibson, K., Dionisi Vici, C. (eds) Physician's Guide to the Diagnosis, Treatment, and Follow-Up of Inherited Metabolic Diseases. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-40337-8_1
Download citation
DOI: https://doi.org/10.1007/978-3-642-40337-8_1
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-40336-1
Online ISBN: 978-3-642-40337-8
eBook Packages: MedicineMedicine (R0)