Abstract
Objectives
The objectives of this study were to determine the value of phenylalanine (Phe) loading for diagnosing dopa-responsive dystonia (DRD) in children.
Methods
We investigated orally administered Phe loading tests (100 mg/kg) in seven patients with confirmed DRD and 17 pediatric patients with clinically suspected but excluded DRD. Results of Phe, tyrosine (Tyr), and biopterin from plasma and dried blood spot (DBS) analyses were correlated, and pediatric cutoff values established.
Results
The peak Phe concentration following a Phe load in the pediatric DRD population is lower than reported in adults. By using adult cutoff values and either Phe/Try ratios or biopterin concentrations only, false positive and false negative results are frequent. Only the combined analysis of the Phe/Tyr ratio and biopterin concentration is reliable in children. In children with DRD, dried blood Phe/Tyr ratio exceeded 4.6 (plasma Phe/Tyr ratio >5.4) after 2 h and biopterin concentration in dried blood remained below 16.2 nmol/L (plasma biopterin <14 nmol/L) 1 h after Phe challenge.
Conclusions
Phe loading is a useful tool for diagnosing DRD in children. Test duration can be reduced to only 2 h, and specific pediatric cutoff values need to be applied. Simultaneous measurements of the Phe/Tyr ratio and biopterin in plasma or DBS are essential in pediatric patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dopa-responsive dystonia (DRD) describes an autosomal-dominant movement disorder. Typically, the age of onset is in childhood; however, patients with adulthood onset have also been identified (Segawa et al. 2003). DRD is caused by mutations in the GCH1 gene coding for the guanosine triphosphate cyclohydrolase I (GTPCH, EC 3.5.4.16), the rate-limiting enzyme in the synthesis of tetrahydrobiopterin (BH4). BH4 is the essential cofactor of phenylalanine (Phe), tyrosine (Tyr), and tryptophane hydroxylases (Thöny et al. 2000). Depletion of BH4 results in insufficient biosynthesis of dopamine and serotonin. Affected children typically present with abnormal gait due to leg dystonia. Later on, dystonia can spread to other limbs and is accompanied by dystonic or postural tremor, torticollis, or writer’s cramp. Symptoms can be accompanied by parkinsonian features such as reduced facial expression or slowing of fine finger movements (Nygaard 1993) and even mimic idiopathic Parkinson’s disease (Grimes et al. 2002). In general, the clinical picture is very heterogeneous, even the characteristic diurnal fluctuation and improvement of symptoms after sleep are not a constant finding.
Patients with DRD show a very favorable response to treatment with L-dopa/carbidopa. However, in treatment trials with L-dopa, etiologically different forms of dystonia cannot be distinguished (Bandmann et al. 1998). Therefore, further biochemical investigations are needed to confirm the diagnosis. In addition to lumbar puncture and analysis of cerebral spinal fluid (CSF), biogenic amines, and pterins, Phe loading tests have proven useful in the differential diagnosis of autosomal-dominant DRD and sepiapterin reductase deficiency (Blau et al. 2001; Hyland et al. 1997; Bandmann et al. 2003; Saunders-Pullmann et al. 2004). During the test, hepatic Phe hydroxylase is stressed by an oral Phe load. Due to the partial BH4 deficiency, conversion of Phe to Tyr is compromised. In addition, the physiological stimulation of BH4 biosynthesis via GTPCH feedback regulatory protein (GFRP) by Phe is absent, and biopterin concentrations remain at a low level under Phe loading. Available data for reliable test interpretation in pediatric patients under Phe loading are limited, and clear cutoff values for the pediatric population are missing (Lopez-Laso et al. 2006). Therefore, the aim of this study was to establish reliable cutoff values for children after Phe loading. Furthermore, we compared Phe, Tyr, and biopterin in plasma and dried blood samples to test the value of filter-paper blood spots as substitute for plasma and investigated the significance of concomitant biopterin determination under Phe loading. Additionally, a shortened test procedure for children was suggested.
Methods
Oral phenylalanine loading
Oral Phe loading with 100 mg Phe/kg body weight (BW) was performed, as previously described (Hyland et al. 1997), except that at base line and at all time points under Phe loading, parallel samples of plasma and blood spots were taken. Plasma was frozen immediately and kept in the dark; DBS were kept in dry conditions at 4°C.
Patients and controls
The results of all 53 Phe loading tests measured between November 2003 and November 2009 in the metabolic laboratory at University Children’s Hospital Heidelberg were re-analyzed. All tests with missing values or incorrectly conducted test procedure were excluded (n = 29). The remaining 24 test results were divided in two subgroups according to clinical data and according to the results of confirmation diagnostics. Subgroup 1 (ten males, seven females; age 0.4 – 18 years, mean 9.0 years) was used as control group. Clinically, these patients presented with multifaceted neurological symptoms including dystonia. Initially, DRD was suspected, but later, excluded by normal pterins in CSF, normal GTPCH enzyme activity, normal GCH1 mutation analysis, or a missing response to an L-dopa treatment trial over 3–4 months at dosages higher than 5 mg/kg per day. Subgroup 2 (three males, four females; age 1.7–14.5 years, mean 8.6 years) comprised all confirmed DRD patients. Confirmation of DRD was defined by detection of a GCH1 mutation or reduced GTPCH enzyme activity in cytokine-stimulated fibroblasts or a significant and long-term response on a treatment trial with L-dopa in those patients who had typical DRD-related changes in CSF neurotransmitter analysis (low levels of homovanillic acid, BH4, and neopterin). In one additional patient, autosomal-recessive GTPCH deficiency without hyperphenylalaninemia was found. The biochemical characteristics of subgroup 2 are summarized in Table 1.
The remaining test results where excluded from the study due to missing clinical information or missing confirmatory investigations. The sample collection for this study was approved by the institutional review board of the medical faculty, University of Heidelberg, Germany (No. 071/2005 + S-033/2007).
Biochemical investigations
Oral Phe loading
Phe and Tyr in DBS were analyzed using tandem mass spectrometry (Micromass Ultima; Metabolic Laboratory, University Children’s Hospital Heidelberg, Germany) following standard methods (Schulze et al. 2003). Biopterin in plasma was measured with high-performance liquid chromatography (HPLC) by standard methods in the Metabolic Laboratory Heidelberg [modified from Blau and Thöny (2008)]. Phe/Tyr and biopterin in plasma and dried blood samples was compared with amino acid results from plasma and dried blood samples; 50 corresponding blood spot and plasma results from patients with phenylketonuria were analyzed. Regression analysis was performed. Similarly, pterins in DBS were measured in eight patients under Phe loading, as described previously by Zurflüh et al. (2005). Total biopterin was calculated as the sum of biopterin and pterin (nmol/l). Results from plasma biopterin measurement were compared with biopterin plus pterin concentrations in DBS.
Statistical analysis
As Kolmogorov–Smirnov tests of Phe/Tyr ratios and biopterin concentrations were nonsignificant for both groups at all five measurement times, data presented in Figs. 1 and 2 were analyzed by two-way analysis of variance (ANOVA) with group as between and time as within subject factors. Post hoc between subject comparisons were carried out by t tests using Bonferroni adjusted significance levels. All calculations were done with SPSS 17.0.
Results
Results of Phe loadings are summarized in Figs. 1 and 2. The maximum Phe concentration achieved under Phe loading in this pediatric population was 1040 μmol/l after 1 h. No differences between DRD and control groups were observed (data not shown). ANOVA of Phe/Tyr ratios (Fig. 1) showed significant main effects for group [F(1;22) =19.14, p < .001, time ((4;88) =41.08, p < .001 and a significant interaction of group by time F(4;88) =9.55 p < .001]. Phe/Tyr ratios of the two groups were equal at t0 [t(1,22) =1.78, n.s.] but significantly different at t1 to t6. The clearest differentiation measured by the standardized mean difference between the two groups was seen after 2 h. Using the previously suggested Phe/Tyr cutoff ratio of higher than 5.25 at 4 h, six of seven DRD patients would have been missed in our study (test sensitivity 14.3 %) (Saunders-Pullmann et al. 2004). ANOVA of biopterin concentrations (Fig. 2) showed significant main effects for group [F(1;21) =9.66, p < .01, time F(4;84) =20.93, p < .0001, and a significant interaction of group by time F(4;84) =10.43 p < .0001 (Fig. 1)]. Biopterin concentrations of the two groups were significantly different at t1 to t4 (all p < 0.05) but not at t0 [t(1,22) =.47, n.s.] and t6 [t(1,22)=.95, n.s.]
Biopterin concentrations reached maximum values after 1 h, showing the clearest differentiation measured by the standardized mean difference at the same time. At 6 h, concentrations of biopterin as well as Phe/Tyr ratios were again almost equal in both groups. In all DRD patients, the biopterin concentration remained below the previously published value of 18 nmol/l (Blau et al. 2003), but two children not affected with DRD did not exceed this value and turned out to be false positives (test specificity 88%).
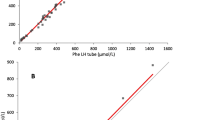
Phe concentrations in DBS and plasma between 12 and 1,400 μmol/L were significantly correlated. Linear regression analysis of DBS Phe on plasma Phe fitted very well (plasma Phe 0.897 × DBS Phe + 40.664; R 2 = 0.92) (Fig. 3a). Similar Tyr concentration in DBS and plasma corresponded between 21 and 186 μmol/l, the regression of DBS values on plasma is clear (plasma Tyr 0.6112 × DBS Tyr + 13.62; R 2 = 0.80) (Fig. 3b). Phe/Tyr plasma cutoffs were defined by converting DBS results by regression equation.
Best correspondence between plasma and dried blood were found for the sum of biopterin plus pterin in DBS and biopterin in plasma. Biopterin concentrations in plasma correlate significantly with biopterin plus pterin concentrations in DBS for concentrations between 3.6 and 53.4 nmol/l, and the regression of DBS values on plasma values was nearly perfect (DBS biopterin + pterin = 1.0931 × plasma Bio + 1.0872; R 2 = 0.9977) (Fig. 3c). Plasma biopterin concentrations are lower than the sum of biopterin and pterin in DBS cards and for comparison must be converted by regression equation.
Discussion
The utility of Phe loading for diagnosing DRD is well documented (Hyland et al. 1997; Bandmann et al. 2003; Saunders-Pullmann et al. 2004). The Phe/Tyr ratio is regarded as a sensitive parameter to distinguish between DRD patients and controls (Hyland et al. 1997). However cutoff values for children have been missing. Our study documents that in the pediatric age, maximum plasma Phe concentrations remain lower than in adults. As a consequence, Phe/Tyr ratios in children are lower than in adults. Using the previously suggested Phe/Tyr cutoff ratio, six out of our seven DRD patients would have been missed. Our pediatric data revealed that the most precise discrimination of DRD patients and controls is achieved by the use of the Phe/Tyr ratio after 2 h. We therefore suggest using this time point for defining a diagnostic cutoff value. By classifying dried blood Phe/Try ratios higher than 4.6 as pathologic, two DRD patients would have been missed (test sensitivity 71%) and one patient from the control group would have been regarded as pathologic (test specificity 94%). Sensitivity or specificity did not improve using other time points, indicating the strong need for additional diagnostic parameters in the Phe-loading test in children.
The value of simultaneous determination of biopterin after Phe loading is not well defined. Hyland at al. regarded biopterin measurement as helpful to distinguish test results of heterozygous patients with phenylketonuria from those with a primary defect in pterin metabolism. They concluded that if plasma biopterin is not available, a repeated Phe loading with prior administration of BH4 is necessary (Hyland et al. 1997). However, subsequent studies showed that simultaneous BH4 administration did not improve test sensitivity and specificity (Bandmann et al. 2003). In addition, false negative normal Phe loadings were reported. In these cases, the additional determination of biopterin levels corrected the false negative result and improved test sensitivity (Saunders-Pullmann et al. 2004).
Our data support the high value of biopterin measurement for the correct interpretation of Phe-loading results, but similar to the Phe/Tyr ratio, specific pediatric cutoff values have to be defined. By using previously published values (Blau et al. 2003), two controls were regarded as affected by DRD. By defining an increased plasma biopterin concentration of higher than 14 nmol/l after 1 h as normal, 100% test sensitivity and specificity were achieved. It is important to note that this interpretation is only possible if the adequate intake of Phe is documented. Consequently, the simultaneous Phe, Tyr, and biopterin measurements during Phe loading are necessary in pediatric patients.
Blood samples were taken at 1, 2, 4, and 6 h after Phe loading. According to our results, measurements of Phe and Tyr in DBS and plasma biopterin at baseline and after 1 and 2 h were sufficient to allow a clear diagnosis in children. Therefore, we recommend a revised shortened test protocol that only includes analysis of Phe and Tyr as well as biopterin from three time points (Fig. 4). Although biopterin alone allows a differentiation between DRD and controls, measurements of Phe/Tyr ratio are needed after 1 and 2 h to confirm adequate Phe intake (Fig. 4).
Apart from less frequent blood sampling, a test procedure of only 2 h results in a shorter fasting time and therefore improved child comfort. Importantly, a previously suggested single point analysis after 4 h should not be recommended in children (Bandmann et al. 2003). So far, the combined measurement of Phe, Tyr, and biopterin require DBS as well as plasma samples. Since plasma samples demand light-protected transport on dry ice, determination from DBS is more convenient and results not only in a faster but also a facilitated test procedure. Our data show that Phe concentrations in DBS and plasma are correlated and confirm the positive correlation between plasma biopterin and blood spot biopterin plus pterin after Phe loading. Consequently, parallel measurement of Phe/Tyr and biopterin from one plasma or dried blood sample is possible. Pediatric cutoff values for both methods were calculated and are integrated in a diagnostic flowchart (Fig. 4).
We conclude that Phe loading in children is a useful test for the step-by-step diagnosis of autosomal-dominant as well as autosomal-recessive DRD. Parallel measurement of Phe/Try ratio and biopterin and applying specific pediatric cutoff values is essential in this age group and results in 100% test sensitivity and specificity. The test procedure can be reduced to 2 h, and parallel measurement of Phe/Tyr and biopterin from one plasma or DBS sample is recommended. In a final step, diagnosis of DRD should be confirmed by evaluation of GTPCH enzyme activity in cytokine-stimulated fibroblasts (Bonafe et al. 2001) and/or by mutation analysis in the GCH1 gene.
References
Segawa M, Nomura Y, Nishiyama N (2003) Autosomal dominant guanosine triphosphate cyclohydrolase I deficiency (Segawa disease). Ann Neurol 54(Suppl 6):S32–S45
Thöny B, Auerbach G, Blau N (2000) Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J 347(Pt 1):1–16
Nygaard TG (1993) Dopa-responsive dystonia. Delineation of the clinical syndrome and clues to pathogenesis. Adv Neurol 60:577–585
Grimes DA, Barclay CL, Duff J, Furukawa Y, Lang AE (2002) Phenocopies in a large GCH1 mutation positive family with dopa responsive dystonia: confusing the picture? J Neurol Neurosurg Psychiatry 72:801–804
Bandmann O, Valente EM, Holmans P et al (1998) Dopa-responsive dystonia: a clinical and molecular genetic study. Ann Neurol 44:649–656
Blau N, Bonafe L, Thöny B (2001) Tetrahydrobiopterin deficiencies without hyperphenylalaninemia: diagnosis and genetics of dopa-responsive dystonia and sepiapterin reductase deficiency. Mol Genet Metab 74:172–185
Hyland K, Fryburg JS, Wilson WG et al (1997) Oral phenylalanine loading in dopa-responsive dystonia: a possible diagnostic test. Neurology 48:1290–1297
Bandmann O, Goertz M, Zschocke J et al (2003) The phenylalanine loading test in the differential diagnosis of dystonia. Neurology 60:700–702
Saunders-Pullmann R, Blau N, Hyland K et al (2004) Phenyalanine loading as a diagnostic test for DRD: interpreting the utility of the test. Mol Genet Metab 83:207–212
Lopez-Laso E, Ormazabal A, Camino R et al (2006) Oral phenylalanine loading test for the diagnosis of dominant guanosine triphosphate cyclohydrolase 1 deficiency. Clin Biochem 39:893–897
Schulze A, Lindner M, Kohlmuller D, Olgemoller K, Mayatepek E, Hoffmann GF (2003) Expanded newborn screening for inborn errors of metabolism by electrospray ionization-tandem mass spectrometry: results, outcome, and implications. Pediatrics 111:1399–1406
Blau N, Thöny B (2008) Pterins and related enzymes. In: Blau N, Duran M, Gibson KM (eds) Laboratory guide to the methods in biochemical genetics. Springer-Verlag, Heidelberg, Berlin, pp 665–702
Zurflüh MR, Giovannini M, Fiori L et al (2005) Screening for tetrahydrobiopterin deficiencies using dried blood spots on filter paper. Mol Genet Metab 86(Suppl 1):S96–S103
Blau N, Duran M, Blaskovics Me (2003) In: Physician's Guide to Laboratory Diagnosis of Metabolic Diseases, Chapter 2, 2nd edn. Springer, London, Berlin, pp. 89-106
Bonafe L, Thony B, Leimbacher W, Kierat L, Blau N (2001) Diagnosis of dopa-responsive dystonia and other tetrahydrobiopterin disorders by the study of biopterin metabolism in fibroblasts. Clin Chem 47:477–485
Acknowledgements
We thank Mrs. Brigitte Schmidt-Mader, Mr. Alexandros Anninos, and Mrs. Bettina Abu Seda for skilful technical assistance. This study was supported in part by the Swiss National Science Foundation grant no. 3100A0-119982/1 (to NB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Jean-Marie Saudubray
Competing interest: None declared.
An erratum to this article can be found at http://dx.doi.org/10.1007/s10545-010-9205-4
Rights and permissions
About this article
Cite this article
Opladen, T., Okun, J.G., Burgard, P. et al. Phenylalanine loading in pediatric patients with dopa-responsive dystonia: revised test protocol and pediatric cutoff values. J Inherit Metab Dis 33, 697–703 (2010). https://doi.org/10.1007/s10545-010-9164-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-010-9164-9