Abstract
Peronosporomycetes are devastating pathogens to numerous crop, ornamental, and native plants. They are phylogenetically distinct from those of fungi and hence most of the fungicides are ineffective against them. A large body of literature reveals that several bacterial genera such as Pseudomonas, Bacillus, Burkholderia, Lysobacter, Enterobacter, etc. exert antagonistic activities against the peronosporomycete phytopathogens in both in vitro and in vivo conditions. These bacterial strains originated from diverse habitats and some of them showed high promise for biocontrol of plant diseases caused by these notorious pathogens. Mechanisms of biocontrol by these bacterial antagonists include (1) antibiosis, including biosurfactant activity, (2) secretion of lytic enzymes, (3) competition for nutrients (C and Fe), (4) high plant and hyphal colonization, (5) hyperparasitism, and (6) development of induced systemic resistance in the host plants. This chapter comprehensively reviews advances of research on biocontrol of peronosporomycete phytopathogens by bacterial antagonists including the mode of actions of the antagonistic principles against the pathogens. Recent advances on genome sequencing of several peronosporomycetes and biocontrol agents will provide basis for better understanding of bacteria–plant–pathogen interactions and development of improved strains that will potentially function as effective biocontrol agents against the notorious peronosporomycete phytopathogens for low input sustainable agriculture.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
7.1 Introduction
The peronosporomycetes are phylogenetic relatives of brown algae and diatoms under the kingdom of Straminipila (Dick 2001). They are devastating pathogens of plants, animals, fishes, crustaceans, and microorganisms (Margulis and Schwartz 2000). Several species of this group of microorganisms such as Phytophthora infestans and Plasmopara viticola are listed among the top ten economically most important plant pathogens, resulting in multibillion-dollar crop losses worldwide (Agrios 1997; Haverkort et al. 2008). Phytophthora spp. have long been recognized worldwide as destroyer of plants. About 80 identified species of Phytophthora are causing some of the world’s most economically important and devastating diseases in over 2,000 plant species (Abad et al. 2008). Among them, Ph. infestans, the cause of potato and tomato late blight disease was responsible for the Great Irish Potato Famine in the mid-nineteenth century. This disease is still widespread throughout potato-growing regions of the world and is virtually impossible to grow potato without some form of late blight disease control. Several other species such as Ph. cinnamomi, Ph. capsici, Ph. megasperma, Ph. parasitica, Ph. erythroseptica, Ph. fragariae, and Ph. palmivora are also extremely destructive on their hosts causing primarily root and lower stem rots, but also some cankers, twig blights, and fruit rots (Yang et al. 1994; Carruthers et al. 1995; Valois et al. 1996; Ko et al. 2009; Schisler et al. 2009; Timmusk et al. 2009). One of the dangerous aspects of Phytophthora pathogens is emergence of new and more virulent species. Record of new species of Phytophthora has become customary from new hosts as well as from new countries or even continents.
The soilborne phytopathogenic peronosporomycetes such as Pythium spp. and Aphanomyces spp. cause devastating damping-off as well as root rot of seedlings in many crop and forest plants (Whipps and Lumsden 1991). They attack seeds after planting and rot them before they germinate. Moreover, root and crown rot caused by Pythium spp. and Aphanomyces spp. has become an increasing problem in different crops (Deora et al. 2006; Timmusk et al. 2009) and resulting inadequate plant stand in both nursery and field. Another historically infamous peronosporomycete is Plasmopara viticola, a causal agent of downy mildew disease of grapevine, which almost inundated the European wine industry in late nineteenth century. This obligate biotrophic pathogen was introduced into Europe from North America in 1976 (Gobbin et al. 2006). Downy mildews caused by various species of biotrophic peronosporomycete genera such as Plasmopara, Basidiophora, Hyaloperonospora, Sclerospora, etc. are serious pathogens of many crops and responsible for substantial amount of annual yield losses (Shetty et al. 1995). Because of the obligate parasitic mode, they are difficult to study at molecular level. Hence, our knowledge on their biology is very limited.
The peronosporomycetes infect their host plants through asexually generated characteristic biflagellate motile zoospores (Fig. 7.1). The zoospores have powerful sensory transduction system to locate and aggregate potential infection sites of the host and then rapidly undergo necessary morphological alterations for invading host tissues (Islam et al. 2001, 2002). The infection cycles of the peronosporomycetes are extremely rapid, which result epidemic for large area of crops within a few days under favorable environment. Despite the economical and environmental importance of the peronosporomycete diseases, they are difficult to control due to their unique cellular features, such as cell wall composition and/or lack of sterol metabolism (Nes 1987). Rapid development of resistance against agrochemical is also complicated in traditional agrochemical-based plant protection approaches against the peronosporomycetes. Moreover, the deleterious effects and consequences of the use of synthetic chemicals to the environment and nontargeted organisms are also discouraging their use against the peronosporomycetes. Therefore, development of effective biologically rational management strategies against the peronosporomycete phytopathogens are badly needed.
Biological control involves the use of organisms, their products, genes, or gene products to control undesirable organisms (pests) and favor desirable organisms, such as crops, trees, beneficial organisms, and insects (National Academy of Sciences 1987). The organism that suppresses the disease or pathogen is referred to as the biological control agent (BCA). In last three decades, we have witnessed a dramatic development in research on biological control of plant diseases including those caused by the peronosporomycetes. Plant pathologists have been fascinated by the perception that disease suppressing soil or antagonistic plant-associated microorganisms could be used as environment-friendly biocontrol agents (Haas and Defago 2005). The concept of biological control is becoming popular not only because of increasing public concern about the use of hazardous chemical pesticides, but also uncertainty or inefficiency of current disease control strategies against the peronosporomycetes (Cook 1993; Islam et al. 2005a, b). Biological control strategies attempt to enhance the activities of BCA either by introducing high populations of a specific BCA or by enhancing the conditions that enable a BCA in their natural habitat to suppress the diseases (Nelson 2004). BCAs are easy to deliver, increase biomass production, and yield and improve soil and plant health (Burr et al. 1978; Kloepper et al. 1980b; Stockwell and Stack 2007). Isolation and characterization of new potential BCAs and understanding their ecology, behavior, and mode of action are considered as major foci in current biocontrol research (Islam et al. 2005b, 2011). The rapid development of convenient techniques in molecular biology has revolutionized this field by facilitating the identification of the underlying molecular mechanism of pathogen suppression (Islam et al. 2005b, 2011; Islam 2008) and by providing means for construction of “superior” BCAs through genetic engineering (Fenton et al. 1992; Bainton et al. 2004).
A large body of literature indicates that biological control agents such as bacterial antagonists can significantly suppress the disease caused by peronosporomycete phytopathogens and increase the yield of crops. Bacterial antagonists commonly studied and deployed for the control of peronosporomycete diseases include Pseudomonas, Bacillus, Burkholderia, Lysobacter, Actinobacter, Enterobacter, Paenibacillus, and Streptomyces (Anjaiah et al. 1998; Handelsman et al. 1990; Liu et al. 2007a; Islam et al. 2004, 2005b, 2011). Suppression of pathogens or diseases by the biocontrol agents is accomplished by several ways, such as production of antibiotics or lytic enzymes (Osburn et al. 1995; Palumbo et al. 2005; Perneel et al. 2008; Islam et al. 2011), competition for specific nutrient (e.g., iron or carbon) (van Dijk and Nelson 1998; Heungens and Parke 2000; Lee et al. 2008), induction of systemic resistance in the host plants (Yan et al. 2002; Zhang et al. 2010), and parasitizing pathogen’s hyphae (Tu 1978) and/or reproductive structures (Khan et al. 1997).
Although several good reviews on biocontrol of plant diseases have been published (McSpadden Gardener and Fravel 2002; Compant et al. 2005; Haas and Defago 2005; Weller 2007), however, there is no review so far been published specifically on biocontrol of peronosporomycete phytopathogens by bacterial antagonists. Recently, potentials for biological control of plant diseases by Lysobacter spp. (Islam 2011) and Bacillus spp. (Borriss 2011) have been reviewed. In this chapter, we attempt to review current knowledge on biocontrol of peronosporomycete phytopathogen by the bacterial antagonists. This review covers activities of BCAs against pathogens in both in vitro and in vivo conditions. The mode of action and application of BCAs in the practical field are also discussed.
7.2 Bioassay Methods to Screen Antagonistic Bacteria, Detect and Identify Antiperonosporomycetal Compounds
Selection of a potential isolate is the first and foremost important step in biological control by bacterial antagonist. Isolation of bacteria in a suitable culture medium from soil, plant, and water generates huge number of taxonomically diverse population. It is always challenging for researchers to find a convenient method to screen these vast population of isolated bacteria and identify the potentially active strain based on in vitro antagonism against a target pathogen. Over the years, some convenient in vitro bioassay methods have been developed in different laboratories. Some widely used bioassay methods are briefly discussed in this section.
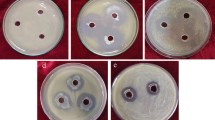
Among the various bioassay methods, dual culture assay is a relatively simple and rapid screening method, which involves the cocultivation of two organisms on a soft agar medium in a Petri dish (Fig. 7.2). The area of the inhibition zone is taken as a measure of antagonistic potential of the isolates. However, microscopic observation on growth inhibited hyphal tips reveals that antagonistic bacteria exert diverse morphological alterations on the approaching hyphae and thus inhibit normal polar growth of the peronosporomycetes which can be seen in naked eye (Deora et al. 2006). The diversity of morphological alterations in the affected hyphae appears to be associated with the mode of action of antagonism by the bacteria (Fig. 7.2).
Morphological alterations due to in vitro interactions between antagonistic bacteria and peronosporomycete phytopathogens in a dual culture on agar plate (adapted from Islam et al. 2005b and Deora et al. 2006). (a), (c)–(h) Aphanomyces cochlioides AC-5, and (b), (i)–(l) Pythium aphanidermatum PA-5. (a) Normal hyphal growth of AC-5; (b) normal hyphal growth of PA-5; (c) inhibition of AC-5 mycelial growth in the presence of Lysobacter sp. SB-K88 (Islam et al. 2005b); (d) curly growth of AC-5 hyphae approaching an SB-K88; (e) Pseudomonas jessenii strain EC-S101: excessive hyphal branching or hyperbranching; (f) Stenotrophomonas maltophilia EC-S105: curling; (g) Delftia sp. EC-107: longer and pointed tip with irregular growth; (h) Bacillus subtilis EC-S108: colonization of bacteria on hyphae; (i) P. jessenii EC-S101: apical branching; (j) Pseudomonas sp. EC-S102: swelling; (k) Pseudomonas sp. EC-S102: extensive vacuolation; (l) Bacillus subtilis EC-S108: decrease in normal branching, swollen hyphae and necrosis. Scale bars, (e)–(l) 50 μm
Other bioassay methods for assessment of activities of BCAs include (1) double layer agar method (Kraus and Loper 1992); (2) homogeneous solution method for motility and viability assay of zoospores (Islam et al. 2005b, 2011); (3) detached leaf assay (Islam et al. 2011); (4) inverted lid method (Ongena et al. 1999); (5) hyphal column assay (Yang et al. 1994); (6) bioassay to detect antiperonosporomycetal substances such as thin layer chromatography (TLC) (Islam et al. 2005b, 2011), high performance liquid chromatography (HPLC) (Nakayama et al. 1999), gas chromatography (GC) (Ko et al. 2009); (7) test with negative mutants (Becker and Cook 1988); (8) molecular detection of antibiotic biosynthesis genes (Chung et al. 2008); (9) use of antibiotic gene transcription in situ (Meyer et al. 2010); (10) lytic enzyme assay (Ko et al. 2009); (11) antiperonosporomycetal protein assay (Woo et al. 2002); (12) detection of siderophores (Schwyn and Neilands 1987); and (13) advanced microscopic study to understand mode of action (Islam 2008, 2010).
7.3 In Vivo Disease Suppression by Various Biocontrol Bacteria
Bacteria from diverse origins and taxonomic genera such as Bacillus, Pseudomonas, Streptomyces, Burkholderia, Lysobacter, and Enterobacter have been shown high potentials to suppress various plant diseases caused by the peronosporomycete phytopathogens (Table 7.1). Literature of some notable groups or genera of biocontrol bacteria are reviewed in this section.
7.3.1 Pseudomonas spp.
Pseudomonas spp. are most researched BCAs against peronosporomycete phytopathogens. Members of this bacterial genus are ubiquitous in soils and rhizosphere of plants. They have many traits that make them one of the best BCAs against various phytopathogens (Weller 2007). It is now over 40 years since Pseudomonas spp. were first recognized as a potential BCA. Within this period, intensive research has given rise to many well-characterized Pseudomonas BCAs (Table 7.1). Among them, the fluorescent Pseudomonas spp. received paramount importance due to their efficacy in biocontrol activity. A well-characterized fluorescent pseudomonad is Ps. fluorescens Pf5, suppresses several Pythium diseases, including damping-off disease in cotton (Howell and Stipanovic 1980) and cucumber caused by Py. ultimum (Kraus and Loper 1992). Another strain of Ps. fluorescens, Pf1 controls damping-off diseases in tomato and hot pepper caused by Py. aphanidermatum (Ramamoorthy et al. 2002). A strain of fluorescent Pseudomonas, DSS73 isolated from the rhizoplane of sugar beet seedlings, displayed suppression of root-pathogenic Py. ultimum by producing biosurfactant antibiotics (Sørensen et al. 2001; Nielsen et al. 2002; Andersen et al. 2003). Ps. fluorescens strain DR54 isolated from sugar beet rhizosphere showed high biocontrol activity against Pythium damping-off in sugar beet (Nielsen et al. 1998). Similarly, Ps. fluorescens strain F113 isolated from sugar beet in Ireland displayed damping-off disease suppression in sugar beet and pea caused by Py. ultimum (Fenton et al. 1992; Delany et al. 2001; Bainton et al. 2004).
An important strain CHA0 of Ps. fluorescens was isolated from roots of tobacco grown near Payern, Switzerland, in a soil naturally suppressive to black root rot of tobacco caused by Thielaviopsis basicola (Stutz et al. 1986). This strain has shown one of the broadest range of potential biocontrol and growth-promoting mechanisms of any PGPR described so far. CHA0 suppresses Pythium damping-off of cucumber and wheat and infection of Arabidopsis by Hyaloperonospora parasitica (Maurhofer et al. 1995; Iavicoli et al. 2003; Meyer et al. 2010). It is also equally effective against nonperonosporomycete fungi, viruses, and nematodes (Keel et al. 1992; Maurhofer et al. 1994; Siddiqui and Shaukat 2003). Application of Ps. fluorescens SS101 to soil or bulbs effectively controls root rot of flower bulb crops caused by Py. intermedium in both laboratory and small-scale field experiments (de Souza et al. 2003b). Strain SS101 when applied to soil controls Pythium-root rot of apple seedlings (Mazzola et al. 2007) and when applied to leaves controls late blight of tomato caused by Ph. infestans (Tran et al. 2007).
Ps. aeruginosa PNA1, isolated from the rhizosphere of chickpea, has widely been shown biocontrol efficacy against a number of phytopathogenic fungi and peronosporomycetes (Anjaiah et al. 1998). This strain demonstrated in vivo biocontrol activity against various peronosporomycetes including Py. splendens on bean (Anjaiah et al. 1998), Py. myriotylum on cocoyam (Tambong and Höfte 2001; Perneel et al. 2008), and Ph. capsici on pepper (Kim et al. 2000). Another strain of Ps. aeruginosa, 7NSK2 isolated from the rhizosphere of barley, promotes the growth of several crops and suppresses Py. splendens-induced damping-off in tomato (Buysens et al. 1996). Isolates of Ps. chlororaphis (previously Ps. aureofaciens) strongly suppressed Py. aphanidermatum in roots of pepper, cucumber, and chrysanthemum (Chatterton et al. 2004; Khan et al. 2003; Liu et al. 2007b), and Ph. megasperma in roots of asparagus (Carruthers et al. 1995) and moderately suppressed Py. dissotocum in roots of hydroponic chrysanthemums (Liu et al. 2007b). Ps. putida strain N1R provides biocontrol of Ps. ultimum on soybean, pea, and cucumber (Paulitz and Loper 1991). Similarly, Ps. jessenii strain EC-S101 and Stenotrophomonas maltophilia EC-S105 efficiently suppressed in vivo damping-off disease caused by Ap. cochloides (Deora et al. 2005). Some pseudomonads have been found to protect plants from various pathogens by inducing systemic resistance. For example, Ps. fluorescens strains UOM and SAR14Ps control Sclerospora graminicola on pearl millet (Raj et al. 2003) and Ps. fluorescens WCS417r controls Hy. Parasitica on Arabidopsis (van der Ent et al. 2008).
7.3.2 Bacillus spp.
The spore-forming bacteria, Bacillus spp. are considered one of the most effective biocontrol candidates for plant diseases caused by peronosporomycetes. A large body of literature is available concerning biological control of peronosporomycete phytopathogens by various strains of Bacillus spp., some important research findings are reviewed (Table 7.1). Bacterial strain antagonistic to a certain peronosporomycete on plate assay may not necessarily be effective in suppressing disease in in vivo or vice versa. For example, Ba. cereus UW85 is not inhibitory to Ph. megasperma f. sp. medicaginis on plates, but its application to the alfalfa seeds suppresses the damping-off disease caused by the same pathogen in the field conditions (Handelsman et al. 1990). In contrast, the same strain was inhibitory to Ph. sojae on plate assay, but increased yields only on the susceptible cultivar where Phytophthora root rot was a factor. At another site where Phytophthora root rot was not a factor, UW 85 increased plant stands significantly over untreated seeds regardless of Phytophthora root rot resistance (Osburn et al. 1995). Both additive and nonadditive responses were also observed when several strains were applied together for disease control. For example, a nonadditive response was demonstrated by Everts and Armentrout (2001) for powdery mildew of pumpkin. Several Bacillus spp. were also found effective in suppressing Phytophthora blight in pepper and squash caused by Ph. capsici. Preinoculation of pepper plants with three strains of Ba. megaterium alone or in combination, significantly reduced disease severity of Phytophthora blight or crown blight caused by Ph. capsici in field experiments (Akgül and Mirik 2008). Several strains of Ba. subtilis isolated from the rhizoplane and rhizosphere pepper have been found useful for suppression of Phytophthora blight of pepper when applied as seed coating (Sid Ahmed et al. 2003a, b; Lee et al. 2008; Chung et al. 2008). The mixture of Ba. safensis T4 + Lysinibacillus boronitolerans SE56 significantly improved control efficacy compared to the individual strain (Zhang et al. 2010).
Several lines of evidence suggest that inoculation of Bacillus spp. may induce systemic resistance in the host plant. Several strains of Ba. safensis, Lysinibacillus boronitolerans, Ba. pumilus, and Ba. macauensis have also been shown to induce protection squash against Phytophthora blight (Zhang et al. 2010). Similarly, Ba. pumilus INR7 + T4 + SE56 and INR7 + Ba. subtilis IN937a + T4 + SE56 tended to induce higher levels of disease reduction compared to individual strains. Ba. pumilus strains INR7 and SE34 were also successful to elicit systemic protection against downy mildew caused by Plasmopara halstedii in sunflower (Nandeeshkumar et al. 2008) and late blight on tomato caused by Ph. infestans (Yan et al. 2002), respectively. Downy mildew, caused by Pl. viticola, was also reduced on grape berry skins and leaves by treatment with Ba. subtilis KS1 (Furuya et al. 2011).
A common soil bacterium, Paenibacillus spp., displayed biocontrol activity against several diseases caused by peronosporomycetes. Paenibacillus sp. strain B2 isolated from the mycorrhizosphere of Sorghum bicolor displayed antagonistic activities against some soilborne pathogens when applied with Glomus mosseae (Budi et al. 1999). Application of strain B2 alone or with G. mosseae to tomato plants significantly reduced root necrosis caused by Ph. parasitica. Besides, this bacterium also enhanced colonization of mycorrhizae in the rhizosphere of tomato. A strain of Pae. illinoisensis KJA-424 isolated from soil in the west coast of Korea reduced root mortality of pepper plants caused by Ph. capsici when it (in 0.2 % colloidal chitin) was applied into the pot soil. Timmusk et al. (2009) reported that pretreatment of Arabidopsis root with some strains of Pae. polymyxa showed significant protection against subsequent infection by Py. aphanidermatum. The survival rates of Py. aphanidermatum infected plants were higher when the seedlings were pretreated with B2 and B5 strains than that of B6 treatment.
7.3.3 Burkholderia spp.
Burkholderia spp. formerly erroneously identified as Pseudomonas spp. showed biocontrol activities against several peronosporomycetal diseases. For example, pea seeds treated with Burkholderia (Pseudomonas) cepacia strains AMMD and AMMDR1 (rifampicin resistant mutant) resulted in increased seedling stand and reduced preemergence damping-off caused by Py. ultimum and Py. sylvaticum. Seed treatment with Bu. cepacia (strain AMMD) and Ps. fluorescens (PRA25) alone or in combination with captan effectively suppressed damping-off disease of pea (Parke et al. 1991). Application of Bu. cepacia increased seedling emergence (40 %) and yield (48 %) compared with captan alone. Strains AMMD or PRA25 significantly suppressed early stage of diseases, while strain AMMD also suppressed the final incidence of disease at harvest over 2 successive years (Bowers and Parke 1993). Bu. cepacia AMMDR1 significantly reduced Py. aphanidermatum postinfection colonization and damping-off of pea seeds, even when the bacteria were applied 12 h after zoospore inoculation (Heungens and Parke 2001). The level of biocontrol of Pythium damping-off by AMMD depended on the host genotype, while the seed treatment with bacteria did not reduce the symptoms of Aphanomyces root rot (King and Parke 1993). Bu. cepacia AMMDR1 significantly reduced colonization of taproots by Ap. euteiches mycelium, when roots were dip inoculated in a concentrated cell suspension (Heungens and Parke 2001). Both root-dip inoculation of bacterial suspension at lower concentrations and seed inoculation resulted in lower numbers of bacteria near the root tip early on in the infection process.
7.3.4 Enterobacter cloacae
Enterobacter cloacae is a common seed-associated bacterium (Hadar et al. 1983), which is an effective biological control agent that suppresses seed infections, protecting a number of plant species from Py. ultimum-induced damping-off (Table 7.1) (Nelson 1988; van Dijk and Nelson 1998, 2000). Seeds previously inoculated with E. clocae strains NRRL B-14095 and NRRL B-14096 suppressed preemergence Pythium damping-off of cucumber compared to control. The performance of biocontrol by E. clocae was varied in different plant species. E. cloacae was equally effective in controlling Pythium damping-off when placed on the seeds of various crops such as carrot, cotton, rye, lettuce, radish, tomato, and wheat (Nelson 1988; Kageyama and Nelson 2003). However, it was ineffective in biocontrol of diseases in corn (Kageyama and Nelson 2003); pea (Hadar et al. 1983; Kageyama and Nelson 2003); and soybean, snap bean, and lima bean.
7.3.5 Lysobacter spp.
The Lysobacter spp. are ubiquitous inhabitants in the diverse environment that have some unique features including gliding motility, high genomic G + C ratio (65–72 %), and brush-like polar fimbriae (Islam et al. 2005a, b). This genus have gained broad interest for several reasons such as (1) rich source for production of a variety of novel antibiotics, such as lysobactins or katanosins (Bonner et al. 1988), cephabacins (Lee et al. 2008), tripropeptins (Hashizume et al. 2001, 2004), and macrocylic lactams such as xanthobaccins (Hashidoko et al. 1999; Nakayama et al. 1999; Yu et al. 2007); (2) production of a wide variety of extracellular cell wall degrading enzymes such as β-lytic proteases (Sid Ahmed et al. 2003b), endopeptidase (Muranova et al. 2004), keratinases, β-1,3 glucanases (Palumbo et al. 2003), cellulase (Ogura et al. 2006), and lysoamidase (Riazanova et al. 2005); (3) ability to suppress plant diseases and colonize plant surfaces (Martin 2002; Islam et al. 2004, 2005b; Islam 2008, 2010); and (4) exhibition of wolf-pack-like micropredatory behavior (Martin 2002; Islam 2010). Some of these unique features of Lysobacter spp. are advantageous for using them as BCAs against phytopathogens (Zhang et al. 2001; Folman et al. 2004; Islam et al. 2005a; Kobayashi et al. 2005; Ji et al. 2008). Among 21 identified species, biocontrol activity of L. enzymogenes strains C3 and 3.1T8 and Lysobacter sp. SB-K8 has extensively been investigated. The biocontrol potentials of Lysobacter spp. including their mode of action have recently been reviewed (Islam 2011).
7.3.6 Actinomycetes
Numerous surveys of soil bacteria have identified considerable number of strains of actinomycetes as potential BCAs (Khan et al. 1997; Filonow and Dole 1999; Crawford et al. 1993; Misk and Franco 2011). One of the advantages of actinomycetes is their ability to produce spore. These spores are long lived and resistant to heat and desiccation, and maintain a stable population over the time. Actinomycetes such as Actinoplanes spp. have shown great promise for reducing Pythium root rot in horticultural plants in the greenhouse. Several strains of Actinoplanes spp. (W57, W257, or 25844) were applied on clay granules at 5 % or 0.5 % w/w to Py. ultimum oospores-infested soil-less potting mix 5 days prior to replanting geranium or poinsettia seedlings. Application of Actinoplanes spp. generally reduced root rot severity and increased plant stand compared to nontreated plants after 6 week grown in a greenhouse (Filonow and Dole 1999). When strain W257 was applied as granules or as a root dip, it was as effective as the fungicide metalaxyl in reducing the root rot. When strains 25844, W57, and W257 were applied as granules at 5 % (w/w) to field plots infested with oospores of Py. ultimum, only strain 25844 consistently increased emergence and reduced root rot of table beets compared to controls (Khan et al. 1997). In the same study, strain 25844 at 1 % (w/w) also increased the emergence of bush beans at 28 days after planting in Py. ultimum-infested plots, but lower rates were found ineffective. Similarly, El-Tarabily et al. (2010) showed that the endophytic actinomycetes such as Ac. campanulatus, Micromonospora chalcea, and Streptomyces spiralis, when applied individually or in combination significantly promoted plant growth and reduced damping-off and crown and root rot of cucumber caused by Py. aphanidermatum under green house conditions. These isolates when applied individually or in combination to cucumber seedlings, also promoted growth and yield and reduced seedling damping-off and root and crown rot of mature cucumber plant in the field (El-Tarabily et al. 2010).
The use of streptomycete actinomycetes as biological control agents against the peronosporomycete disease has received considerable attention (Crawford et al. 1993; Valois et al. 1996; Xiao et al. 2002; Joo 2005; Lee et al. 2005; Misk and Franco 2011; Abdalla et al. 2011; Islam et al. 2011) (Table 7.2). Eleven strains of actinomycetes belong to the genus Streptomyces significantly reduced the root rot index caused by Ph. fragariae var. rubi when inoculated on raspberry plantlets (Valois et al. 1996). In contrast, antiperonosporomycetal compounds originating from actinomycetes appear to be selectively active against Phytophthora and Pythium. Spraying tomato seedlings with culture broth of Streptomyces sp. AMG-P1 resulted highly inhibitory effect against late blight disease caused by Ph. infestans at 500 μg freeze-dried weight per milliliter, whereas its antibiotic paromomycin showed potent in vivo activity against red pepper and tomato late blight diseases with 80 and 99 % control value, respectively, at 100 μg/ml (Lee et al. 2005).
The use of Streptomyces spp. in biocontrol of plant diseases depends on their potential inhibitory effects on the pathogen. Eight isolates having potential pathogen-inhibitory capabilities were subsequently tested for their ability to control Phytophthora root rots on alfalfa and soybean in sterilized vermiculite and naturally infested field soil. The Streptomyces isolates significantly reduced root rot severity in alfalfa and soybean caused by Ph. medicaginis and Ph. sojae, respectively (Xiao et al. 2002). Similarly, in a greenhouse experiment, Strepmyces sp. BSA25 and WRA1 with the highest antagonistic capabilities against broad spectrum of pathogens were tested for their ability to control Phytophthora root rot of chickpea caused by Ph. medicaginis and found that both isolates promote vegetative growth of chick pea and successfully suppressed Phytophthora root rot when coinoculated with either Mesorhizobium ciceri WSM1666 or Kaiuroo 3 (Misk and Franco 2011).
7.3.7 Disease Suppression by Other BCAs
There are a few other bacterial genera which have been proved to be effective BCAs against peronosporomycete pathogens, but have been used in a limited study. Tu (1978) reported that Phytophthora root rot of soybean was lessened in the green house when rhizobia were applied to the potted soil immediately after planting. Serratia plymuthica HRO-C48 isolated from the rhizosphere of oilseed rape was effective in suppressing damping-off of cucumber caused by Py. aphanidermatum (Pang et al. 2009). Stenotrophomonas maltophilia W81, isolated from a sugar beet rhizosphere is capable of conferring protection against Py. ultimum-mediated damping-off (Dunne et al. 1997).
7.4 Mechanism of the Biological Control
Biological control of peronosporomycete phytopathogens is a multifaceted process in which several mechanisms are involved. This section reviews current knowledge on widely recognized mechanisms of disease suppression by the biocontrol bacteria.
7.4.1 Direct Antagonism or Antibiosis
A condition in which one or several metabolites that are excreted by an organism have a harmful effect on other organisms is known as antibiosis (Haas and Defago 2005). The microbial metabolites that can suppress growth and reproduction or kill other microorganisms at low concentration are known as antibiotics. A large number of structurally diverse chemical compounds (antibiotics) have been identified as principles of biocontrol of peronosporomycetal diseases by antagonistic bacteria (Figs. 7.3, 7.4, 7.5, and 7.6). The action mechanisms of antibiotics are very diverse and sometime very specific. Indeed, antibiosis is one of the most-studied mechanisms of biological control by bacterial antagonists. Antibiotics such as DAPG, phenazines, pyoluteorin, hydrogen cyanide, oomycin A, anthranilate, and cyclic lipopeptides have been reported to involve in suppression of peronosporomycete phytopathogens by Pseudomonas spp. (Table 7.2). The polyketide phenolic antibiotic, DAPG is produced by different strains of Ps. fluorescens to suppress the growth of peronosporomycetes (Fig. 7.3) (Shanahan et al. 1992; Fenton et al. 1992; Keel et al. 1992; Maurhofer et al. 1995; Delany et al. 2001; Iavicoli et al. 2003; Bainton et al. 2004; Islam and Fukushi 2010; Islam and von Tiedemann 2011).
Light and scanning electron micrographs showing Aphanomyces cochlioides zoospore-lytic activity of xanthobaccin A isolated from the biocontrol bacterium Lysobacter sp. SB-K88 (adapted from Islam et al. 2005b). (a) Micrograph of a biflagellate A. cochlioides zoospore (untreated control); (b) no lysis of zoospore in control. A small portion of (10–15 %) of motile zoospores in the control dish were stopped and changed into round cystospores and then settled to the bottom of the dish; (c) complete lysis of all halted zoospores by xanthobaccin A at 1.0 μg/ml
DAPG has shown a wide range of inhibitory activities such as antiviral, antibacterial, antifungal, antihelminthic, and phytotoxic properties (Bainton et al. 2004). Production of DAPG is considered as one of the major determinants of disease suppression by Ps. fluorescens. It inhibits a diverse group of peronosporomycetes such as Py. ultimum (Fenton et al. 1992; Shanahan et al. 1992; Delany et al. 2001), Ap. cochloides (Islam and Fukushi 2010), Peronospora parasitica (Iavicoli et al. 2003), Pl. viticola (Islam and von Tiedemann 2011). DAPG-induced disease suppression is associated with alteration or disruption of a variety of cellular peronosporomycetes. For example, mycelial growth inhibition of Py. ultimum and Ap. cochlioides through excessive branching and curling (Shanahan et al. 1992; Islam and Fukushi 2010); disruption of the organization of cytoskeletal filamentous actin in Ap. cochlioides hyphae (Islam and Fukushi 2010); disorganization in hyphal tips of Py. ultimum var. sporangiiferum, including alterations (proliferation, retraction, and disruption) of the plasma membrane, vacuolization, and cell content disintegration (de Souza et al. 2003a); inhibition of zoosporogenesis and the motility of zoospores of Ap. cochlioides and Pl. vitivola (Islam and von Tiedemann 2011); and induction of systemic resistance in the host plants (Iavicoli et al. 2003). DAPG has recently been reported to inhibit the mitochondrial function in yeast (Gleeson et al. 2010). As cleavage of nuclei and dramatic differentiation of sporangia during zoosporogenesis require supply of energy from the mitochondria, impairment of the mitochondrial function in the Pl. viticola sporangia and zoospores by DAPG might be associated with suppression of zoospore release and motility inhibition of zoospores, respectively (Islam and von Tiedemann 2011). To understand structure–activity relationships, Islam and von Tiedemann (2011) tested several phloroglucinol derivatives, namely, phloroglucinol (PG), monoacetylphloroglucinol (MAPG), 2,4,6-triacetylphloroglucinol (TAPG), and 2,4-dipropylphloroglucinol (DPPG) structurally related to the DAPG on zoosporogenesis and motility behavior of two peronosporomycetes, Pl. viticola and Ap. cochlioides (Fig. 7.3). According to their bioassay results, the activities of the tested compounds ranked DPPG > TAPG > DAPG > MAPG > PG for both zoosporogenesis and motility inhibition of the zoospores. It appeared that (1) the degree of substitution of hydrogen atoms in the benzene ring of phloroglucinol by acyl groups (acetyl or propyl) increased bioactivity; and (2) substitution with a larger aliphatic group (propyl) showed higher activity than the shorter aliphatic group (acetyl).
Phenazines are low molecular weight nitrogen-containing heterocyclic antimicrobial compound consisting of brightly colored pigment produced by the bacterial genera Pseudomonas (Gurusiddaiah et al. 1986; Carruthers et al. 1995; Anjaiah et al. 1998; Perneel et al. 2008). Phenazine compounds have antibiotic activity against a wide range of bacterial and fungal pathogens including several peronosporomycetes that cause important root diseases of plants (Carruthers et al. 1995; Anjaiah et al. 1998). Two phenazine antibiotics, phenazine-1-carboxylic acid (PCA) and phenazine-1-carboxamide (oxychlororaphine), and an anthranilate produced by P. aeruginosa PNA1 showed a dominant role in suppressing Pythium damping-off diseases in chick pea, bean, and lettuce (Fig. 7.3) (Anjaiah et al. 1998; Perneel et al. 2008). PCA produced by Ps. fluorescens 2-79 (Gurusiddaiah et al. 1986) and Ps. chlororaphis Tx-1 (Carruthers et al. 1995) showed excellent activity against Py. aristosporum and Ph. megasperma, respectively. The mode of action of phenazines in antiperonosporomycete interactions includes mycelial growth inhibition of Pythium (Anjaiah et al. 1998; Perneel et al. 2008; Gurusiddaiah et al. 1986), and Phytophthora (Carruthers et al. 1995) and aggregation of cell content and vacuolization of Pythium hyphae (Perneel et al. 2008). Another antibiotic, pyoluteorin (4,5-dichloro-1H-pyrrol-2yl-2.6-dihydroxyphenyl ketone) is a chlorinated polyketide antibiotic secreted by the rhizosphere bacterium Ps. fluorescens Pf-5 (Howell and Stipanovic 1980; Kraus and Loper 1992) and Ps. fluorescens CHA0 (Maurhofer et al. 1994, 1995). This antibiotic inhibits growth of mycelia of a seed- and root-rotting peronosporomycete, Py. ultimum (Howell and Stipanovic 1980; Kraus and Loper 1992). Ps. fluorescens strain Hv37aR2 produces oomycin A, which is linked to in vivo biocontrol of Py. ultimum infection on cotton (Howie and Suslow 1991).
Zwittermicin A is a linear aminopolyol, which is involved in suppression of Phytophthora diseases on alfalfa and soybean by Ba. cereus UW85 (Handelsman et al. 1990; Silo-Suh et al. 1994; Osburn et al. 1995). It inhibits elongation of germ tube of zoospores and mycelial growth of Phytophthora spp. (Osburn et al. 1995). Four compounds, namely, phenylacetic acid (PA), hydrocinnamic acid (HCA), 4-hydroxyphenylacetic acid (HAA), and 4-hydroxyphenylacetate methyl ester (HPME) were isolated from the culture broth of Burkholderia sp. strains MP-1, PA, HCA, and HPME, which moderately inhibited Ph. capsici. However, HAA isolated from the culture supernatant of Lysobacter antibioticus HS124 induces abnormal hyphae of Ph. capcisi (Ko et al. 2009).
The biocontrol bacterium Lysobacter sp. SB-K88 suppresses damping-off disease in sugar beet and spinach caused by Ap. cochlioides and Pythium sp. through production of at least three lytic antibiotics, xanthobaccin A, B, and C (Nakayama et al. 1999; Islam et al. 2005b). Direct application of purified xanthobaccin A to seeds suppressed damping-off disease in sugar beet in soil naturally infested with Pythium spp. (Nakayama et al. 1999). The predominant antibiotic, xanthobaccin A produced by SB-K88 inhibits mycelial growth, impairs motility, and causes lysis of zoospores of Aphanomyces cochlioides (Nakayama et al. 1999; Islam 2008, 2010) (Figs. 7.4 and 7.5).
The mode of action of this macrocyclic lactam antibiotic includes disruption of ultrastructure and organization of filamentous actin in the cells of Ap. cochlioides (Islam 2008). The plane structure of xanthobaccin A is the same as that of a known antibiotic maltophilin, which was isolated from a rhizobacterium of rape Stenotrophomonas maltophilia R3089 (Jacobi et al. 1996). Both xanthobaccin A and maltophilin belong to a group of tetramic acid containing macrocyclic lactam antibiotics. An analogue of xanthobaccin A, dihydromaltophilin was identified as a heat stable and potent antiperonosporomycetal compound in the culture fluid of L. enzymogenes strain C3 (Yu et al. 2007). This compound exhibits a wide range of antimicrobial activities and shows a novel mode of action by disrupting the biosynthesis of a distinct group of sphingolipids (Giesler and Yuen 1998).
Suppression of Py. aphanidermatum damping-off in cucumber by Serratia plymuthica HRO-C48 is found to be responsible for its ability to produce antibiotic pyrrolnitrin (Pang et al. 2009). The pyrrolnitrin belongs to phenylpyrrole group and inhibits growth, synthesis of protein, RNA, DNA, and uptake of metabolites of pathogens (Pang et al. 2009).The aminoglycoside antibiotic, paromomycin is detected in Streptomyces sp. AMG-P1 as a candidate for the control of tomato and potato diseases caused by Pythium spp. and Ph. infestans (Lee et al. 2005). A new antiperonosporomycetal compound, khatmiamycin was recently isolated from the culture broth of a terrestrial Streptomyces sp. ANK313, which exhibits potent motility inhibitory (100 %) and lytic (83 ± 7 %) activities against zoospores of the grapevine downy mildew pathogen, Pl. viticola (Fig. 7.6) (Abdalla et al. 2011).
Similarly, four isocoumarins have been isolated from the terrestrial Streptomyces sp. ANK302, namely 6,8-dimethoxy-3-methylisocoumarin, 6,8-dihydroxy-3-methylisocoumarin, 6,8-dihydroxy-7-methoxy-3-methylisocoumarin, and 6,7,8-trimethoxy-3-methylisocoumarin displayed varying levels of motility inhibitory effects against Pl. viticola zoospores (Zinada et al. 2011). Although mechanism is not known, several other antibiotics such as nonactin, oligomycin F, and antimycin A isolated from marine Streptomyces spp. also displayed potent motility inhibitory and lytic activities against zoospores of the grapevine downy mildew pathogen, Pl. viticola (Fig. 7.7) (Islam et al. unpublished).
Recently, staurosporine was identified as the active principle in the ethyl acetate extracts of a marine Streptomyces sp. strain B5136 that rapidly impaired the motility of zoospores of the grapevine downy mildew pathogen Pl. viticola (Fig. 7.6) (Islam et al. 2011). The indolocarbazole antibiotic, staurosporine is a known broad-spectrum inhibitor of protein kinases, including protein kinase C (PKC). To understand the role of specific protein kinase in the maintenance of flagellar motility of zoospores, Islam et al. (2011) tested 22 known kinase inhibitors. Interestingly, the PKC inhibitor chelerythrine was the most potent to arrest the motility of zoospores at concentrations starting from 5 nM. Inhibitors that targeted kinase pathways other than PKC pathways did not practically show any activity in impairing zoospore motility. Both staurosporine and chelerythrine also inhibited the release of zoospores from the Pl. viticola sporangia in a dose-dependent manner. In addition, staurosporine completely suppressed downy mildew disease in grapevine leaves at 2 μM, suggesting the potential of small-molecule PKC inhibitors for the control of peronosporomycete phytopathogens (Fig. 7.8). This study for the first time discovered that PKC is as a key signaling mediator associated with zoosporogenesis and the maintenance of flagellar motility in peronosporomycete zoospores (Islam et al. 2011). Interestingly, this finding parallels earlier work on the role of PKC in flagellar motility of mammalian sperm and spermatozoa of aquatic vertebrates (Rotem et al. 1990; White et al. 2007). Because motility is critical for the life cycles and pathogenicity of pathogens, elucidation of the details of signal transduction pathways might help us to design strategies for biorational management of the notorious peronosporomycete phytopathogens.
Suppression of sporangial growth of a downy mildew pathogen, Plasmopara viticola on grapevine leaf disks by varying doses of staurosporine isolated from Streptomyces sp. B 5136 (adapted from Islam et al. 2011). Application of staurosporine at 2 mM concentration completely suppresses downy mildew disease on grapevine leaf. Staurosporine was suspended in aqueous DMSO (1 %) and an appropriate dose was sprayed on leaf disks placed on 1.5 % water agar 12 h before (pre-) or after (post) inoculation with P. viticola sporangia (5 × 103/ml). Inoculated leaf disks were incubated at 25 °C in 95 % relative humidity for 6 days
7.4.2 Biosurfactants as Antiperonosporomycetal Agent
Several strains of Ps. fluorescens were reported to produce antibiotics with surface-active properties. These antibiotics are designated as biosurfactants, which belong to a family of closely related cyclic lipopeptides (CLP). CLP has been shown destructive effects on zoospores of Phytophthora and Pythium spp. (Stanghellini and Miller 1997; Nielsen et al. 1999; de Souza et al. 2003b; de Bruijn et al. 2007; Perneel et al. 2008). Massetolide A, a cyclic lipopeptide with a nine-amino-acid peptide ring linked to 3-hydroxydecanoic acid was isolated from the culture broth of Ps. fluorescens strain SS101 (de Souza et al. 2003b). It is a metabolite with versatile functions causing lysis of zoospores, providing significant control of Ph. infestans, both locally and systemically via induced resistance and contribute to the colonization of tomato plants by Ps. fluorescens SS101 (Tran et al. 2007). Further study by van de Mortel et al. (2009) demonstrated that massetolide A induced the formation of transmembrane pores with an estimated size of between 1.2 and 1.8 nm. Zoospores were found to be most sensitive to massetolide A followed by mycelium and cysts. Massetolide A significantly reduced sporangium formation and caused increased branching and swelling of hyphae. Interestingly, a loss-of-function transformant of Ph. infestans lacking the G-protein subunit was more sensitive to massetolide A, whereas a gain-of-function transformant required a higher massetolide A concentration to interfere with zoospore aggregation which suggests that the cellular responses of Ph. infestans to this cyclic lipopeptide are, in part, dependent on G-protein signaling. Genome-wide expression profiling by microarray analysis may help to unravel the mode of action of massetolide on Ph. infestans.
Another metabolite, viscosinamide is an important component of activity of Ps. fluorescens DR54 against Py. ultimum (Nielsen et al. 1998, 1999). It aids in surface colonization of plant roots and soil surfaces and as antibiotic, reduces growth and aerial mycelium development of Py. ultimum and Rhizoctonia solani (Nielsen et al. 1999). Some strains of Pseudomonas sp. produce amphisin, lokisin, hodersin, and tensin (Nielsen et al. 2002). The ability of Ps. fluorescens DSS73 to efficiently control root-pathogenic Py. ultimum is shown to arise from secreting amphisin (Andersen et al. 2003). The biosurfactants, rhamnolipids produced by Ps. aeruginosa PNA1 suppressed plant-pathogenic peronosporomycetes through lysis of zoospore and inhibition of mycelia growth (Kim et al. 2000; Perneel et al. 2008). Although an increasing number of CLPs with surfactant properties have been described in Pseudomonas spp., a few are also produced by Bacillus spp. Ba. subtilis ATCC 6633 produces surfactant mycosubtilin, a member of the iturin family (Leenders et al. 1999). Leclère et al. (2005) showed that a derivative of the Ba. subtilis strain BBG100 that overproduces mycosubtilin showed increased activity against Pythium on tomato seedlings. Iturin A, another antimicrobial lipopeptide structurally very similar to mycosubtilin has been reported to be produced by several Bacillus strains. This compound showed strong inhibitory activity against various peronosporomycetes (Chung et al. 2008; Furuya et al. 2011). Iturin A is also produced by a strain of Acinetobacter (Liu et al. 2007a). The cell free filtrate of Acinetobacter sp. LCH001 was strongly inhibitory against several phytopathogens including Ph. capsici, F. graminearum, and R. solani, and the bioactive compounds identified were as isomers of iturin A, namely, iturin A2, iturin A3, and iturin A6.
7.4.3 Lytic Enzyme as a Means of Biocontrol
Production of extracellular lytic enzymes has been implicated in plant protection by many biocontrol bacteria (Table 7.3). Exposure of phytopathogens to lytic enzymes can result in the degradation of the polymeric compounds such as chitin, proteins, cellulose, hemicellulose, glucans, and DNA of cell walls of the pathogen and use these as a carbon and energy source (Leah et al. 1991). Proteases, chitinases, glucanases, endopeptidase, lipases, lysoamidase, phospholipases, keratinases, lactamases, and phosphatases produced by bacterial antagonists can degrade structural matrix of cell wall of many phytopathogens including the peronosporomycetes (Lim et al. 1991; Fridlender et al. 1993; Valois et al. 1996; Dunne et al. 1997; Nielsen et al. 2002; Andersen et al. 2003; Hong and Meng 2003; Koch et al. 2002; Palumbo et al. 2005).
It has been reported that some fluorescent pseudomonad produced protease, siderophore, and HCN acted as antimicrobial agents against Pythium sp. and Ph. nicotianae. Suppression of late blight disease in pepper through secretion of lytic enzymes such as protease, chitinase, β-1,3-glucanase, and lipase in concert with the release of antibiotic compound 4-hydroxyphenylacetic acid by L. antibioticus strain HS124 has been demonstrated (Ko et al. 2009). However, chitinases are less essential in causing lysis of cell walls of the peronosporomycetes as they do not contain significant amount of chitin. For example, Stenotrophomonas maltophilia strain W81 produces extracellular enzymes chitinase and protease; however, commercially purified chitinase or cell-free supernatants from cultures of the protease-negative mutant W81M1 or the chitinase- and protease-negative mutant W81A1 had no effect on integrity of the essentially chitin-free Pythium mycelium and did not prevent subsequent growth of this peronosporomycete (Dunne et al. 1997). The proteolytic enzyme was identified as serine protease and the bacterial mutants capable of over producing serine protease showed improved biocontrol activity against Pythium spp. on sugar beet (Dunne et al. 2000). Serine protease is antiperonosporomycetal against Py. ultimum, causing irreversible loss of mycelial growth ability, degradation of proteinaceous cell-wall components, and leakage of cell constituents (Dunne et al. 1997, 2000). Similarly, the ability of strain DSS73 to inhibit the growth of the root pathogenic peronosporomycete, Py. ultimum is believed to originate from the production and excretion of proteases and other biocontrol traits by the bacterium (Nielsen et al. 2002; Koch et al. 2002).
Bacterial enzymes with glucanolytic activity have a very significant role in the suppression of peronosporomycete diseases because β-glucans constitute 80–90 % of the wall dry weight of the peronosporomycete phytopathogens. L. enzymogenes strain C3 produces multiple extracellular β-1,3-glucanases encoded by the gluA, gluB, and gluC genes and are thought to contribute to the biological control activity against Bipolaris leaf spot of tall fescue and Pythium damping-off of sugar beet (Palumbo et al. 2005). Bu. cepacia synthesizes β-1,3-glucanase that destroys the integrity of R. solani, S. rolfsii, and Py. ultimum cell walls (Fridlender et al. 1993). Valois et al. (1996) reported that the antagonistic actinomycetes that suppressed the mycelial growth of Ph. fragariae var. rubi were shown to produce glucanases cleaving β-1,3, β-1,4, and β-1,6. These enzymes could hydrolyze glucans from cell wall structure of growing mycelia of Pythium and Phytophthora, and cause lysis of hyphal cells (Valois et al. 1996; Hong and Meng 2003; Palumbo et al. 2005). Streptomyces sp. AP77 produces an extracellular protein to suppress Pythium. Surprisingly, this anti-Pythium protein, designated as SAP, had neither Pythium cell wall-degrading activity nor any other polysaccharolytic activity, implying that it has a unique inhibitory function different from those of the polysaccharolytic enzymes used in the higher terrestrial plants (Woo et al. 2002).
7.4.4 Competition Between Plant-Associated Bacteria and Peronosporomycete Phytopathogens
Rhizosphere is a battle field or playground for diverse microorganisms including plant pathogens and beneficial bacteria. Competition between plant-associated bacteria and phytopathogens has long been thought to be an important means of suppressing plant diseases. In rhizosphere and spermosphere habitats, common critical resources are shared by both the pathogen and introduced microbial biocontrol strains. They compete with each other for nutrient and/or space. Root and seed exudates are primary source of nutrient for the rhizosphere microorganisms. Rhizosphere competence implies that bacterial antagonists are well adapted to their utilization (Lugtenberg et al. 1999). Germination and hyphal growth of pathogen propagules are stimulated by root and seed exudates (Stanghellini and Burr 1973; Nelson 1990). However, degradation of exudate stimulants by introduced microbial strain limits germination of pathogen propagules and hyphal growth of the pathogen. As a result, chances of the disease development are reduced. For example, biocontrol of Py. ultimum by pseudomonads could be mediated through competition for seed volatiles. Hyphal growth from soilborne sporangia of Py. ultimum is stimulated by volatile compounds such as ethanol and acetaldehyde from the germinating seeds of pea and soybean.
However, this stimulation is reduced when seeds are treated with Ps. putida NIR. NIR uses these volatiles as carbon source and reduce their concentration in the spermosphere through metabolism. Heungens and Parke (2000) reported that B. cepacia AMMDR1 controls Py. aphanidermatum largely through antibiosis, but competition for zoospore-attracting compounds can also contribute to the effect. The bacterium AMMDR1 metabolizes the zoospore attractant. Zoospores of Pythium spp. are also attracted to water soluble sugars, amino acids, and fatty acids exuded by seeds or seedling (van Dijk and Nelson 1998). van Dijk and Nelson (1998) have shown that E. cloacae strain EcCt-501 can utilize seed exudates from a number plant species as a sole carbon source and reduces stimulatory activity of exudate to Py. ultimum sporangia by metabolizing the active stimulatory molecules including linoleic acid present in the exudates. Therefore, this trait is important for biological control of Pythium seed rot by this bacterial antagonist.
Fatty acids from seeds and roots are required to elicit germination responses of P. ultimum (van Dijk and Nelson 2000). Two mutants of E. cloacae EcCT-501R3, Ec31 (fadB) and EcL1 (fadL), reduced in β-oxidation and fatty acid uptake, respectively, fail to metabolize linoleic acid, to inactivate the germination-stimulating activity of cotton seed exudate and linoleic acid, and to suppress Pythium seed rot (van Dijk and Nelson 2000). This suggests that E. cloacae prevents Py. ultimum seed infections by preventing the germination of Py. ultimum sporangia through efficient metabolism of fatty acid components of seed exudate. However, the success of E. cloacae as a biological control organism is directly related to its ability to rapidly interfere with the early responses of Pythium propagules to germinating seeds. E. cloacae fails to suppress Pythium damping-off, if bacterial cells are added after full sporangial activation, but suppresses Py. ultimum seed infections if sporangial activation and germination happens within the first 30–90 min after sowing (Windstam and Nelson 2008a). It has been reported that E. cloacae can reduce the stimulatory activity of cucumber but not corn seed exudates (Kageyama and Nelson 2003). This is due to exudate inactivation by E. cloacae occurs in the cucumber spermosphere but not in the corn spermosphere (Windstam and Nelson 2008a). Other components of the seed exudates such as elevated level of sugars in the corn spermophere prevent degradation of long-chain unsaturated fatty acids by E. cloacae, leading to its failure to suppress Py. ultimum sporangial activation, germination, and subsequent disease development (Windstam and Nelson 2008b).
7.4.5 Production of Siderophores by Biocontrol Bacteria
Siderophores are small, high-affinity iron chelating compounds secreted by grasses and microorganisms such as bacteria and fungi. Iron is an important mineral element essential for growth of all living organisms including microorganisms. The scarcity of bioavailable iron in soil habitats and on plant surfaces leads to a furious competition (Loper and Henkels 1997). Under iron-limiting conditions, some antagonistic bacteria produce low molecular weight compounds called siderophores to competitively acquire ferric ion (Whipps 2001). These metabolites chelate the ferric ion and serve as vehicles for the transport of Fe(III) into bacterial cells. There are various types of bacterial siderophores, which differ in their abilities to sequester iron. However, in general, they deprive pathogenic peronosporomycetes of this essential element since their siderophores have lower affinity (Loper and Henkels 1999; O’Sullivan and O’Gara 1992). Some plant growth promoting microorganisms increase plant growth by supplying the plant with sequestered iron where microbial siderophores are used by plants as a source of iron. In a number of cases, the growth promotion and biocontrol effect of rhizosphere-inhabiting bacteria has been attributed to siderophore-mediated iron acquisition (Kloepper et al. 1980a; Loper 1988). Siderophore production was detected in the isolates of Ba. subtilis that increase shoot and root length of red pepper plants and suppress Phytophthora blight of the plants (Lee et al. 2008).
Becker and Cook (1988) reported that the plant growth-promoting activity of some strains of fluorescent pseudomonads on wheat results from ability of the strains to suppress Pythium by production of siderophores. Siderophore, pyoverdin production has been proved to be important in the biological control of Pythium-induced damping-off of cotton by Ps. fluorescens 3551(Loper 1988) and in the inhibition of Ph. parasitica by P. fluorescens and P. putida in vitro (Yang et al. 1994). Ps. aeruginosa 7NSK2 improves the growth of several crops (Höfte et al. 1991) by producing three siderophores, salicylic acid (Buysens et al. 1996), pyochelin (Höfte et al. 1993), and the fluorescent pyoverdin (Höfte et al. 1993) under iron limiting condition. Production of either pyochelin or pyoverdin by 7NSK2 is necessary to achieve high levels of protection against Py. splendens-induced postemergence damping-off in tomato (Buysens et al. 1996). The action of pyoverdin and pyochelin seems to be interchangeable because a mutant producing only pyochelin or pyoverdin is equally antagonistic and with both mutants, wild-type levels of protection are obtained. Mycelial growth (Loper 1988) but not sporangial germination (Paulitz and Loper 1991) of Pythium spp. is inhibited by iron starvation. Streptomyces species are known for the production of hydroxamate type siderophores, which inhibit phytopathogen growth by competing for iron in rhizosphere soils (Khamna et al. 2009). Suppression of Ph. medicaginis as well as enhancement of plant growth by actinobacterial strains is attributed not only to their antibiotic production, but also to the ability to produce siderophores (Misk and Franco 2011).
7.4.6 Induced Systemic Resistance
Some biocontrol bacteria elicit a phenomenon that is known as induced systemic resistance (ISR) in the host plant. ISR of plant against pathogen is a widespread phenomenon that has been intensively investigated with respect to the underlying signaling pathways as well as to its potential use in plant protection (Heil and Bostock 2002). Elicited by a local infection or colonization of nonpathogenic bacteria, plants respond with a salicylic‐dependent signaling cascade that leads to the systemic expression of a broad spectrum and long‐lasting disease resistance that is efficient against peronosporomycetes, fungi, bacteria, and viruses. Changes in cell wall composition, de novo production of pathogenesis‐related proteins such as chitinases and glucanases, and synthesis of phytoalexins are associated with resistance (Kloepper and Tuzun 1996; Van Loon et al. 1998).
Bacterial agents mediated ISR against peronosporomycete pathogens such as Ph. infestans, Ph. capsici, Py. aphanidermatum, Pl. halstedii, Sclerospora graminicola, H. parasitica, and Peronospora tabacina have been demonstrated in many plant species including tomato, sunflower, squash, chili, pearl millet, apple seedling, tobacco, and Arabidopsis (Umesha et al. 1998; Ongena et al. 1999, 2005; Yan et al. 2002; Zhang et al. 2001, 2010; Van der Ent et al. 2008; Mazzola et al. 2007; Muthukumar et al. 2011). Zhang et al. (2010) reported that some strains of Bacillus spp. are effective in inducing ISR against Ph. capsici on squash, and improved disease control can be achieved by multiplexing them.
Two strains of plant growth-promoting rhizobacteria, Ba. pumilus SE34 and Ps. fluorescens 89B61, elicited systemic protection against late blight on tomato (Yan et al. 2002). Induced resistance by SE34 and 89B61 is associated with reduction in disease severity and germination of sporangia and zoospore on the leaf surface. Although physical separation between tested bacteria and target pathogens are necessary for ISR to be elicited, strain SE34 is detected in the leaves, suggesting the involvement of additional mechanism beside ISR during SE34-mediated protection (Yan et al. 2002). Localized stimulation of one part of a plant can result in the systemic expression of resistance in other parts; therefore, it has been hypothesized that a signal is generated and mobilized from the initial infection site (Dean and Kuc 1986).
Salicylic acid (SA), jasmonic acid (JA), and ethylene apparently are involved in signaling pathways. Both Ba. pumilus SE34 and Ps. fluorescens 89B61 elicit ISR in a JA-dependent manner. From a molecular point of view, the onset of rhizobacteria-mediated ISR has not generally been associated with major changes in gene expression. The nonpathogenic rhizobacterial strain Ps. fluorescens WCS417r has been shown to trigger ISR against H. parasitica in Arabidopsis (Ton et al. 2002). However, root inoculation with Ps. fluorescens WCS417r does not lead to an accumulation in the roots or in the leaves of the SA-responsive genes PR-1, PR-2, and PR-5, of the ET-inducible gene Hel, of the ET- and JA- responsive genes ChiB and Pdf1.2, or of the JA-inducible genes Atvsp, Lox1, Lox2, Pal1, and Pin2. A change could only be observed in the potentiation of the expression of JA-dependent Atvsp after pathogen challenge of ISR-expressing plants (van Wees et al. 1999). Higher level of antimicrobial phenolics were accumulated in cucumber plants treated with Ps. fluorescens BTP1 and M3 and challenged with Py. aphanidermatum (Ongena et al. 1999). Similarly, potentiated activities of phenylalanine ammonia lyase (PAL), peroxidase (PO), polyphenol oxidase (PPO), and phenolics were observed in Ps. fluorescens Pf1 pretreated tomato and hot pepper plants challenged with Py. aphanidermatum (Ramamoorthy et al. 2002). Combined application of talc-based formulation of Ps. fluorescens EBL 20-PF and Trichoderma viridae and challenge inoculated with Py. aphanidermatum recorded maximum induction of PO, PPO, PAL, β-1,3-glucanase, and the accumulation of phenolics in chili plants (Muthukumar et al. 2011).
Further studies using H. parasitica have shown that WCS417r-mediated ISR is elicited through JA-, ET-, and NPR1-dependent defenses (Ton et al. 2002). T-DNA knockout mutants myb72-1 and myb72-2 are incapable of mounting ISR against the pathogen H. parasitica, indicating that MYB72 is essential to establish ISR against this pathogen (van der Ent et al. 2008). MYB72 is an ethylene-inducible R2R3-MYB-like transcription factor gene and is specifically activated in the roots upon colonization by WCS417r (Verhagen et al. 2004). Root inoculation of Arabidopsis thaliana ecotype Columbia with Ps. fluorescens CHA0r also partially protect leaves from the H. parasitica where production of DAPG is thought to be the determinant of Ps. fluorescens CHA0r for this ISR (Iavicoli et al. 2003). Although DAPG is known for its antibiotic property, in this experiment DAPG produced by bacteria leads to physiological changes that subsequently induce ISR. Another study on pea plants indicates that DAPG can act also as a plant hormone-like substance, inducing physiological and morphological changes that enhance nodulation by Rhizobium. ISR induced by CHA0r depends on NPR1 and JA signaling pathways, indicating that ISR induced by strains WCS417r and CHA0r against H. parasitica differ with respect to the sensitivity to ET.
Bacteria-mediated ISR against peronosporomycete phytopathogen is also associated with changes in plant defense-related enzymes (Ongena et al. 2005; Muthukumar et al. 2011). Disease protection by Ba. subtilis M4 against Py. aphanidermatum in tomato is associated with significant changes in gene transcription in the host plant (Ongena et al. 2005). These enzymes are known to have antifungal properties and thought to play an important role in plant defense by restricting the growth and development of pathogens (Boller 1992). Although several hundred articles on ISR have been published, however, many questions are still unanswered and require further investigation. Strong efforts are required to identify the compounds causing resistance, and future studies should quantify these compounds in combination with the biologically detectable resistance to characterize the induced stage (Heil and Bostock 2002).
7.4.7 Hyperparasitism
Parasitism is a symbiosis in which two phylogenetically unrelated organisms coexist over a prolonged period of time. An avirulent pathogen could be hyperparasite on more virulent pathogens. The activities of various hyperparasites that parasitize virulent plant pathogens can lead to biocontrol. Several bacterial antagonists have been reported to be hyperparasites on several peronosporomycetes. Actinoplanes spp. are filamentous bacteria that produce minute sporangia, which when hydrated release motile spores capable of parasitizing Pythium spp. or related microorganisms. Khan et al. (1993) reported that oospores of Py. aphanidermatum, Py. arrhenomanes, Py. irregulare, Py. myriotylum, and Py. ultimum were parasitized by Ac. azureus, Ac. brasiliensis, Ac. caeruleus, Ac. ferrugineus, Ac. ianthinogenes, Ac. italicus, Ac. minutisporangius, Ac. rectilineatus, Ac. teichomyceticus, Ac. utahensis, Ac. violaceous, Ac. yunnahenis, plus 15 strains of unspeciated Actinoplanes. Parasitized oospores had disorganized cytoplasms and hyphae of Actinoplanes sp. emerging from them. Filonow and Dole (1999) showed that strains of Actinoplanes spp. that are hyperparasites of oospores of Pythium spp. reduce root rot severity and increase plant stand of poinsettia and geranium.
Ac. missouriensis and Pseudomonas spp. infect oospores of Ph. megasperma var. sojae, Ph. cactorum, Pythium sp., and Ap. euteiches in natural soil, reducing populations of oospores in soil. Tu (1978) reported that Rh. japonicum reduced Phytophthora root rot of soybean by parasitizing hyphae of the peronosporomycete. The bacteria colonize growing hyphal tips and prevent contact between Ph. megasperma and host root tissue, and thereby reduce the chance of Ph. megasperma infection. Rhizobium reduces fungal sporulation and causes extensive surface and internal colonization of mycelia of Ph.megasperma and Py. ultirnum (Tu 1978).
7.4.8 Plant and Hyphal Colonization
Antagonistic bacteria are thought to protect against root pathogens more effectively if they have a strong ability to colonize the root system (Weller 1988). Islam et al. (2005a, b) investigated plant colonization behavior of a Lysobacter sp. SB-K88 by the aid of scanning electron microscopy (SEM). SB-K88 colonizes both the root and leaf surfaces in a characteristic perpendicular fashion. In colonized regions, a semitransparent film apparently enveloping the root and microcolonies was observed on the sugar beet root surface (Islam et al. 2005a). That Lysobacter strain also efficiently colonized the roots of several plants, including spinach, tomato, A. thaliana, and Amaranthus gangeticus. Interestingly, the SB-K88 also colonized Ap. cochlioides hyphal surface in the same perpendicular manner when grown together on liquid medium. Detailed transmission electron microscopic analysis revealed that the SB-K88 has long (~6 μm) brush-like, fragile fimbriae at one pole of the dividing bacterial cells. As fimbriae are known to function in bacteria to adhere to the substrates, Islam et al. assumed that brush-like fimbriae help SB-K88 to attach perpendicularly on plant and hyphal cell walls as well as for gliding motility. Presence of fimbriae appears to be characteristic structural features of bacteria having gliding motility (Spormann 1999).
In cotton, control of Pythium seed rot and preemergence damping-off by E. cloacae and E. herbicola strains were correlated with suppression of seed colonization by Pythium spp. (Nelson 1988). Seed pericarps are favorable ecological niches for species of Pseudomonas. Ps. fluorescens-putida ML5 and Ps. putida R20 readily colonize pericarp of sugar beet seed. Occupation of this competitive site in spermosphere accounts for the effectiveness of these strains in reducing the incidence of seed rot and damping-off caused by Py. ultimum (Osburn et al. 1989). Fukui et al. (1994a, b) also demonstrated the importance of high initial pericarp colonization by Ps. fluorescens for antagonism against Py. ultimum. The dynamics of bacterial colonization of plant tissues vary among bacteria as influenced by various environmental factors (Loper et al. 1985). For Ps. fluorescens B5, the total population size per plant and downward colonization of the root (below 40 mm depth) increased significantly with increasing its inoculum density applied to the seeds, while for Ps. corrugata 2140, no significant influence of initial inoculum density on root colonization was observed (Schmidt et al. 2004a). Soil matrix potential and temperature had pronounced influence on seed or root colonization and biological control of Pythium spp. by pseudomonads (Mathre et al. 1994; Schmidt et al. 2004b). Population density of Ps. fluorescens B5 per seedling as well as downward colonization and biocontrol performance by B5 were significantly reduced at high temperatures (25–35 °C), while Ps. aureofaciens AB254 (Mathre et al. 1994) showed good biological control against Py. ultimum at temperatures above 22 °C. This strain may therefore complement Ps. fluorescens B5 in a combined inoculum, although compatibility would have to be confirmed.
In addition to the colonization of plant roots, the colonization of hyphae could be an important mechanism for maintaining a close association between antagonistic bacteria and peronosporomycetal pathogens. Biological control of Pythium seed rot and preemergence damping-off of cotton by E. cloacae and Erwinia herbicola have been attributed to the ability of bacteria to attach to the hyphae and to inhibit the growth of Py. ultimum (Nelson 1988). Ps. putida 06909 grew extensively on hyphae of Ph. parasitica that cause citrus root rot and inhibited, but did not kill, the pathogen in vitro (Yang et al. 1994). Hyphal colonization-deficient Tn5 mutants of P. fluorescens and P. putida were nonflagellated and were defective in colonization and inhibition of colonies of Ph. parasitica in vitro, confirming the linkage between the loss of flagella and the loss of the ability to colonize and inhibit peronosporomycetal mycelia. The loss of flagella in Tn5 mutants of P. fluorescens has also been associated with an inability to colonize potato roots (de Weger et al. 1987). In another study, nonflagellated Tn5 mutants of P. fluorescens were defective in adhesion to sand, suggesting that the flagella, or other outer membrane proteins associated with flagella, were involved in adhesion to surfaces (Deflaun et al. 1990). Islam et al. (2005b) demonstrated that a biocontrol strain Lysobacter sp. SB-K88 equally colonize both plant roots and hypahe of damping-off pathogen Aphanomyces cochlioides in perpendicular fashion. The SB-K88 does not show penetration of hyphal cells, however, disrupts ultrastructure and cytoskeleton of the Ap. cochlioides cells by secreting macrocyclic lactam antibiotics.
7.5 Conclusion and Perspectives
The peronosporomycetes have remarkable biological features including β-1,3-glucan polymers and cellulose made cell wall, energy storage carbohydrate mycolaminarin, diploidy at the vegetative stage, motile zoospores, and sexual cycle, which make them ubiquitous inhabitants in the diverse environment. Application of bacterial antagonists for the management of peronosporomycetal diseases of various plants is strongly connected to our understanding of bacterial diversity, host specificity, mechanism of action, active ingredient identification, formulation, and application. Information on potential biocontrol agents, their in vitro antagonistic activity, detection and isolation techniques of antipathogenic compounds, mode of action, in vivo disease suppressive activity, and practical formulation will facilitate to more efficient application methods of inoculant strains and strategy to reduce disease caused by peronosporomycete phytopathogens. A wide diversity of bacterial strains have been reported with antagonistic activity and some have shown high promise in vivo biocontrol effect on economically important peronosporomycete plant diseases through different mechanisms including antibiosis, high plant colonization, induced systematic resistance, and lytic enzyme production. This wealth of information has provided a firm foundation for broader incorporation of these bacterial antagonists into sustainable strategies for the management of peronosporomycete phytopathogens.
Although knowledge on ecological impact of biocontrol agents as alternative means of chemical-based plant disease management has been enhanced in the past decades, however, the greatest challenge facing BCAs is to release them for practical use. BCAs are needed to be transformed from niche market products into mainstream crop protection agents. More technical developments are desired to recognize the high-throughput production process of quality biocontrol products and to identify factors that affect efficacy and shelf life of these living biopesticides. Aside from the fundamental research for biocontrol agent identification, application, and efficacy improvement, continued effort should be taken to establish close linkage between researchers and entrepreneurs to facilitate technology promotion and acceptance by end users. Recent advances on whole genome sequences of several peronosporomycetes and biocontrol bacteria will provide the future basis for a comprehensive understanding of BCA–plant–pathogen interactions and development of improved strains with customized properties that will potentially function as effective biocontrol agents in low input sustainable agriculture.
References
Abad ZG, Abad JA, Coffey MD, Oudemans PV, et al. (2008) Phytophthora bisheria sp. nov., a new species identified in isolates from the Rosaceous raspberry, rose and strawberry in three continents. Mycoligia 100:99–110
Abdalla MA, Win HY, Islam MT, von Tiedemann A, Schüffler A, Laatsch H (2011) Khatmiamycin, a motility inhibitor and zoosporicide against the grapevine downy mildew pathogen Plasmopara viticola from Streptomyces sp. ANK313. J Antibiot. 64:655–659
Agrios GN (1997) Plant pathology. Academic, San Diego, CA
Akgül DS, Mirik M (2008) Biocontrol of Phytophthora capsici on pepper plants by Bacillus Megnaterium strains. J Plant Pathol 90(Suppl 1):29–34
Andersen JB, Koch B, Nielsen TH, Sørensen D, Hansen M, Nybroe O, Christophersen C, Sørensen J, Molin S, Givskov M (2003) Surface motility in Pseudomonas sp DSS73 is required for efficient biological containment of the root-pathogenic microfungi Rhizoctonia solani and Pythium ultimum. Microbiology 149:37–46
Anjaiah V, Koedam N, Nowak-Thompson B, Loper JE, Höfte M, Tambong JT, Cornelis P (1998) Involvement of phenazines and anthranilate in the antagonism of Pseudomonas aeruginosa PNA1 towards Fusarium spp. and Pythium spp. Mol Plant Microbe Interact 11:847–854
Bainton NJ, Lynch JM, Naseby D, Way JA (2004) Survival and ecological fitness of Pseudomonas fluorescens genetically engineered with dual biocontrol mechanisms. Microbiol Ecol 48:349–57
Becker JO, Cook RJ (1988) Role of siderophores in suppression of Pythium species and the production of increased-growth response of wheat by fluorescent pseudomonads. Phytopathology 78:778–782
Berger F, Li H, White D, Frazer R, Leifert C (1996) Effect of pathogen inoculum, antagonist density, and plant species on biological control of Phytophthora and Pythium damping-off by Bacillus subtilis CotI in high-humidity fogging glasshouses. Phytopathology 86:428–433
Boller T (1992) Antimicrobial functions of the plant hydrolases, chitinase and β-1,3-glucanase. In: Fritig B, Legrand M (eds) Mechanisms of plant defense responses. Kluwer Academic, Dordrecht, pp 391–400
Bonner DP, O’Sullivan J, Tanaka SK, Clark JM, Whitney RR (1988) Lysobactin, a novel antibacterial agent produced by Lysobacter sp. II. Biological properties. J Antibiot (Tokyo) 41:1745–1751
Borriss R (2011) Use of plant-associated Bacillus strains as biofertilizers and biocontrol agents in agriculture. In: Maheshwari DK (ed) Bacteria in agrobiology: plant growth responses. Springer, Berlin, pp 41–76
Bowers JH, Parke JL (1993) Epidemiology of Pythium damping-off and Aphanomyces root rot of peas after seed treatment with bacterial agents for biological control. Phytopathology 83:466–1473
Budi SW, van Tuinen D, Martinotti G, Gianinazzi S (1999) Isolation from the sorghum bicolor mycorrhizosphere of a bacterium compatible with arbuscular mycorrhiza development and antagonistic towards soilborne fungal pathogens. Appl Environ Microbiol 65:5148–5150
Burr TJ, Schroth MN, Suslow T (1978) Increased potato yields by treatment of seed pieces with specific strains of Pseudomonas fluorescens and P. putida. Phytopathology 68:1377–1383
Buysens S, Heungens K, Poppe J, Hofte M (1996) Involvement of pyochelin and pyoverdin in suppression of Pythium induced damping-off of tomato by Pseudomonas aeruginosa 7NSK2. Appl Environ Microbiol 62:865–71
Callan NW, Mathre DE, Miller JB (1990) Bio-priming seed treatment for biological control of Pythium ultimum preemergence damping-off in sh2 sweet corn. Plant Dis 74:368–372
Carruthers FL, Shum-Thomas T, Conner AJ, Mahanty HK (1995) The significance of antibiotic production by Pseudomonas aureofaciens PA 147–2 for biological control of Phytophthora megasperma root rot of asparagus. Plant Soil 170:339–344
Chatterton S, Sutton JC, Boland GJ (2004) Timing Pseudomonas chlororaphis applications to control Pythium aphanidermatum, Pythium dissotocum, and root rot in hydroponic peppers. Biol Control 30:360–373
Chung S, Kong H, Buyer JS, Lakshman DK, Lydon J, Kim S-D, Roberts D (2008) Isolation and partial characterization of Bacillus subtilis ME488 for suppression of soilborne pathogen of cucumber and pepper. Appl Microbiol Biotechnol 80:115–123
Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Cook RJ (1993) Making greater use of introduced microorganisms for biological control of plant pathogens. Annu Rev Phytopathol 31:53–80
Crawford DL, Lynch JM, Whipps JM, Ousley MA (1993) Isolation and characterization of actinomycete antagonists of a fungal root pathogen. Appl Environ Microbiol 59:3899–3905
de Bruijn I, de Kock MJD, Yang M, de Waard P, van Beek TA, Raaijmakers JM (2007) Genome-based discovery, structure prediction and functional analysis of cyclic lipopeptide antibiotics in Pseudomonas species. Mol Microbiol 63:417–428
de Souza JT, Arnould C, Deulvot C, Lemanceau P, Gianinazzi-Pearson V, Raaijmakers JM (2003a) Effect of 2, 4-diacetylphloroglucinol on Pythium: cellular responses and variation in sensitivity among propagules and species. Phytopathology 93:966–975
de Souza JT, de Boer M, de Waard P, van Beek TA, Raaijmakers JM (2003b) Biochemical, genetic and zoosporicidal properties of cyclic lipopeptide surfactants produced by Pseudomonas fluorescens. Appl Environ Microbiol 69:7161–7172
de Weger LA, van der Vlugt CIM, Wljfjes AHM, Bakker PAHM, Schlppers B, Lugtenberg B (1987) Flagella of a plant-growth-stimulating Pseudomonas fluorescens strain are required for colonization of potato roots. J Bacteriol 169:2769–2773
Dean RA, Kuc J (1986) Induced systemic protection in cucumber: the source of the “signal”. Physiol Mol Plant Pathol 28:227–236
DeFlaun MF, Tanzer AS, McAteer AL, Marshall B, Levy SB (1990) Development of an adhesion assay and characterization of an adhesion-deficient mutant of Pseudomonas fluorescens. Appl Environ Microbiol 56:112–119
Delany IR, Walsh UF, Ross I, Fenton AM, Corkery DM, Gara FO (2001) Enhancing the biocontrol efficacy of Pseudomonas fluorescens F113 by altering the regulation and production of 2,4-diacetylphloroglucinol. Plant Soil 232:195–205
Deora A, Hashidoko Y, Islam MT, Tahara S (2005) Antagonistic rhizoplane bacteria induce diverse morphological alterations in Peronosporomycete hyphae during in vitro interaction. Eur J Plant Pathol 112:311–322
Deora A, Hashidoko Y, Islam MT, Aoyama Y, Ito T, Tahara S (2006) An antagonistic rhizoplane bacterium Pseudomonas sp. strain EC-S101 physiologically stresses a spinach root rot pathogen Aphanomyces cochlioides. J Gen Plant Pathol 72:57–74
Deora A, Hatano E, Tahara S, Hashidoko Y (2010) Inhibitory effects of furanone metabolites of a rhizobacterium, Pseudomonas jessenii, on phytopathogenic Aphanomyces cochlioides and Pythium aphanidermatum. Plant Pathol 59:84–99
Dick MW (2001) The Peronosporomycetes. In: McLaughlin DJ, McLaughlin EG, Lemke PA (eds) Systematics and evolution, Part A, vol VII. Springer, Berlin, pp 39–72
Dunne C, Crowley JJ, Moënne-Loccoz Y, Dowling DN, de Bruijn FJ, O’Gara F (1997) Biological control of Pythium ultimum by Stenotrophomonas maltophilia W81 is mediated by an extracellular proteolytic activity. Microbiology 143:3921–3931
Dunne C, Moenne-Loccoz Y, de Bruijn FJ, O’Gara F (2000) Overproduction of an inducible extracellular serine protease improves biological control of Pythium ultimum by Stenotrophomonas maltophilia strain W81. Microbiology 146:2069–2078
El-Tarabily KA, Hardy GEStJ, Sivasithamparam K (2010) Performance of three endophytic actinomycetes in relation to plant growth promotion and biological control of Pythium aphanidermatum, a pathogen of cucumber under commercial field production conditions in the United Arab Emirates. Eur J Plant Pathol 128:527–539
Everts KL, Armentrout DK (2001) Evaluation of the effectiveness of bio-fungicides, ground cover and host resistance in reducing diseases of pumpkin. Biol Cult Tests 17:V18
Fenton AM, Stephens PM, Crowley J, O’Callaghan M, O’Gara F (1992) Exploitation of genes involved in 2, 4-diacetylphloroglucinol biosynthesis to confer a new biocontrol capability to a Pseudomonas strain. Appl Environ Microbiol 58:3873–3878
Filonow BA, Dole MJ (1999) Biological control of Pythium damping-off and root rot of greenhouse grown geranium and poinsettias. Proc Okla Acad Sci 79:29–32
Folman LB, Postma J, Van Veen JA (2003) Characterization of Lysobacter enzymogenes (Chtistensen and Cook 1978) strain 3.1 T8, a powerful antagonist of fungal diseases of cucumber. Microbiol Res 158:107–115
Folman LB, De Klein MJEM, Postma J, van Veen JA (2004) Production of antifungal compounds by Lysobacter enzymogenes isolate 3.1 T8 under different conditions in relation to its efficacy as a biocontrol agent of Pythium aphanidermatum in cucumber. Biol Control 31:145–154
Fridlender M, Inbar J, Chet I (1993) Biological control of soilborne plant pathogens by a β-1, 3 glucanase-producing Pseudomonas cepacia. Soil Biol Biochem 25:1121–1221
Fukui R, Campbell GS, Cook J (1994a) Factors influencing the incidence of embryo infection by Pythium spp. during germination of wheat seeds in soils. Phytopathology 84:695–702
Fukui R, Poinar EI, Bauer PH, Schroth MN, Hendson M, Wang X-L, Hancock JG (1994b) Spatial colonization patterns and interactions of bacteria on inoculated sugar beet seed. Phytopathology 84:1338–1345
Furuya S, Mochizuki M, Aoki Y, Kobayashi H, Takayanagi T, Shimizu M, Suzuki S (2011) Isolation and characterization of Bacillus subtilis KS1 for the biocontrol of grapevine fungal diseases. Biocontrol Sci Technol 21:705–720
Georgakopoulos DG, Fiddaman P, Leifert C, Malathrakis NE (2002) Biological control of cucumber and sugar beet damping-off caused by Pythium ultimum with bacterial and fungal antagonists. J Appl Microbiol 92:1078–1086
Giesler LJ, Yuen GY (1998) Evaluation of Stenotrophomonas maltophilia strain C3 for biocontrol of brown patch disease. Crop Prot 17:509–513
Gleeson O, O’Gara F, Morrissey JP (2010) The Pseudomonas fluorescens secondary metabolite 2, 4-diacetylphloroglucinol impairs mitochondrial function in Saccharomyces cerevisae. Antonie van Leeuwenhoek 97:261–273
Gobbin D, Rumbou A, Linde CC, Gessler C (2006) Population genetic structure of Plasmopara viticola after 125 years of colonization in European vineyards. Mol Plant Pathol 7:519–531
Gravel V, Martinez C, Antoun H, Tweddell RJ (2005) Antagonist microorganisms with the ability to control Pythium damping-off tomato seeds in rockwool. BioControl 50:771–786
Gurusiddaiah S, Weller DM, Sarkar A, Cook RJ (1986) Characterization of an antibiotic produced by a strain of Pseudomonas fluorescens inhibitory to Gaeumannomyces graminis var. tritici and Pythium spp. Antimicrob Agent Chemother 29:488–495
Haas D, Defago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3:307–319
Hadar Y, Harman GE, Taylor AG, Norton JM (1983) Effects of pregermination of pea and cucumber seeds and seed treatment with Entorobacter cloacae on rots caused by Pythium spp. Phytopathology 73:13322–13325
Hagedorn C, Gould WD, Bradinelli RT (1989) Rhizobacteria of cotton and their repression of seedling disease pathogens. Appl Environ Microbiol 55:2793–2797
Handelsman J, Raffel SJ, Mester EH, Wunderlich L, Grau CR (1990) Biological control of damping-off of alfalfa seedlings with Bacillus cereus UW85. Appl Environ Microbiol 56:713–718
Hashidoko Y, Nakayama T, Homma Y, Tahara S (1999) Structure elucidation of xanthobaccin A, a new antibiotic produced from Stenotrophomonas sp. strain SB-K88. Tetrahedron Lett 40:2957–2960
Hashizume H, Igarashi M, Hattori S, Hori M, Hamada M, Takeuchi T (2001) Tripropeptins, novel group antibiotic produced by Lysobacter sp. BMK333-48 F3. J Antibiot (Tokyo) 57:394–399
Hashizume H, Hattori S, Igarashi M, Akamatsu Y (2004) Tripropeptin E, a new tripropeptin antimicrobial agents produced by Lysobacter sp. I. Taxonomy, isolation and biological activities. J Antibiot (Tokyo) 54:1054–1059
Hatano E, Hashidoko Y, Deora A, Fukushi Y, Tahara S (2007) Isolation and structure elucidation of peronosporomycetes hyphal branching-inducing factors produced by Pseudomonas jessenii EC-S101. Biosci Biotechnol Biochem 71:1601–1605
Haverkort AJ, Boonekamp PM, Hutten R, Jacobsen E, Lotz LAP, Visser RGF, Kessel GT, van der Vossen EAG (2008) Societal costs of late blight in potato and prospects of durable resistance through cisgenic modification. Potato Res 51:47–57
Heil M, Bostock RM (2002) Induced systemic resistance (ISR) against pathogens in the context of induced plant defenses. Ann Bot 89:502–512
Heungens KK, Parke JL (2000) Zoospore homing and infection events: effects of the biocontrol bacterium Burkholderia cepacia AMMDR1 on two oomycete pathogens of pea. Appl Environ Microbiol 66:5192–5200
Heungens KK, Parke JL (2001) Post-infection biological control of oomycete pathogens of pea by Burkholderia cepacia AMMDR1. Phytopathology 91:383–391
Höfte M, Seong KY, Jurkevitch E, Verstraete W (1991) Pyoverdin production by the plant growth beneficial Pseudomonas strain 7NSK2: ecological significance in soil. Plant Soil 130:249–258
Höfte M, Buysens S, Koedam N, Cornelis P (1993) Zinc affects siderophore-mediated high affinity iron uptake systems in the rhizosphere Pseudomonas aeruginosa 7NSK2. Biometals 6:85–9
Hong T-Y, Meng M (2003) Biochemical characterization and antifungal activity of an endo-1, 3-β-glucanase of Paenibacillus sp. isolated from garden soil. Appl Microbiol Biotechnol 61:472–478
Howell CR, Stipanovic RD (1980) Suppression of Pythium ultimum-induced damping-off of cotton seedlings by Pseudomonas fluorescens and its antibiotic, pyoluteorin. Phytopathology 70:712–715
Howie WJ, Suslow TV (1991) Role of antibiotic biosynthesis in the inhibition of Pythium ultimum in the cotton spermosphere and rhizosphere by Pseudomonas fluorescens. Mol Plant Microbe Interact 4:393–399
Iavicoli A, Boutet E, Buchala A, Métraux JP (2003) Induced systemic resistance in Arabidopsis thaliana in response to root inoculation with Pseudomonas fluorescens CHA0. Mol Plant Microbe Interact 16:851–858
Islam MT (2008) Disruption of ultrastructure and cytoskeleton network is involved with biocontrol of damping-off pathogen Aphanomyces cochlioides by Lysobacter sp. SB-K88. Biol Control 46:312–321
Islam MT (2010) Mode of antagonism of a biocontrol bacterium Lysobacter sp. SB-K88 toward a damping-off pathogen Aphanomyces cochlioides. World J Microbiol Biotechnol 26:629–637
Islam MT (2011) Potentials for biological control of plant diseases by Lysobacter spp., with special reference to strain SB-K88. In: Maheshwari DK (ed) Bacteria in agrobiology: plant growth responses. Springer, Berlin, pp 335–363
Islam MT, Fukushi Y (2010) Growth inhibition and excessive branching in Aphanomyces cochlioides induced by 2,4-diacetylphloroglucinol is linked to disruption of filamentous actin cytoskeleton in the hyphae. World J Microbiol Biotechnol 26:1163–1170
Islam MT, von Tiedemann A (2011) 2,4-Diacetylphloroglucinol suppresses zoosporogenesis and impairs motility of Peronosporomycete zoospores. World J Microbiol Biotechnol 27:2071–2079
Islam MT, Ito T, Tahara S (2001) Morphological studies on zoospores of Aphanomyces cochlioides and changes during the interaction with host materials. J Gen Plant Pathol 67:255
Islam MT, Hashidoko Y, Ito T, Tahara S (2002) Microscopic studies on the attachment and differentiation of zoospores of the phytopathogenic fungus Aphanomyces cochlioides. J Gen Plant Pathol 68:111–117
Islam MT, Hashidoko Y, Deora A, Ito T, Tahara S (2004) Interactions between rhizoplane bacteria and a phytopathogenic Peronosporomycete Aphanomyces cochlioides in relation to the suppression of damping-off disease in sugar beet and spinach. IOBC/WPRS Bull 27:255–260
Islam MT, Hashidoko Y, Deora A, Ito T, Tahara S (2005a) Suppression of damping-off disease in host plants by the rhizoplane bacterium Lysobacter sp. strain SB-K88 is linked to plant colonization and antibiosis against soilborne Peronosporomycetes. Appl Environ Microbiol 71:3776–3786
Islam MT, Hashidoko Y, Deora A, Ito T, Tahara S (2005b) Lysobacter–Aphanomyces interactions: an ecological role for biocontrol of damping-off disease. Nippon Nogei Kagakkai Taikai KoenYoshishu 2005:221
Islam MT, von Tiedemann A, Laatsch H (2011) Protein kinase C is likely to be involved in zoosporogenesis and maintenance of flagellar motility in the Peronosporomycete zoospores. Mol Plant Microbe Interact 24:938–947
Jacobi M, Winkelmann G, Kaiser D, Kempter C, Jung G, Berg G, Bahl H (1996) Maltophilin: a new antifungal compound produced by Stenotrophomonas maltophilia R3089. J Antibiot 11:1101–1104
Jayaraj J, Radhakrishnan NV (2008) Enhanced activity of introduced biocontrol agents in solarized soils and its implications on the integrated control of tomato damping-off caused by Pythium spp. Plant Soil 304:189–197
Ji GH, Wei LF, He YQ, Wu YP, Bai XH (2008) Biological control of rice bacterial blight by Lysobacter antibioticus strain 13-1. Biol Control 45:288–296
Joo G-J (2005) Production of an anti-fungal substance for biological control of Phytophthora capsici causing phytophthora blight in red-peppers by Streptomyces halstedii. Biotechnol Lett 27:201–205
Kageyama K, Nelson EB (2003) Differential inactivation of seed exudate stimulation of Pythium ultimum sporangium germination by Enterobacter cloacae influences biological control efficacy on different plant species. Appl Environ Microbiol 69:1114–1120
Keel C, Schnider U, Maurhofer M, Voisard C, Burger D, Haas D, Défago G (1992) Suppression of root diseases by Pseudomonas fluorescens CHA0: importance of the bacterial secondary metabolite 2, 4-diacetylphloroglucinol. Mol Plant Microbe Interact 5:4–13
Khamna S, Yokota A, Lumyong S (2009) Actinomycetes isolated from medicinal plant rhizosphere soils: diversity and screening of antifungal compounds, indole-3-acetic acid and siderophore production. World J Microbiol Biotechnol 25:649–655
Khan NI, Filonow AB, Singleton LL, Payton ME (1993) Parasitism of oospores of Pythium spp. by strains of Actinoplanes spp. Can J Microbiol 39:964–972
Khan NI, Filonow AB, Singleton LL (1997) Augmentation of soil with sporangia of Actinoplanes spp. for biological control of Pythium damping-off. Biocontrol Sci Technol 7:11–22
Khan A, Sutton JC, Grodzinski B (2003) Effects of Pseudomonas chlororaphis on Pythium aphanidermatum and root rot in peppers grown in small-scale hydroponic troughs. Biocontrol Sci Technol 13:615–630
Kim BS, Lee JY, Hwang BK (2000) In vivo control and in vitro antifungal activity of rhamnolipid B, a glycolipid antibiotic, against Phytophthora capsici and Colletotrichum orbiculare. Pest Manag Sci 56:1029–1035
Kim YC, Jung H, Kim KY, Park SK (2008) An effective biocontrol bioformulation against Phytophthora blight of pepper using growth mixtures of combined chitinolytic bacteria under different field conditions. Eur J Plant Pathol 120:373–382
King EB, Parke JL (1993) Biocontrol of Aphanomyces root rot and Pythium damping-off by Pseudomonas cepacia strain AMMD on four pea cultivars. Plant Dis 77:1185–1188
Kloepper JW, Tuzun S (1996) Induced systemic to diseases and increased plant growth by growth-promoting rhizobacteria under field conditions. Phytopathology 81:1508–1516
Kloepper JW, Leong J, Teintze M, Schroth MN (1980a) Pseudomonas siderophores: a mechanism explaining disease-suppressive soils. Curr Microbiol 4:317–320
Kloepper JW, Schroth MN, Miller TD (1980b) Effects of rhizosphere colonization by plant growth-promoting rhizobacteria on potato plant development and yield. Phytopathology 70:1078–1082
Ko HS, Jin RD, Krishnan HB, Lee SB, Kim KY (2009) Biocontrol ability of Lysobacter antibioticus HS124 against Phytophthora blight is mediated by the production of 4-hydroxyphenylacetic acid and several lytic enzymes. Curr Microbiol 59:608–615
Kobayashi DY, Reedy RM, Palumbo JD, Zhou JM, Yuen GY (2005) A clp gene homologue belonging to the Crp gene family globally regulates lytic enzyme production, antimicrobial activity, and biological control activity expressed by Lysobacter enzymogenes strain C3. Appl Environ Microbiol 71:261–269
Koch B, Nielsen TH, Sørensen D, Andersen JB, Christophersen C, Molin S, Givskov M, Sørensen J, Nybroe O (2002) Lipopeptide production in Pseudomonas sp. strain DSS73 is regulated by components of sugar beet seed exudates via the Gac two-component regulatory system. Appl Environ Microbiol 68:4509–4516
Kraus J, Loper JE (1992) Lack of evidence for a role of antifungal metabolite production by Pseudomonas fluorescens Pf-5 in biological control of Pythium damping-off of cucumber. Phytopathology 82:264–71
Leah RH, Tommerup S, Svendsen I, Murphy J (1991) Biochemical molecular characterization of three barley seed proteins with antifungal properties. J Biol Chem 266:1564–1573
Leclère V, Bechet M, Adam A, Guez JS, Wathelet B, Ongena M, Thonart P, Gancel F, Chollet-Imbert M, Jacques P (2005) Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Appl Environ Microbiol 71:4577–4584
Lee HB, Kim Y, Kim JC, Choi GJ, Park S-H, Kim C-J, Jung HS (2005) Activity of some aminoglycoside antibiotics against true fungi, Phytophthora and Pythium species. J Appl Microbiol 99:836–843
Lee KJ, Kamala-Kannan S, Sub HS, Seong CK, Lee GW (2008) Biological control of Phytophthora blight in red pepper (Capsicum annuum L.) using Bacillus subtilis. World J Microbiol Biotechnol 24:1139–1145
Leenders F, Stein TH, Kablitz B, Franke P, Vater J (1999) Rapid typing of Bacillus subtilis strains by their secondary metabolites using matrix assisted laser-desorption/ionization mass spectrometry of intact cells. Rapid Commun Mass Spectrom 13:943–949
Lim HS, Kim YS, Kim SD (1991) Pseudomonas stutzeri YPL-1 genetic transformation and antifungal mechanism against Fusarium solani, an agent of plant root rot. Appl Environ Microbiol 57:510–516
Liu CH, Chen X, Liu TT, Lian B, Gu YC, Caer V, Xue YR, Wang BT (2007a) Study of the antifungal activity of Acinetobacter baumannii LCH001 in vitro and identification of its antifungal components. Appl Microbiol Biotechnol 76:459–466
Liu W, Sutton JC, Grodzinski B, Kloepper JW, Reddy MS (2007b) Biological control of Pythium root rot of Chrysanthemum in small-scale hydroponic units. Phytoparasitica 35:159–17
Loper JE (1988) Role of fluorescent siderophore production in biological control of Pythium ultimum by a Pseudomonas fluorescens strain. Phytopathology 78:166–172
Loper JE, Henkels MD (1997) Availability of iron to Pseudomonas fluorescens in rhizosphere and bulk soil evaluated with an ice nucleation reporter gene. Appl Environ Microbiol 63:99–105
Loper JE, Henkels MD (1999) Utilization of heterologous siderophores enhances levels of iron available to Pseudomonas putida in the rhizosphere. Appl Environ Microbiol 65:5357–5363
Loper JE, Haack C, Schroth MN (1985) Population dynamics of soil Pseudomonads in the rhizosphere of potato (Solanum tuberosum L). Appl Environ Microbiol 49:416–422
Lugtenberg BJ, Kravchenko LV, Simons M (1999) Tomato seed and root exudate sugars: composition, utilization by Pseudomonas biocontrol strains, and role in rhizosphere colonization. Environ Microbiol 1:439–446
Mao W, Lumsden RD, Lewis JA, Hebbar PK (1998) Seed treatment using pre-infiltration and biocontrol agents to reduce damping-off of corn caused by species of Pythium and Fusarium. Plant Dis 82:294–299
Mao S, Lee SJ, Hwangbo H, Kim YW, Park KH, Cha GS, Park RD, Kim KY (2006) Isolation and characterization of antifungal substances from Burkholderia sp. culture broth. Curr Microbiol 53:358–364
Margulis L, Schwartz KV (2000) Five kingdoms: an illustrated guide to the phyla of life on earth. W.H. Freeman, New York
Martin MO (2002) Predatory prokaryotes: an emerging research opportunity. J Mol Microbiol Biotechnol 4:467–477
Mathre DE, Callan NW, Johnston RH, Miller JB, Schwend A (1994) Factors influencing the control of Pythium ultimum-induced seed decay by seed treatment with Pseudomonas aureofaciens AB254. Crop Prot 13:301–307
Maurhofer M, Keel C, Haas D, Defago G (1994) Pyoluteorin production by Pseudomonas fluorescens strain CHA0 is involved in the suppression of Pythium damping-off of cress but not of cucumber. Eur J Plant Pathol 100:221–232
Maurhofer M, Keel C, Haas D, Défago G (1995) Influence of plant species on disease suppression by Pseudomonas fluorescens CHA0 with enhanced antibiotic production. Plant Pathol 44:44–50
Mazzola M, Zhao X, Cohen MF, Raaijmakers JM (2007) Cyclic lipopeptide surfactant production by Pseudomonas fluorescens SS101 is not required for suppression of complex Pythium spp. populations. Phytopathology 97:1348–1355
McSpadden Gardener BB, Fravel DR (2002) Biological control of plant pathogens: research, commercialization, and application in the USA. Plant Health Prog. doi:10.1094/PHP-2002-0510-01-RV
Meyer JB, Lutz MP, Frapolli M, Péchy-Tarr M, Rochat L, Keel C, Défago G, Maurhofer M (2010) Interplay between wheat cultivars, biocontrol pseudomonads, and soil. Appl Environ Microbiol 76:6196–6204
Milus EA, Rothrock CS (1997) Efficacy of bacterial seed treatments for controlling Pythium root rot of winter wheat. Plant Dis 81:180–184
Misk A, Franco C (2011) Biocontrol of chickpea root rot using endophytic actinobacteria. BioControl 56:811–822
Muranova TA, Krasovskaya LA, Tsfasman IM, Stepnaya OA, Kulaev IS (2004) Structural investigations and identification of the extracellular bacteriolytic endopeptidase L1 from Lysobacter sp. XL1. Biochemistry (Mosc) 69(5):501–505
Muthukumar A, Eswaran A, Sangeetha G (2011) Induction of systemic resistance by mixtures of fungal and endophytic bacterial isolates against Pythium aphanidermatum. Acta Physiol Plant. doi:10.1007/s11738-011-0742-8
Nakayama T, Homma Y, Hashidoko Y, Mizutani J, Tahara S (1999) Possible role of xanthobaccins produced by Stenotrophomonas sp. strain SB-K88 in suppression of sugar beet damping-off disease. Appl Environ Microbiol 65:4334–4339
Nandeeshkumar P, Ramachandrakini K, Prakash HS, Niranjana SR, Shetty HS (2008) Induction of resistance against downy mildew on sunflower by rhizobacteria. J Plant Interact 3:256–262
National Academy of Sciences (1987) Research briefings 1987: Report of the research briefing panel on biological control in managed ecosystems. National Academy Press, Washington, DC
Nelson EB (1988) Biological control of Pythium seed rot and preemergence damping-off of cotton with Enterobacter cloacae and Erwinia herbicola applied as seed treatments. Plant Dis 72:140–142
Nelson EB (1990) Exudate molecules initiating fungal responses to seeds and roots. Plant Soil 129:61–73
Nelson EB (2004) Microbial dynamics and interactions in the spermosphere. Ann Rev Phytopathol 42:271–309
Nes WD (1987) Biosynthesis and requirement of sterols in the growth and reproduction of the oomycetes. In: Fuller G, Nes WD (eds) Ecology and Metabolism of Plant Lipids. American Chemical Society, Symposium Series 325, Washington DC, pp. 304–328
Nielsen MN, Sørensen J, Fels J, Pedersen HC (1998) Secondary metabolite- and endochitinase-dependent antagonism toward plant-pathogenic microfungi of Pseudomonas fluorescens isolates from sugar beet rhizosphere. Appl Environ Microbiol 64:3563–3569
Nielsen TH, Christophersen C, Anthoni U, Sørensen J (1999) Viscosinamid, a new cyclic depsipeptide with surfactant and antifungal properties produced by Pseudomonas fluorescens DR54. J Appl Microbiol 86:80–90
Nielsen TH, Sørensen D, Tobiasen C, Andersen JB, Christophersen C, Givskov M, Sørensen J (2002) Antibiotic and biosurfactant properties of cyclic lipopeptides produced by fluorescent Pseudomonas spp. from the sugar beet rhizosphere. Appl Environ Microbiol 68:3416–3423
O’Sullivan DJ, O’Gara F (1992) Traits of fluorescent Pseudomonas spp. involved in suppression of plant root pathogens. Microbiol Rev 56:662–676
Ogura J, Toyoda A, Kurosawa T, Chong AL, Chohnan S, Masaki T (2006) Purification, characterization, and gene analysis of cellulase (Cel8A) from Lysobacter sp. IB-9374. Biosci Biotechnol Biochem 70:2420–2428
Ongena M, Daay F, Jacques P, Thonart P, Benhamou N, Paulitz TC, Cornelis P, Koedam NM, Bélanger RR (1999) Protection of cucumber against Pythium root rot by fluorescent pseudomonads: Predominant role of induced resistance over siderophores and antibiosis. Plant Pathol 48:66–76
Ongena M, Duby F, Jourdan E, Beaudry T, Jadin V, Dommes J, Thonart P (2005) Bacillus subtilis M4 decreases plant susceptibility towards fungal pathogens by increasing host resistance associated with differential gene expression. Appl Microbiol Biotechnol 67:692–698
Osburn RM, Schroth MN, Hancock JG, Hendson M (1989) Dynamics of sugar beet colonization by Pythium ultimum and Pseudomonas species: effects on seed rot and damping-off. Phytopathology 79:709–716
Osburn RM, Milner JL, Oplinger ES, Smith RS, Handelsman J (1995) Effect of Bacillus cereus UW85 on the yield of soybean at two field sites in Wisconsin. Plant Dis 79:551–556
Palumbo JD, Sullivan RF, Kobayashi DY (2003) Molecular characterization and expression in Escherichia coli of three β-1,3-glucanase genes from Lysobacter enzymogenes Strain N4-7. J Bacteriol 185:4362–4370
Palumbo JD, Yuen GY, Jochum CC, Tatum K, Kobayashi DY (2005) Mutagenesis of beta-1, 3-glucanase genes in Lysobacter enzymogenes strain C3 results in reduced biological control activity toward Bipolaris leaf spot of tall fescue and Pythium damping-off of sugar beet. Phytopathology 95:701–707
Pang Y, Liu X, Ma Y, Chernin L, Berg G, Gao K (2009) Induction of systemic resistance, root colonization and biocontrol activities of the rhizospheric strain of Serratia plymuthica are dependent on N-acyl homoserine lactones. Eur J Plant Pathol 124:261–268
Parke JL, Rand RE, Joy AE, King EB (1991) Biological control of Pythium damping-off and Aphanomyces root rot of peas by application of Pseudomonas cepacia or Ps. fluorescens to seed. Plant Dis 75:987–992
Paulitz TC, Loper JE (1991) Lack of a role for fluorescent siderophore production in the biological control of Pythium damping off of cucumber by a strain of Pseudomonas putida. Phytopathology 81:930–935
Perneel M, D’Hondt L, De Maeyer K, Adiobo A, Rabaey K, Hofte M (2008) Phenazines and biosurfactants interact in the biological control of soil-borne diseases caused by Pythium spp. Environ Microbiol 10:778–788
Postma J, Stvens LH, Wiegors GL, Davelaar E, Nijhuis EH (2009) Biological control of Pythium aphanidermatum in cucumber with a combined application of Lysobacter engymogenes strain 3.1T8 and chitosan. Biol Control 48:301–309
Raj SN, Deepak SA, Basavaraju P, Shetty HS, Reddy MS, Kloepper JW (2003) Comparative performance of formulations of plant growth promoting rhizobacteria in growth promotion and suppression of downy mildew in pearl millet. Crop Prot 22:579–588
Ramamoorthy V, Raguchander T, Samiyappan R (2002) Enhancing resistance of tomato and hot pepper to Pythium diseases by seed treatment with fluorescent Pseudomonads. Eur J Plant Pathol 108:429–441
Riazanova LP, Ledova LA, Tsurikova NV, Stepnaia OA, Sinitsyn AP, Kulaev IS (2005) Effect of the proteolytic enzymes of Bacillus licheniformis and the lysoamidase of Lysobacter sp. XL1 on Proteus vulgaris and Proteus mirabilis. Prikl Biokhim Mikrobiol 41(5):558–563
Rotem R, Paz GF, Homonnai ZT, Kalina M, Naor Z (1990) Protein kinase C is present in human sperm: possible role in flagellar motility. Proc Natl Acad Sci USA 87:7305–7308
Schisler DA, Slininger PJ, Miller JS, Woodell LK, Clayson S, Olsen N (2009) Bacterial antagonists, zoospore inoculum retention time and potato cultivar influence pink rot disease development. Am J Potato Res 86:102–111
Schmidt CS, Agostini F, Leifert C, Killham K, Mullins CE (2004a) Influence of inoculum density of the antagonistic bacteria Pseudomonas fluorescens and Pseudomonas corrugata on sugar beet seedling colonisation and suppression of Pythium damping off. Plant Soil 265:111–122
Schmidt CS, Agostini F, Leifert C, Killham K, Mullins CE (2004b) Influence of soil temperature and matric potential on sugar beet seedling colonization and suppression of Pythium damping-off by the antagonistic bacteria Pseudomonas fluorescens and Bacillus subtilis. Phytopathology 94:351–363
Schmidt CS, Agostini F, Simon A-M, Whyte J, Leifert JTC, Killham K, Mullins C (2004c) Influence of soil type and pH on the colonisation of sugar beet seedlings by antagonistic Pseudomonas and Bacillus strains, and on their control of Pythium damping-off. Eur J Plant Pathol 110:1025–1046
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Shanahan P, O’Sullivan DJ, Simpson P, Glennon JD, O’Gara F (1992) Isolation of 2,4-diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Appl Environ Microbiol 58:353–358
Shetty SA, Shetty HS, Mathur SB (1995) Downy mildew of pearl millet. Technical Bulletin, Downy Mildew Research Laboratory, Department of Studies in Applied Botany, University of Mysore, Manasagangotri, Mysore, India
Sid Ahmed A, Ezziyyani M, Egea-Gilabert C, Candela ME (2003a) Selecting bacterial strains for use in the biocontrol of diseases caused by Phytophthora capsici and Alternaria alternata in sweet pepper plants. Biol Plant 47:569–574
Sid Ahmed A, Ezziyyani M, Pérez-Sanchez C, Candela ME (2003b) Effect of chitin on biological control activity of Bacillus spp. and Trichoderma harzianum against root rot disease in pepper (Capsicum annuum) plants. Eur J Plant Pathol 109:418–426
Siddiqui MA, Shaukat SS (2003) Suppression of root-knot disease by Pseudomonas fluorescens CHA0 in tomato: importance of bacterial secondary metabolite, 2, 4-diacetylpholoroglucinol. Soil Biol Biochem 35:1615–1623
Silo-Suh LA, Lethbridge BJ, Raffel SJ, He H, Clardy J, Handelsman J (1994) Biological activities of two fungistatic antibiotics produced by Bacillus cereus UW85. Appl Environ Microbiol 60:2023–2030
Sørensen D, Nielsen TH, Christophersen C, Sørensen J, Gajhede M (2001) Cyclic lipodecapeptide amphisin from Pseudomonas sp. strain Dss73. Acta Crystallogr C Cryst Struct Commun 57:1123–1124
Spormann AM (1999) Gliding motility in bacteria: insights from studies of Myxococcus xanthus. Microbiol Mol Biol Rev 63:621–641
Stanghellini ME, Burr TJ (1973) Germination in vivo of Pythium aphanidermatum in oospores and sporangia. Phytopathology 63:1493–1496
Stanghellini ME, Miller RM (1997) Biosurfactants; their identity and potential efficacy in the biological control of zoosporic plant pathogens. Plant Dis 81:4–12
Stephan D, Schmitt A, Carvalho SM, Seddon B, Koch E (2005) Evaluation of biocontrol preparation and plant extracts for the control of Phytophthora infestans on potato leaves. Eur J Plant Pathol 112:235–246
Stockwell VO, Stack JP (2007) Using Pseudomonas spp. for integrated biological control. Phytopathology 97:244–249
Stutz EW, Defago G, Kern H (1986) Naturally occurring fluorescent pseudomonads involved in suppression of black root of tomato. Phytopathology 80:1050–1053
Tambong JT, Höfte M (2001) Phenazines are involved in biocontrol of Pythium myriotylum on cocoyam by Pseudomonas aeruginosa PNA1. Eur J Plant Pathol 107:511–521
Timmusk S, van West P, Gow NAR, Paul Huffstutler R (2009) Paenibacillus polymyxa antagonizes oomycete plant pathogens Phytophthora palmivora and Pythium aphanidermatum. J Appl Microbiol 106:1473–1481
Ton J, Van Pelt JA, Van Loon LC, Pieterse CMJ (2002) Differential effectiveness of salicylate-dependent and jasmonate/ethylene-dependent induced resistance in Arabidopsis. Mol Plant Microbe Interact 15:27–34
Tran HTT, Ficke A, Asiimwe T, Hofte M, Raaijmakers JM (2007) Role of the cyclic lipopeptide surfactant massetolide A in biological control of Phytophthora infestans and colonization of tomato plants by Pseudomonas fluorescens. New Phytol 175:731–42
Tu JC (1978) Protection of soybean from severe Phytophthora root rot by Rhizobium. Physiol Plant Pathol 12:237–240
Umesha S, Dharmesh SM, Shettyt SA, Krishnappa M, Shetty HS (1998) Biocontrol of downy mildew disease of pearl millet using Pseudomonas fluorescens. Crop Prot 17:387–392
Valois D, Fayad K, Barasubiye T, Gagnon M, Déry C, Brzezinski R, Beaulieu C (1996) Glucanolytic actinomycetes antagonistic to Phytophtora fragariae var. rubi, the causal agent of raspberry root rot. Appl Environ Microbiol 62:1630–1635
van de Mortel JE, Tran H, Govers F, Raaijmakers JM (2009) Cellular responses of the late blight pathogen Phytophthora infestans to cyclic lipopeptide surfactants and their dependence on G proteins. Appl Environ Microbiol 75(15):4950–4957
van der Ent S, Verhagen BWM, Van Doorn R, Bakker D, Verlaan MG, Pel MJC, Joosten RG, Proveniers MCG, Van Loon LC, Ton J, Pieterse CMJ (2008) MYB72 is required in early signaling steps of rhizobacteria-induced systemic resistance in Arabidopsis. Plant Physiol 146:1293–1304
van Dijk K, Nelson EB (1998) Inactivation of seed exudate stimulants of Pythium ultimum sporangium germination by biocontrol strains of Enterobacter cloacae and other seed-associated bacteria. Soil Biol Biochem 30:183–192
van Dijk K, Nelson EB (2000) Fatty acid competition as a mechanism by which Enterobacter cloacae suppresses Pythium ultimum sporangium germination and damping-off. Appl Environ Microbiol 66:5340–5347
van Loon LC, Bakker PAHM, Pieterse CMJ (1998) Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36:453–483
van Wees SCM, Luijendijk M, Smoorenburg I, van Loon LC, Pieterse CMJ (1999) Rhizobacteria-mediated induced systemic resistance (ISR) in Arabidopsis is not associated with a direct effect on expression of known defense-related genes but stimulates the expression of the jasmonate-inducible gene Atvsp upon challenge. Plant Mol Biol 41:537–549
Verhagen BWM, Glazebrook J, Zhu T, Chang H-S, Van Loon LC, Pieterse CMJ (2004) The transcriptome of rhizobacteria-induced systemic resistance in Arabidopsis. Mol Plant Microbe Interact 17:895–908
Wakelin SA, Walter M, Jaspers M, Stewart A (2002) Biological control of Aphanomyces euteiches root-rot of pea with spore-forming bacteria. Australas Plant Pathol 31:401–407
Wang H, Hwang SF, Chang KF, Turnbull GD, Howard RJ (2003) Suppression of important pea diseases by bacterial antagonists. BioControl 48:447–460
Weller DM (1988) Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu Rev Phytopathol 26:379–407
Weller DM (2007) Pseudomonas biocontrol agents of soilborne pathogens: looking back over 30 years. Phytopathology 97:250–256
Whipps JM (2001) Microbial interactions and biocontrol in the rhizosphere. J Exp Bot 52:487–511
Whipps JM, Lumsden DR (1991) Biological control of Pythium species. Biocontrol Sci Technol 1:75–90
White D, de Lamirande E, Gagnon C (2007) Protein kinase C is an important signaling mediator associated with motility of intact sea urchin spermatozoa. J Exp Biol 210:4053–4064
Williams GE, Asher MJC (1996) Selection of rhizobacteria for the control of Pythium ultimum and Aphanomyces cochlioides on sugarbeet seedlings. Crop Prot 15:479–48
Windstam S, Nelson EB (2008a) Differential interference with Pythium ultimum sporangium activation and germination by Enterobacter cloacae in the corn and cucumber spermospheres. Appl Environ Microbiol 74:4285–4291
Windstam S, Nelson EB (2008b) Temporal release of fatty acids and sugars in the spermosphere: impacts on Enterobacter cloacae-induced biological control. Appl Environ Microbiol 74:4292–4299
Woo J-H, Kitamura E, Myouga H, Kamei Y (2002) An antifungal protein from the marine bacterium Streptomyces sp. strain AP77 is specific for Pythium porphyrae, a causative agent of red rot disease in Porphyra spp. Appl Environ Microbiol 68:2666–2675
Xiao K, Samac DA, Kinkel LL (2002) Biological control of Phytophthora root rots on alfalfa and soybean with Streptomyces. Biol Control 23:285–295
Yan Z, Reddy MS, Ryu C-M, McInroy JA, Wilson M, Kloepper JW (2002) Induced systemic protection against tomato late blight elicited by plant growth-promoting rhizobacteria. Phytopathology 92:1329–1333
Yang C-H, Menge JA, Cooksey DA (1994) Mutations affecting hyphal colonization and pyoverdine production in Pseudomonads antagonistic toward Phytophthora parasitica. Appl Environ Microbiol 60:473–481
Yu F, Zaleta-Rivera K, Zhu X, Huffman J, Millet JC, Harris SD, Yuen G, Li XC, Du L (2007) Structure and biosynthesis of heat-stable antifungal factor (HSAF), a broad-spectrum antimycotic with a novel mode of action. Antimicrob Agents Chemother 51:64–72
Zhang Z, Yuen GY, Sarath G, Penheiter A (2001) Chitinases from the plant disease biocontrol agent, Stenotrophomonas maltophilia C3. Phytopathology 91:204–211
Zhang S, White LW, Martinez MC, McInroy JA, Kloepper JW, Klessen W (2010) Evaluation of plant growth-promoting rhizobacteria for control of Phytophthora blight on squash under greenhouse conditions. Biol Control 53:129–135
Zinada DS, Shaabana KA, Abdalla MA, Islam MT, Schüffler A, Laatsch H (2011) Bioactive isocoumarins from a terrestrial Streptomyces sp. ANK302. Nat Prod Commun 6:45–48
Acknowledgements
The first author (MTI) is thankful to Prof. Satoshi Tahara and Prof. Yasuyuki Hashidoko of Hokkaido University, Japan, for their supports during his postdoc research under a JSPS fellowship. The Ministry of Science and Information and Communication Technology of the Government of Peoples Republic of Bangladesh (project Nos. BS-77 and ES-12) also deserve special thanks for partial financial supports.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Islam, M.T., Hossain, M.M. (2013). Biological Control of Peronosporomycete Phytopathogen by Bacterial Antagonist. In: Maheshwari, D. (eds) Bacteria in Agrobiology: Disease Management. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-33639-3_7
Download citation
DOI: https://doi.org/10.1007/978-3-642-33639-3_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-33638-6
Online ISBN: 978-3-642-33639-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)