Abstract
Phytophthora blight of pepper caused by Phytophthora capsici has devastating consequences when combined with other pathogens, including Rhizoctonia solani, Fusarium oxysporum, and Fusarium solani. In order to develop a field-effective biocontrol strategy against Phytophthora blight of pepper, three chitinolytic bacteria, Serratia plymuthica strain C-1, strongly antagonistic to P. capsici, Chromobacterium sp. strain C-61, strongly antagonistic to R. solani, and Lysobacter enzymogenes strain C-3, antagonistic to R. solani and Fusarium spp., were selected. In pot studies, application of cultures combining the three bacterial strains effectively suppressed Phytophthora blight more than application of any single bacterial strain. Bioformulations developed from growth of the strains in a simple medium containing chitin under large batch conditions resulted in effective control in field applications. Efficacy of the bioformulated product depended on both the dose and timing of application. Although the undiluted product suppressed Phytophthora blight under all field conditions, a 10-fold diluted product was effective in solar-sterilized greenhouses and in fields with crop rotation. These results suggest that the developed product could be a new effective system to control Phytophthora blight disease in pepper.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytophthora blight of pepper, caused by Phytophthora capsici, is one of the most devastating soil-borne diseases in Korea (Kim 1993). This pathogen attacks the roots, stems, leaves, and fruit of pepper, but the most severe yield loss is by wilting and death of foliage due to rot of root and stem around the soil line (Park and Kim 1989). Various methods that utilize fungicides, antagonists, cultural practices and resistant cultivars have been evaluated in attempts to develop effective control strategies against Phytophthora blight (Hwang and Kim 1995). Several actinobacteria, other bacteria and fungi were selected as biocontrol agents because they inhibit the growth of P. capsici (Ahmad et al. 1999; Ahn and Hwang 1992; Rajkumar et al. 2005; Shen et al. 2002). The efficacy of biocontrol agents against Phytophthora blight of pepper was enhanced by the addition of organic materials to the soil (Nam et al. 1988), use of the agents with other materials (Lee et al. 1999) and the method of application (Park and Kim 1989).
Although most studies have focused strictly on the suppression of P. capsici growth, it has been found that Phytophthora blight of pepper was more severe when plants were also challenged with Rhizoctonia solani, Fusarium oxysporum, and Fusarium solani (Park and Kim 1991). Therefore, a more effective biocontrol strategy against Phytophthora blight of pepper may require suppression of the total pathogen complex. Additionally control may be more effective when a combination of biocontrol agents is used (Boer et al. 1999; Domenech et al. 2006; Raupach and Kloepper 1998) along with cultural or physical practices (Fravel 2005) such as timing of application (Carisse and Rolland 2004).
Biocontrol may involve the use of cultures that possess antifungal compounds such as lytic chitinases and antibiotics. For example, Lysobacter enzymogenes, formerly classified as Stenotrophomonas maltophilia (Sullivan et al. 2003), secretes lytic enzymes such as chitinase, β-1, 3-glucanase, protease, and lipase (Dunne et al. 1997; Zhang and Yuen 2000a, b), as well as an antifungal antibiotic (Jakobi and Winkelmann 1996). Serratia plymuthica also synthesizes chitinases (Frankowski et al. 2001) and antibiotics (Shoji et al. 1989). Our previous work showed that Chromobacterium sp. strain C-61 produced antifungal chitinases involved in the control of R. solani (Park et al. 1995).
Even though pot studies reveal several good candidates for biocontrol of Phytophthora blight, there are limited reports of effective biocontrol in the field. Chitin and its derivatives can be used as a nutrient to grow selective biocontrol agents in a cost-effective way. Chitin supplies are abundant in nature and include crab shells (Wang et al. 1999). The addition of chitin to soil accelerated growth of chitinolytic bacteria (Chae et al. 2006; Hallmann et al. 1999; Manjula and Podile 2001). Culture suspensions from chitin-amended medium have better control efficacy because of their strong production of lytic enzymes and antibiotics (Chae et al. 2006; Zhang and Yuen 2000a). Therefore, we have studied chitin-degrading bacteria that displayed strong antifungal activities against the pathogens contributing to the Phytophthora blight complex on pepper.
Our goal was to develop a formulated product based on a cost-effective growth medium containing chitin as a substrate for effective biocontrol. We selected a combination of three chitinolytic bacteria with strong inhibitory activity against P. capsici as well as R. solani, F. oxysporum, and F. solani. The formulated bacterial culture was tested to determine the timing of application and dosage that were sufficient for the suppression of Phytophthora blight of pepper. Efficacy was studied in fields with rotating and continuous cropping of pepper as well as in greenhouse conditions.
Materials and methods
Plant pathogens and antagonistic bacteria
Phytophthora capsici, R. solani, F. oxysporum and F. solani were previously isolated from roots of wilted pepper (Park and Kim 1991). These pathogens were grown on potato dextrose agar (PDA, Difco, Ditroit, MI) at 28°C. The chitinolytic Chromobacterium strain C-61 was isolated previously from soil in Korea (Park et al. 2005). Other chitinolytic bacteria were isolated from soils of different origin through the plating of diluted suspensions of soil samples on synthetic medium containing colloidal chitin (Sneh 1981). The chitinolytic bacterial strains were detected as those that produced clear zones on the medium containing colloidal chitin. These isolated bacteria were transferred to the edges of PDA plates, and agar disks (0.5 cm diam) bearing mycelium of each pathogen were placed in the centre of the plate. Those bacterial cultures that antagonized growth of all three fungal pathogens were selected after incubation at 28°C for 4 or 7 days. Their antagonistic activities were evaluated by determining the extent of inhibition of growth of the fungal mycelia. The selected antagonistic bacteria were grown on nutrient agar (NA, Difco, Detroit, MI) or nutrient broth (NB, Difco, Detroit, MI) at 28°C, and were stored at −70°C in NB containing 20% glycerol. The chitinolytic strains, C-1 and C-3, were selected from these studies. The C-1 isolate was identified as Serratia plymuthica, and the C-3 isolate was identified as Lysobacter enzymogenes from their 16S rRNA gene sequences, as described previously (Park et al. 1995).
Selection of a medium for bioformulation of the selected bacteria
To select a cost-effective medium that would grow the three chitinolytic bacteria but did not support growth of the four Phytophthora blight pathogens, three chitin-based formulations were examined. A minimal medium [3 g (NH4)2SO4, 4 g KH2PO4, 3 g K2HPO4, and 0.2 g MgSO4 7H2O l−1 distilled water], was used without dilution or at 1/5 and 1/25 dilutions; crude chitin (0.2%) was added to each medium. The chitinolytic bacteria, S. plymuthica C-1 and Chromobacterium sp. C-61 were grown in NB for 12 h and L. enzymogenes strain C-3 for 36 h. A 20 μl aliquot of each bacterial culture was transferred to 100 ml of the three chitin media and incubated on a rotary shaker (180 rpm) at 28°C. Bacterial cell growth was determined by dilution plating bacterial suspensions on NA containing appropriate antibiotics: ampicillin, polymyxin B, and vancomycin (each 50 μg ml−1) for C-61; ampicillin, streptomycin, and kanamycin (each 50 μg ml−1) for C-3; and ampicillin, novobiocin, and polymyxin B (each 25 μg ml−1) for C-1. The plates were incubated at 28°C for 4 days before colony-forming units (cfu) were counted.
To determine how the three chitin-based growth media supported the growth of the pathogens in the disease complex, a PDA agar disk (1.0 cm in diam) bearing mycelia of P. capsici, R. solani, F. oxysporum and F. solani was added to 100 ml aliquots of medium. The growth of each plant pathogen at 28°C on a rotary shaker was evaluated for up to 30 days visually by turbidity and by spreading culture suspensions on PDA to confirm growth of the anticipated fungus.
Preparation of bioformulated product on a large scale and quantification of chitinase
Larger-scale growth of the three chitinolytic bacteria was achieved by the transfer of 100 ml of 12–36 h NB culture of each bacterial strain to 500 l of the 1/5 diluted chitin medium. Bacterial cfu in the culture fluids were determined by dilution plating at 5-day intervals during growth at 28 ± 2°C with stirring in a fermenter (Heuksalim, Chungbuk, Korea). The chitinase activity in culture fluids was determined with 4-methylumbelliferyl-d-N, N-diacetylchitobioside [4-MU-(GlcNAc)2] (Sigma, St. Louis, MO) as the substrate. Assays were performed in microplates by the addition of a filter-sterilized culture filtrate (10 μl) to 100 μl 0.1 mM 4-MU-(GlcNAc)2 in 100 mM KH2PO4 buffer (pH 7.0). After incubation for 20 min at 37°C, 0.1 ml of 0.2M Na2CO3 was added and absorbance was measured with excitation at 360 nm and emission at 440 nm on a Bio-Tek FLX-8000 (Biotek, Vermont, USA). One unit of chitinase activity was defined as the activity required to liberate one micromole of 4-methylumbelliferone min−1 ml−1 of culture supernatant (Park et al. 2005).
Biocontrol studies: pot experiments
The biocontrol efficacy of a single strain or combined bacterial strains was assessed in 10 day-old cultures grown in flask with 1/5 diluted chitin medium. The inoculum was added to a growth matrix infested with P. capsici alone or mixtures of the four pathogens. Fifty ml of each bacterial culture was applied by soil-drench and water was applied as a control. To prepare the pathogen inocula, 40 g of oatmeal (The Quaker Oats Co. Chicago, IL, USA) was mixed with 160 g of sandy loam soil (greenhouse soil), and 200 g batches were sterilized in 500 ml flasks by autoclaving at 121°C for 90 min. Fungal mycelium was raised on PDA plates for 7 days, and homogenized in a Waring blender containing 50 ml of sterile water for each plate. This inoculum was transferred to 200 g of the sterilized oatmeal-amended soil in the flask, and then incubated for 15 days at 28°C. The infested matrix was sieved using a 0.25 mm mesh sieve and was mixed with autoclaved sandy loam soil (1 part by weight/99 parts by weight). This infested mixture was transferred to rectangular plastic pots (15 × 6 × 10 cm) and 60 day-old seedlings of pepper (var. Cheondoong) were planted in two rows with five plants per row. As a control, seedlings were transplanted into the autoclaved sandy loam soil. The number of wilted plants was measured daily for 20 days as an assessment of the disease index. Biocontrol values for the treatments were calculated using the following formula; [(% diseased plants in non-inoculated soil – % diseased plants in pathogen-inoculated soil)/(% diseased plants in non-inoculated soil)] × 100. Four experiments were conducted in a greenhouse with three replicates per treatment with 50 plants.
Biocontrol studies: field experiments
The biocontrol efficacy of a product prepared with inocula of the three bacterial strains and grown together for 10 days in 1/5 diluted chitin medium (bioformulated product) was examined in 2006 in two fields: one field had a crop rotation of sweet potato and soybean preceding pepper in the fourth year, whereas the second field had been continuously cultivated with pepper for 3 years. The SinTaeyang pepper variety was used, with plants being transplanted on April 20. Each field was divided, and efficacy of undiluted or 10-fold diluted culture fluids was examined by applications with two different time schedules: on April 20, June 15, and July 25 and on June 15, July 5, and July 25 for both field plots. Water was applied as a control. Experimental plots were arranged in a randomized complete block design (RCBD) with three replicates. Approximately 75 l were applied to each 150 m2 plot using a power-driven sprayer. The peppers were cultivated using ordinary farming practices. Disease incidence of Phytophthora blight was rated on June 15, July 10, and August 5 by counting plants showing wilt symptoms.
In one greenhouse experiment, soil was sterilized by solarization as described by Katan (1981) with addition of nitrolime (100 kg/10 a) for 1 month. The second greenhouse that had been used for continuous pepper cultivation for 3 years had no soil sterilization. Pepper seedlings (var. Cheondoong) were transplanted on May 1. Undiluted and 10-fold diluted products were applied using a power-driven sprayer three times at 20-day intervals from the transplanting date using doses of 50 l for each 100 m2 plot. Water was applied as the control. Experimental plots were arranged in a RCBD with three replicates of each treatment. The peppers were cultivated using standard farming practices without any applications of fungicides and insecticides. Phytophthora blight was investigated at 10-day intervals from June 10 in the continuous-cultivation greenhouse, and 10-day intervals from July 1 in the solarized greenhouse.
Statistical analysis
Data were analyzed by ANOVA by using SPSS 12.OK for Windows software (SPSS Institute, Republic of Korea). The significance of the effects of bacterial treatment was determined by Duncan’s multiple range test (P = 0.05).
Results
Selection of effective chitin-degrading bacteria to inhibit growth of Phytophthora blight pathogens
More than 200 chitin-degrading bacterial strains were isolated from various soils from Jeonnam Providence in the Republic of Korea. Three chitin-degrading rhizobacteria were selected for further study. These isolates were C-1 from a forest soil, C-3 from a pear orchard, and C-61 from eggplant-cultivated soil. The C-61 was previously isolated and identified as chitinolytic Chromobacterium strain C-61 (Park et al. 1995). The 16S rRNA sequence of strain C-3 showed 99.51% identity with L. enzymogenes, and that of strain C-1 showed 99.67% identity with S. plymuthica (data not shown). Strains C-61 and C-3 are deposited in the Korean Agriculture Culture Collection Centre (KACC, Suwon, Republic of Korea) as KACC 91199P and KACC 91200P, respectively.
The C-1 chitin-degrading strain inhibited only P. capsici. Strain C-61 was less effective at inhibiting P. capsici than C-1, but inhibited the growth of R. solani. Strain C-3 weakly inhibited growth of all fungal pathogens including P. capsici, R. solani, F. oxysporum, and F. solani (Fig. 1 and Table 1). Chitinolytic activity, as measured by zones of inhibition on the chitin-amended growth medium, was greatest for Chromobacterium sp. C-61, and strains L. enzymogenes C-3 and S. plymuthica C-1 had similar activities (Fig. 1 and Table 1).
Inhibitory effect of bacteria against soil-borne plant pathogens of pepper on PDA (a) and their chitinolytic ability on chitin-agar plates (b). Chitin-degrading bacteria: Ly, Lysobacter enzymogenes strain C-3; Se, Serratia plymuthica strain C-1; Ch, Chromobacterium sp. strain C-61. Phytophthora blight pathogens: Ph, Phytophthora capsici; Rh, Rhizoctonia solani; Fo, Fusarium oxysporum; Fs, Fusarium solani
Enhancement of biocontrol efficacy by combination of the selected bacterial isolates
The incidence of wilting for pot-grown pepper plants was significantly higher when the growth matrix contained a mixture of four pathogens (95%) than when only P. capsici was present (52%; Table 2). All combinations of bacterial cultures suppressed the wilting symptoms significantly. When applied singly, strain C-1 showed the highest level of control. Efficacy of the culture of strain C-1 was not significantly different than that of the combinations C-1 with C-61 or C-3, or combination of the three strains; C-1, C-61 and C-3, when P. capsici only was challenged. When the four plant pathogens were present together, the best efficacy was from the combination of three strains (Table 2).
Development of a chitin-based growth medium for mass production of biocontrol agents
To select the most effective growth medium for the biocontrol activity of the chitinolytic bacterial strains, each strain was grown individually in three different concentrations of the chitin-based formulation. Growth of all three bacterial strains was faster in the undiluted chitin medium; 1012 cfu bacterial cells ml−1, were observed in undiluted chitin medium three days after bacterial inoculation compared to 108–109 cfu ml−1 in the diluted-chitin medium (Fig. 2). By days 5 and 6 in 1/5 and 1/25 diluted-chitin medium, bacterial cells reached 1012 cfu ml−1 (Fig. 2). Growth of the three bacteria was much lower when chitin in water was provided as the sole nutrient source without addition of minimal salts. All four plant pathogens; R. solani, F. oxysporum, F. solani, and P. capsici, grew on the undiluted chitin medium, but did not grow on the 1/5 and 1/25 diluted-chitin medium (data not shown).
Growth of Serratia plymuthica C-1 (a), Chromobacterium sp. C-61 (b) or Lysobacter enzymogenes C-3 (c) on chitin-based media contained 0.2% chitin. Undiluted (filled diamond), 1/5 diluted (filled square) and 1/25 diluted (open square) chitin media were used for each bacterial inoculum; bacterial cultures were grown in a flask with shaking incubation. This experiment was repeated three times with similar data; data from one study are provided
For mass production of the bioformulated product, three bacterial strains were co-cultivated in a fermenter containing 1/5 diluted-chitin medium. The highest cell densities of the bacterial strains were reached between 10 and 15 days after inoculation; at 10 days after incubation, 7 × 109 cfu ml−1 for C-1, 1 × 1010 cfu ml−1 for C-3, and 9 × 108 cfu ml−1 for strain C-61 (Table 3). Chitinase activities of the bacterial strains reached the maximum level between 10 and 20 days after bacterial inoculation (Table 3).
Biocontrol efficacy of the bioformulated product against Phytophthora blight of pepper in different field conditions
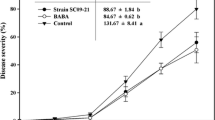
The biocontrol efficacy was evaluated with the batch cultures containing the three bacterial strains in 1/5 diluted-chitin medium (bioformulated product). Disease incidence of Phytophthora blight was earlier and higher in the field under continuous pepper cultivation than in the field with crop rotation (Table 4). Disease incidence of Phytophthora blight was suppressed in both fields by the application of the bioformulated product. In the crop rotation field, applications of both the undiluted and 1/10 diluted bioformulated product were effective. In the continuously cultivated field, only the undiluted bioformulated product showed effective control (Table 4).
Phytophthora blight of pepper also occurred earlier and higher in the greenhouse soils continuously cultivated with pepper than in the greenhouse with solarized soil. In the greenhouse with solarized soils, disease incidence of Phytophthora blight was significantly reduced by applications of both undiluted and 1/10 diluted bioformulated product. Biocontrol effectiveness was dose-dependent (Table 5).
Discussion
In this study, three bacterial strains, S. plymuthica strain C-1, L. enzymogenes strain C-3 and Chromobacterium sp. strain C-61, showed biocontrol activity against the disease complex causing pepper wilt both under laboratory conditions and in field and greenhouse cultivation. Each of the three bacterial strains produced chitinases in culture but showed differential inhibition of the pepper pathogens in in vitro assays. Here, we demonstrated that the biocontrol potential of S. plymuthica strain C-1 was augmented by combination with the two other chitinolytic soil bacteria, strains C-3 and C-61, especially when the plants were challenged with the fungal pathogen complex.
Our findings for S. plymuthica are in agreement with the results of studies with another Serratia isolate in showing suppression of P. capsici symptoms (Shen et al. 2005). Lysobacter strains inhibited a wide range of fungal pathogens: Pythium spp. (Folman et al. 2003), R. solani (Giesler and Yuen 1998; Zhang et al. 2001), Bipolaris sorokiniana (Zhang and Yuen 2000b), and Uromyces appendiculatus (Yuen et al. 2001). Chitinases from Chromobacterium sp. strain C-61 and L. enzymogenes played an important role in the inhibition of R. solani (Park et al. 1995) and B. sorokiniana (Zhang and Yuen 2000b), respectively. Growth of Fusarium spp. strains also was chitinase-sensitive (Ajit et al. 2006; Morales et al. 2006).
Zhang and Yuen (2000a) suggested that the total growth mixture containing cells and culture fluids was practical for biocontrol. We formulated a cost-effective growth medium containing chitin that when diluted fivefold or greater did not support growth of the four pathogens found as a complex in the roots of diseased pepper (Park and Kim 1991), although bacterial growth was maintained.
Although chitinases in the formulated product may play a role in the inhibition of R. solani and Fusarium spp., they are unlikely to be effective against P. capsici, because cell walls of oomycetes consist mainly of cellulose and β-glucans (Ainsworth et al. 1973). The production by strains C-1, C-3, and C-61 of other antifungal substances that could contribute to their effectiveness is now being studied. Another strain of L. enzymogenes produced β-1, 3-glucanase, protease, and lipase (Dunne et al. 1997; Zhang and Yuen 2000a, b), as well as an antibiotic, maltophilin (Jakobi and Winkelmann 1996). Antibiotic production by S. plymuthica also was reported previously (Shoji et al. 1989). We believe that the combination of antifungal factors and chitinase contributed to the suppression of disease observed following the application of the bacterial formulation to the pepper seedlings. We are currently investigating the importance of chitinolysis in disease control by mutational analysis of chitinase production in the bacterial strains.
We anticipate that in field and greenhouse cultivation, application of the bioformulated products that still contain chitin and chitinoligomers would support the growth of indigenous chitinolytic bacteria. Addition of chitin-amended compost in soil (Chae et al. 2006) and chitin-supplemented formulations enhanced population levels of a chitinolytic bacterium in soil (Manjula and Podile 2001).
We demonstrated the efficacy of the bioformulated product under several field conditions. Efficacy was related to the extent to which pathogen inoculum was present based on farming practices. Earlier application at a higher dose would be needed under conditions in which the pathogen population is high. Under managed conditions where pathogen pressure is reduced by crop rotation or solarization (Gamliel and Katan 1991; Rupe et al. 1997), the bioformulated product was demonstrated to be effective at a lower dose, thus, lowering the cost-benefit ratio. We are currently commercializing the developed formulation to benefit the commercial pepper crop.
In conclusion, we developed a field-effective biocontrol strategy against Phytophthora blight of pepper. Growth of a combination of three chitinolytic bacterial strains grown in a chitin-based growth medium provided cost-effective biocontrol for the Phytophthora blight complex under greenhouse and field conditions. The efficacy of the bioformulated product for the control of Phytophthora blight in pepper could be further enhanced by combinations with crop rotation and/or soil sterilization by solarization and timing of application.
References
Ahmad, A. S., Sanchez, C. P., Egea, E., & Candela, M. (1999). Evaluation of Trichoderma harzianum for controlling root rot caused by Phytophthora capsici in pepper plants. Plant Pathology, 48, 58–65.
Ahn, S. J., & Hwang, B. K. (1992). Isolation of antibiotic-producing actinomycetes antagonistic to Phytophthora capsici from pepper-growing soils. Korean Journal of Mycology, 20, 259–268.
Ainsworth, G. C., Sparrow, F. K., & Sussman, A. S. (1973). The fungi, vol. IV (pp. 62–165). New York: Academic.
Ajit, N. S., Verma, R., & Shanmugam, V. (2006). Extracellular chitinases of fluorescent pseudomonads antifungal to Fusarium oxysporum f. sp. dianthi causing carnation wilt. Current Microbiology, 52, 310–316.
Boer, M., Sluis, I., Von Loon, L. C., & Bakker, P. A. H. M. (1999). Combining Pseudomonas fluorescent spp. strains to enhance suppression of Fusarium wilt of radish. European Journal of Plant Pathology, 105, 201–210.
Carisse, O., & Rolland, D. (2004). Effect of timing of application of the biological control agent Microsphaeropsis ochracea on the production and ejection pattern of ascospores by Venturia inaequalis. Phytopathology, 94, 1305–1314.
Chae, D. H., Jin, R. D., Hwangbo, H., Kim, Y. W., Kim, Y. C., Park, R. D., et al. (2006). Control of late blight (Phytophthora capsici) in pepper plant with a compost containing multitude of chitinase-producing bacteria. BioControl, 51, 339–351.
Domenech, J., Reddy, M. S., Kloepper, J. W., Ramos, B., & Gutierrez-Mañero, J. (2006). Combined application of the biological product LS213 with Bacillus, Pseudomonas or Chryseobacterium for growth promotion and biological control of soil-borne diseases in pepper and tomato. BioControl, 51, 245–258.
Dunne, C., Crowley, J. J., Monne-Loccoz, Y., Dowling, D. N., de Bruijn, F. J., & O’Gara, F. (1997). Biological control of Pythium ultimum by Stenotrophomonas maltophilia W81 is mediated by an extracellular proteolytic activity. Microbiology, 143, 3921–3931.
Folman, L. B., Postma, J., & Van Veen, J. A. (2003). Characterization of Lysobacter enzymogenes (Christensen and Cook 1978) strain 3.1T8, a powerful antagonist of fungal diseases of cucumber. Microbiological Research, 158, 1–9.
Frankowski, J., Lorito, M., Scala, F., Schmid, R., Berg, G., & Bahl, H. (2001). Purification and properties of two chitinolytic enzymes of Serratia plymuthica HRO-C48. Archives of Microbiology, 176, 421–426.
Fravel, D. R. (2005). Commercialization and implementation of biocontrol. Annual Review of Phytopathology, 43, 359–377.
Gamliel, A., & Katan, J. (1991). Involvement of fluorescent pseudomonads and other microorganisms in increased growth response of plants in solarized soils. Phytopathology, 81, 494–502.
Giesler, L. J., & Yuen, G. Y. (1998). Evaluation of Stenotrophomonas maltophilia strain C3 for biocontrol of brown patch disease. Crop Protection, 17, 509–513.
Hallmann, J., Rodríguez-Kábana, R., & Kloepper, J. W. (1999). Chitin-mediated changes in bacterial communities of the soil, rhizosphere and within roots of cotton in relation to nematode control. Soil Biology and Biochemistry, 31, 551–560.
Hwang, B. K., & Kim, C. H. (1995). Phytophthora blight of pepper and its control in Korea. Plant Diseases, 79, 221–227.
Jakobi, M., & Winkelmann, G. (1996). Maltophilin: a new antifungal compound produced by Stenotrophomonas maltophilia R3089. The Journal of Antibiotics, 49, 1101–1104.
Katan, J. (1981). Solar heating (solarization) of soil for control of soilborne pests. Annual Reviews of Phytopathology, 19, 211–236.
Kim, C. H. (1993). Current status of fungal and bacterial diseases of hot pepper and their control measures. Journal of Korean Capsicum Research Cooperation, 2, 1–11.
Lee, J. Y., Kim, B. S., Lim, S. W., Lee, B. K., Kim, C. H., & Hwang, B. H. (1999). Field control of Phytophthora blight of pepper plants with antagonistic rhizobacteria and DL-b-Amino-n-Butyric acid. The Plant Pathology Journal, 15, 217–222.
Manjula, K., & Podile, A. R. (2001). Chitin-supplemented formulations improve biocontrol and plant growth promoting efficiency of Bacillus subtilis AF 1. Canadian Journal of Microbiology, 47, 618–625.
Morales de la Vega, L., Barboza-Corona, J. E., Aguilar-Uscanga, M. G., & Ramirez-Lepe, M. (2006). Purification and characterization of an exochitinase from Bacillus thuringiensis subsp. aizawai and its action against phytopathogenic fungi. Canadian Journal of Microbiology, 52, 651–657.
Nam, C. G., Jee, H. J., & Kim, C. H. (1988). Studies on biological control of Phytophthora blight of Red-pepper II. Enhancement of antagonistic activity by soil amendment with organic materials. Korean Journal of Plant Patholology, 4, 313–318.
Park, J. H., & Kim, H. K. (1989). Biological control Phytophthora crown and root rot of greenhouse pepper with Trichoderma harzianum and Enterobacter agglomerans by improved method of application. Korean Journal of Plant Pathology, 5, 1–12.
Park, S. K., & Kim, K. C. (1991). Pathogenicities of pathogens and disease complex associated with wilt of hot-pepper plants cropped in plastic house. Korean Journal of Plant Pathology, 7, 28–36.
Park, S. K., Lee, M. C., & Harman, G. E. (2005). The Biocontrol activity of Chromobacterium sp. strain C-61 against Rhizoctonia solani depends on the productive ability of chitinase. The Plant Pathology Journal, 21, 275–282.
Park, S. K., Lee, H. Y., & Kim, K. C. (1995). Role of chitinase produced by Chromobacterium violaceum in the suppression of Rhizoctonia damping-off. Korean Journal of Plant Pathology, 11, 304–311.
Rajkumar, M., Lee, W. H., & Lee, K. J. (2005). Screening of bacterial antagonists for biological control of Phytophthora blight of pepper. Journal of Basic Microbiology, 45, 55–63.
Raupach, G. S, & Kloepper, J. W. (1998). Mixtures of plant growth-promoting rhizobacteria enhance biological control of soilborne diseases and potential extension to systemic and foliar diseases. Phytopathology, 88, 1158–1164.
Rupe, J. C., Robbins, R. T., & Gbur, Jr., E. E. (1997). Effect of crop rotation on soil population densities of Fusarium solani and Heterodera glycines and on the development of sudden death syndrome of soybean. Crop Protection, 16, 575–580.
Shen, S. S., Choi, O. H., Park, S. H., Kim, C. G., & Park, C. S. (2005). Root colonizing and biocontrol competency of Serratia plymuthica A21-4 against Phytophthora blight of pepper. The Plant Pathology Journal, 21, 64–67.
Shen, S. S., Kim, J. W., & Park, C. S. (2002). Serratia plymuthica strain A21-4: a potential biocontrol agent against Phytophthora blight of pepper. The Plant Patholology Journal, 18, 138–141.
Shoji, J., Hinoo, H., Sakazaki, R., Kato, T., Hattori, T., Matsumoto, K., et al. (1989). Isolation of CB-25-I, an antifungal antibiotic, from Serratia plymuthica. The Journal of Antibiotics, 42, 869–874.
Sneh, B. (1981). Use of Rhizosphere chitinolytic bacteria for biological control of Fusarium oxysporum f.sp. dianthi in carnation. Phytopathologische Zeitschrift, 100, 251–256.
Sullivan, R. F., Holtman, M. A., Zylstra, G. J., White, J. F., & Kobayashi, D. Y. (2003) Taxonomic positioning of two biological control agents for plant diseases as Lysobacter enzymogenes based on phylogenetic analysis of 16S rDNA, fatty acid composition and phenotypic characteristics. Journal of Applied Microbiology, 94, 1079–1086.
Wang, S. L., Yieh, T. C., & Shih, I. L. (1999). Production of antifungal compounds by Pseudomonas aeruginosa K-187 using shrimp and crab shell powder as a carbon source. Enzyme and Microbial Technology, 25, 142–148.
Yuen, G. Y., Steadman, J. R., Lindgren, D. T., Schaff, D., & Jochum, C. (2001). Bean rust biological control using bacterial agents. Crop Protection, 20, 395–402.
Zhang, Z., & Yuen, G. Y. (2000a). Effects of culture fluids and preinduction of chitinase production on biocontrol of bipolaris leaf spot by Stenotrophomonas maltophilia C3. Biological Control, 18, 277–286.
Zhang, Z., & Yuen, G. Y. (2000b). The role of chitinase production by Stenotrophomonas maltophilia strain C3 in biological control of Bipolaris sorokiniana. Phytopathology, 90, 384–389.
Zhang, Z., Yuen, G. Y., Sarath, G., & Penheiter, A. R. (2001). Chitinases from the plant disease biocontrol agent, Stenotrophomonas maltophilia C3. Phytopathology, 91, 204–211.
Acknowledgements
This work was supported by a grant from the Technology Development Programme for Agriculture and Forestry, Ministry of Agriculture and Forestry, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, Y.C., Jung, H., Kim, K.Y. et al. An effective biocontrol bioformulation against Phytophthora blight of pepper using growth mixtures of combined chitinolytic bacteria under different field conditions. Eur J Plant Pathol 120, 373–382 (2008). https://doi.org/10.1007/s10658-007-9227-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-007-9227-4