Abstract
The radiobiological characteristics of the different dose components of BNCT exposure are examined. Dose-rate determines the biological effectiveness of γ-rays, due to the capacity of cells to repair DNA damage from this low-LET radiation. Photon dose-rate has been largely overlooked in the application of BNCT. Recoil protons vary in their relative biological effectiveness (RBE) as a function of neutron energy and tissue endpoint. Thus the energy spectrum of a beam will influence the RBE of this component of the dose. Protons, of the energy produced by nitrogen capture, have not been studied. In practice protons from nitrogen capture have been combined with the recoil proton contribution into a total neutron dose. The relative biological effectiveness of the products of the boron capture reaction are a composite of the RBE of the short range products and the biodistribution of the boron, referred to collectively as the compound biological effectiveness (CBE) factor. The caution needed in the application of these factors for different normal tissues and tumors is discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Fast Neutron
- Boron Concentration
- Boron Neutron Capture Therapy
- Relative Biological Effectiveness
- Boron Compound
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The first treatments of patients with glioblastoma, in the early 1950s on the Medical Research Reactor at Brookhaven National Laboratory, were carried out using a thermal neutron beam and borax as the boron carrier [22]. Those treatments were undertaken after extremely limited preclinical animal studies. Published are scant details of experiments outlining the uptake of borax in transplanted, methylcholanthrene induced brain tumors in mice, relative to plasma and normal brain [22]. Comparable data were also published for individual patients [42] along with attempts to compute radiation energies and to estimate effects in normal and neoplastic brain tissue. However, the understanding of the increased biological effectiveness of particle irradiation relative to x- or γ-rays was in its infancy at this time. Although never published, a small study was carried out on dogs (Calvo, personal communication 1996) to assess the safety of the proposed clinical irradiation protocol. Four animals were exposed to thermal neutrons after the administration of borax; they remained fit and well after 48 h, and thus, the treatment was judged to be safe.
While such an approach might appear strange in the present era, it should be recognized that none of the present concepts underlining the radiobiological basis of radiotherapy were established at that time and then even conventional radiotherapy had developed largely on the basis of anecdotal evidence from observations on patients exposed to x-rays. Thus, the early attempts at boron neutron capture therapy (BNCT) were understandably inadequate in terms of the ability to estimate and predict responses from such a complex, mixed field, of irradiation. The use of an inadequate boron compound and a poorly penetrating thermal neutron beam were additional drawbacks of those earlier studies.
A greater understanding of the increased biological effectiveness of particle radiation, relative to x- or γ-rays, has come from studies related to the application of fast neutron radiotherapy [23]. However, yet again, the initial clinical attempts at fast neutron therapy were compromised by a lack of radiobiological knowledge. The early investigators were well aware that fast neutrons were biologically more effective than x-rays and animal experiments were done to determine the relative biological effectiveness (RBE), the ratio of absorbed doses from the novel experimental radiation and x-rays required to produce the same biological effect. However, patients were still seriously overdosed in the initial patient studies [78]. Later, it became evident that this was because the initial neutron experiments were carried out using large single doses and that the RBE values obtained were applied to patients treated with fractionated irradiation doses. It was not recognized at that time that the increase in RBE with decreasing dose per fraction is a relatively large effect [25].
While relevant and extensive preclinical studies are now often the norm and should indeed be mandatory, the problems of the past should serve as a pointer to the future use of BNCT, particularly for new applications. Failure to take full account of the current understanding of radiobiological principles or the application of inappropriate radiobiological weighting factors to new applications could result in clinical under- or overdosage of patients and significantly delay the full clinical application of this potentially useful radiotherapy modality.
2 Basic Radiobiological Considerations
BNCT considered as a mixed field irradiation exposure, the principle components of which include, in addition to the alpha particles and lithium ions resulting from the boron neutron capture reaction:
γ-rays both incident within the neutron beam and those induced by the neutron hydrogen capture reaction:
plus protons, either as recoil protons from fast neutron interactions, largely with hydrogen, or from neutron capture with tissue nitrogen:
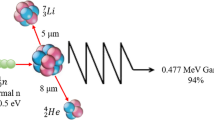
These various absorbed dose components contributing to the total radiation dose are usually assumed to act independently from each other in treatment planning such that the total photon-equivalent dose (D w) is given by the equation: Dw = (absorbed γ-ray dose • DRF) + (absorbed recoil proton dose • RBEn) + (absorbed nitrogen capture dose • RBEN) + (absorbed 10B capture dose • CBE) where DRF is the dose-reduction factor for γ-rays, which varies with dose-rate; RBEn is the relative RBE of fast neutrons; RBEN the equivalent value for protons from the nitrogen capture reaction; and CBE the compound biological effectiveness factor which is a compilation of the RBE of alpha particles and 7Li ions and the microdistribution of 10B in a particular tissue. Due to the short range of these particles in tissue, 9 and 5 μm, respectively, the biological effect of energy deposition depends critically on both the gross and microscopic localization of boron in tissues and cells. For example, the energy from the boron neutron capture reactions occurring within the lumen of a blood vessel may be completely dissipated within the blood volume and in a sense “wasted.”
For this reason, the radiobiological properties of each dose component need to be examined separately, although consideration should also be given to the possibility that there may be an interaction between high linear energy transfer (LET) components of the total dose and the low-LET, γ-rays.
2.1 Radiobiological Properties of γ-Rays
The radiobiological effects of low-LET x- or γ-rays have been extensively investigated since they are the predominant radiation used in radiation therapy. The radiobiological property of most significance for BNCT, where currently single exposures are widely used, is the variation in the biological effectiveness of γ-rays with dose-rate. This dose-rate effect is due to the repair of sublethal radiation damage to DNA with time. For prolonged exposures to low dose-rate γ-rays, the effectiveness is decreased compared to γ-rays delivered at a dose-rate of 1 Gy/min or more. This can be clearly illustrated by examining the effects of different dose-rates on the clonogenic survival of cells in vitro [4]. For a given absorbed radiation dose, the level of cell survival increases as the dose-rate is reduced (Fig. 17.1). In this example, for a clonogenic cell survival of 1%, the DRF for an equivalent effect would be <0.7 for dose-rates of <0.16 Gy/min (DRF: the ratio of doses at high dose-rate to low dose-rate required to produce the same biological effect). The γ-ray dose-rates for the present generation of clinical epithermal neutron beams are in the range 0.16–0.086 Gy/min, clearly indicating that these γ-rays would be less biologically effective than those delivered at approximately 1 Gy/min, that is, a DRF of <1.0. The relative positions of the dose-rates for γ-rays of three epithermal neutron beams that have been used in clinical trials, Helsinki (FiR1), Petten (HFR), and Brookhaven National Laboratory (BMRR), are indicated in this example, and the detailed γ-ray characteristics are given in Table 17.1.
In vitro cell survival curves for Chinese hamster cells after irradiation with 60Co γ-rays at various dose-rates. The dose-reduction factor is the ratio of doses to produce the same effect from different dose-rates, for example, A/B for a dose rate of 0.009 Gy/min. The dose-rates of γ-rays in typical clinical epithermal beams (e.g., FiR1, HFR, BMRR) are in the range 0.16–0.009 Gy/min (Reproduced from Hopewell et al. [39]; with permission)
When BNCT is used for the treatment of glioblastoma, then the dose-limiting normal tissue is the central nervous system (CNS). The radiation response of this tissue, along with many other tissues has been shown to depend on dose-rate and as a consequence the time of exposure. In the case of the spinal cord, a useful model to study for CNS responses, the dose-rate effect is demonstrated by an increase in the dose associated with a 50% incidence of radiation-induced myelopathy (ED50), as the dose-rate is decreased in two species, the rat and the pig (Fig. 17.2). When these ED50 values are normalized, relative to the value for irradiation at the highest dose-rate in this instance of 1.8 Gy/min, there is a linear relationship between dose-rate (log scale) and the DRF. For dose-rates <0.1 Gy/min, comparable to existing epithermal neutron beams, the DRF is <0.7 as is illustrated in the examples in Table 17.1. The dose-rate effect for γ-rays was not taken into account in the initial studies in many centers, including Petten and BMRR. The likely implications of this are discussed below.
Variation in the ED50 for radiation-induced myelopathy in the pig (Δ) and rat (□) as a function of dose-rate. The ED50 values for dose rates of <1.0 Gy/min are expressed as a ratio of the highest dose-rate of 1.8 Gy/min (Dose reduction factor – DRF). The DRF (○) was linearly related to the dose-rate, correlation coefficient 0.996 (Reproduced from Hopewell et al. [39]; with permission)
The effects of dose-rate, based on extensive historical data [21], were considered in recent experimental animal studies to determine weighting factors for normal lung tissue [45], just as they were for studies of CNS toxicity in experiments on dog brain at the FiR1 reactor [6]. When overall exposure times are short (<10 min) as in some biological studies with thermal neutron beams [20, 59, 60], then, and only then, can the effects of repair of sublethal damage be considered sufficiently small to be ignored.
Recently, models have been developed to enable the calculation of equivalent doses based on the kinetics of repair of sublethal irradiation damage for photon irradiation [54]. This allows the calculation of equivalent photon doses for different overall irradiation times, for single exposures, and for fractionated irradiation with incomplete repair intervals. The kinetics of repair of sublethal damage have also been established for CNS tissue following exposure to a wide range of exposure times using irradiation sources of differing dose-rates [75]. This is characterized by a short and a long repair parameter with half-times of 0.19 and 2.16 h, respectively.
2.2 Radiobiological Properties of Fast Neutrons
Studies into the relative biological effectiveness of fast neutrons have been carried out in relation to neutron therapy. Neutron facilities for therapy vary in the energy spectrum of the neutrons produced. The implications of this have been compared using a range of in vitro and in vivo assays. The most extensive set of comparative studies has used the mouse intestinal crypt assay [31]. These studies showed up to a 50% difference in biological effectiveness when other neutron beams were compared to a relatively high average energy reference beam. The higher the mean energy of the neutron beam, the lower the RBE.
In vitro studies, using V79 cells, with relatively monoenergetic neutron sources, have shown a defined relationship between neutron energy and RBE [34]. For this cell line and for damage assessed at the doses required to reduce the surviving fraction to 37% (D37), neutrons with an energy of 0.3–0.4 MeV appeared to be the most biologically effective with an RBE of ∼6.0. As the energy of the neutrons was increased, the RBE value decreased, appearing to reach a minimum value of 1.7 for neutron energies in the range 5–15 MeV (Fig. 17.3). For neutron energies lower than 0.3 MeV, the RBE also declined, being less than 4.0 for 0.1 MeV (100 keV) neutrons. In an unrelated study with 24 keV neutrons from a filtered reactor beam, the trend continued for the same endpoint [58]. The recoil protons from these lower energy neutrons would be expected to have the highest LET.
Variation in the RBE, based on a surviving fraction of 37% (D37) of V79 cells, with neutron energy (Data from Hall et al. [34] (●))
These in vitro studies also showed that the RBE depends on the level of effect at which doses are compared. Based on the linear quadratic (LQ) model of cell survival, there will be an upper limit to the RBE for the different energy neutrons and this will be the ratio of the values of α, the initial slope of the cell survival curves [8] such that:
where α H and α L are the values of alpha for fast neutrons and photons, respectively.
This dependence of the RBE on the level of effect is reflected in the increase in RBE with decreasing dose/fraction in dose fractionation studies [23]. This represents the decreasing level of effect produced by a reduction in the dose/fraction as fraction numbers are increased. RBE values can also be highly dependent on the particular tissue studied by a factor of ∼2 [23]. Thus, the use of a single value for the RBE of a particular radiation quality is most unlikely to be applicable to all tissues.
2.3 Radiobiological Properties of Protons from the Nitrogen Capture Reaction
The protons produced as a consequence of the thermal neutron/nitrogen capture reaction have a low energy of 580 keV and thus have very high-LET characteristics. There are no ways of directly determining the RBE of this energy of protons from a mixed field irradiation involving either thermal or epithermal neutron irradiations, and hence, for practical reasons, the nitrogen capture dose is frequently included with the fast neutron dose, as a combined beam high-LET dose.
Only a limited number of studies have been undertaken using monoenergetic protons at similar energies [5, 72]. Those studies, like those with fast neutrons, were carried out using V79 cells. For protons of decreasing energy (increasing LET) from 7.4 to 1.16 MeV, the RBEmax increased from a little over 1.0 up to a maximum value of ∼7.0. For lower proton energies of 0.84 and 0.73 MeV, the RBEmax declined progressively from the maximum value (Fig. 17.4). In order to be able to compare the RBE values from these studies with those involving different energies of fast neutrons, the D37 was calculated from the LQ cell survival curve parameters reported by the authors. This information is plotted on the same figure for comparison. The D37 RBE values for V79 cells show the same general pattern of change with increase in LET but are lower than the corresponding RBEmax values. These RBE values, based on the D37, appeared to be lower than might have been anticipated from the fast neutron studies (Fig. 17.3). This observation brings into question any assumption that the biological weighting factor used for recoil protons should be the same as that for fast neutrons and for protons from the nitrogen capture reaction, a common assumption in BNCT treatment planning.
2.4 Implications for the Weighting of Dose for Epithermal Neutron Beams
For reactor-based epithermal neutron beams, where there is likely to be major differences in the dose-rate of the γ-ray dose component, and also in the fast neutron spectra of the incident beam, there would appear to be a need to compare their biological effectiveness, as was the case for fast neutron therapy facilities [31]. The short-term crypt colony assay, which proved to be so useful in comparing the biological effectiveness of different fast neutron beams, has been used to examine the RBE of a number of thermal/epithermal neutrons beams. In an initial publication, the RBE of the epithermal neutron beam at the Massachusetts Institute of Technology (MIT) was determined at two depths in a phantom, 2.5 and 9.7 cm [32], and was found to decrease with depth, reflecting the reduced contribution of the high-LET component to the total dose at the greater depth. An attempt was made to estimate the RBE of the high-LET component of the total dose at these two depths. However, for that analysis, the authors used a DRF of 1.0 for the γ-ray dose component, despite the fact that the photon dose-rate at the greater depth in particular was low (∼0.15 Gy/min) compared to that of the photon irradiation used in the control experiments (0.83 Gy/min). In their paper, the authors did express concern that all radiobiological mechanisms had not been taken into account in the analysis. Although not as extensive as for the dose-rate information for the spinal cord and lung, information does exist for the effects of dose-rate on jejunum crypt survival [26]. Those studies suggest that a DRF of ∼0.7 would have been more appropriate to use in the calculations of the high-LET dose component RBE at a depth of 9.7 cm. At a depth 2.5 cm a larger DRF (<1.0) would be applicable. Use of more appropriate weighting factors for the γ-ray dose component would have resulted in a higher estimate of the weighting factor for the high-LET dose component. These details may not be that important because within a given facility, the overall RBE of the beam at a given depth for a specified tissue does not change. The problem arises when these separate weighting factors of an individual beam’s dose components are used to estimate the photon-equivalent dose in a different facility where not only the γ-ray dose-rate may be different but also the fast neutron spectra. This view is supported by a more recent publication [33] where the results from the crypt colony assay are compared for 7 BNCT facilities worldwide.
It has already been mentioned that, although a low DRF of 0.45 (relative to a high dose-rate of 1.8 Gy/min ) has been estimated to be appropriate for the CNS for use with the epithermal neutron beam at the BMRR and elsewhere, a value of 1.0 was actually used (Table 17.1). In a separate analysis [6], the dose-related incidence of a number of different endpoints, after epithermal neutron irradiation of dog brain on the FiR1 beam, were converted into photon-equivalent doses based on the weighting factors derived from studies on dogs and used clinically for the epithermal neutron beam at BMRR, namely 1.0 for low-LET γ-ray component and 3.2 for the high-LET component of the beam [30]. The use of the above weighting factors, adopted for use with the BMRR beam, for the FiR1 beam consistently produced an overestimate of the equivalent photon dose received when compared with the actual data for dogs irradiated with 6 MV x-rays. This is illustrated for the endpoint of single and multiple permanent contrast-enhancing lesions on MRI in Fig. 17.5. The average overestimate of the photon-equivalent dose using the BMRR weighting factors for the FiR1 beam was 12%. These data illustrate the inherent dangers of applying weighting factors derived in one epithermal neutron beam to another neutron beam, no matter how similar the two beams may appear from the point of view of the absorbed dose components.
Dose-related incidence of either (a) single or (b) multiple contrast-enhanced lesions on T1-weighted magnetic resonance images in the brain of dogs after irradiation with either epithermal neutrons (■) from the FiR1 beam or from 6 MeV x-rays (●). The photon-equivalent doses for the different physical epithermal beam doses used in this study were also calculated using the weighting factors developed for the BMRR (□). These weighted doses produced ED50 (± SE) values that were significantly higher than the experimentally observed ED50 values for photon irradiation (Reproduced from Hopewell et al. [39]; with permission)
This view is strongly supported by recent re-evaluation (Millar, personal communication, 2010), where the kinetics of repair of sublethal damage for CNS tissue have been considered in the calculation of equivalent x-ray doses for the different experiments that were carried out to establish weighting factors for the FiR1 and BMRR epithermal neutron beams. For example, for the endpoint of multiple contrast-enhancing lesions in the brain of dogs, the x-ray ED50 for that effect of 15.01 Gy was given in 18.75 min (0.8 Gy/min). The experimentally derived iso-effective dose for the FiR1 beam (∼80% photons) would have been delivered in 158 min. The calculated dose, equivalent to 15.01 Gy of x-rays in 18.75 min, delivered in 158 min, is 19.31 Gy based on repair kinetic parameters. This indicates a DRF of 0.78, higher than the value of 0.6 used in an earlier publication [6]. In the original calculation of the DRF, the actual reference dose-rate of 0.8 Gy/min was not used and, based on the information provided in Fig. 17.2, the dose-rate of the epithermal neutron beam was compared with a reference dose rate of 1.8 Gy/min to give the DRF. Using the revised DRF of 0.78, calculated using the parameters for the kinetics of repair in CNS tissue, where the times for repair of sublethal damage were matched for the beam and x-rays alone, a lower estimate of the RBE of the high-LET component of the FiR1 beam was obtained, 3.3 as compared with the original 3.9 [6].
For the experimental studies on the BMMR, historical data based on the results from CT and not MRI to recognize changes in the brain of Beagle dogs were used [24]. The x-ray doses in this study were given at a higher dose-rate of 3 Gy/min. To calculate weighting factors for the BMRR epithermal beam using the endpoint of lethal necrosis, the ED50 for this effect was 14.8 Gy (4.9 min exposure) of x-rays compared with 9.23 Gy (158 min exposure) for the BMMR epithermal beam. There is also a need for caution in accepting these data since only two dogs were irradiated at this epithermal beam dose and also because Labradors and not Beagle dogs were used. Despite the limitations of the BMMR study, calculations, based on the same repair parameter, the x-ray equivalent dose for 14.8 Gy given in 4.9 min was 20.24 Gy in 158 min, DRF 0.73. The associated RBE of the high-LET component of the BMMR epithermal neutron beam (∼73% photons) is thus 4.1, higher than that for the FiR1 beam. These weighting factors are closer to the alternative weighting factors of 0.6 and 4.4 for the low- and high-LET components of the BMMR beam, originally proposed by Gavin et al. [30], but never adopted.
The difference in the DRF for the photon components of the dose in the FiR1 and BMMR epithermal neutron beams of 0.78 and 0.73, respectively, is due to the difference in the original exposure times required to give the reference x-ray only doses in the two experiments. Had the reference x-ray dose, in the above example for multiple contrast-enhancing changes on MRI, of 15.01 Gy given in 18.75 min (0.8 Gy/min) been delivered at 3 Gy/min as in the BMMR related study, then the equivalent x-ray dose would have been reduced to 14.15 Gy (given in 4.7 min), and as a result, the DRF would be the same: 0.73. This finding points to the importance of taking into account the significance of the fast component of repair for reference x-ray exposure times of 10–30 min.
An alternative approach for the comparison of the biological effectiveness of the high-LET components of the dose, both within the same beam and between beams, involved the use of a simple in vitro cell survival model. This was initially described in 2001 [48]. Briefly, V79 cells were irradiated in suspension at different depths (20–65 mm) in a water-filled cylindrical phantom.
Over the period of irradiation, the temperature of the water was kept at 4 °C. This prevented the repair of sublethal irradiation damage over the variable exposure times both within the same beam and for different beams. Thus, the need to correct for the variable dose-rates of the γ-ray components to the dose was avoided, DRF 1.0.
The usefulness of this model can best be illustrated by reference to a recent comparison of the biological effectiveness of the moderated accelerator-based epithermal neutron beam at the University of Birmingham, UK, and the reactor-based epithermal neutron beam at Studsvik Medical, Sweden [49]. At all depths in the phantom, the biological effectiveness of the Studsvik beam, for a given total absorbed dose, were always greater than that for the Birmingham beam. For both beams, the survival data obtained for irradiation at 50 and 65 mm depths in the phantom were comparable and have been combined in this analysis. Cell survival curves were not always complete down to a surviving fraction level of 0.1%, especially for the Birmingham beam. This was due to the very low dose-rates (0.58–1.04 Gy/h, depending on depth in the phantom) resulting in very long exposure times, compared with the comparatively higher dose-rates at Studsvik (8.2–16.2 Gy/h). Extrapolation of the cell survival curves was based on the linear and quadratic parameter fits to data points available (Fig. 17.6). The ratio of doses for the same level of cell survival was independent of the depth in the phantom, 1.3, 1.3, and 1.33 for a depth of 20, 35, and 50 plus 65 mm, respectively. However, the dose ratio did depend on the survival level used for the comparison, for example, ranging from 1.41 at 10% survival to 1.25 at 0.1% survival. These differences seem to be related to the differences in the neutron spectra, particularly the fast neutron contribution to the total dose, in the Studsvik beam (Fig. 17.7). The difference in the fast neutron contribution to the total absorbed dose was 51% at 20 mm and 83% at 65 mm depth, while the difference in the total high-LET contribution was 24–26%. Comparable neutron spectra at 50 and 65 mm depth are consistent with the similar cell survival data at these two depths for both beams. Similar differences in the high-LET content of the beam as a function of depth might be a simple explanation as to why the dose ratios for a given level of cell survival were independent of the depth in the phantom. However, more intercomparisons are required before any definitive general conclusions can be drawn.
Variation in survival of V79 cells irradiated with epithermal neutrons from either the Studsvik reactor-based beam (■, □) or the Birmingham accelerator-based beam (●,○). The curves are shown for irradiation at (a) 20 mm, (b) 35 mm, (c) 50 mm (■, ●) plus 65 mm (□, ○) depths in the phantom. Error bars represent ± SE (Reproduced from Hopewell et al. [39]; with permission)
MCNP model calculations for the neutron spectra of the Studsvik reactor- (●--● ) and Birmingham accelerator-based (□--□ ) epithermal neutron beams at depths of 20, 35, 50, and 65 mm in a phantom, the depths used for V79 cell irradiations. Each data point represents the midpoint in an energy bin of finite width (Modified from Mason [49], modified by Mason and Giusti, personal communication 2006)
3 Radiobiological Properties of Boron Capture Agents
The poor selectivity for tumor of the boron compounds used in the early BNCT clinical trials was recognized as one of the factors contributing to their unsuccessful outcome. A search for better boron delivery agents was initiated in the 1960s. The amino acid, p-boronophenylalanine (BPA) and the sulfhydryl borane (Na2B12H11SH, or BSH) were two of the compounds evaluated in those studies. Compound development continues to be an active area of BNCT research.
However, given the degree of characterization required, in particular, toxicity evaluation and radiobiological studies, it would take several years for any new compound to enter a clinical trial. At this time, BPA and BSH are the only two boron compounds in use for clinical BNCT, hence, much of this section of the chapter will necessarily focus on the radiobiological studies that have been reported for these two agents. The approaches used to characterize these compounds for both normal tissues and tumors should also be applied to newer agents.
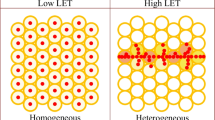
The absorbed dose to tissues resulting from 10B capture is a function of the neutron fluence and the 10B concentration in blood and in the parenchymal tissue surrounding the blood vessels. For the compounds BPA and BSH, the dose contribution from the 10B(n,α)7Li reaction for normal tissues is routinely calculated based on the blood 10B concentration over the course of irradiation. No direct account is taken of the 10B content of the parenchyma or of the vascular endothelial cells in any dose calculations. This is because it is not yet possible to measure normal tissue concentrations of 10B during the course of irradiation. Noninvasive imaging techniques are under development to achieve this objective [41, 56]. However, there would be a requirement to measure tissue boron levels if, for example, compounds such as one from a family of porphyrins, copper tetra-phenyl-carboranyl porphyrin (CuTCPH), were developed. These compounds are retained in tumor and normal tissues to variable degrees but are cleared from the blood [57]. The use of blood boron levels, which may be approaching the lower limit of accurate measurement, may then be misleading. However, for BPA and BSH, the physical absorbed dose delivered to normal tissues has historically been described in terms of the absorbed dose delivered to the blood and the CBE factors calculated are defined so as to be the multiplicative factor that transforms the blood dose into the biologically effective normal tissue dose. Thus, if in the future, measurements of boron levels in normal tissues become available, these CBE factors should not be used. If the normal tissue blood ratio is <1.0, then the photon-equivalent dose to the normal tissue, from the 10B(n,α)7Li reaction, would be underestimated, while if the ratio was >1.0, then the photon-equivalent dose would be overestimated. The photon-equivalent dose, from the 10B(n,α)7Li reaction, has been overestimated in some publications. For example, in a therapeutic study involving the treatment of spontaneous nasal planum squamous cell carcinoma in cats [82], the photon-equivalent doses to the skin and oral mucosa were calculated using the available CBE factors based on blood boron levels. The tissue boron levels and not the blood boron levels were used in this example, and thus, the dose contribution from the 10B(n,α)7Li reaction was overestimated because normal tissue blood ratios were >1.0.
Experimentally derived CBE factors must always be used with caution in clinical treatment protocols. The biodistribution profile of a given boron delivery agent needs to be as thoroughly characterized as possible in the relevant animal models and in patients. In particular, the vascular/nonvascular 10B partition ratio in the animal model used to derive the CBE factor must be similar to the ratio in patients at the time of irradiation. It must be emphasized that comparability of 10B biodistributions is a prerequisite to translating an animal model-derived CBE factor to the clinical situation. At the low doses of BPA (250–290 mg BPA/kg body weight) that were used in the BNL clinical trial, the 10B distribution in human brain was similar to that measured during radiobiological studies in the rat and dog from which the clinical CBE factors were estimated [11, 17].
Given the historical focus on the use of BNCT for treatment of brain tumors (primarily glioblastoma multiforme), the evaluation of effects on the important dose-limiting normal tissues, namely skin, central nervous system (CNS), and oral mucosa have assumed central importance.
3.1 Normal Tissue Effects
3.1.1 Skin
The response of rat skin to BNC irradiation using BPA or BSH has been studied using the thermal beam of the BMRR [59]. The CBE factors for BPA using the early endpoint of moist desquamation and the late endpoint of dermal necrosis were 3.7 ± 0.7 and 0.73 ± 0.42, respectively. The CBE factors for BSH with moist desquamation and dermal necrosis endpoints were 0.55 ± 0.06 and 0.86 ± 0.08, respectively. It is evident from these findings that the microdistribution of these two compounds had a marked effect on the CBE factor obtained. BPA would appear to accumulate in metabolically active basal stem cells within the epidermis, which would account for the very high CBE factor. For the endpoint of dermal necrosis, where the vascular endothelium represents the likely target cell population, the CBE factor values for BPA and BSH were comparable. Observations using neutron activation autoradiography suggest that BPA and BSH have a similar microdistribution in the dermis (Morris, unpublished data, 1999). The clinical implications of these findings are that, per unit boron concentration, BSH mediated BNCT causes less damage to the epidermis than BPA mediated BNCT.
The biological effect of BPA based BNCT on human malignant melanoma using thermal neutrons has produced important information on the effect of this treatment on human skin [27]. Based on boron measurements in blood and skin, these investigators estimated the boron concentration in the skin at the time of BNCT to be between 1.3 and 1.5 times the concurrent level in the blood.
The CBE factor has been derived for the skin in other species. A value of 2.4 has been reported for BPA using acute reactions in hamster skin as the endpoint [37]. The results of dog irradiations with epithermal neutrons at the BMRR indicated a CBE factor for BSH of 0.5 [29].
For the porphyrin CuTCPH, a promising experimental boron delivery agent, the CBE factor was 1.8, for the early endpoint of moist desquamation, calculated in the standard way, using the boron concentration in the blood [66]. In the CuTCPH study, irradiation was carried out when the blood boron level was low (∼1.5 μg/g), 72 h after the initiation of a 48 h infusion of the compound. The CBE factor was much lower, 0.1, if the boron concentration in the skin was used in the calculations. This suggests that although there was a significant accumulation of boron in the skin at the time of irradiation, there were relatively low levels of boron in epidermal cells, and that the bulk of the boron was in the dermis.
3.1.2 CNS
The development of late changes in the CNS after radiation exposure has traditionally been described in terms of damage to specific target cell populations, the loss of which is responsible for specific functional and histologically identifiable injury. Conflicting theories have considered either the vascular endothelial cell or elements of the CNS parenchyma, or both, to be the critical target cells. More recently, radiation damage to the vasculature during BNCT has been shown to be the probable cause of necrosis in the rat spinal cord, suggesting that the vascular endothelium is the primary radiation target in the CNS [20, 61].
The spinal cord of rats has been used to study the response of normal CNS to BNCT [60, 63]. The late effects seen in the spinal cord following single BNCT exposures are similar to those seen in the brain [30, 61]. The radiosensitivity of the rat brain and spinal cord to fractionated irradiation are also comparable [83]. The late end point of limb paralysis (myeloparesis) for the evaluation of radiation-induced spinal cord damage is clearly defined while histological and morphometric endpoints that have been used to assess damage to the brain are time consuming.
The CBE factors for BPA and BSH, measured experimentally, do indeed reflect the different biodistributions of these two boron compounds in the sense that BPA crosses the blood brain barrier while BHS does not. In the case of BSH, the CBE factor was calculated to be 0.53 ± 0.10 [60]. This value is approximately three times lower than that for BPA (1.34 ± 0.13) at a comparable blood 10B concentration of ∼20 μg 10B/g. The threefold difference in the CBE factors was of the same order as that predicted by Rydin et al. [76] who calculated that the fraction of the dose received by the vessel wall would be one-third to one-fifth that delivered to an infinite pool of blood, depending on the diameter of a vessel, based solely on geometry.
In the derivation of CBE factors for potential use in clinical protocols, it is advisable to use a wide range of blood 10B concentrations. Studies carried out using BSH indicated that the CBE factor estimate remains constant at about 0.5 for blood 10B levels ranging from 20 to 120 μg/g [62]. However, progressive escalation of the blood dose of BPA to deliver blood 10B concentrations in the range 20–90 μg/g resulted in CBE factors that varied from 0.66 to 1.34 [63]. Irradiations of the spinal cord in these studies took place 1 h after the administration of the BPA. At this time point, major differences were found in the relative distribution of 10B in the blood and the CNS parenchyma, such that for the highest blood 10B concentration (90 μg/g), the ratio of the level of 10B in the blood to that in the CNS parenchyma was a factor of 3.5 higher than at the lowest blood 10B concentration (20 μg 10B/g). This major change in the partition ratio of 10B between the blood and parenchyma at the time of irradiation was the reason for the observed variations in the calculated CBE factors for different concentrations of 10B in the blood [63]. In the clinical situation, BPA is administered more slowly (e.g., 2 h i.v. infusion), and as a result, it is unlikely that there would be a pronounced change in the 10B concentration ratio between the blood and the CNS parenchyma at higher BPA doses than those currently used. These experimental data emphasize the importance of thorough preclinical and clinical biodistribution studies in protocols involving escalation of the dosage of a boron compound or an alteration in the infusion schedule. In addition, the dependence of CBE factors on experimental conditions makes it critical that studies intended to provide CBE factors for clinical use must be designed to approximate the clinical situation as closely as possible.
Studies of the response of the brain of dogs to single-dose BNCT were carried out using the epithermal neutron beams at the BMRR and at the high flux reactor at Petten using BPA or BSH, respectively [28, 30, 40]. The attenuation of the epithermal neutrons, as a function of depth, produced a nonuniform dose across the brain. The BNCT doses used for comparison to the published x-ray data [24] were defined as the average absorbed dose (Gy) in the volume of the dog brain that received between 90 and 100% of the maximum brain dose. This corresponded to a volume of about 30 cm3, or about 20% of the brain volume. There were differences in the volumes and dose distributions between the 4 MeV x-rays used in the historical studies and the BNCT irradiations; however, the volume that received the prescribed doses were relatively large and potential volume effects on the iso-effect dose (ED50 for abnormalities noted on MRI scans and severe neurological deficit requiring euthanasia) could be excluded. The CBE factors, determined by comparing effects on the normal brain to the published data for hemi-brain irradiation with 4 MeV x-rays [24], were 1.1 for BPA and 0.3–0.5 for BSH [28, 30, 40]. These CBE factors are in agreement with the values derived independently for the rat spinal cord [60]. It should be noted that the historical photon results were based on the results of CT scans and not MRI, and although considerable thought was given to the comparability of effects using these two methods, this is the type of comparison that should be avoided if at all possible. As already indicated, historical photon controls were not used for the determination of the RBE of the FiR1 epithermal neutron beam [6]. This enabled the same MRI and histological methods to be used to evaluate dose-related changes in dog brain from both the epithermal neutron beam and the photon irradiation controls.
For CuTCPH, the CBE factors calculated using the same rat spinal cord as in the studies described previously were 4.4 and 3.8, calculated on the basis of the boron concentration in the blood or the parenchyma, respectively [66]. This finding is in accord with measurements of the boron concentration in the parenchyma of the cord, which were higher than in the blood, tissue: blood concentration ratio 1.9:1. These relatively high CBE factors could also indicate a selective accumulation of this boronated porphyrin in the walls of blood vessels in the CNS, damage to which is believed to be responsible for late CNS morbidity [20]. There is general evidence to indicate that porphyrins have a significant affinity for blood vessels (e.g., [7, 68]).
3.1.3 Oral Mucosa
Only a limited number of reports have documented the CBE factors (based solely on the 10B concentration in the blood) for the oral mucosa, using ulceration of the ventral surface of the tongue of rats as the end point. For the boron delivery agent BPA, a CBE factor of 4.9 was estimated [19]. This CBE factor is considerably higher than that reported for BSH (CBE factor ∼0.3) in the same model [64]. These major differences in the CBE factor indicate variations in the microdistribution of these two boron delivery agents in the mucosal epithelium. Average boron measurements (DCP-AES) of whole tissue samples from the ventral surface of the tongue showed appreciable uptake of 10B (∼21 μg/g) at 3 h after administration of BSH at a dose of 55 mg/kg [64]. A similar finding was reported for BPA administered at a 10B concentration similar to that used for BSH, where the level of 10B in the ventral surface of the tongue at 3 h after administration was estimated to be ∼23 μg/g [64, 65].
These data, while indicating that the overall concentrations of 10B in the tongue were similar for both boron delivery agents, provide no information with regard to the biodistribution profile in different anatomical regions of the tissue. Ion microscopy studies of 10B in tissues [64, 65] have enabled the microdistribution of this element to be analyzed with considerably greater precision than previously possible. Ion microscopy analysis revealed that the level of 10B in the mucosal epithelium was very low after BSH administration [64]. In contrast, the 10B content of the mucosal epithelium was ∼3.5 times higher in rats receiving BPA. In the case of BSH, the majority of 10B was located in the lamina propria and not in the mucosal epithelium. This differs from the findings for BPA where the 10B content of the mucosal epithelium was fairly similar to that in the lamina propria; the boron concentration ratios for mucosal epithelium: lamina propria were 1:6 for BSH and 1:1.5 for BPA. Also, 10B accumulation in the mucosal epithelium was five times higher with BPA than BSH. These data indicated that a major factor contributing to the difference in the CBE factors for BSH and BPA, despite similar gross boron tissue concentrations, was the relatively low uptake of BSH in the mucosal epithelium.
When using CuTCPH as the boron capture agent, gross levels of boron in the oral mucosa of the rat were found to be high, with a mucosa: blood ratio of 49:1 [67]. Irradiation with thermal neutrons in the presence of CuTCPH gave a CBE factor of ∼0.04, using ulceration as the endpoint. This value was calculated using measurements of the estimated content of boron in the oral mucosa at the time of irradiation [67]. If the absorbed dose from the 10B(n,α)7Li reaction was calculated on the basis of the boron concentration in the blood at the time of irradiation, the CBE factor was appreciably increased to ∼1.7 [67]. However, as mentioned previously, the boron levels in blood were very low at the time of irradiation. Indeed, the boron concentrations in the blood were close to the level of detection in this irradiated series of animals. As a consequence, the CBE factor calculated on the basis of blood levels can only be viewed as a rough approximation.
3.2 Tumor Response
Experimental therapeutic studies related to BNCT have been carried out in a variety of animal tumor models. BPA mediated BNCT has been shown to inhibit the growth of melanoma, producing high local tumor control rates, in mice, hamsters, and pigs [13, 55]. BPA has also proved to be effective in BNCT studies on hamster derived melanoma, grown as xenografts, in the rabbit eye [71]. The first successful treatment of a brain tumor (rat 9L gliosarcoma) was carried out by Joel et al. in the late 1980s using the dimeric form of BSH as the boron capture agent and the BMRR thermal neutron beam [44]. This was followed, in 1992, by another study involving the first irradiation of the 9L gliosarcoma in the rat with BPA-mediated BNCT [14]. These experiments were repeated in 1994 using an improved BPA delivery system of BPA complexed with fructose (BPA-F) to increase the solubility. This produced long-term tumor-free animal survival rates approaching 100% [16]. Additional studies by Saris et al. [77], with the murine GL 261 glioma, and Matalka et al. [51], with a human melanoma cell line (MRA 27) implanted in the brain of nude rats, also demonstrated the efficacy of BPA mediated BNCT. More recently, the preferential uptake of BPA and BSH by the rat F98 glioma has been considerably enhanced by the disruption of the blood brain barrier following the intracarotid injection of mannitol [84, 85]. Dramatic further improvements in F98 tumor growth inhibition were subsequently observed in BNCT studies, using a combination of both BPA and BSH [1] using the blood brain barrier disruption approach. Since this earlier work, multiple translational studies have been performed in different tumor models, using different boron compounds, routes of compound administration, and a range of administration strategies [3].
While the CBE factors for the skin, CNS, and oral mucosa are fairly well defined for both BPA and BSH, those for use in estimating the photon-equivalent dose to tumor are much less certain. For BPA, CBE factors of 4.0, 3.8, and 3.6 were obtained for a surviving fraction of 10, 1, and 0.1 %, respectively, for the rat 9L gliosarcoma model, based on the in vivo irradiation of intracranially implanted tumors in situ, with removal immediately after irradiation and the assay of clonogenic cell survival in vitro [15]. CBE factors of 1.3, 1.2, and 1.2 were also derived in the same way for BSH for the 9L gliosarcoma. Again, as would be expected, slightly higher values were obtained for the highest level of cell survival. This later study was based primarily on values for the oxidized dimeric form of BSH (BSSB). The CBE factors for both BSH and BSSB evaluated for cultured cells in vitro were much higher both for BSSB (range 3.6–3.1) and BSH (range 3.2–2.8) [15]. This difference reflects the likely heterogeneous boron distribution in solid tumors relative to cells in culture and warns against the application of any CBE values derived totally from cells in vitro to an in vivo situation. Ideally, CBE factors for boron capture agents such as BPA and BSH should be derived directly using an in vivo assay. This is difficult for the intracranially implanted 9L gliosarcoma because of the high risk of normal tissue complications that would result from the large single dose of x-rays that would be needed to control this tumor. An assessment of local tumor control after x-irradiation is an essential requirement for the calculation of a CBE factor.
An assumed requirement for an effective boron capture agent is that the compound should accumulate in tumor relative to blood and normal tissues. However, the preferential uptake of the boron compounds by tumor tissue does not explain differences in CBE values between tumor tissue and normal tissue. Potentially, differences in the microdistribution determined by features such as tumor cell metabolism and tumor cell geometry (large nuclei, changes in nucleus/cytoplasm ratio, etc.) vs. normal cell metabolism and geometry account for differences in CBE values. Historically, calculations of the physical radiation dose from the 10B(n,α)7Li neutron capture reaction have been made based on an estimate of the gross boron concentration in tumor tissue. Since this cannot be routinely measured directly, biodistribution studies need to be carried out in advance of experimental animal or patient studies to determine the tumor: blood or tumor: normal tissue concentration ratios for boron. The tumor: blood boron ratio is then used to estimate tumor boron levels from measurements of blood samples at the time of irradiation, which in the case of the boron compound currently used clinically, occurs within a few hours of compound administration. This is different from studies in normal tissues where the boron concentration in the blood at the time of irradiation is used directly to calculate an absorbed dose. This is then converted into a weighted dose using the appropriate CBE factor. Examples of published tumor:blood boron concentration ratios include those for the rat 9L gliosarcoma, which are 3.3 ± 0.5 for BPA (oral administration) and 3.2 ± 0.4 for BPA-F. For BSH and its dimeric form, BSSB, the corresponding values were much lower, 0.71 ± 0.2 and 0.76 ± 0.2, respectively [38]. This low ratio, suggesting no selective uptake relative to blood, was not considered to be a drawback because this compound, which does not cross the intact blood brain barrier, had been developed specifically for the treatment of primary gliomas. The tumor:brain boron concentration ratios for these two compounds, BHS and BSSB, were very high, that is, ∼8 and ∼17, respectively; these boron carriers cross easily through the disrupted blood brain barrier into tumor tissue [38]. These numbers have to be viewed with caution because boron measured in brain, or indeed in other normal tissues, may reflect the amount of boron present in the residual blood in any tissue sample. The concentration ratios reported above in the rat 9L gliosarcoma model are comparable with those obtained for gliomas, based on pharmacokinetic studies in man [18, 79].
More recently, biodistribution studies have been carried out on chemically induced primary squamous cell carcinomas in the hamster cheek pouch. For compounds such as GB-10 (Na 102 B10H10), which is related to BSH, the distribution of boron in tumor, relative to adjacent normal tissues, is not influenced by a selective blood vessel barrier of the type found in the CNS. The tumor:blood boron concentration ratios ranged from 2.8 to 4.8 for BPA and from 0.8 to 1.0 for GB-10, depending on the dose and administration protocol but not in a clearly defined way [35]. Thus, based on these two tumor models, uptake of the current group of clinically used boron carriers and related compounds by tumor relative to blood does not change significantly with tumor model. However, it must be emphasized that this in itself does not indicate the same biological effectiveness. There is an urgent need to establish CBE factors for different tumor types and to avoid the use of CBE factors derived for the 9L gliosarcoma for other tumor types.
Another boron carrier for which comparative data exist for these two tumor types is CuTCPH. Tumor:blood boron concentration ratios ranged from 80:1 in subcutaneously implanted 9L gliosarcoma to 16:1 in the same tumor implanted intracerebrally [57]. In squamous cell carcinomas of the hamster cheek pouch, even higher values of 99:1 were obtained [47]. These high tumor: blood boron concentration ratios were all measured 3 days after the first administration of a series of i.p. injections of the compound, delivered over periods up to 48 h. They were associated with very low blood boron concentrations, which cannot be reliably used to estimate tumor boron levels in a clinical scenario. These high tumor:blood boron concentration ratios measured in the hamster cheek pouch model do not necessarily correlate with high levels of local tumor control. A combination of high absolute boron concentration values and a favorable compound localization, that is, a high CBE factor are pivotal to obtaining the greatest biological effect. This fact stresses the need for radiobiological studies to evaluate the potential therapeutic efficacy of a particular boron carrier. The therapeutic effect of boron carriers must not be predicted on the basis of tumor:blood boron concentration ratios alone.
The importance of the use of in vivo models to assess the likely effectiveness of BNCT mediated by a boron compound has recently been illustrated by studies with GB-10 on induced squamous cell carcinomas in the hamster cheek pouch [81]. As indicated above, GB-10 does not target these tumors selectively and yet GB-10 mediated BNCT still produced a 70% overall initial tumor response rate with no damage to the normal tissues of the cheek pouch. Analysis using light microscopy has indicated that GB-10 mediated BNCT damages the abnormal tumor blood vessels, but spares blood vessels in precancerous and normal tissue. The stroma of the tumor was characterized by marked hemorrhaging, caused by the rupture of blood vessel walls, congestion, and edema. No damaged or ruptured blood vessels were detected in any of the fields of precancerous or normal tissue examined throughout the follow-up period of 30 days. Blood vessels of tumors are believed to be structurally and functionally abnormal. Tumor blood vessels have been reported to be dilated and altered in the sense that their walls exhibited fenestrations, vesicles, and transcellular holes. There were widened interendothelial cell junctions and a discontinuity or absence of the basement membrane [9]. Regardless of the effectiveness of GB-10 mediated BNCT, the working hypothesis is that GB-10 leaks from the abnormal tumor blood vessels into the extracellular space and accumulates in the vicinity of endothelial cells. In addition, in terms of purely physical geometric considerations, the dose distribution in dilated tumor vessels would be closer to the charged particle equilibrium distribution than in normal (narrower) blood vessels where a boron concentration gradient will exist between blood adjacent to the luminal wall of the blood vessel, the endothelial cell, and the surrounding tissue. With GB-10 located in the blood and in the extracellular space around blood vessels, a selective tumor effect would result from selective blood vessel damage rather than from the selective uptake of the boron compound by the tumor [81]. This proposed mechanism is in contrast to the traditional BNCT paradigm which ascribes selective tumor damage to selective boron uptake by tumors. Furthermore, it illustrates the limitations of gross bio-distribution studies and the need to perform in vivo BNCT studies to evaluate the potential therapeutic efficacy of a boron compound.
As indicated previously, achieving high tumor:normal tissue and tumor:blood mean boron concentration ratios is clearly an asset. However, using this approach alone, BNCT will not be optimized unless at least the majority of all the tumor clonogenic cells are targeted, regardless of their position in the tumor and metabolism and degree of differentiation or proliferation. Given that tumors are very often heterogeneous, targeting of all the appropriate tumor cell populations is an acknowledged challenge in oncology. In the case of BNCT, the tumor cell populations that are poorly loaded with boron will be significantly underdosed. The combined administration of boron compounds with different uptake properties should contribute to a more homogeneous targeting within a heterogeneous tumor and in this way to the therapeutic efficacy of BNCT [2, 35, 36, 70, 80]. Studies with induced squamous cell carcinoma in the hamster cheek pouch model have highlighted the difficulty of achieving complete remissions, within 30 days, in larger tumors (>100 mm3) treated with BPA-mediated BNCT alone [46]. Conversely, BNCT mediated by GB-10 and BNCT mediated by a combination of GB-10 and BPA induced complete remission in some of these large tumors [81]. Improved tumor response could be partially ascribed to improved tumor cell targeting by a combined compound administration protocol [36]. The combined administration of GB-10 and BPA achieved a statistically significant 1.8-fold increase in the targeting homogeneity of boron, over GB-10 alone, and a statistically significant 3.3-fold increase in targeting homogeneity of boron over BPA alone [36]. This conclusion was based on a reduced coefficient of variation in the gross measurements in multiple samples in individual animals. Thus, in this case at least, combined administration of two boron compounds with different properties and uptake mechanisms (BPA and GB-10) improved targeting homogeneity. Moreover, GB-10 mediated BNCT, in particular, may contribute, via a selective effect on tumor blood vessels, to the treatment of larger tumors that are more difficult to treat on a “cell by cell” basis. GB-10 and BPA could combine vascular targeting and cellular targeting, respectively, to achieve an improved tumor response in a similar manner to what was recently been reported for dual-mode photodynamic therapy [10].
An additional potential advantage of combined boron compound administration protocols lies in the finding that they can deliver increased absolute amounts of boron to tumor tissue [35]. For similar tumor:normal tissue ratios, high, non-toxic, absolute 10B concentrations are an advantage because they allow for shorter irradiation times and a concomitant reduction in the background dose from the neutron beam [12]. In addition to the multiple variables described above that influence the value of CBE factors for tumors, for a single boron carrier, little is known about the interaction between boron carriers administered concomitantly in different proportions. The improvement in targeting homogeneity described above, for example, would conceivably result in a synergistic therapeutic effect when boron carriers are administered jointly. Within this context, the CBE value of a combination of boron compounds must be determined in vivo and cannot be calculated based on the individual CBE values for each boron compound. The fact that tumor control by BNCT mediated by certain boron compounds might be due to vascular damage rather than direct killing of tumor cells and available evidence that targeting of heterogeneous tumor cell populations plays a pivotal role in the biological effect of BNCT [81] also questions the validity of some CBE values determined using in vivo irradiation/ in vitro assays [19] since this excludes the evaluation of any effect on tumor response specifically involving the tumor vasculature.
All of the above considerations stress the need to establish CBE factors under conditions that resemble, as closely as possible, the clinical scenario that the experiments are attempting to characterize.
4 Future Research Requirements
4.1 Interaction Between High and Low-LET Radiations
In the routine practice of BNCT, it is, as indicated previously, assumed that the different components of the mixed field irradiation act independently of each other. However, only a relatively small increase in the biological effectiveness of γ-rays, when given in combination with a high-LET radiation relative to γ-rays alone, would significantly reduce the apparent RBE/CBE of the high-LET components of this mixed beam irradiation. While of considerable importance for the understanding of BNCT, the potential interaction between high- and low-LET radiations has not been extensively studied nor directly investigated in relation to BNCT. The only studies that have been undertaken, with relevance to this question, have been the sequential irradiation of V79 cells with fixed doses of either fast neutrons or 238Pu α-particles (140 keV/μm), prior to exposure to high dose-rate x-rays [52, 53].
For high dose-rate x-rays and α-particles given totally separately, the RBE of the α-particles, relative to x-rays, was approximately 6.0, 3.0, and 2.4 for clonogenic cell survival levels of 50, 10, and 1%, respectively (Fig. 17.8a). When a fixed dose of 0.5 Gy of α-particles, which reduced clonogenic cell survival by 50%, was given immediately prior to x-rays, the resulting x-ray cell survival curve was still curvilinear. Normalization of the data back to an initial 100% survival showed the x-ray (with 0.5 Gy of α-particles) cell survival curve to be unchanged relative to x-rays alone (Fig. 17.8b). This was not the case when the initial α-particle dose was increased to a fixed dose of either 2.0 or 2.5 Gy. The RBE of x-rays combined with the higher dose of α-particles was ≥1.15 when compared with x-rays given alone (Fig. 17.8c, d). McNally et al. [53] concluded that “alpha particles cause damage capable of interacting with x-ray damage.” However, the relationship is not a simple one; it depends on the relative mix of high- and low-LET radiation. These results raise important questions although results from studies with fixed doses of α-particles or neutrons do not necessarily predict the response of cells and tissues when a fixed percentage of high-LET radiations (recoil protons from fast neutrons, protons from nitrogen capture and α-particles, and lithium ions from the 10B capture) is given concomitantly with γ-rays, sometimes at relatively low dose rates. Carefully designed investigations are needed to address this issue, using a proportion of high-LET radiation to the total absorbed dose, of the same order of magnitude as that present in tissue during BNCT exposures. In a recent study [73] in which V79 cells were concomitantly irradiated with x-rays and alpha particles, no evidence for any interaction was found. However, in this study, the percentage contribution of alpha particles to the total absorbed dose was <19%, and thus, the results obtained are still consistent with the earlier studies of McNally et al. [53]. This as been reaffirmed by a very recent study [50] where evidence for the interaction between gamma-rays and fast neutrons, in a mixed field irradiation with epithermal neutrons, was not only shown to be dependent on the proportion dose of contribution from the high-LET dose component but also suggested that it might also be influenced by the energy spectrum of the fast neutron dose component.
Clonogenic cell survival curves for V79 cells after irradiation with either (a) x-rays [3 Gy/min] or α-particles [0.35 Gy/min; 140 keV/μm] alone or with a fixed dose of α-particles, (b) 0.5 Gy, (c) 2.0 Gy, or (d) 2.5 Gy followed by a variable dose of x-rays. For these combined irradiations, the curve for each irradiation type is given as a reference. For these combined irradiations, the actual data (O-O) has been normalized to 100 % cell survival (-). The RBE values result from the comparison of this normalized data with x-irradiation alone (Reproduced from Hopewell et al. [39]; with permission)
4.2 Use of Existing Boron Compounds for New Medical Applications
The determination of CBE factors, which represent the biological effectiveness of the 10B(n,α)7Li reaction products in specific normal tissues at risk as a result of any new application, should be a mandatory part of any development program. Reports of adverse normal tissue reactions with any new radiotherapy modality are a cause for concern and a justifiable reason for the closure of studies, as has proven to be the case for BNCT in the past. CBE factors for a specific normal tissue, using existing boron carriers, cannot safely be applied to other normal tissues.
An example that perhaps demonstrates these concerns was the extrapolation of weighted doses, obtained from the clinical studies at Brookhaven National Laboratory with an epithermal neutron beam, for the safe treatment of patients with glioblastoma with BPA-mediated BNCT, to the extracorporeal treatment of livers in patients with colorectal metastases using the same neutron capture agent [74]. An additional complication in these investigations was that a thermal, and not an epithermal neutron beam, was used. This was judged to be acceptable, and it was suggested to be a conservative choice because the RBE of 14.3 MeV D-T generated fast neutrons for liver tissue was more than 30% greater in comparison with that of thermal neutrons for brain tissue. The end point selected for the liver was the clonogenic survival of hepatocytes in vitro [43], which has no proven link with the development of late radiation damage to liver and where the RBE will depend on the level of cell survival at which damage was assessed. The brain endpoint was based on the ED50 for radiation myelopathy in rats [59, 60]. This endpoint is associated with an approximately fixed level of cell survival in endothelial cells, and not parenchyma cells [20], making this a very poor comparison. Although it was recognized that a limiting tolerance dose was needed to be established for the liver using appropriate animal experiments [69], the same assumptions were still used in subsequent dose modeling studies. In the absence of appropriate data, there is always the tendency for early proposals to be perpetuated. In such situations where there is considerable uncertainty in the radiobiological parameters, it is much more appropriate to quote total absorbed doses, including the breakdown of that total absorbed dose into its different components. Otherwise, important information for potential future use will be lost.
4.3 Use of Novel Boron Compounds and Alternative Neutron Sources
Experience from fast neutron therapy has shown that even small changes in the neutron spectra can result in a change in the relative RBE of a particular beam [31]. With the currently available beams for BNCT, there is at present insufficient information to be able to predict the likely biological effectiveness of any new neutron source. More studies of the type that used V79 cells to compare the biological effectiveness of the fast neutron component of epithermal beams at Studsvik and Birmingham are required. This would avoid the difficulties in interpretation brought about by other confounding variables, such as the variations in effects resulting from the dose-rate of the γ-ray dose component. Simple short-term studies, such as those proposed by Gueulette et al [32], would also provide a guide to the relative effectiveness of different beams.
For new compounds, the key issue is the determination of the CBE factor for the specific normal tissues at risk of developing adverse effects and depends on the tumor site treated for which a particular boron compound is proposed. These studies do not need to be undertaken on an epithermal beam, which for most tissues may require the use of a large animal model. The use of a well characterized thermal beam would be suitable, preferably one where exposure times are short so the confounding effects of a low dose-rate from the γ-ray dose component can be avoided.
BNCT may be one of the most complicated therapeutic modalities ever to reach the stage of human clinical trials. As the application of BNCT continues to expand into new tumor sites, the radiobiological principles discussed in this chapter assume central importance. It is essential that all available radiobiological information from prior BNCT experience, as well as from other modalities such as fast neutrons, be evaluated and integrated into the plans for future BNCT development.
Those who cannot remember the past are condemned to repeat it.
George Santayana
References
Barth RF, Yang WL, Rotaru JH et al (1997) Neutron-capture therapy of brain-tumours – enhanced survival following intracarotid injection of either sodium borocaptate or boronophenylalanine with or without blood–brain-barrier disruption. Cancer Res 57:1129–1136
Barth RF, Yang W, Coderre JA (2003) Rat brain tumor models to assess the efficacy of boron neutron capture therapy: a critical evaluation. J Neurooncol 62:61–74
Barth RF, Coderre JA, Vicente MG et al (2005) Boron neutron capture therapy of cancer: current status and future prospects. Clin Cancer Res 11:3987–4002
Bedford JS, Mitchell JB (1973) Dose-rate effects in synchronous mammalian cells in culture. Radiat Res 54:316–327
Belli M, Cherubini R, Finotto S et al (1989) RBE-LET relationship for the survival of V79 cells irradiated with low energy protons. Int J Radiat Biol 55:93–104
Benczik J, Seppälä T, Snellman M et al (2003) Evaluation of the relative biological effectiveness of a clinical epithermal neutron beam using dog brain. Radiat Res 159:199–209
Berenbaum MC, Hall GW, Hoyes AD (1986) Cerebral photosensitisation by haematoporphyrin derivative. Evidence for an endothelial site of action. Br J Cancer 53:81–89
Cárabe-Fernández A, Dale RG, Jones B (2007) The incorporation of the concept of minimum RBE (RBEmin) into the linear-quadratic model and the potential for improved radiobiological analysis of high-LET treatments. Int J Radiat Biol 83:27–39
Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407:249–264
Chen B, Pogue BW, Hoopes PJ et al (2005) Combining vascular and cellular targeting regimens enhances the efficacy of photodynamic therapy. Int J Radiat Oncol Biol Phys 61:1216–1226
Coderre JA (1992) A phase 1 biodistribution study of p-boronophenylalanine. In: Moss R, Gabel D (eds) Boron neutron capture therapy: towards clinical trials of glioma with BNCT. Plenum Press, New York, pp 111–121
Coderre JA, Morris GM (1999) The radiation biology of boron neutron capture therapy. Radiat Res 151:1–18
Coderre JA, Slatkin DN, Micca PL et al (1991) Boron neutron capture therapy of a murine melanoma with para-boronophenylalanine – dose response analysis using a morbidity index. Radiat Res 128:177–185
Coderre JA, Joel DD, Micca PL et al (1992) Control of intracerebral gliosarcomas in rats by boron neutron capture therapy with p-boronophenylalanine. Radiat Res 129:290–296
Coderre JA, Makar MS, Micca PL et al (1993) Derivations of relative biological effectiveness for the high-LET radiations produced during boron neutron capture irradiations of the 9L rat gliosarcoma in vitro and in vivo. Int J Radiat Oncol Biol Phys 27:1121–1129
Coderre JA, Button TM, Micca PL et al (1994) Neutron capture therapy of the 9L rat gliosarcoma using the p-boronophenylalanine-fructose complex. Int J Radiat Oncol Biol Phys 30:643–652
Coderre JA, Elowitz EE, Chadha M et al (1997) Boron neutron capture therapy of glioblastoma multiforme using the p-boronophenylalanine-fructose complex and epithermal neutrons: trial design and early clinical results. J Neurooncol 33:141–152
Coderre JA, Chanana AD, Joel DD et al (1998) Biodistribution of boronophenylalanine in patients with glioblastoma multiforme: boron concentration correlates with tumor cellularity. Radiat Res 149:163–170
Coderre JA, Morris GM, Micca PL et al (1999) The effects of boron neutron capture irradiation on oral mucosa: evaluation using a rat tongue model. Radiat Res 152:113–118
Coderre JA, Morris GM, Micca PL et al (2006) Late effects of radiation on the central nervous system: role of vascular endothelial damage and glial stem cell survival. Radiat Res 166:495–503
Down JD, Easton DF, Steel GG (1986) Repair in the mouse lung during low dose-rate irradiation. Radiother Oncol 6:29–42
Farr LE, Sweet WH, Robertson JS et al (1954) Neutron capture therapy with boron in the treatment of glioblastoma multiforme. Am J Roentgenol Radium Ther Nucl Med 71:279–293
Field SB (1976) An historical survey of radiobiology and radiotherapy with fast neutrons. Curr Top Radiat Res Q 11:1–86
Fike JR, Cann CE, Davis RL et al (1984) Computed tomography analysis of the canine brain: effects of hemi-brain x irradiation. Radiat Res 99:294–310
Fowler JF (1982) Workshop summary. Int J Radiat Oncol Biol Phys 8:2207–2210
Fu KK (1991) Influence of dose rate on normal tissue tolerance. In: Gutin PH, Leibel SA, Sheline GE (eds) Radiation injury to the nervous system. Raven, New York, pp 69–90
Fukuda H, Hiratsuka J, Honda C et al (1994) Boron neutron capture therapy of malignant melanoma using 10B-paraboronophenylalanine with special reference to evaluation of radiation dose and damage to the skin. Radiat Res 138:435–442
Gabel D, Philipp KH, Wheeler FJ et al (1998) The compound factor of the 10B(n, α)7Li reaction from borocaptate sodium and the relative biological effectiveness of recoil protons for induction of brain damage in boron neutron capture therapy. Radiat Res 149:378–386
Gavin PR, Wheeler FJ, Huiskamp R et al (1992) Large animal studies of normal tissue tolerance using an epithermal neutron beam and borocaptate sodium. In: Moss R, Gabel D (eds) Boron neutron capture therapy: towards clinical trials of glioma. Plenum Press, New York, pp 197–209
Gavin P, Kraft S, Huiskamp R, Coderre J (1997) A review: CNS effects and normal tissue tolerance in dogs. J Neurooncol 33:71–80
Gueulette J, Beauduin M, Grégoire V et al (1996) RBE variation between fast neutron beams as a function of energy. Intercomparison involving 7 neutron therapy facilities. Bull Cancer Radiother 83(Suppl):55s–63s
Gueulette J, Binns PJ, De Coster BM et al (2005) RBE of the MIT epithermal neutron beam for crypt cell regeneration in mice. Radiat Res 164:805–809
Gueulette J, Liu H-M, Jiang S-H et al (2006) Radiobiological characterization of the epithermal neutron beam produced at the Tsing Hua open-pool reactor (THOR) for BNCT: comparison with other BNCT facilities. Ther Radiol Oncol 13:135–146
Hall EJ, Novak JK, Kellerer AM et al (1975) RBE as a function of neutron energy. I. Experimental observations. Radiat Res 64:245–255
Heber E, Trivillin VA, Nigg D et al (2004) Biodistribution of GB-10 (Na 102 B10H10) compound for boron neutron capture therapy (BNCT) in an experimental model of oral cancer in the hamster cheek pouch. Arch Oral Biol 49:313–324
Heber EM, Trivillin VA, Nigg DW et al (2006) Homogeneous boron targeting of heterogeneous tumors for boron neutron capture therapy (BNCT): chemical analyses in the hamster cheek pouch oral cancer model. Arch Oral Biol 51:922–929
Hiratsuka J, Fukuda H, Kobayashi T et al (1991) The relative biological effectiveness of B-10-neutron capture therapy for early skin reaction in the hamster. Radiat Res 128:186–191
Hopewell JW, Morris GM, Coderre JA (1994) Determination of radiobiological parameters for the safe clinical application of BNCT. In: Auterinen I, Kallio M (eds) Proceedings of the CLINCT BNC T Workshop. Helsinki University of Technology Report TKK-F-A718, pp 86–93
Hopewell JW, Benczik J, Mason A (2009) Radiobiology program requirements for boron neutron capture therapy at a nuclear research reactor. In: Sauerwein WAG, Moss RL (eds) Requirements for boron neutron capture therapy (BNCT) at a nuclear research reactor. European Commission Joint Research Centre, Institute for Energy, Petten, The Netherlands pp 50–61
Huiskamp R, Gavin PR, Coderre JA et al (1996) Brain tolerance in dogs to boron neutron capture therapy with borocaptate sodium (BSH) or boronophenylalanine (BPA). In: Mishima Y (ed) Cancer neutron capture therapy. Plenum Press, New York, pp 591–596
Imahori Y, Ueda S, Ohmori Y et al (1998) Fluorine-18-labeled fluoroborono-phenylalanine PET in patients with glioma. J Nucl Med 39:325–333
Javid M, Brownell GL, Sweet WH (1952) The possible use of neutron-capturing isotopes such as boron 10 in the treatment of neoplasms. II. Computation of the radiation energies and estimates of effects in normal and neoplastic brain. J Clin Invest 31:604–610
Jirtle RL, DeLuca PM, Hinshaw WM et al (1984) Survival of parenchymal hepatocytes irradiated with 14.3 MeV neutrons. Int J Radiat Oncol Biol Phys 10:895–899
Joel DD, Fairchild RG, Laissue JA et al (1990) Boron neutron capture therapy of intracerebral rat gliosarcomas. Proc Natl Acad Sci USA 87:9808–9812
Kiger JL, Kiger WS 3rd, Riley KJ et al (2008) Functional and histological changes in rat lung after boron neutron capture therapy. Radiat Res 170:60–69
Kreimann EL, Itoiz ME, Longhino L et al (2001) Boron neutron capture therapy for the treatment of oral cancer in the hamster cheek pouch model. Cancer Res (Advances in Brief) 61:8638–8642
Kreimann EL, Miura M, Itoiz ME et al (2003) Biodistribution of a carborane-containing porphyrin as a targeting agent for boron neutron capture therapy of oral cancer in the hamster cheek pouch. Arch Oral Biol 48:223–232
Mansfield C, Hopewell JW, Beynon TD et al (2001) A biological comparison of neutron beams used for BNCT research. In: Hawthorne F et al (eds) Frontiers in neutron capture therapy. Kluwer Academic/Plenum Publishers, New York, pp 407–411
Mason AJ (2005) A comparison of epithermal neutron beams for BNCT. Ph.D. thesis, University of Birmingham, Birmingham
Mason AJ, Giusti V, Green S et al (2011) Interaction between the biological effects of high- and low-LET radiation dose components in a mixed field exposure. Int J Radiat Biol 87:1162–1172
Matalka KZ, Bailey MQ, Barth RF et al (1993) Boron neutron capture therapy of intracerebral melanoma using boronophenylalanine as a capture agent. Cancer Res 53:3308–3313
McNally NJ, de Ronde J, Hinchliffe M (1984) The effect of sequential irradiation with X-rays and fast neutrons on the survival of V79 Chinese hamster cells. Int J Radiat Biol Relat Stud Phys Chem Med 45:301–310
McNally NJ, de Ronde J, Folkard M (1988) Interaction between X-ray and α-particle damage in V79 cells. Int J Radiat Biol Relat Stud Phys Chem Med 53:917–920
Millar WT, Hopewell JW (2007) Effects of very low dose-rate 90Sr/90Y exposure on the acute moist desquamation response of pigskin: comparison based on predictions from dose fractionation studies at high dose rate with incomplete repair. Radiother Oncol 83:187–195
Mishima Y, Ichihashi M, Nakanishi T et al (1983) Cure of malignant melanoma by single thermal neutron capture treatment using melanoma seeking compounds: 10B/melanogenesis interaction to in vitro/in vivo radiobiological analysis to preclinical studies. In: Fairchild RG, Brownell G (eds) Proceedings of the first international symposium on neutron capture therapy. Brookhaven National Laboratory, Upton, pp 355–364
Mishima Y, Imahori Y, Honda C et al (1997) In vivo diagnosis of human melanoma with positron emission tomography using specific melanoma-seeking 18F-DOPA analogue. J Neurooncol 33:163–169
Miura M, Joel DD, Smilowitz HM et al (2001) Biodistribution of copper carboranyltetraphenylporphyrins in rodents bearing an isogeneic or human neoplasm. J Neurooncol 52:111–117
Morgan GR, Mill AJ, Roberts CJ et al (1988) The radiobiology of 24 keV neutrons. Measurement of the relative biological effect free-in-air, survival and cytogenetic analysis of the biological effect at various depths in a polyethylene phantom and modification of the depth-dose profile by boron 10 for V79 Chinese hamster and HeLa cells. Br J Radiol 61:1127–1135
Morris GM, Coderre JA, Hopewell JW et al (1994) Response of rat skin to boron neutron capture therapy with p-boronophenylalanine or borocaptate sodium. Radiother Oncol 32:144–153
Morris GM, Coderre JA, Hopewell JW et al (1994) Response of the central nervous system to boron neutron capture irradiation: evaluation using rat spinal cord model. Radiother Oncol 32:249–255
Morris GM, Coderre JA, Bywaters A et al (1996) Boron neutron-capture irradiation of the rat spinal-cord – histopathological evidence of a vascular-mediated pathogenesis. Radiat Res 146:313–320
Morris GM, Coderre JA, Hopewell JW et al (1996) Boron neutron capture irradiation of the rat spinal cord: effects of variable doses of borocaptate sodium. Radiother Oncol 39:253–259
Morris GM, Coderre JA, Micca PL et al (1997) Central nervous system tolerance to boron neutron capture therapy with p-boronophenylalanine. Br J Cancer 76:1623–1629
Morris GM, Smith DW, Patel H et al (2000) Boron microlocalisation in oral mucosal tissue: implications for boron neutron capture therapy. Br J Cancer 82:1764–1771
Morris GM, Coderre JA, Smith DR (2001) A rat model of oral mucosal response to boron neutron capture therapy. In: Hawthorne F et al (eds) Frontiers in neutron capture therapy. Kluwer Academic/Plenum Publishers, New York, pp 1273–1277
Morris GM, Coderre JA, Hopewell JW et al (2003) Porphyrin-mediated boron neutron capture therapy: evaluation of the reactions of skin and central nervous system. Int J Radiat Biol 79:149–158
Morris GM, Coderre JA, Micca PL et al (2005) Porphyrin-mediated boron neutron capture therapy: a preclinical evaluation of the response of the oral mucosa. Radiat Res 163:72–78
Nelson JS, Liaw LH, Orenstein A et al (1988) Mechanism of tumor destruction following photodynamic therapy with hematoporphyrin derivative, chlorin, and phthalocyanine. J Natl Cancer Inst 80:1599–1605
Nievaart VA, Moss RL, Kloosterman JL et al (2006) Design of a rotating facility for extracorporal treatment of an explanted liver with disseminated metastases by boron neutron capture therapy with an epithermal neutron beam. Radiat Res 6:81–88
Ono K, Masunaga S, Suzuki M et al (1999) The combined effect of borono phenylalanine and borocaptate in boron neutron capture therapy for SCCVII tumors in mice. Int J Radiat Oncol Biol Phys 43:431–436
Packer S, Coderre JA, Saraf S et al (1992) Boron neutron capture therapy of anterior chamber melanoma with p-boronophenylalanine. Invest Ophthalmol Vis Sci 33:395–403
Perris A, Pialoglou P, Katsanos AA et al (1986) Biological effectiveness of low energy protons. I. Survival of Chinese hamster cells. Int J Radiat Biol Relat Stud Phys Chem Med 50:1093–1101
Phoenix B, Green S, Hill MA et al (2009) Do the various radiations present in BNCT act synergistically? Cell survival experiments in mixed alpha-particle and gamma-ray fields. Appl Radiat Isot 67:S318–S320
Pinelli J, Altieri S, Fossati F et al (2001) Operational modalities and effects of BNCT on liver metastases of colon adenocarcinoma. In: Hawthorne F et al (eds) Frontiers in neutron capture therapy. Kluwer Academic/Plenum Publishers, New York, pp 1427–1440
Pop LA, Millar WT, van der Plas M et al (2000) Radiation tolerance of rat spinal cord to pulsed dose rate (PDR-) brachytherapy: the impact of differences in temporal dose distribution. Radiother Oncol 55:301–315
Rydin RA, Deutsch OL, Murray BW (1976) The effect of geometry on capillary wall dose for boron neutron capture therapy. Phys Med Biol 21:134–138
Saris SC, Solares GR, Wazer DE et al (1992) Boron neutron capture therapy for murine malignant gliomas. Cancer Res 52:4672–4677
Stone RS (1948) Neutron therapy and specific ionization. Am J Roentgenol Radium Ther 59:771–785
Stragliotto G, Fankhauser H, Gutin PH et al (1995) Biodistribution of boron sulfhydryl for boron neutron capture therapy in patients with intracranial tumors. Neurosurgery 36:285–293
Trivillin VA, Heber EM, Itoiz ME et al (2004) Radiobiology of BNCT mediated by GB-10 and GB-10 + BPA in experimental oral cancer. Appl Radiat Isot 61:939–945
Trivillin VA, Heber EM, Nigg DW et al (2006) Therapeutic success of boron neutron capture therapy (BNCT) mediated by a chemically non-selective boron agent in an experimental model of oral cancer: a new paradigm in BNCT radiobiology. Radiat Res 166:387–396
Trivillin VA, Heber EM, Rao M et al (2008) Boron neutron capture therapy (BNCT) for the treatment of spontaneous nasal planum squamous cell carcinoma in felines. Radiat Environ Biophys 47:147–155
van der Kogel AJ (1991) Central nervous system radiation injury in small animal models. In: Gutin PH, Leibel SA, Sheline GE (eds) Radiationinjury to the nervous system. Raven, New York, pp 91–111
Yang W, Barth RF, Carpenter DE et al (1996) Enhanced delivery of boronophenylalanine for neutron capture therapy by means of intracarotid injection and blood–brain barrier disruption. Neurosurgery 38:985–992
Yang W, Barth RF, Rotaru JH et al (1997) Boron neutron capture therapy of brain tumours: enhanced survival following intracarotid injection of sodium borocaptate with or without blood–brain barrier disruption. Int J Radiat Oncol Biol Phys 37:663–672
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Hopewell, J.W., Morris, G.M., Schwint, A.E., Coderre, J.A. (2012). Boron Neutron Capture Therapy: Application of Radiobiological Principles. In: Sauerwein, W., Wittig, A., Moss, R., Nakagawa, Y. (eds) Neutron Capture Therapy. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-31334-9_17
Download citation
DOI: https://doi.org/10.1007/978-3-642-31334-9_17
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-31333-2
Online ISBN: 978-3-642-31334-9
eBook Packages: MedicineMedicine (R0)