Abstract
Locally, industrial and volcanic exhalation of vanadium oxides can cause a vanadium overload in soil surface areas. In an oxic environment, vanadium compounds will be oxidized to vanadate(V), which is soluble and thus drains into the ground water. Since vanadate(V) acts as a phosphate antagonist, it is potentially toxic. Soil bacteria such as Geobacter metallireducens and Shewanella oneidensis reduce vanadate to insoluble and comparatively harmless vanadium(IV) hydroxide. The remobilization of vanadium(IV) can occur by strong chelators excreted by other bacteria such as Azotobacter. In lead water pipe scales, insoluble vanadinite deposits form, from which the drinking water contaminants lead(II) and vanadate(V) are remobilized under acidic conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
11.1 Introduction
Vanadium is the 21st element in abundance in Earth’s crust. Although more than 120 minerals are known, the main amount of vanadium is distributed in such a way that this element is ubiquitous and omnipresent. Most vanadium minerals have formed by geological processes, but a biogenic formation by several strains of bacteria, employing vanadium(V) as an electron acceptor in respiration, is also conceivable. Vanadium is further enriched in fossil “fuels,” where it accumulates in the form of vanadyl porphyrins. Burning of these fuels, exposure to vanadium oxides in the course of mining and processing of vanadium ores, and re-mobilization of vanadate from vanadinite (a lead vanadate) accumulated in the scales of lead water pipes, are the main sources for potential hazardous vanadium exposure.
Vanadium compounds are classified as mutagenic and teratogenic, and potentially carcinogenic. Toxic levels of vanadium in humans are, except in case of direct exposure, not easily achieved. The main health problems appear to arise from the inhalation of vanadium pentoxide. The structural similarity of vanadate and phosphate, plus the stability (in contrast to phosphate) of penta-coordinated vanadium, give rise to a vanadate–phosphate antagonism and synergism which can be both beneficial (in the sense of vanadate as a regulator of the phosphate metabolism) and detrimental (by inhibition of phosphate-activated substrates and phosphate-dependent enzymes). The inhibition of a protein–tyrosine phosphatase by vanadate, lending vanadium compounds insulin-enhancing properties, is an example for an inhibition with benign consequences. Along with possible health hazards arising from the vanadate–phosphate antagonism, vanadium compound are involved in the production as well as the annihilation of reactive oxygen species, and thus, potentially, in the proliferation as well as in the slowdown of the growth of tumor cells. Trapping of alkyl radicals by vanadium compounds points into the same direction.
The essentiality of vanadium for life, in particular for human life, has not yet been established, although, based on the regulatory function of vanadate in the phosphate metabolism, vanadium is likely to be an essential trace element. Vanadium(V) (in the form of vanadate) is more toxic than vanadium(IV) (in the form of the vanadyl ion and its compounds). The lower toxicity of vanadium(IV) is a result of the insolubility of vanadyl hydroxide. VV and VIV readily interconvert under physiological conditions. Vanadium(III) does not appear to play a pivotal role.
Vanadium overloads, due to acute exposure to vanadium compounds, are comparatively rapidly removed from serum and tissues; here, half-lives amount to a few hours at the worse. Vanadate is built in into the hydroxyapatite lattice of the bones, a fact which increases the overall half-life of vanadium to a couple of days, corresponding to a residence time of about a month. Acute intoxication can be treated with reducing agents such as ascorbic acid, with medications working both as reductants and chelators, such as catechins (an oligophenol), and with efficient chelating agents, e.g., desferoxamine.
11.2 The Aqueous Chemistry of Vanadium
Under physiological conditions, vanadium can occur in the oxidation states +III, +IV, and +V, which are also the oxidation states of vanadium in terrestrial minerals (Sect. 11.3). Vanadium(II) is unstable under oxic conditions. VIV and VV are by far the most important oxidation states in aqueous media, and thus in living organisms. Under normal physiological conditions, VIII, when generated by sufficiently strong reductants or by disproportionation of VIV, is short-lived and rapidly re-oxidized to higher oxidation states. An exception are the vanadocytes of ascidians, where VIII is stable in the highly acidic cytosol of these blood cells (Sect. 11.3).
Vanadium(V) may occur in cationic form (monooxidovanadium(V) VO3+ or dioxidovanadium(V) VO +2 ) and in anionic form as vanadate. While the cationic variants are soluble only when coordinated to – and thus “protected” (against hydrolysis) by – sufficiently strong ligands, the bare vanadates are readily soluble in water. Oxygen has a high affinity to VV and VIV; the cationic species are therefore almost always present in the form of these oxido ions. Eventually, deoxygenation of VO2+ can occur, e.g., by interaction with thiols such as provided by cysteine residues RSH in peptides and proteins (11.1). In blood serum, most of the vanadium in the +IV and +V states is coordinated to (apo)transferrin and, to a much lesser extent, to serum albumin.
Although “nonoxido” vanadium(IV) exists in nature (see amavadin; Sect. 11.3), the by far more common form of vanadium(IV) is VO2+, embedded in the center of coordination compounds. “Free” VO2+ is hardly available under physiological conditions, i.e., in the pH range 5–8, because it forms almost insoluble oxidovanadium hydroxides “VO(OH)2,” commonly termed vanadyl hydroxides in the literature. The solubility of vanadyl hydroxide lies in the nanomolar range; the species present in solution is the anion [VO(OH)3]−. This tetrahedral vanadate(IV) anion is iso-structural with the simplest vanadate(V) anion [VO2(OH)2]− ≡ H2VO −4 (at pH 7), and phosphate [PO3(OH)]2− ≡ HPO 2−4 (again at pH 7). The reason for the vanadate–phosphate antagonism, to be addressed in Sect. 11.3, roots in this structural similarity.
The speciation of vanadate(V) very much depends on the pH and the concentration. At pH 7 and concentrations in the micro and sub-micromolar range (and hence at physiological conditions), the dominant species is diprotonated monovanadate H2VO −4 . The pK a for the protonation equilibrium \( {{\hbox{H}}_2}{\hbox{V}}{{\hbox{O}}_4}^{-} \leftrightarrows {\hbox{HV}}{{\hbox{O}}_4}^{2 - } + {{\hbox{H}}^{+} } \) at an ionic strength of 0.15 mM (blood serum) is 8.5 (Schmidt et al. 2001). At concentrations exceeding ca. 0.1 mM, divanadate HnV2O7 (4−n)− (n = 1, 2) and tetravanadate V4O12 4− form in the pH range 5–9. At still higher concentrations, pentavanadate V5O15 5− comes in, and at pH < 6.3, decavanadates HnV10O28 (6−n)− (n = 1–3) are present. At pH > 6.3, decavanadate is thermodynamically unstable; it is, however, kinetically stable to the extent that its decay into vanadates of lower nuclearity takes several hours. The speciation diagrams in Fig. 11.1 convey an impression of the vanadate species present in water as a function of pH and concentration. In the mixed vanadate–phosphate system, the labile mixed species H2VPO7 2− is present (Andersson et al. 2005).
Speciation diagrams of vanadate for c(V) = 1 mM (left) and 1 μM (right) at an ionic strength of 0.15 mM Na(Cl) and a temperature of 25°C; x V is the mole fraction. V+ = [VO2(H2O)4]+, V10 = decavanadate, V4 = tetravanadate, V2 = divanadate, V− = H2VO −4 , V2− = HVO 2−4 . Courtesy L. Pettersson, Umeå University (Sweden)
Vanadate and vanadyl are linked by the redox couple depicted in (11.2). The redox potential at pH 7, EpH = 7 = −0.34 V, compares to that of NAD+/NADH (EpH = 7 = −0.315 V). Vanadate(V) is readily reduced to VO2+ under physiological conditions by reductants such as glutathion and NADH, while VO2+ in turn is readily oxidized in the presence of oxygen or reactive oxygen species (ROS). VO2+ by itself can be involved in the generation of ROS such as superoxide (11.3), and hydroxyl radicals (Sakurai 1994) (11.4).
11.3 Occurrence and Biological Usage
The abundance of vanadium in Earth’s crust, 0.013% w/w, compares to that of carbon (0.02%) and nitrogen (0.017%). In seawater, where vanadium is present in the form of ion pairs Na+H2VO −4 at a concentration of ca. 30 nM, it is the second-to-most abundant transition metal, only outplayed by molybdenum (100 nM, present in the form of molybdate). The comparatively high abundance of V and Mo in sea water and other oxic aqueous systems reflects the fact that these two anionic transition metal species are easily soluble in water. In volcanic regions, concentrations of vanadate in subsoil water can go up to 120 μg L−1 (2.4), exceeding the notification level, 15 μg vanadium per liter, set by, e.g., the California Office of Environmental Health Hazard Assessment.
In rock, vanadium is an omnipresent trace element. In vanadium-based minerals, vanadium occurs in the oxidation states +III, +IV, and +V. Representative examples are roscoelite K(Al,VIII)2(OH,F)2[AlSi3O10], patronite VIV(S2)2 and vanadinite PbCl2⋅3Pb3[VVO4]2. In addition, vanadium(+II) has been retrieved in meteorites, such as in chondrules of the Vigarano meteorite, where octahedral magnesium sites in the mineral forsterite (Mg2SiO4) are partially replaced by V2+ (Giuli et al. 2006). Vanadium minerals are mainly formed in the course of geological processes, but biogenic formation of some minerals, based on oxidovanadium(IV) hydroxide, is an additional option (vide infra). Further, vanadium can accumulate in fossilized bio-mass, such as peat, coal, crude oil, bitumen, and oil-shales. Venezuelan crude oil can contain up to 0.12% vanadium. This enrichment reflects a secondary process: the extraction of vanadium from vanadium-bearing rock as oil passes through these rocks. Crude oil contains porphinogens, originating from hemes and chlorophyll-derived porphyrins of the fossilized organisms, and these porphyrinogenic systems are excellent ligands which extract vanadium from rock and firmly complex the VO2+ cation.
Certain bacteria, such as the soil bacterium Shewanella oneidensis, can use vanadate as an electron acceptor in respiration (Carpentier et al. 2005), Fig. 11.2. The reduction occurs at the outer cell wall, and the reduction product is vanadyl hydroxide of the approximate formula VO(OH)2. Around pH 7, vanadyl hydroxide is an almost insoluble compound, which eventually can mineralize and thus give rise to the formation of minerals resembling sherwoodite, a mixed-valence (VIV/VV) calcium–aluminum polyoxidovanadate. The electrons for the reduction are delivered via the oxidation of lactate to pyruvate in the cytosolic membrane. The electron transport across the periplasm is accomplished by oligomeric heme proteins, {Fe2+/3+}; for the reaction sequence see (11.5). Other bacteria, e.g., Pseudomonas isachekovii, use molecular hydrogen and carbon monoxide as reductants (Antipov et al. 2000) (11.6).
Electron transfer pathway in the membrane of Shewanella oneidensis (upper left): Electrons and protons delivered by the oxidation of lactate to pyruvate (box at lower left) are picked up by a quinone (Q) and delivered to cytochrome-c type hemoproteins, which finally carry the reduction equivalents to the outer membrane, where VV is reduced to VIV. The reduction of vanadate is coupled H+ influx into the cytosol and ATP synthesis at the inner membrane
The bacterium Geobacter metallireducens has been employed to effectively remove vanadate from groundwater contaminated by mining activities, employing acetate for electron delivery (Ortiz-Bernad et al. 2004) (11.7). The insoluble compound formed in this process resembles the mineral sincosite CaV2(OH)4[PO4]2⋅3H2O, symbolized by {VO(OH)2} in (11.7).
Other microorganisms, such as the proteobacterium Azotobacter vinelandii and the cyanobacterium Anabaena variabilis use vanadium as a constituent of the cofactor, a Fe7VS9 cluster, of a vanadium-dependent nitrogenase for the ATP-driven conversion of N2 + H+ to ammonium ions and molecular hydrogen (Eady 2003; Rehder 2008a) (11.8), and eventually for the production of alkanes and alkenes from carbon monoxide and hydrogen (Lee et al. 2010). For the latter reaction, see the non-stoichiometric (11.9). The reactions are analogous to the Haber–Bosch and Fischer–Tropsch syntheses, respectively, but proceed, in contrast to these industrial processes, at ambient pressure and temperature. A. vinelandii synthesizes the vanadophore azotochelin (Bellenger et al. 2007), 1 in Fig. 11.3, which is an efficient transporter for vanadium(V).
Another vanadium-based group of enzymes, the vanadate-dependent haloperoxidases, are present in marine algae, and in fungi and lichen. In these organisms, vanadate H2VO –4 is coordinated to an imidazol nitrogen of a histidine in the active site protein pocket. The enzyme catalyzes the two-electron oxidation of halide, Hal− (Cl−, Br−, I−), to a Hal+ species such as hypohalous acid, (11.10a) for Hal = Br, which then halogenates organic substrates RH, (11.10b). The enzymes also catalyze the oxidation of (prochiral) sulfides to (chiral) sulfoxides (11.11). Oxidant is hydrogen peroxide. The active center of these peroxidases, with vanadium(+V) in a trigonal–bipyramidal {O=VO(OH)2N} coordination environment (2 in Fig. 11.3), is analogous to the respective center in vanadate-inhibited phosphatases, cf. Sect. 11.4.
Vanadium is further accumulated in mushrooms of the genus Amanita, e.g., in the toad stool (fly agaric) Amanita muscaria, and further by some ascidians (sea squirts) and fan worms. The non-oxido vanadium(+IV) compound isolated from A. muscaria, amavadin (3 in Fig. 11.3), contains vanadium coordinated to (S,S)-N-hydroxyimino-2,2′-diisopropionic acid (Berry et al. 1999), an organic derivative of hydroxylamine. Given the versatility of vanadium in redox catalysis, amavadin may be a relic of a vanadium-dependent oxidase or oxygenase, used by the mushrooms in an earlier stage of their evolution (Rehder 2008a). Many ascidians take up vanadate from sea-water, reduce it to VO2+ by means of a reductase with NADPH as the cofactor, and bind VO2+ to lysine residues of a (Cys)2-rich protein called vanabin (Ueki et al. 2009). The vanadyl cation is further reduced to vanadium(III) and stored, mainly in the form of [VO(H2O)4(HSO4)]+, in the highly acidic medium of special ascidian blood cells termed vanadocytes. The highest vanadium concentration, 0.35 M and hence an accumulation from sea water by a factor of 108, has been measured in Ascidia gemmata. The function of vanadium in these marine animals has remained elusive during the almost 100 years that have elapsed since its discovery in ascidians.
11.4 Physiological Effects and Toxicity
The total body pool of vanadium is low; depending on the source of information, it varies between ca. 0.1 and 1 mg (Wenning and Kirsch 1988; Léonard and Gerber 1994), corresponding to an approximate concentration of c = 0.03–0.3 μM. The average vanadium concentration in blood is 2.3 μg L−1 (c = 45 nM) (Leuschner et al. 1994). Vanadium contents in food are typically around 30 μg V kg−1 food (Wenning and Kirsch 1988), while drinking water commonly contains less than 10 μg L−1 (c < 0.2 μM) (Cohen 1996). In basaltic volcanic areas, the concentration can go up by an order of magnitude. Breathable air contains 0.25–75 ng V m−3 in rural areas, and 60–300 ng m−3 in urban settings (Cohen 1996). The daily overall intake, dominated by dietary sources, amounts to 0.01–2 mg vanadium (Cohen 1996; Scior et al. 2005). Less than 1% of this remains absorbed: vanadium, in particular in its oxidation state +IV, is effectively excreted through feces and urine. The no-effect level is an intake of about 10 mg kg−1 day−1; hence, normal diet-related exposure to vanadium does not affect our health.
High exposure risks (>30 mg m−3 in the breathable air), coupled with potentially severe health problems, go along with the mining and milling of vanadium containing ores, the production of vanadium metal, vanadium oxides used in redox catalysis and batteries (Tracey et al. 2007), and fly ashes from oil firing. The latter problem arises from the sometimes high amounts of vanadium (present as vanadyl porphyrins) in crude oil; cf. Sect. 11.3. The worldwide industrial emission of vanadium into the atmosphere totaled 71,000 tons per year in 1995 (Nriagu and Pirrone 1998), which may be extrapolated to 85,000 tons in 2010. This compares to an estimated world-wide emission of vanadium from natural sources (continental dust, volcanic activity, forest fires) of approximately 10 tons per year.
The toxicity of vanadium compounds is generally low. Orally applied, pentavalent compounds (vanadates) are more toxic than tetravalent compounds (vanadyl) because the latter form almost insoluble VO(OH)2 in the gastro-intestinal tract. In the US, vanadyl sulfate VOSO4 is a common supplement, in daily doses up to 60 mg, for athletes for an alleged increase their muscle mass (Barceloux 1999). Vanadium pentoxide V2O5, the main aerial contaminant, is potentially mutagenic and teratogenic. In mice, V2O5 causes pulmonary inflammation and tumor promotion (Rondini et al. 2010). In rats, vanadium compounds cause DNA cleavage, likely as a results of the intermittent formation of reactive hydroxyl radicals (Sakurai 1994); (11.4) in Sect. 11.2. Vanadium oxides may therefore be considered “suspected carcinogenic compounds” for humans: In workers exposed to the inhalation of V2O5, its metabolites cause oxidation of DNA, affect DNA repair, and induce the formation of tumor-associated antigens (Ehrlich et al. 2008). On the other hand, vanadium compounds such as sodium vanadate, VO(acac)2 (acac = acetylacetonate(1-)), and VO(maltolate)2 (bis(maltolato-oxidovanadium(IV), BMOV) inhibit the proliferation of hepatoma cells to a higher extent than of hepatic cells, which is paralleled by lower levels of ROS in hepatoma than in hepatic cells (Wang et al. 2010).
Human vanadium poisoning symptoms are mainly restricted to the conjunctivae and respiratory system, renal and gastrointestinal irritation. Exposure can thus give rise to conjunctivitis, rhinitis, pulmonary inflammation resulting in bronchitis and asthma-like diseases, and dysfunctions of the digestive system. The limit value for immediate danger to health for an average human is about 7 mg V in the case of intravenous application, and 35 mg V m−3 in breathing air. The following compilation lists selected official exposure limits (MAC) and LD50/LC50 values. MAC refers to the maximum allowable concentration at the workplace (40-h week, 8-h time-weighted average). LD50 and LC50 indicate the level of a harmful substance (in mg per kg body weight) causing the death of 50% of the test animals by oral (LD) or inhalative (LC) administration, respectively.
-
V2O5: MAC: 0.05 mg m−3
-
V2O5: LD50 (rat, oral) 10 mg kg−1
-
V2O5: LD50 (mouse, oral): 5 mg kg−1
-
V2O5: LC50 (rat) 126 mg m−3, 6 h exposure
A biological threshold limit of 50 μg V g−1 of creatinine in urine collected at the end of the work week has been adopted in the US. Vanadium is also toxic for aquatic organisms: LD50 values are 4.8 mg L−1 for soft water, and 30 mg−1 for hard water. The lower toxicity in hard water reflects the formation of sparingly soluble calcium vanadates.
Irritation of the conjunctivae and pulmonary systems either directly by vanadium oxides (V2O5, V2O4, V2O3) and/or the oxidovanadium moieties VO +2 , VO3+, and VO2+ formed by solubilization of the oxides in the physiological systems goes along with the generation of reactive oxygen species (H2O2, and the radicals \( {{\hbox{O}}_{{2}{^\bullet} }}^{-} \) and •OH), considered to be the actual agents responsible for tissue impairment, either by oxidative damage and/or intervention with the phosphorylation of signaling and transcription pathways (Rondini et al. 2010). In hepatic cells, N-acetylcysteine can ameliorate this (indirect) cytotoxicity (Wang et al. 2010). While oxidovanadium species are usually considered to generate ROS, they can also consume ROS, e.g., in the course of the oxidation of vanadyl to vanadate(V) reversal of (11.4) in Sect. 11.2. Further, trivanadate V3O 3−9 , and VO +2 , complexed to sylicylidenehydrazide ligands, have been shown to consume alkylating toxins (Hamilton et al. 2006; Fautch et al. 2009), thus preventing DNA alkylation and, concomitantly, cancer risk.
Physiological effects of vanadium applied in the form of vanadate or simple vanadium complexes also arise from its direct intervention with phosphatases, phosphorylation enzymes, kinases, ribonucleases, and the phosphate metabolism in general (Tracey et al. 2007; McLauchlan et al. 2010). These interventions go back to the structural similarity between vanadate VO(OH) −3 /H2VO −4 and phosphate HPO 2−4 on the one hand (cf. also Sect. 11.1), and the ability of the transition metal vanadium to enlarge its coordination sphere and thus to form stable penta- and hexa-coordinated coordination compounds on the other hand. In contrast, penta-coordinated phosphorus only exists as a transitory species in, e.g., phosphatase reactions. In Fig. 11.4, this is pictured for the pentavalent transition state formed in the course of the phosphoester cleavage by a phosphatase with histidine in the active center, and the inhibition of this hydrolysis through the build-in of vanadate into the active site of the enzyme. The awareness of the role of vanadate as a phosphatase inhibitor goes back to the discovery of the switch-off of the Na+, K+-pump by sodium vanadate (Cantley et al. 1977). Trace amounts of vanadium have since been proposed to be an essential regulatory nutrient for most if not all living beings. The omnipresence of oxidic vanadium in soil, and of vanadate in the aqueous medium, has likely provoked an adaption – in terms of beneficial use – to vanadium already in the primordial development of life (Rehder 2008b). This is also suggested by the similarity of the active centers of vanadate-dependent haloperoxidases and vanadate-inhibited phosphatases (2 in Fig. 11.3). Interestingly, vanadate-inhibited phosphatases can exhibit some haloperoxidase activity (Tanaka et al. 2002).
The vanadate–phosphate analogy (Stankiewicz and Tracey 1995; Crans et al. 2004; Steens et al. 2009) is also the key to the potency, in the treatment of diabetes mellitus, of vanadate, vanadyl and simple vanadium compounds such as BMOV and its ethyl analog, bis(ethylmaltolato)oxidovanadium BEOV. Here, vanadium acts as a regulator of glucose homeostasis and inhibits free fatty acid release. The active species likely is vanadate H2VO −4 , a key “end-products” in physiological turn-over of vanadium compounds (vide infra). The mode of action possibly is by inhibition of a protein tyrosine phosphatase at the cytosolic site of the cellular insulin receptor and/or the activation of a tyrosine kinase in the signaling path (Sakurai et al. 2006). So far, BEOV has been the only vanadium compound to be subjected to clinical tests (phase II). The tests have, however, been abandoned due to renal problems with some of the probands.
Other beneficial modes of action of vanadate compounds, as tested ex vivo (with cell cultures) and, in part, in vivo (with animals) include the treatment of certain cancer forms (such as leukemia, Ehrlich ascites tumors, carcinomas of the lungs, prostate, testes, ovaria, and liver), amoebiasis (Maurya et al. 2006), tuberculosis, HIV, and herpes. VO2+ complexed to sugars such as the disaccharide trehalose promotes both, glucose consumption in osteoblasts and inhibition of the proliferation of tumoral osteoblasts (Barrio et al. 2003; Etcheverry et al. 2009).
11.5 Uptake, Speciation, Excretion, and Detoxification
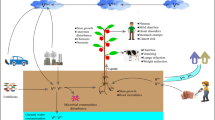
The main pathways for uptake, speciation, distribution, and excretion of vanadium compounds are illustrated in Fig. 11.5. Dietary vanadium may be vanadate, vanadyl (VO2+), e.g., in the form of VOSO4, or vanadium compounds with organic ligands (symbolized {V} in Fig. 11.5), such as VO(acac)2 and BEOV. Inhaled vanadium species, mainly ingested by the pulmonary tissue, commonly are particulate vanadium oxides (V2O5, V2O4, V2O3) or essentially oxidic vanadium minerals. Basic dietary vanadium species will be oxidatively converted to vanadate(V) in the oral cavity, transformed to decavanadate or even VO +2 (Sect. 11.1) in the acidic medium of the stomach, and reconverted to vanadate and vanadyl under the slightly alkaline and reducing conditions in the small intestines. Vanadium complexes may or may not survive the medial conditions of the gastrointestinal tract. In case they are unstable under the conditions prevailing there (such as the low pH in the stomach), decomposition and generation of vanadate/vanadyl takes place. Once transferred into the blood, most of the vanadium is complexed by transferrin (Tf) and apoTf. The complexation constant for the binding of VO2+ to apoTf, as defined by (11.12), is 10−14.7 (Kiss et al. 2006), i.e., only those complex {V} species survive the transport through the blood which are thermodynamically more stable than VO2+-Tf, or whose degradation is kinetically hampered. VO2+-Tf can be taken up by the cells via endocytosis; vanadate may also directly enter the cells through phosphate channels. Once arrived within the tissue cells, even stable complexes will be converted to basic inorganic vanadium compounds. Given the commonly reducing conditions in the cytosol, provided by reductants such as NAD(P)H, FADH2, glutathione, ascorbate, and catecholamines, the predominant cytosolic species likely is VO2+, stabilized to some extent by coordination to cytosolic constituents capable of ligating vanadium (and here again, glutathione and ascorbate come in; Rehder et al. 2002). Where VO2+ becomes involved in the production of ROS (11.1), or where ROS are formed from other sources, vanadate(V) H2VO −4 will form.
The retention time of vanadium in tissue is up to 30 min (Yasui et al. 2002). Part of the vanadium is, however, stored in the bones, where it’s half-life is 4–5 days (Setyawati et al. 1998): VO2+ is absorbed to the bone surface, while H2VO −4 is incorporated in the hydroxyapatite lattice, where it can replace phosphate (Rehder 2008a; Etcheverry et al. 2009). The final excretion of resorbed vanadium is essentially via the kidneys, i.e., most of the vanadium is recovered in the urine.
Vanadium concentrations in serum after intravenous injection to humans in the form of vanadate in a 20% albumin infusion (containing a total amount of 47.6 μg V) declined rapidly within a few hours (<30% after 24 h). Subsequently, serum vanadium concentrations dropped more slowly, approaching zero after a month (Heinemann et al. 2003). In general, vanadium’s toxicological and pharmacological potential very much depends on the mode of vanadium intake/application (intravenous, subcutaneous, oral, inhalation, absorption through mucosae), individual response, and modulating factors such as age, sex, exercise, stress, and the nutritional state (Thompson et al. 1998).
While global anthropogenic vanadium emission is not likely to constitute a significant health risk, local exposure of workers in industrial enterprises processing vanadium ores and compounds can result in severe health problems caused by vanadium toxication (see Sect. 11.4). Also, local increase of vanadium contents in soil, originating from the deposition of vanadium oxides in the course of industrial activities or excessive local burning of fossil fuels, may cause a potential health problem for the population in the respective area. Vanadium may further be mobilized from its deposits, and thus arrive in surface and ground water, eventually ending up in drinking water. Mobilization can come about by solubilization of oxidic vanadium, i.e., the formation of water-soluble vanadate, in particular in acidic soils, and by complexation and thus solubilization of vanadyl VO2+ in non-oxic environments by siderophores, humic acids, and other organic constituents with suitable ligand properties. Even at vanadium levels <15 μg L−1, the notification level set for drinking water by the US Environmental Protection Agency, accumulation of vanadium up to 2% by weight in the corrosion deposits in lead drinking water pipes can occur – a potential reservoir for human exposure by municipal water systems, if this vanadium becomes re-mobilized by alterations in the drinking water characteristics (Gerke et al. 2009).
The formation of vanadinite, PbCl2⋅3Pb3[VVO4]2, or Pb5[VO4]3Cl for short, and the re-mobilization of vanadate can be formulated as depicted in the equilibrium (11.13), the Pb2+ ions being delivered via, e.g., divalent lead (hydroxy)carbonates and lead phosphate in the lead pipe. Equation (11.13) also demonstrates that remobilization of vanadate from insoluble vanadinite takes place as the medium becomes sufficiently acidic. At higher phosphate concentrations, chloropyromorphite Pb5[PO4]3Cl is more stable than vanadinite (Gerke et al. 2009); excess phosphate thus can destabilize vanadinite. Hence, while the formation of vanadinite deposits in the pipe scales is a means of detoxification, a decrease of pH or the presence of excess phosphate (such as provided by orthophosphate-based corrosion inhibitors) can result in re-toxification of drinking water (11.14).
Most cases of vanadium toxication will recover on removal from exposure and symptomatic treatment. In case of vanadium ingestion, application of ascorbic acid, followed by 2,3-mercapto-1-propanol in a later stage has been recommended (International Programme on Chemical Safety, Health and Safety Guide, no. 42, 1990). Both agents are likely to effect reduction of vanadium(V) to vanadium(IV), which transforms into insoluble and hence essentially harmless vanadyl hydroxides in the small intestines. By extrapolation from animal studies (mice, rats, rabbits, calves), treatment of toxic effects related to vanadium might be achieved by drinking green tea (Soussi et al. 2009), or by treatment with chelating agents such as ethylenediaminetetraacetate (EDTA) (Domingo et al. 1986; Gummow et al. 2006), deferoxamine mesylate (Domingo et al. 1986), or 4,5-dihydroxy-1,3-benzene disulphonate (Tiron) (Shrivastava et al. 2007); Fig. 11.6. Green tea from Camellia sinensis contains oligophenols such as catechins (4 in Fig. 11.6), which can act as antioxidants and as chelators for metal ions such as VO2+, VO3+, and VO +2 . Similarly, Tiron (5) combines these two properties, while EDTA (6) and deferoxamine (7 in Fig. 11.6) are strong chelating agents, which are also used in masking other metal ions.
11.6 Conclusion
The categorization of vanadium, by the WHO and national health organizations, as mutagenic, teratogenic, and potentially cancerogenic affords special awareness in handling this element, and precautions when exposed to it. In the light of the potential hazards, the approval, in North America, of vanadyl sulfate as a food additive (“vanadyl fuel”) consumed by athletes for a putative increase of the muscle mass appears to be an unorthodox issue. On the other hand, since less than 1% of bare vanadyl VO2+ is absorbed, its adverse effect should be minimal.
On a general basis, exposure to vanadium present in the environment (drinking water, food, aerial dust) is a negligible problem. Acute vanadium poisoning has so far only been observed with workers directly exposed to inhalation of vanadium oxide at the working place, and with animals injected or fed high doses of vanadium compounds. As far as workplace exposure is concerned, a maximum allowable concentration (MAC value) of 0.05 g V m−3 has been assessed. The increasing use of vanadium compounds in catalysis (e.g., V2O4 in the production of sulfuric acid, vanadate esters and ester chlorides in polymerization catalysis), in silver vanadium oxide batteries, and in vanadium steels, may henceforward increase the contamination, by vanadium, of water resources and the atmosphere. Oncoming developments for industrial applications of vanadium include vanadiumoxide-based nanotubes, -rods and -wires, metal-organic frameworks (MOFs), composite vanadates/silicates, and large polyoxidometalates (POMs). Another source for potentially increasing vanadium pollution and hence increasing health hazards is the burning of petrol and other fossil fuels, which can contain high amounts of porphyrinogenic vanadium compounds. Finally, remobilization of vanadate from vanadinite accumulating in the scales of lead water pipes, is a potential problem. For the decontamination of soil and wet areas containing an overload of vanadium (V), bacteria which reduce VV (V2O5, vanadate) to insoluble vanadyl hydroxide are an option. Geobacter metallireducens and Shewanella oneidensis are promising candidates.
As is common with elements which, at higher doses and under specific conditioning are toxic, beneficial effects, e.g., the treatment of cancer, amoebiasis, and diabetes mellitus may come in. Bis(ethylmaltolato)oxidovanadium(IV), BEOV, has been a bearer of hope in this respect for the treatment of diabetes for a couple of years. This potential insulin-enhancing drug has faced a draw-back on occasion of clinical phase II tests – which fact does not imply that similar vanadium compounds, or novel developments based on vanadium will be more successful and hence beneficial.
References
Andersson I, Gorzsás A, Kerezsi C, Tóth I, Pettersson L (2005) Speciation of the aqueous H+/H2VO −4 /H2O2/phosphate system. Dalton Trans 3658–3666
Antipov AN, Lyalikova NN, L’vov NP (2000) Vanadium-binding protein excreted by vanadate-reducing bacteria. IUBMB Life 49:137–141
Barceloux DC (1999) Vanadium. J Toxicol Clin Toxicol 37:265–278
Barrio DA, Williams PAM, Cortizo AM, Etcheverry SB (2003) Synthesis of a new vanadyl(IV) complex with trehalose (TrVO): insulin-mimetic activities in osteoblast-like cells in culture. J Biol Inorg Chem 8:459–468
Bellenger JP, Arnaud-Neu F, Asfari Z, Myeni SCB, Stiefel EI, Kraepiel AML (2007) Complexation of oxoanions and cationic metals by the biscatecholate siderophore azotochelin. J Biol Inorg Chem 12:367–376
Berry RE, Armstrong EM, Beddoes RL, Collison D, Ertok SN, Halliwell M, Garner CD (1999) The structural characterization of amavadin. Angew Chem Int Ed Engl 38:795–798
Cantley LC Jr, Josephson L, Warner R, Yanagisawa M, Lechene C, Guidotti G (1977) Vanadate is a potent (sodium, potassium ion)-dependent ATPase inhibitor in ATP derived from muscle. J Biol Chem 252:7421–7423
Carpentier W, De Smet L, Van Beeumen J, Brigé A (2005) Respiration and growth of Shewanella oneidensis MR-1 using vanadate as the sole electron acceptor. J Bacteriol 187:3293–3301
Cohen MD (1996) Vanadium and its immunotoxicology. Toxicol Ecotoxicol News 3:132–135
Crans D, Smee JJ, Gaidamauskas E, Yang L (2004) The chemistry of vanadium and the biological activities exerted by vanadium compounds. Chem Rev 104:849–902
Domingo JL, Llobet JM, Tomas JM, Corbella J (1986) Influence of chelating agents on the toxicity, distribution and excretion of vanadium in mice. J Appl Toxicol 6:337–341
Eady RR (2003) Current status of structure function relationships of vanadium nitrogenase. Coord Chem Rev 237:23–30
Ehrlich VA, Nersesyan AK, Hoelzl C, Ferk F, Bichler J, Valic E, Schaffer A, Schulte-Herrmann R, Fenech M, Wagner K-H, Knasmüller S (2008) Inhalative exposure to vanadium pentoxide causes DNA damage in workers: results of a multiple end point study. Environ Health Perspect 116:1689–1693
Etcheverry SB, Ferrer EG, Gonzales-Baró AC, Parajón-Costa BS, Williams PAM (2009) Vanadis’ charms: from the mythology to the bioinorganic chemistry. J Argent Chem Soc 97:127–150
Fautch JM, Fanwick PE, Wilker JJ (2009) Oxidovanadium complexes for the consumption of alkylating toxins. Eur J Inorg Chem 33–37
Gerke TL, Scheckel KG, Schock MR (2009) Identification and distribution of vanadinite (Pb5(V5+O4)3Cl) in lead pipe corrosion by-products. Environ Sci Technol 43:4412–4418
Giuli G, Pratesi G, Eeckhout SG, Paris E (2006) Divalent vanadium in Vigarano CV3 meteorite chondrules. Proceedings of the 69th annual meeting of the meteoritical society meeting, Zurich, Switzerland, p 5148, 6–11 Aug 2006
Gummow B, Botha CJ, Williams MC (2006) Chronic vanadium poisoning in calves and its treatment with calcium disodium ethylenediaminetetraacetate. Vet Res Commun 30:807–822
Hamilton EE, Fanwick PE, Wilker JJ (2006) Alkylation of inorganic oxo compounds, and insight on preventing DNA damage. J Am Chem Soc 128:3388–3395
Heinemann G, Fichtl B, Vogt W (2003) Pharmacokinetiks of vanadium in humans after intravenous administration of vanadium containing albumin solution. J Clin Pharmacol 55:241–245
Kiss T, Jakusch S, Bouhsina S, Sakurai H, Enyedy A (2006) Binding constant of VIVO to transferrin. Eur J Inorg Chem 3607–3613
Lee CC, Hu Y, Ribbe MW (2010) Vanadium nitrogenase reduces CO. Science 329:642
Léonard A, Gerber GB (1994) Mutagenicity, carcinogenicity and teratogenicity of vanadium compounds. Mutat Res 317:81–88
Leuschner J, Haschke H, Sturm G (1994) New investigations on acute toxicities of vanadium oxides. Monatsh Chem 125:623–646
Maurya MR, Kumar A, Azam A, Bader C, Rehder D (2006) Dioxo- and oxovanadium(V) complexes of thiohydrazone ONS donor ligands: synthesis, characterization, reactivity, and antiamoebic activity. Inorg Chem 45:1260–1269
McLauchlan CC, Hooker JD, Jones MA, Dymon Z, Backhus EA, Greiner BA, Dorner NA, Youkhana MA, Manus LM (2010) Inhibition of acid, alkaline, and tyrosine (PTP1B) phosphatases by novel vanadium complexes. J Inorg Biochem 104:274–281
Nriagu JO, Pirrone N (1998) Emission of vanadium into the atmosphere. In: Nriagu JO (ed) Vanadium in the environment, part 1. Wiley, New York
Ortiz-Bernad I, Anderson RT, Vrionis HA, Loveley DR (2004) Vanadium respiration by Geobacter metallireducens: novel strategy for in situ removal of vanadium from groundwater. Microbiology 70:3091–3095
Rehder D (2008a) Bioinorganic vanadium chemistry. Wiley, Chichester
Rehder D (2008b) Is vanadium a more versatile target in the activity of primordial life forms than hitherto anticipated? Org Biomol Chem 6:957–964
Rehder D (2010) Leben ohne Vanadium? Bioanorganische Chemie des Vanadiums. Chem Unserer Zeit 44:322–331
Rehder D, Costa Pessoa J, Geraldes CFGC, Castro MMCA, Kabanos T, Kiss T, Meier B, Micera G, Pettersson L, Rangel M, Salifoglou A, Turel I, Wang D (2002) In vitro study of the insulin-mimetic behaviour of vanadium(IV, V) co-ordination compounds. J Biol Inorg Chem 7:384–396
Rondini EA, Walters DM, Bauer AK (2010) Vanadium pentoxide induces pulmonary inflammation and tumor promotion in a strain-dependent manner. Part Fiber Toxicol 7:9
Sakurai H (1994) Vanadium distribution in rats and DNA cleavage by vanadyl complex: implications for vanadium toxicity and biological effects. Environ Health Perspect 102:35–36
Sakurai H, Kato A, Yoshikawa Y (2006) Chemistry and biochemistry of insulin-mimetic vanadium and zinc complexes. Trial for treatment of diabetes mellitus. Bull Chem Soc Jpn 79:1645–1664
Schmidt H, Andersson I, Rehder D, Pettersson L (2001) A potentiometric 51V NMR study of the aqueous H+/H2VO −4 /H2O2/L-α-alanyl-L-histidine system. Chem Eur J 7:251–257
Scior T, Guevara-García A, Bernard P, Do Q-T, Domeyer D, Laufer S (2005) Are vanadium compounds drugable? Structure and effects of antidiabetic vanadium compounds: a critical review. Mini Rev Med Chem 5:995–1008
Setyawati IA, Thompson KH, Yuen VG, Sun Y, Battel M, Lyster DM, Vo C, Ruth TJ, Zeissler S, McNeill JH, Orvig C (1998) Kinetic analysis and comparison of uptake, distribution and excretion of 48V labeled compounds in rats. J Appl Physiol 84:569–575
Shrivastava S, Jadon A, Shukla S, Mathur R (2007) Chelation therapy and vanadium: effect on reproductive organs in rats. Ind J Exp Biol 45:515–523
Soussi A, Murat JC, Gaubin Y, Croute F, Soleilhavoup JP, El Feki A (2009) Green tea drinking reduces the effects of vanadium poisoning in rat kidney. Food Sci Technol Res 15:413–422
Stankiewicz PJ, Tracey AS (1995) Stimulation of enzyme activity by oxovanadium complexes. In: Sigel H, Sigel A (eds) Vanadium and its role in life, vol 31, Metal ions in biological systems. Marcel Dekker, New York
Steens N, Ramadam AM, Parac-Vogt TN (2009) When structural and electronic analogy lead to reactivity: the unprecedented phosphodiesterase activity of vanadates. Chem Commun 965–967
Tanaka N, Dumay V, Liao Q, Lange AJ, Wever R (2002) Bromoperoxidase activity of vanadate-substituted acid phosphatases from Shigella flexneri and Salmonella enterica ser typhimurium. Eur J Biochem 269:2162–2167
Thompson KH, Battell M, McNeill JH (1998) Toxicology of vanadium in mammals. In: Nriagu JO (ed) Vanadium in the environment, part 2. Wiley, New York
Tracey AS, Willsky GR, Takeuchi ES (2007) Vanadium, chemistry, pharmacology and practical applications. CRC, Boca Raton
Ueki T, Kawakami N, Toshishige M, Matsuo K, Gekko K, Michibata H (2009) Characterization of vanadium-binding sites of the vanadium-binding protein vanabin 2 by site-directed mutagenesis. Biochim Biophys Acta 1790:1327–1333
Wang Q, Liu T-T, Fu Y, Wang K, Yang X-G (2010) Vanadium compounds discriminate hepatoma and normal hepatic cells by differential regulation of reactive oxygen species. J Biol Inorg Chem 15:1087–1097
Wenning R, Kirsch N (1988) Vanadium. In: Seiter HG, Sigel H, Sigel A (eds) Handbook of inorganic compounds. Marcel Dekker, New York
Yasui H, Tamura A, Takino T, Sakurai H (2002) Structure-dependent metallokinetics of antidiabetic vanadyl-picolinate complexes in rats: studies on solution structure, insolinomimetic activity and metallokinetics. J Inorg Biochem 91:327–338
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Rehder, D. (2011). Transport, Accumulation, and Physiological Effects of Vanadium. In: Sherameti, I., Varma, A. (eds) Detoxification of Heavy Metals. Soil Biology, vol 30. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-21408-0_11
Download citation
DOI: https://doi.org/10.1007/978-3-642-21408-0_11
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-21407-3
Online ISBN: 978-3-642-21408-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)