Abstract
Azotochelin is a biscatecholate siderophore produced by the nitrogen-fixing soil bacterium Azotobacter vinelandii. The complexation properties of azotochelin with a series of oxoanions [Mo(VI), W(VI) and V(V)] and divalent cations [Cu(II), Zn(II), Co(II) and Mn(II)] were investigated by potentiometry, UV–vis and X-ray spectroscopy. Azotochelin forms a strong 1:1 complex with molybdate (log K = 7.6 ± 0.4) and with tungstate and vanadate; the stability of the complexes increases in the order Mo < V < W (log K Moapp = 7.3 ± 0.4; log K Vapp = 8.8 ± 0.4 and log K Wapp = 9.0 ± 0.4 at pH 6.6). The Mo atom in the 1:1 Mo–azotochelin complex is bound to two oxo groups in a cis position and to the two catecholate groups of azotochelin, resulting in a slightly distorted octahedral configuration. Below pH 5, azotochelin appears to form polynuclear complexes with Mo in addition to the 1:1 complex. Azotochelin also forms strong complexes with divalent metals. Of the metals studied, Cu(II) binds most strongly to azotochelin \({(\log \beta_{{{\text{CuLH}}^{{2 -}}}}=-12.9\pm 0.1)}\), followed by Zn(II) \({(\log \beta _{{{\text {ZnL}}^{{3 -}}}} =-24.1\pm 0.14, \log \beta _{{{\text {ZnLH}}^{{2 -}}}} =-17.83\pm 0.09)}\), Mn(II) \({(\log \beta _{{{\text {MnL}}^{{3 -}}}} = -29, \log\beta_{{{\text {MnLH}}^{{2-}}}}=-18.6\pm 0.8, \log \beta _{{{\text {MnLH}}_{2} ^{-}}} =-11.5\pm 0.7)}\) and Co(II) \({(\log \beta _{{{\text {CoLH}}^{{2 -}}}}= -23.0\pm0.3, \log \beta _{{{\text {CoLH}}_{2} ^{-}}}=-13.5\pm 0.2)}\). Since very few organic ligands are known to bind strongly to oxoanions (and particularly molybdate) at circumneutral pH, the unusual properties of azotochelin may be used for the separation and concentration of oxoanions in the laboratory and in the field. In addition, azotochelin may prove useful for the investigation of the biogeochemistry of Mo, W and V in aquatic and terrestrial systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most of the metals essential for life exist in cationic form in aqueous solutions, but a few of them, such as Mo, V and W, are present as oxoanions at biologically relevant pH values. Molybdenum is an essential component of the molybdenum nitrogenase that catalyzes the fixation of atmospheric N2 into ammonium. It is also found in various oxidoreductases, including nitrate reductase and nitrite oxidoreductase, which are other important enzymes in the nitrogen cycle [1]. Vanadium, in addition to being found in various bromoperoxidases and iodoperoxidases, is part of the alternative nitrogenase system that is expressed by some nitrogen fixers when molybdenum is unavailable [2–7]. A number of tungsten enzymes are known, most of which catalyze redox reactions of very low potentials, and a few hyperthermophilic archaea seem to have an absolute requirement for this element [8, 9]. Because tungstate has a chemical structure similar to that of molybdate, it is also a natural competitor for molybdate uptake by bacteria. In nitrogen-fixers, the replacement of molybdenum by tungsten in nitrogenase results in an inactive form of the enzyme [10–13]. Clearly, a better understanding of the speciation and bioavailability of Mo, V and W in the environment, and of the requirements of nitrogen-fixing bacteria for these elements, is needed to clarify their importance in the nitrogen cycle. In the past, these questions have presented a particular experimental challenge [14–17], in part because of the lack of a strong complexing agent for oxoanions.
Early reports indicated that azotochelin, a biscatecholate siderophore [18] produced by the nitrogen-fixing soil bacterium Azotobacter vinelandii binds strongly to molybdate and might be a “molybdophore” [19–22]. Although this hypothesis has never been confirmed [23], it emphasizes the need for a detailed study of the complexation properties of azotochelin in aqueous solutions.
Here, we report on the acid–base properties of azotochelin [N,N-di(2,3-dihydroxybenzoyl)-l-lysine] and on the formation of its complexes with molybdate, tungstate, vanadate and four bioactive metal cations often used in culture media: Cu(II), Zn(II), Mn(II) and Co(II). The stoichiometry and stability constants of the complexes were investigated by UV–vis spectroscopy and potentiometry. The influence of azotochelin on the speciation of Fe(III) in the presence of EDTA was also examined. The molecular interactions between azotochelin and molybdate and the structure of the resulting complexes in solution were characterized by X-ray absorption spectroscopy.
Materials and methods
Preparation of azotochelin
Azotochelin was prepared according to published procedures [24, 25]. The structure and the purity of the final product were confirmed by 1H NMR using a Bruker 400-MHz SY400 spectrometer and by mass spectrometry using a ZAB HF V6 analytical spectrometer.
Preparation of K2[MoO2(cat)2]·H2cat·H2O
Crystals of the Mo(VI)–catechol complex K2[MoO2(cat)2]·H2cat·H2O, whose structure was reported by Tkachev and Atovmyan [26], were prepared according to published procedures [27].
Stability constant determination
Ultrapure water was used for all complexation studies. The concentration of azotochelin in stock solutions was determined by UV–vis absorption, using the extinction coefficient determined by titration of an azotochelin solution with molybdate. Metal stock solutions were prepared by weighing known amounts of Na2MoO4·2H2O, Na2WO4·2H2O, NaVO3, CuCl2·2H2O, CoCl2·6H2O, MnCl2·4H2O, ZnSO4·7H2O, FeCl3·6H2O, NaH2PO4, Na2SO4 and Na2HAsO2. All metal stock solutions were purchased either from Sigma-Aldrich or from Fisher-Bioblock. Metal stock solutions, except those of molybdate, tungstate and vanadate, were standardized by the classic titration using EDTA and appropriate colored indicators. The concentrations of the stock solutions of Mo, W and V were verified by inductively coupled plasma mass spectrometry.
The stability constants of the azotochelin complexes with Cu(II), Co(II), Zn(II) and Mn(II) were determined potentiometrically by competition with the proton. The complexation of Zn(II) by azotochelin was further investigated by UV–vis spectrophotometry, as was the complexation of Mo(VI), W(VI), V(V) and Fe(III).
Potentiometric studies
Measurements were performed under an Ar atmosphere, using a combined glass electrode (Mettler Toledo) connected to an automatic titrator (Metrohm 716 DMS Titrino) equipped with a magnetic stirrer and a thermostated bath (25 ± 0.1 °C). The electrode was calibrated daily by titration of a 1 mM solution of HCl with a 10 mM solution of KOH, previously standardized against potassium phthalate. In the course of a titration, the amount of base added to the solution between two pH measurements was variable, and decreased near the equivalence points. In this procedure the reaction time, after base addition and before pH measurement, was 50 s.
The protonation constants of azotochelin were determined by titration of an azotochelin solution (5 × 10−4 M azotochelin and 1 equiv HCl in 0.1 M KCl) with a concentrated KOH solution standardized against potassium phthalate.
The stability constants β of the 1:1 azotochelin complexes with the cationic metals Cu(II), Co(II), Mn(II) and Zn(II) were determined by acid–base titration of solutions containing 1 equiv of azotochelin and 1 equiv of metal [metal concentration and ligand concentration both 5 × 10−4 M for Cu(II), Co(II) and Mn(II) and both 10−3 M for Zn(II)].
The titration data were interpreted using MINEQL [28], FITEQL [29, 30] SIRKO [31] and Hyperquad [32]. The autoprotolysis constant used for water was pK W = 13.78. The protonation constants for azotochelin were held constant during the refinement procedure for the determination of the stability constants β. The inorganic speciation of the metals in solution was calculated using the published constants [33].
Spectrophotometric studies
The stability constants of the azotochelin complexes with V(V), W(VI), Mo(VI) and Zn(II), as well as the relative affinity of Fe(III) for azotochelin and EDTA, were investigated by spectrophotometry. The experiments were performed with a Shimadzu UV-2101PC spectrophotometer equipped with a thermostated cell compartment (25 °C) and UVPC 32 software. The chemical speciation of V(V), W(VI), Mo(VI) and Zn(II) in solution was calculated using the published constants [33–35].
In a first set of experiments, 2 ml of a solution of 3 × 10−4 M azotochelin in N-(2-hydroxyethyl)piperazine-N′-ethanesulfonic acid (HEPES) (or phosphate) buffer with 0.1 M KCl was pipetted into a 1-cm spectrophotometric cell. The absorption spectra were recorded between 280 and 650 nm after additions of aliquots of a 1.8 × 10−3 M stock solution of the metal [V(V), W(VI), Mo(VI) or Zn(II)]. The aliquots were added directly into the cell. The final metal-to-ligand ratio was between 2.5 and 3. The complexation of V(V) and W(VI) by azotochelin was investigated at pH 6.6 (10−2 M HEPES buffer). The complexation of Mo was studied over a range of pH extending from 3 to 12, using HEPES (10−2 M) or phosphate (0.1 M) buffers. The complexation of Zn by azotochelin was investigated by spectrophotometry at pH 6.00, 6.30 and 6.51 (0.1 M HEPES buffer) in order to validate the constants log β Zn1 and log β Zn0 determined by potentiometry and ensure the best possible accuracy of these constants. (The 6.0–6.5 pH range corresponds to the pH region where the value of the constant for the Zn–azotochelin complex, K Znapp , allows direct investigation by UV–vis spectrophotometry.) In addition to apparent complexation constants, these experiments yielded the extinction coefficients for azotochelin and its metal complexes (Table 1).
Some of the constants were refined in competition experiments between two metals for azotochelin. These experiments were carried out for the couples Mo(VI) and Zn(II), Mo(VI) and V(V), and Mo(VI) and W(VI). For example, to determine K Moapp (pH 6.6), the constant for the Mo(VI)–azotochelin complex at pH 6.6, 50-μL aliquots of a 1.8 × 10−3 M stock solution of zinc sulfate were added to 2 ml of an equimolar (3 × 10−4 M) aqueous solution of azotochelin and sodium molybdate (pH 6.6 and I = 0.1 M) directly into a 1-cm cell. The absorption spectrum of the solution was recorded between 280 and 650 nm after addition of each aliquot. Data analysis with Hyperquad yielded \({K^{{\text {Zn/Mo}}}={{K^{{\text {Zn}}}_{{\text {app}}}}}/{{K^{{\text {Mo}}}_{{\text {app}}}}}.}\)
The constant K Moapp was then calculated as log K Moapp = log K Znapp − log K Zn/Mo.
The apparent stability constants for the V–azotochelin and W–azotochelin complexes, K Vapp and K Wapp , were determined at pH 6.6 in duplicate competition experiments between Mo and V and between Mo and W, respectively, following the procedure described above. The constants were calculated as log K Vapp = log K V/Mo + log K Moapp and log K Wapp = log K W/Mo + log K Moapp . The relative affinity of Fe(III) for azotochelin and EDTA at pH 6.7 (0.1 M phosphate buffer) was evaluated by comparing the UV–vis spectra of aqueous solutions of (1) iron and azotochelin (5 × 10−5 M FeCl3, 10−4 M azotochelin), (2) iron and EDTA (5 × 10−5 M FeCl3, 10−4 M EDTA) and (3) iron, azotochelin and EDTA (5 × 10−5 M FeCl3, 10−4 M azotochelin, 10−4 M EDTA).
Structural characterization of the Mo–azotochelin complex in solution by X-ray spectroscopy
The speciation of aqueous molybdate and its complexation with azotochelin were evaluated with X-ray absorption spectroscopy. Solutions of sodium molybdate (Na2MoO4·2H2O, 4 mM) and mixtures of sodium molybdate and azotochelin (MoT = 4 mM, LT = 5 mM) were prepared in Milli-Q water; the pH of the solutions was adjusted with HCl or NaOH. The solutions were then analyzed by X-ray absorption near-edge structure (XANES) spectroscopy. The spectra at the molybdenum K edge were collected at the Stanford Synchrotron Radiation Laboratory (Menlo Park, CA, USA) using beamline 4–3 and a Si(220) monochromator. The monochromator was detuned by 25% at the Mo absorption edge and set at 20,300 eV. Slit sizes were 1 mm × 20 mm and the I o, I 1 and I 2 chambers were filled with argon. The X-ray absorption spectra were collected in fluorescence mode using a germanium detector.
The XANES spectrum of solid crystals of K2[MoO2(cat)2]·H2cat·H2O was also collected for comparison purposes. The spectra were analyzed with WinXAS [36].
Mo extended X-ray absorption fine structure (EXAFS) spectra of aqueous Mo–azotochelin complex were collected on beamline 2–3 at the Stanford Synchrotron Radiation Laboratory using a Si(220) monochromator crystals. The spectra were collected for up to about 800 eV above the absorption edge. The experimental setup used for these studies was the same as that of the XANES spectra described above. The EXAFS spectra were processed using the EXAFSPAK software package. The structural environment of Mo in the aqueous complex was obtained by fitting the experimental EXAFS spectra with the theoretically derived phase and amplitude functions for O, C and Mo backscatterers.
Results and discussion
Acid–base
Although there are five acid–base groups in azotochelin (one carboxylate and four catecholate; Structure 1), we were able to determine only four constants with accuracy (Table 2). The first three pK a values of azotochelin were previously reported by Duhme et al. [22]. There is good agreement with the pK a values determined in this study, although our value for the first pK a (pK a1 = 3.3 ± 0.1), which corresponds to the deprotonation of the carboxylic group, is somewhat lower than the published constant (pK a1 = 3.65 ± 0.05, Table 2). The second and third pK a values (pK a2 = 7.47 ± 0.04 and pK a3 = 8.55 ± 0.02), corresponding to the deprotonation of the first and second catecholate groups, are essentially the same the constants of Duhme et al. (Table 2). The acid–base constant of the third catecholate group, pK a4 = 10.9 ± 0.1, was determined for the first time, while the fifth pK a value, for the deprotonation of the last catecholate group, could not be measured, and must be higher than 12.
Mo(VI) complexation
The study of the complexation of Mo(VI) and of the other oxoanions [W(VI) and V(V)] by azotochelin using potentiometry was difficult because (1) the formation of the 1:1 Mo–azotochelin complex does not release any protons (see Eq. 2) and (2) the formation of polyoxoanions at low pH complicates the interpretation of the data. The complexation of molybdate by azotochelin was thus investigated by UV–vis spectroscopy and X-ray spectroscopy over a pH range extending from 3 to 12. As detailed later, we found that Mo forms at least two types of complexes with azotochelin: a 1:1 complex that is dominant at neutral pH and a polynuclear complex (most likely a 6:3 Mo–azotochelin complex) that controls Mo speciation at acidic pH and high Mo concentration.
The 1:1 Mo–azotochelin complex
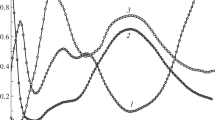
As seen in Fig. 1, at neutral pH (6 < pH < 8), molybdenum and azotochelin form a strong complex (C1) that absorbs in the UV and the visible, with two absorption maxima at 320 and 385 nm. The absorption band at 320 nm most likely correspond to a π to π* transition localized on azotochelin, while the intense visible band at 385 nm (responsible for the yellow–orange color of Mo–azotochelin solutions) corresponds to a ligand-to-metal charge transfer. At each of the pH values investigated (pH 6, 6.6 and 7), the spectral variations induced by the addition of molybdate to an azotochelin solution stop at a metal-to-ligand ratio close to 1, indicating the formation of a very stable 1:1 complex. The overall apparent stability constant (K Moapp ) is defined as
where [C1] is the concentration of the 1:1 Mo–azotochelin complex (measured by absorbance) and [Mo]T and [L]T are the total concentrations of molybdenum and azotochelin in solution, respectively. But the Mo–azotochelin complex is so stable that the reaction between Mo and azotochelin is essentially quantitative, and the exact value for K Moapp cannot be determined by a simple titration of the ligand with the metal. The difficulty was resolved in a competition experiment between Mo(VI) and Zn(II) for azotochelin. The constants for the formation of the Zn(II)–azotochelin complexes were determined independently by potentiometry and UV–vis spectroscopy (Table 3 and below) and indicated that Zn(II) may be a good competitor for the Mo–azotochelin complex at the pH values of interest. In addition, the spectroscopic features of the Zn–azotochelin complexes are distinct from those of the Mo–azotochelin complex. It was thus possible to follow the formation of the Zn complexes by UV–vis spectrophotometry during the titration of a Mo–azotochelin solution with Zn(II) (Fig. 2), leading to the complexation constants \({\log K^{{\text {Zn/Mo}}} = \log{\left({{{K^{{\text {Zn}}}_{{\text {app}}}}}/{{K^{{\text {Mo}}}_{{\text {app}}}}}} \right)}= - 1.77}\) at pH 6.6 and log K Zn/Mo = − 0.61 at pH 7.0. On this basis we calculate log K Zn/Motherm = − 28.5 ± 0.3 for the competition reaction (Eq. 1):
The apparent constant for the Zn(II)-azotochelin complex is log K Znapp = 5.6 ± 0.14 at pH 6.6 and is log K Znapp = 7.0 ± 0.14 at pH 7.0 (Table 3), and the apparent complexation constant for the Mo–azotochelin complex is calculated as log K Moapp = log K Znapp − log K Zn/Mo = 7.3 ± 0.4 at pH 6.6 and log K Moapp = 7.6 ± 0.4 at pH 7.0. These high values of K Moapp are consistent with our direct titration data and with those of Duhme et al. [22], which are essentially identical to ours. These authors, however, reported a low value of log K Moapp (log K Moapp = 4 at pH 6.6) which does not provide a good fit for the data. For comparison, the apparent stability constant for the complex of Mo with piperazine-1,4-bis(N-methylacetohydroxamic acid) (PiPD-MAHA, a dihydroxamate ligand) is log K = 2.5 at pH 6.6 [37].
UV–vis absorption spectra of a 2.5 × 10−4 M solution of azotochelin with 4.95 × 10−4 M molybdate at various pH values. The spectra at pH 6, 6.6 and 7 are characteristic of the 1:1 Mo–azotochelin complex, while the spectra at pH 3 and 4 correspond to the formation of a polymeric (possibly 6:3) Mo–azotochelin complex
Calculated (line) and measured (diamonds) absorbances at 412 nm of a Mo(VI) and azotochelin solution (3.0 × 10−4 M Mo, 2.2 × 10−4 M azotochelin) titrated with Zn(II) (1.81 × 10−3 M Zn) at pH 7.0. The absorbance was calculated with Hyperquad (log K Znapp = 7.0, log K Moapp = 7.6). Insert: Evolution of the UV–vis absorption spectrum of a solution of molybdate and azotochelin (2.2 × 10−4 M azotochelin, 3.0 × 10−4 M Mo) following additions of Zn(II) (pH 7, 25 °C)
Finally, UV–vis spectroscopy (as well as X-ray spectroscopy, see below) indicate that, as was previously shown [22], molybdate and azotochelin react between pH 6 and 7 following the reaction
On the basis of the apparent complexation constants for the Mo–azotochelin complex and of the acid–base constants of azotochelin, the thermodynamic constant for this reaction is calculated to be log K Motherm = 7.6 ± 0.4.
The UV–vis spectra indicated little or no change in the structure of the Mo–azotochelin complex between pH 6 and 7 (Fig. 1). The analysis of the Mo–azotochelin complex by XANES spectroscopy allowed the precise assessment of its structure at pH 6.6. The XANES spectrum of a solution where all the molybdenum is complexed with azotochelin (5 mM azotochelin, 4 mM Na2MoO4·2H2O, pH 6.6 adjusted with NaOH) was found to be essentially identical to the spectrum of a dioxo biscatecholate Mo(VI) complex anion (K2[MoO2(cat)2]·H2cat·H2O) whose structure was determined independently by X-ray crystallographic analysis of a single crystal [26, 38, 39] (Fig. 3). This indicates that, in the Mo-azotochelin complex as in the K2[MoO2(cat)2]·H2cat·H2O crystal, the two oxo groups bound to the Mo atom are in a cis position. In theory, the lysine side chain linking the catechol groups in azotochelin could participate in the coordination of Mo, either through its acid group or by hydrogen bonding between the amide hydrogens and their adjacent catecholate oxygens [40]. It appears clearly that the acid group does not bind to Mo, since this would result in a significant change in the XANES spectrum of the complex compared with the (K2[MoO2(cat)2]·H2cat·H2O) crystal.
X-ray absorption near-edge structure spectra of a molybdate and azotochelin solution (MoT = 4 mM, LT = 5 mM, pH 6.6) and of crystals of a dioxo biscatecholate Mo(VI) complex, K2[MoO2(cat)2]·H2cat·H2O. Insert: Structure of the Mo–azotochelin complex at pH 6.6; H atoms have been omitted for clarity. The structure takes into account the formation of hydrogen bonds between the amide protons and the ortho-phenolate oxygens, which has been found for similar compounds in the solid state [40], but has not been demonstrated in solution
The structure of the Mo–azotochelin complex (pH 6.6) was further examined using Mo EXAFS spectra (data not shown). The Fourier transforms of the Mo EXAFS spectra exhibit strong scattering from neighboring O atoms as a broad feature, and a weak feature from farther C (or other light atoms). When these shells were fit with the theoretically derived phase and amplitude functions for different backscatterers, such as O, C and Mo, the first shell showed scattering from 2.14 O atoms at 1.72 Å, 2.72 O atoms at 2.06 Å and 4 C atoms at 2.97 Å. The Debye–Waller parameter for these shells was 0.003, 0.0066 and 0.005 Å2, respectively. These structural parameters were obtained when all the fitting parameters (bond distance, coordination number, Debye–Waller parameter) were allowed to float. On the basis of these initial fits, the EXAFS spectra were processed further by fixing the coordination numbers of the first (1.72 Å), second (2.06 Å) and third (2.97 Å) shells at 2, 4 and 4, respectively. Fixing of the coordination numbers has no effect on the overall quality of the fit, bond distances and the Debye-Waller parameter (except for the second shell, which increased to 0.011 Å2). These results indicate that Mo is coordinated in a distorted octahedron with two distinct O distances. The third-shell environment could not be evaluated thoroughly because of weak scattering from light atoms. These EXAFS spectra also did not indicate any scattering from nearby Mo atoms, which suggests the absence of Mo polymers.
At pH 8 and above, XANES data (not shown) indicate the formation of the same 1:1 molybdenum complex that exists at neutral pH, but the determination of the overall apparent complexation constant is difficult because of the rapid degradation of azotochelin over time (possibly due to oxidation of the catecholate groups and/or hydrolysis).
The 6:3 Mo–azotochelin complex
While the UV–vis absorption spectra of solutions with Mo and azotochelin in a 2:1 molar ratio at pH 6, 6.6 and 7 are identical, and correspond to the formation of a very stable 1:1 complex (MoO2L3−), the absorption spectra of the same solution at pH 5 and lower are different, indicating the formation of at least one other complex, C2, in addition to the 1:1 complex (Fig. 1).
Three azotochelin solutions at pH 5.0, 4.0 and 3.0 were titrated with sodium molybdate; as seen in Fig. 4, azotochelin appears to be titrated (as shown by a slight decrease in absorbance) for a Mo-to-azotochelin ratio of 2:1, indicating the formation of a complex containing two Mo for one azotochelin. Data analysis at these pH values (Fig. 4; Table 4) indicates that the C2 complex has an approximate stoichiometry of six Mo for three azotochelin, corresponding to the reaction
As seen in Fig. 4, the absorbance data at low pH can be fitted with the apparent constants listed in Table 4, but the calculated line does not fit the data as well we may have expected from the other fits (compare, e.g., Fig. 4 and Figs. 2, 5, 6), and it is possible that other complexes with a slightly different stoichiometry are also present in solution. Since H2Mo6O 4−21 is the most abundant polymeric species of Mo at the concentrations and pH values that we have been investigating [34], it is plausible that the 6:3 Mo–azotochelin complex consists of three azotochelins attached to a Mo6O21 polymeric core. This hypothesized structure is consistent with the XANES spectra of Mo–azotochelin solutions at acidic pH values (data not shown).
Clearly, the relative stabilities of the polynuclear and 1:1 complexes are dependent on the total Mo and azotochelin concentrations in solution. For example, at pH 4, for LT = 100 μM and MoT = 50 μM, the 6:3 complex accounts for about 34% of the molybdate. But the concentration of the 6:3 complex drops quickly with decreasing Mo concentrations, and for molybdate concentrations below 10 μM, less than 0.1% of the molybdate is in the 6:3 complex. Obviously, at the low Mo(VI) concentrations encountered in terrestrial or aquatic systems, only the 1:1 complex needs to be taken into account even at low pH.
V(V) and W(VI) complexation
The stability constants for the complexes of azotochelin with W(VI) and V(V) at pH 6.6 were determined in a series of competition experiments with molybdate. Whereas the UV–vis spectrum of the 1:1 Mo–azotochelin complex shows two clear absorption maxima, corresponding to a π to π* transition on the ligand and to a ligand-to-metal charge transfer in the complex, these two transitions overlap in the W(VI) and V(V) complexes; the UV–vis spectra show only one absorption maximum in the near UV (inserts in Figs. 5, 6). As a result, tungstate and vanadate solutions with azotochelin have a light-yellow hue.
Calculated (line) and measured (diamonds) absorbances at 412 nm of a Mo(VI) and azotochelin solution (1.64 × 10−4 M Mo, 1.30 × 10−4 M azotochelin) titrated with W(VI) (2.1 × 10−4 M W) at pH 6.6. The absorbance was calculated with Hyperquad (log K Moapp = 7.3, log K Wapp = 9.0). Insert: Evolution of the UV–vis absorption spectrum of a solution of molybdate and azotochelin (1.30 × 10−4 M azotochelin, 1.64 × 10−4 M Mo) following additions of tungstate (pH 6.6, 25 °C)
Calculated (line) and measured (diamonds) absorbances at 412 nm of a Mo(VI) and azotochelin solution (1.15 × 10−4 M Mo, 1.13 × 10−4 M azotochelin) titrated with V(V) (6.5 × 10−4 M V) at pH 6.6. The absorbance was calculated with Hyperquad (log K Moapp = 7.3, log K Vapp = 8.8). Insert: Evolution of the UV–vis absorption spectrum of a solution of molybdate and azotochelin (1.13 × 10−4 M azotochelin, 1.15 × 10−4 M Mo) following additions of vanadate (pH 6.6, 25 °C)]
Tungstate added to a solution of molybdate and azotochelin displaced Mo from the Mo–azotochelin complex, indicating that tungstate is able to compete with molybdate for azotochelin (Fig. 5). This experiment was duplicated and data analysis yielded K W/Mo, where log K W/Mo = log K Wapp − log K Moapp = 1.68 ± 0.03. The apparent formation constant for the tungstate–azotochelin complex, K Wapp , can be calculated as log K Wapp = 9.0 ± 0.4 at pH 6.6. The tungstate complex thus appears to be more stable than the molydate complex at this pH. In a matching competition experiment, we added molybdate to an equimolar solution of tungstate and azotochelin. After 3 h, no spectral variations were observed, even at high Mo-to-W ratios, when all the azotochelin should be in the form of the Mo–azotochelin complex (data not shown). It thus appears that the dissociation kinetics of the W–azotochelin complex is slow, as has been observed before for other tungstate complexes [34].
The complexation constant of vanadate with azotochelin, K Vapp , was determined at pH 6.6 in a competition experiment with molybdate (Fig. 6). The data analysis yielded log K V/Mo = 1.44 ± 0.04 and log K Vapp = 8.8 ± 0.4, a value close to the constant for the W–azotochelin complex.
It may be noted that the additions of arsenate, sulfate or phosphate anions to azotochelin solutions at pH 6.6 led to no variation in the UV–vis spectrum of azotochelin, suggesting that, as expected, these anions are not complexed by azotochelin.
Zn(II), Mn(II), Co(II) and Cu(II) complexation
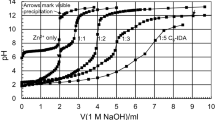
The complexation constants of azotochelin with Zn(II), Co(II), Mn(II) and Cu(II) were determined by potentiometry (Fig. 7), and the fit of the titration curves led to the overall stability constants, β z , corresponding to the following general equilibrium:
These constants were calculated using the average values of the protonation constants reported in Table 2. Although we obtained a satisfactory fit of the potentiometric data over the entire pH range for Zn(II), Co(II) and Mn(II), we could not fit the Cu(II) data above pH 7, possibly owing to an oxidation of the ligand by Cu(II) at high pH [41]. The nature of the complexes depends on the cation: ML3− and MLH2− with Zn2+; ML3−, MLH2− and MLH2 − with Mn2+; and MLH2− and MLH2 − with Co2+ (Table 3). The addition of Zn(II), Mn(II) or Co(II) (metal-to-ligand concentration ratio of 1) to the azotochelin solution does not affect the titration curve before the first equivalence point (which corresponds to the titration of 1 equiv of HCl and 1 equiv of the carboxylic acid group), suggesting that the catecholate groups on azotochelin react with these metals with no participation of the carboxyl group.
Experimental (squares) and calculated (line) potentiometric titration of a solution of ZnCl2 (1.09 mM), azotochelin (0.77 mM) and HCl (1.08 mM) in 0.1 M KCl. The initial volume was 10 ml, and the solution was titrated with a 66.6 mM solution of KOH. TOTH is [HCl]T − [base]T, where [HCl]T and [base]T are the concentrations of acid and base added to the solution, respectively. The line was calculated with the constants in Table 3
Of all the metal ions studied, Cu(II) forms the strongest complex with azotochelin. For instance, in an equimolar (3 × 10−4 M) solution of azotochelin and Cu(II), at pH 6.0, virtually all Cu(II) is complexed with azotochelin, while only 40% of Zn2+, 30% of Mn2+ and 25% of Co2+ are complexed.
The stability constants of iron complexes with azotochelin could not be determined directly by the same potentiometric method, probably because of slow kinetics [42]. In order to estimate the effect of azotochelin on the concentration of dissolved ferric hydrolysis species in the presence of EDTA, we studied the speciation of Fe(III) in a solution containing 10−4 M azotochelin, 10−4 M EDTA and 5 × 10−5 M FeCl3 at pH 6.7 (0.1 M phosphate buffer). The absorption spectrum of the solution can be reproduced by adding the absorption spectrum of azotochelin to that of the Fe–EDTA complex, indicating that the Fe–azotochelin complex does not form (Fig. 8). Since the apparent stability constant of the Fe–EDTA complex at pH 6.7 is log K Fe–EDTAapp = 13.80 [33], the apparent stability constant for the Fe–azotochelin complex must be such that log K FeLapp < 12.7 for a 1:1 complex, or \({\log K^{{{\text {Fe}}_{2}{\text {L}}_{3}}}_{{\text {app}}} < 34.5}\) if a 2:3 complex forms, as is often observed for complexes of iron with tetradentate ligands [43–45].
UV–vis absorption spectra at pH 6.7 (0.1 M phosphate buffer) of solutions of a Fe(III) and azotochelin (FeT = 5 × 10−5 M, LT = 10−4 M), b Fe(III) and EDTA (FeT = 5 × 10−5M, EDTA = 10−4 M) and c Fe(III), azotochelin and EDTA (FeT = 5 × 10−5 M, LT = 10−4 M, EDTA = 10−4 M). Insert: comparison of the absorption spectrum of solution c (full line) with a hypothetical spectrum calculated by adding the spectrum of solution b to the spectrum of a 10−4 M solution of azotochelin (dotted line)
Conclusion
Since very few ligands are known to form strong complexes with molybdate near neutral pH, the unusual high affinity of azotochelin for molybdate, vanadate and tungstate may be useful for a variety of applications. For example, azotochelin could be coupled to a solid matrix in a chromatographic column and used either to isolate oxoanions from solution or to remove traces of Mo to provide a Mo-free medium.
Azotochelin could also be valuable to further our understanding of the importance of metals in biogeochemical cycles. For example, there are indications that Mo may limit important reactions in the terrestrial nitrogen cycle [14–17, 46–50], but the Mo requirements of terrestrial nitrogen-fixers and denitrifiers are still poorly known. Azotochelin may be an adequate ligand to buffer molybdate concentrations in culture media, thus playing for molybdate (and for the other oxoanions tungstate and vanadate) a role similar to that of EDTA for cationic metals [51–56]. This study revives the hypothesis of azotochelin being a “molybdophore” [19, 22], as well as a siderophore for the nitrogen-fixer Azotobacter vinelandii [42, 57–59]. Azotochelin (or a similar biscatecholate ligand) could thus open the important but so far underexplored field of the role that limitation and/or toxicity of oxoanions may have on microorganisms.
References
Stiefel EI (2002) In: Sigel A, Sigel H (eds) Molybdenum and tungsten. Their roles in biological processes, vol 39. Dekker, New York, pp 1–30
Kimblin C, Bu XH, Butler A (2002) Inorg Chem 41:161–163
Carter-Franklin JN, Butler A (2004) J Am Chem Soc 126:15060–15066
Bishop PE, Premakumar R, Dean DR, Jacobson MR, Chisnell JR, Rizzo TM, Kopczynski J (1986) Science 232:92–94
Hemrika W, Renirie R, Macedo-Ribeiro S, Messerschmidt A, Wever R (1999) J Biol Chem 274:23820–23827
Messerschmidt A, Wever R (1996) Proc Natl Acad Sci USA 93:392–396
Eady RR (2003) Coord Chem Rev 237:23–30
Kletzin A, Adams MWW (1996) FEMS Microbiol Rev 18:5–63
Chan MK, Mukund S, Kletzin A, Adams MWW, Rees DC (1995) Science 267:1463–1469
Keeler RF, Varner JE (1957) Arch Biochem Biophys 70:585–590
Benemann JR, Smith GM, Kostel PJ, McKenna CE (1973) FEBS Lett 29:219–221
Hales BJ, Case EE (1987) J Biol Chem 262:16205–16211
Siemann S, Schneider K, Oley M, Müller A (2003) Biochemistry 42:3846–3857
Hallenbeck PC, Benemann JR (1980) FEMS Microbiol Lett 9:121–124
Kahn D, Hawkins M, Eady RR (1982) J Gen Microbiol 128:779–787
Lei S, Pulakat L, Gavini N (1999) Biochem Biophys Res Commun 264:186–190
Jacobson MR, Premakumar R, Bishop PE (1986) J Bacteriol 167:480–486
Stintzi A, Barnes C, Xu J, Raymond KN (2000) Proc Natl Acad Sci USA 97:10691–10696
Page WJ, von Tigerstrom M (1982) J Bacteriol 151:237–242
Patel U, Baxi MD, Modi VV (1988) Curr Microbiol 17:179–182
Saxena B, Vithlani L, Modi VV (1989) Curr Microbiol 19:291–295
Duhme AK, Hider RC, Naldrett MJ, Pau RN (1998) J Biol Inorg Chem 3:520–526
Cornish AS, Page WJ (2000) Appl Environ Microbiol 66:1580–1586
Chimiak A, Neilands JB (1984) Struct Bonding 58:89–96
Merz KW, Fink J (1956) Arch Pharm 289:347–358
Tkachev VV, Atovmyan LO (1975) Sov J Coord Chem Engl Transl 1:715–720
Griffith WP, Pumphrey CA, Rainey TA (1986) J Chem Soc Dalton Trans 6:1125–1128
Westall JC, Zachary JL, Morel FMM (1976) Technical Report 18. MIT, Cambridge
Westall JC (1982) Report 82–02. Department of Chemistry, Oregon St University, Corvallis
Herbelin AL, Westall JC (1999) Report 99-01. Department of Chemistry, Oregon St University, Corvallis
Vetrogon VI, Lukyanenko NG, Schwing-Weill MJ, Arnaud-Neu F (1994) Talanta 41:2105–2112
Gans P, Sabatini A, Vacca A (1996) Talanta 43:1739–1753
Martell AE, Smith RM (1974–1989) Critical stability constants. Plenum, New York
Cruywagen JJ (2000) Adv Inorg Chem 49:127–182
Cruywagen JJ, Draaijer AG, Heyns JBB, Rohwer EA (2002) Inorg Chim Acta 331:322–329
Ressler T (1998) J Synchrotron Radiat 5:118–122
Farkas E, Csoka H, Gama S, Santos MA (2000) Talanta 57:935–943
Lu X, Liu S, Mao X, Bu X (2001) J Mol Struct 562:89–94
Atovmyan LO, Sokolova Y, Tkachev VV (1970) Dokl Phys Chem Sect 195:1355–1356
Duhme AK (1997) J Chem Soc Dalton Trans 773–778
Torreggiani A, Trinchero A, Tamba M, Taddei P (2005) J Raman Spectrosc 36:380–388
Cornish AS, Page WJ (1998) Microbiology 144:1747–1754
Boukhalfa H, Crumbliss AL (2002) BioMetals 15:325–339
Hou Z, Raymond KN, O‘Sullivan B, Esker TW, Nishio T (1998) Inorg Chem 37:6630–6637
Carrano CJ, Cooper SR, Raymond KN (1979) J Am Chem Soc 101:599–604
Eady RR, Robson RL, Richardson TH, Miller RW, Hawkins M (1987) Biochem J 244:197–207
Eady RR, Robson RL (1984) Biochem J 224:853–862
Bishop PE, Jarlenski DML, Hetherington DR (1982) J Bacteriol 150:1244–1251
Lei S, Pulakat L, Gavini N (2000) FEBS Lett 482:149–153
Self WT, Grunden AM, Hasona A, Shanmugam KT (2001) Res Microbiol 152:311–321
Anderson MA, Morel FMM (1982) Limnol Oceanogr 27:789–813
Sunda WG, Huntsman SA (1997) Nature 390:389–392
Sunda WG, Huntsman SA (1995) Mar Chem 50:189–206
Sunda WG, Swift DG, Huntsman SA (1991) Nature 351:55–57
Timmermans KR, Stolte W, de Baar HJW (1994) Mar Biol 121:389–396
Wilhelm SW, Trick CG (1994) Limnol Oceanogr 39:1979–1984
Page WJ (1995) BioMetals 8:30–36
Duhme AK, Hider RC, Khodr HH (1997) Chem Ber/Recueil 130:969–973
Knosp O, von Tigerstrom M, Page WJ (1984) J Bacteriol 159:341–347
Acknowledgements
The authors wish to thank Norbert Clauer for his help throughout this work. This study was supported by grants from the NSF (CHE-0221978, Center for Environmental Bioinorganic Chemistry) and the French Department of Research, as well as a fellowship from the French Department of Education to J.P.B.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bellenger, JP., Arnaud-Neu, F., Asfari, Z. et al. Complexation of oxoanions and cationic metals by the biscatecholate siderophore azotochelin. J Biol Inorg Chem 12, 367–376 (2007). https://doi.org/10.1007/s00775-006-0194-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-006-0194-6