Abstract
This chapter is an update of the chapter which appeared in The Mycota, Vol. X in 2002. Not many novel compounds with nematicidal and insecticidal activities have been described in the meantime. Therefore, we have shifted focus from the chemical diversity of fungal metabolites and their producing organisms towards novel insights into the mode of action and the ecological significance of the compounds, e.g. their role for insect pathogens during colonization of the host.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 I. Introduction
In our last review (Anke and Sterner 2002), published in the first edition of this volume, we focused on the chemical diversity of nematicidal and insecticidal fungal metabolites and the producing organisms. Since then, only a few novel compounds have been described. However, many publications have appeared which describe the biological activities of some of the described compounds (other than insecticidal/nematicidal) or their mode of action. In addition, the ecological significance of some of the compounds has been partly elucidated, e.g. their role in insect pathogens during the colonization of the host. Therefore this chapter also includes some of these aspects.
2 II. Novel Compounds and Their Producers
2.1 A. Peptides, Cyclic Peptides, and Cyclic Depsipeptides
Cyclic peptides and depsipeptides produced by fungi, among them insect pathogenic fungi (e.g. members of the genera Aschersonia, Beauveria, Isaria, Metarhizium, Paecilomyces, Verticillium), and their occurrence have been summarized in four comprehensive review articles (Anke and Sterner 2002; Zimmermann 2007a, b; Anke and Antelo 2009).
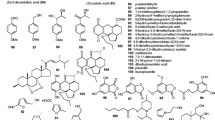
Since 2000, new producers of bioactive depsipeptides have been reported, for example, Beauveria fellina strains of marine origin (Lira et al. 2006), Verticillium sp. FKI-1033 (Monma et al. 2006), Aspergillus carneus (Capon et al. 2003), Torrubiella luteorostrata and its anamorph Paecilomyces cinnamomeus (both isolated from a scale insect; Isaka et al. 2007), Verticillium hemipterigenum (Nilanonta et al. 2003; Supothina et al. 2004), an Aureobasidium species from the tropical rain forest (Boros et al. 2006), an unidentified endophytic fungus (Huang et al. 2007), and a soil-borne Phoma species (Aoyagi et al. 2007). For a compilation of beauvericins and enniatins produced by Cordyceps species and their anamorphs as well as other insect pathogens, see Isaka et al. (2005a, b). Pseudodestruxins were found in Nigrosabulum globosum (Che et al. 2001) and reviews on destruxins and the producing organisms were published by Pedras et al. (2002) and Zimmermann (2007b). Some of the relevant compounds are listed in Table 7.1.
Chemical screening by HPLC-MS techniques led to the identification of five novel beauverolides from Beauveria bassiana and Paecilomyces spp. (Kuzma et al. 2001; Jegorov et al. 2004). Specific protocols for the detection and quantification of insecticidal cyclodepsipeptides in fungal cultures are now available (Jegorov et al. 2003).
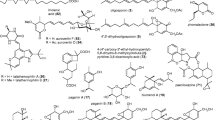
Besides cyclopeptides and depsipeptides, fungi can also produce linear peptides with insecticidal activity; recently reported examples are efrapeptin G and methylated peptides from a marine fungus associated with a sponge, neoefrapeptins from Geotrichum candidium (Nagaraj et al. 2001; Fredenhagen et al. 2006) and the Aib-containing cicadapeptins I and II from Cordyceps heteropoda (Krasnoff et al. 2005). Structures of some linear peptides are shown in Fig. 7.1, cyclic peptides and depsipeptides in Figs. 7.2 and 7.3. The neoefrapeptins comprise a large group of ten 16-residue and two 13-residue peptides containing also non-proteinogenic amino acids (Fredenhagen et al. 2006).
From protoplasts of a monokaryotic strain of the basidiomycete Omphalotus olearius, five novel hydroxylated omphalotin derivatives (omphalotin E–I) were isolated, complementing the already known omphalotins.
Interestingly the monokaryotic strain grew faster and produced higher amounts of these compounds than the dikaryotic parental strain from which it was obtained (Liermann et al. 2009). All O. olearius strains, irrespective of their geographical origin, seem to produce omphalotin derivatives (Anke et al., unpublished data). In fruiting bodies, omphalotins could not be detected. Omphalotins E-I exhibited similar nematicidal activities against Meloidogyne incognita (Kofoid & White) as omphalotin A. Antibacterial or antifungal activities were not detected and none of the compounds showed cytotoxic effects towards mouse leukemia cells (L1012 cells) or human colon adenocarcinoma cells (Colo 320 cells) at concentrations up to 50 μg/ml. The novel compounds were seemingly produced at the expense of the known omphalotins, including omphalontin A, the amount of which decreased drastically towards the end of the fermentation (Liermann et al. 2009).
2.2 B. Novel Metabolites and New Derivatives of Insecticidal or Nematicidal Metabolites
A screening of some 500 endophytic fungi for the production of nematicidal metabolites led to the selection of 17 strains with selective activity towards Meloidogyne incognita, while Caenorhabditis elegans was less affected (Schwarz et al. 2004). In five strains identified as Phomposis phaseoli and Melanconium betulinum (four strains), the nematicidal principle was found to be the simple metabolite 3-hydroxypropionic acid (Schwarz et al. 2004).
In the Sarcosomataceae (teleomorphs of the genera Galiella, Urnula, Strumella), the production of the galiellalactone precursors pregaliellalactone (Fig. 7.4) and structurally related hexaketides accounted for the nematicidal activity. Interestingly an endophytic strain of Cistus salviifolius, which according to its 18S rDNA sequences also belongs to the Sarcosomataceae, produced the same compounds (Köpcke et al. 2002a, b). A further nematicidal metabolite, MK7924 (Fig. 7.5), with weak activity against Aspergillus niger and Caenorhabditis elegans was isolated from Coronophora gregaria (Kumazawa et al. 2003).
Paraherquamides H, I (from Penicillium cluniae). Pregaliellalactone (from Galiella rufa). Quinolonone B, yaequinolones D, F (from Penicillium sp. FKI-2140). Penipratynolene and 2,6-pyridinedicarboxylic acid (from Penicillium bilaiae). Quinolactacide (from Penicillium citrinum). Peniprequinolone (from Penicllium simplicissium). Nafuredin-γ (from Aspergillus niger)
Quinolactacide (Fig. 7.4), an insecticidal quinolone, was isolated from solid-state cultures of Penicillium citrinum (Abe et al. 2005). Using Artemia salina as test organism, seven novel yaequinolones were isolated from Penicillium sp. FKI-2140, together with nine known and structurally related compounds, among them quinolinones A and B, penigequinolones A and B, and peniprequinolone (Uchida et al. 2006a, b).
Quinolone derivatives were also obtained from other Penicillium species, e.g. P. janczewskii (He et al. 2005) and P. simplicissimum (Kusano et al. 2000). Peniprequinolone, in addition to its strong insecticidal properties, inhibits the root-lesion nematode Pratylenchus penetrans (Kusano et al. 2000). Some of the quinolones and their producers are listed in Table 7.1, and their structures are given in Fig. 7.4. Another Penicillium metabolite active against the nematode Pratylenchus penetrans is penipratynolene (Nakahara et al. 2004). The compound was isolated together with 6-methoxy-carbonylpicolinic acid and 2,6-pyridinedicarboxylic acid (Fig. 7.4) from Penicillium bilaiae.
New nodulisporic acids were isolated from a Nodulisporium sp., namely nodulisporic acids B, B1, B2, and C, C1, C2, and Δ23 nodulisporic acid C4 (Ondeyka et al. 2002, 2003; Singh et al. 2004). The compounds are active against fleas on dogs (Shoop et al. 2001) and comprise complex chemical structures (see Fig. 7.5). From Penicillium expansum Link MK-5, communesins C, D, and E (Fig. 7.5) were obtained, along with two known communesins (A, B). All novel communesins showed selective insecticidal activity against silkworms (Hayashi et al. 2004).
The large family of paraherquamide metabolites has gained new members from P. cluniae Quintanilla: paraherquamide H and I (Fig. 7.4) together with five known derivatives. Structure–activity relationships revealed paraherqueamide E to be the most potent member, with a LD50 of 0.089 μg/nymph of the milkweed bug Oncopeltus fasciatus Dallas (López-Gresa et al. 2006).
Nafuredin is one of the few insecticidal metabolites that does not contain nitrogen, i.e. it is not an alkaloid. The substance was isolated from a marine-derived Aspergillus niger strain using mitochondria of Ascaris suum as test system with the aim to find inhibitors of NADH-fumarate reductase from helminths (Omura et al. 2001). By weak alkaline treatment the compound was converted to its γ-lactone (Fig. 7.4) which also inhibits NADH-fumarate reductase (Shiomi et al. 2005).
Several reports on insecticidal activities of extracts obtained from fungal culture filtrates have been published without identification of the active components (Meyer et al. 2004; Mohanty and Prakash 2008). Thus, Chen et al. (2003) reported on an extract obtained from an endophytic fungus with high activities against Heliothis armigera; the metabolites isolated, however, were not insecticidal (Yasui et al. 2006).
Many insecticidal or nematicidal metabolites, despite their complex structures, have been chemically synthesized during the last few years, for example, 9-prenylpaxilline (Smith and Cui 2003), marcfortine A, and structurally related metabolites of the paraherquamide group, many of which have potent insecticidal and antihelmintic properties (Williams 2002; Williams et al. 2003; Trost et al. 2007): quinolactacide (Abe et al. 2006), nafuredin-γ (Nagamitsu et al. 2003), and verticilide (Momna et al. 2006).
3 III. Biological Activities and Mode of Action
Cyclic depsipeptides and their biological activities were thoroughly reviewed by Sarabia et al. (2004), insecticidal and other biological activities of destruxins, isariins, enniatins, and beauverolides by Anke and Sterner (2002), by Anke and Antelo (2009), and by Zimmermann (2007a, b).
Sirodesmin PL produced by Leptosphaeria maculans was found to have phytotoxic, antibacterial, and insecticidal properties (Rouxel et al. 1988; Boudart 1989). Serinocyclin A isolated from Metarhizium anisopliae condia produced a sublethal locomotory defect in mosquito larvae (Krasnoff et al. 2007). Argifin and argadin, two cyclopentapeptides from a Gliocladium sp. and a Clonostachys sp. turned out to be potent inhibitors of chitinase B from the gram-negativ bacterium Serratia marcescens (Arai et al. 2000). When injected into cockroach larvae, their moult was arrested (Houston et al. 2002).
Like many peptaibols, the efrapeptins act as channel-forming ionophores and have insecticidal, antimalarial, and antiprotozoal activities (Nagaraj et al. 2001). In addition, they inhibit exocytosis but not endocytosis in eukaryotic cells (Muroi et al. 1996) and exhibit antibacterial and antifungal activities (Bandani et al. 2000).
Efrapeptins bind strongly to V-ATPases in the brush border membrane of the mid-gut of the wax moth Galleria mellonella (Bandani et al. 2001). Leucinostatins A and B originally isolated from Penicillium lilacinum [now Paecilomyces lilacinus (Thom) Samson] were found to have nematicidal activities (Arai et al. 1973; Park et al. 2004). Like other peptaibols they act on bacteria, are uncouplers in the inner mitochondria membrane and inhibit mitochondrial ATP synthesis. Recently interesting and potent in vivo and in vitro antitrypanosomal activities of these compounds have been reported. Despite its toxicity, the strong in vivo antitrypanosomal activity of leucinostatin B makes the compound a promising lead structure for the development of drugs with a new type of action mode (Ishiyama et al. 2009).
Among nine beauverolides tested for acyl-CoA:cholesterol acyltransferase (ACAT) inhibitory activity in CHO-cells expressing ACAT1 or ACAT2, beauverolides I and III inhibited ACAT1 rather selectively, and no antimicrobial or cytotoxic activities were detected.
Both compounds produced by the entomopathogenic fungus Beauveria bassiana exert anti-atherogenic activity in low-density lipoprotein receptor- and apolipoprotein E-knockout mice without any side effects and thus may serve as lead structures for new anti-atherosclerotic agents (Namatame et al. 2004). Contrary to the beauverolides, related beauvericin was clearly cytotoxic (Matsuda et al. 2004; Ohshiro et al. 2007). Cell lines derived from insects, e.g. Spodoptera frugiperda SF-9 cells, were also inhibited by beauvericin (Calo et al. 2003; Fornelli et al. 2004). The compounds induced rapid cell death in Xenopus oocytes via influx of Ca2+ (Tang et al. 2005).
Furthermore, significant effects were detected for destruxin E on insect haemocytes (Vey et al. 2002) and for different destruxins, e.g destruxins A, B, and E, on human and insect cell lines (Skrobek and Butt 2005). Verticilide from moulds of the genus Verticillium inhibits the binding of ryanodine to its receptor (RyR) and, hence, has insecticidal activity (Monma et al. 2006).
The target of PF1022A, a fungal cyclo-octadepsipetide, is a latrophilin-like receptor from the parasitic nematode Haemonchus contortus (Saeger et al. 2001). Emodepsin, a semi-synthetic depsipeptide derived from PF1022A, has already been successfully used against helminths in veterinary medicine (Conder et al. 1995; Dyker et al. 2004; Samson-Himmelstjerna et al. 2005). Due its limited availability and therefore a rather high price, its use is restricted to small pets. PF1022A is a metabolite of an endophytic fungus from the ornamental plant Camellia japonica (Sasaki et al. 1992; Scherkenbeck et al. 2002). Based on its 18S rRNA gene sequence, the endophyte was tentatively identified as a member of the ascomycetous family Xylariaceae close to Xylaria polymorpha and Rosellinia necatrix (Miyado et al. 2000).
Selective nematicidal properties were only reported for the omphalotins with high inhibitory activity towards Meloidogyne incognita and lower activities against Caenorhabditis elegans (Mayer et al. 1999). The nematicidal properties of hydroxylated omphalotins, some of which can be produced by monokaryotic strains generated from dikaryotic parent mycelia, were found to be higher than those of the unsubstituted compound, but unfortunately they were not stable (Büchel et al. 1998; Liermann et al. 2009). Their mode of action has not yet been elucidated.
Paraherquamides, potent anthelmintic agents isolated from various Penicillium species, were reported to possess promising activities against drug-resistant intestinal parasites (Williams et al. 2003).
Quinolactacide was also strongly active against Myzus persicae, and 250 ppm were lethal to 88% of the aphides tested; and, at a concentration of 500 ppm, 42% mortality towards the diamondback moth (Plutella xylostella) was recorded (Abe et al. 2006).
Nafuredin is a selective inhibitor of the helminth complex I (NADH-fumarate reductase). It showed only very weak inhibition of the mammalian complex I (NADH ubiquinone reductase from bovine liver) but was selectively active in vivo against the stomach worm Haemonchus contortus in sheep (Omura et al. 2001). The γ-lactone was almost equally active in the helminth complex I assay but less active in the in vivo tests (Shiomi et al. 2005). The differences between the human and helminth complex I make this an interesting target for the development of novel selective drugs against parasitic nematodes.
4 IV. Ecological Significance
Many secondary metabolites play a crucial role for fungi in their natural habitats. For example, endophytic fungi of grasses belonging to the genera Neotyphodium/Epichloe confer protection from mammalian and insect herbivores, or enhanced resistance to nematodes and phytopathogenic fungi (Schardl et al. 2004; Panaccione et al. 2006). Some of these beneficial effects are due to secondary metabolites.
Loline and peramine have been identified among the fungal metabolites with insecticidal activities in the plant host. Another metabolite from Epichloe typhina, epichlicin, efficiently prevents the germination (IC50 value of 22 nM) of Cladosporium phlei spores, a host plant pathogen (Seto et al. 2007). Likewise, sirodesmin PL or zearalenone and other mycotoxins produced by Leptoshaeria maculans and Fusarium species, respectively, were detected in the plant hosts (Laser et al. 2003; Elliott et al. 2007). Some endophytic fungi produce 3-hydroxypropionic acid as a nematicidal principal (Schwarz et al. 2004). This might be the natural nematicide with the simplest chemical structure; however it remains to be elucidated whether the compound is also produced in planta.
The function of shearamide A, an insecticidal cyclopeptide isolated from the ascostromata of Eupenicillium shearii (Belofsky et al. 1998), and likewise sclerotiamide from Aspergillus sclerotiorum sklerotia (Whyte et al. 1996), may be to protect the reproductive or survival structures of the fungi against insects or nematodes, similar to ergopeptides in the sclerotia of Claviceps species (Leistner and Steiner 2009).
Investigations on the role of destruxins in the pathogenicity of Metarhizium anisopliae against three species of insects revealed a direct relationship between the titre of destruxins produced by the strains in vitro and their destructive action (Kershaw et al. 1999).
However, in M. anisopliae mutants, incapable of destruxin production, virulence towards Galleria mellonella was unaltered (Amiri-Besheli et al. 2000). In the plant pathogenic mould Alternaria brassicae, destruxin B is a host-specific toxin. In three Brassica species the degree of their sensitivity to destruxin B positively correlated with their degree of susceptibility (Pedras et al. 2002).
From cultures of a number of fungi producing cyclic depsipeptides (e.g. Beauveria bassiana), also dipeptides composed of the same amino acids as the depsipeptides were isolated.
Other insect pathogens like Verticillium species and Metarhizium anisopliae as well as plant pathogenic fungi, e.g. Colletotrichum gloeosporioides, Exserohilum holmi, Gliocladium deliquenscens, Alternaria and Trichoderma spp. were also found to produce dipeptides. An unidentified endophyte from mangrove leafs (Rhizophora spp.) produced two cyclic depsipeptides and three diketopiperazines (Huang et al. 2007).
It might be interesting to elucidate the insecticidal and nematicidal activities exhibited by cocktails of all these secondary metabolites of a pathogen. Such synergistic effects of different fungal metabolites have been largely neglected so far. In general, the role of dipeptides and depsipeptides in insect and plant pathogenicity is not fully understood. Further, it is intriguing that depsipeptides are widespread in phytopathogens (e.g. Cochliobolus with anamorphs Helminthosporium and Bipolaris, Calonectria with its anamorph Cyclindrocladium, as well as Fusarium and Alternaria), insect pathogens (Aschersonia, Beauveria, Cordyceps, Diheterospora, Fusarium, Hirsutella, Isaria, Metharizium, Paecilomyces, Tolypocladium, Verticillium), and others (Zimmermann 2007a, b; Buckingham 2008). As molecular tools become more and more available, this question may be correctly addressed and respectively answered in the near future.
The efrapeptins produced by insect pathogenic Tolypocladium species are also produced in vivo; the amounts, however, were found to be too small to cause the death of insects. Therefore, it was suggested that the compounds act in concert with additional, not yet known, pathogenicity factors (Bandani et al. 2000).
For the function of enzymes in entomopathogenic fungi and their role in disease development, see Khachatourians and Qazi (2008) in this context. The effects of secondary metabolites on the enzymes involved in pathogenesis in plants and insects alike is another innovative field, in which interesting results still wait to be elucidated.
5 V. Conclusions
Natural products derived from plants, animals, and microorganisms constitute only 7.6% of the global insecticides market (Elbert et al. 2007). However, more than 50 000 microbial metabolites are known. Half of them exhibit bioactivities, first and foremost antibiotic activity. Fungal metabolites represent about 40% of these natural products (Bérdy 2005; Buckingham 2008). So far, no secondary metabolite from a fungus has been developed into a marketable insecticidal or nematicidal product, despite the fact that most insect pathogens are fungi and many have successfully been screened for the production of “soft pesticides”. However, as it appears, many of the compounds isolated from these fungi are rather toxic, like the destruxins or efrapeptins. While they fulfil their ecological role very well, i.e. killing the host animal, they do not meet the requirements of modern agricultural pesticides regarding selectiveness, low costs, and environmental safety. Nevertheless, the example of PF1022A shows that fungal metabolites can be developed into drugs useful in agriculture and veterinary medicine. The many synthetic efforts using natural products as lead structures also point in this direction. Taking into account the overall number of fungal species, which is estimated to be around 1.5 million (Hawksworth 2001), and comparing this number with the number of about 50 000 species screened so far, it seems to be only a matter of time until the first insecticide from a fungus is introduced into the market.
References
Abe M, Imai T, Ishii N, Usui M, Okuda T, Oki T (2005) Quinolactacide, a new quinolone insecticide from Penicillium citrinum. Biosci Biotechnol Biochem 69:1202–1205
Abe M, Imai T, Ishii N, Usui M (2006) Synthesis of quinolactacide via an acyl migration reaction and dehydrogenation with manganese dioxide, and its insecticidal activities. Biosci Biotechnol Biochem 70:303–306
Amiri-Besheli B, Khambay B, Cameron S, Deadman ML, Butt TM (2000) Inter- and intra-specific variation in destruxin production by insect pathogenic Metarhizium spp., and its significance to pathogenesis. Mycol Res 104:447–452
Anke H, Antelo L (2009) Cyclic peptides and depsipeptides from fungi. In: Anke T, Weber D (eds) Physiology and genetics. Mycota XV. Springer, Berlin Heidelberg New York, pp 273–296
Anke H, Sterner O (2002) Insecticidal and nematicial metabolites from fungi. In: Osiewacz HD (ed) Industrial applications. Mycota X. Springer, Berlin Heidelberg New York, pp 109–127
Aoyagi A, Yano T, Kozuma S, Takatsu T (2007) Pleofungins, novel inositol phosphorylceramide synthase inhibitors, from Phoma sp. SANK 13899. J Antibiot 60:143–152
Arai N, Shiomi K, Iwai Y, Omura S (2000) Argifin, a new chitinase inhibitor, produced by Gliocladium sp. FTD-0668. II. Isolation, physico-chemical properties, and structure elucidation. J Antibiot 53:609–614
Arai T, Mikami Y, Fukushima K, Utsumi T, Yazawa K (1973) A new antibiotic, leucinostatin, derived from Penicillium lilacinum. J Antibiot 26:157–161
Bandani AR, Khambay BPS, Faull JL, Newton R, Deadman M, Butt TM (2000) Production of efrapeptins by Tolypocladium species and evaluation of their insecticidal and antimicrobial properties. Mycol Res 104:537–544
Bandani AR, Amiri B, Butt TM, Gordon-Weeks R (2001) Effects of efrapeptin and destruxin, metabolites of entomogenous fungi, on the hydrolytic activity of a vacuolar type ATPase identified on the brush border membrane vesicles of Galleria mellonella midgut and on plant membrane bound hydrolytic enzymes. Biochim Biophys Acta 1510:367–377
Belofsky G N, Gloer JB, Wicklow DT, Dowd PF (1998) Shearamide A: a new cyclic peptide from the ascostromata of Eupenicillium shearii. Tetrahed Lett 39:5497–5500
Bérdy J (2005) Bioactive microbial metabolites. J Antibiot 58:1–26
Boros C, Smith CJ, Vasina Y, Che Y, Dix AB, Darveaux B, Pearce C (2006) Isolation and identification of the icosalides – cyclic peptolides with selective antibiotic and cytotoxic activities. J Antibiot 59:486–494
Boudart G (1989) Antibacterial activity of sirodesmin PL phytotoxin: application to the selection of phytoxin-deficient mutants. Appl Enivron Microbiol 55:1555–1559
Büchel, E, Martini U, Mayer A, Anke H, Sterner O (1998) Omphalotins B, C, and D, nematicidal cyclopeptides from Omphalotus olearius. Absolute configuaration of omphalotin A. Tetrahedron 54:5345–5352
Buckingham J (ed) (2008) Dictionary of natural products on DVD, ver 17.1. Chapman and Hall/CRC, Boca Raton
Calo L, Fornelli F, Nenna S, Tursi A, Caiaffa MF, Macchia L (2003) Beauvericin cytotoxicity to the invertebrate cell line SF-9. J Appl Genet 44:515–520
Capon RJ, Skene C, Stewart M, Ford J, O’Hair RAJ, Williams L, Lacey E, Gill JH, Heiland K, Friedel T (2003) Aspergillicins A-E: five novel depsipeptides from the marine-derived fungus Aspergillus carneus. Org Biomol Chem 1:1856–1862
Che Y, Swenson DC, Gloer JB, Koster B, Malloch D (2001) Pseudodestruxins A and B: new cycllic depsipeptides from the coprophilous fungus Nigrosabulum globosum. J Nat Prod 64:555–558
Chen G, Lin Y, Wen L, Vrijmoed LLP, Jones EBG (2003) Two new metabolites of a marine endophytic fungus (No. 1893) from an estuarine mangrove on the South China Sea coast. Tetrahedron 59:4907–4909
Chen SY, Dickson DW, Mitchell DJ (2000) Viability of Heterodera glycines exposed to fungal filtrates. J Nematol 32:190–197
Conder GA, Johnson SS, Nowakowski DS, Blake TE, Dutton FE, Nelson SJ, Thomas EM, Davis JP, Thompson DP (1995) Anthelmintic profile of the cyclodepsipeptide PF1022A in in vitro and in vivo models. J Antibiot 48:820–823
Dyker H, Harder A, Scherkenbeck J (2004) Chimeric cyclodepsipeptides as mimetics for the anthelmintic PF1022A. Biorg Med Chem Lett 14: 6129–6130
Elbert A, Nauen R, McCaffery A (2007) IRAC, resistance and mode of action classification of insecticides. In: Krämer W, Schirmer U (eds) Modern crop protection compounds. Wiley-VCH, Weinheim, pp 753–771
Elliott CE, Gardiner DM, Thoma G, Cozijnsen A, van de Wouw A, Howlett BJ (2007) Production of the toxin sirodesmin PL by Leptosphaeria maculans during infection of Brassica napus. Mol Plant Pathol 8:791–802
Feifel SC, Schmiederer T, Hornbogen T, Berg H, Süssmuth RD, Zocher R (2007) In vitro synthesis of new enniatins: Probing the α-D-hydroxy carboxylic acid binding pocket of the multienzyme enniatin synthetase. ChemBioChem 8:1767–1770
Fornelli F, Minervini F, Logrieco A (2004) Cytotoxicity of fungal metabolites to lepidopteran (Spodoptera frugiperda) cell line (SF9). J Invert Pathol 85:74–79
Fredenhagen A, Molleyres LP, Böhlendorf B, Laue G (2006) Structure determination of neofrapeptins A to N: peptides with insecticidal activity produced by the fungus Geotrichum candidum. J Antibiot 59:267–280
Glinski M, Hornbogen T, Zocher R (2001) Enzymatic synthesis of fungal N-methylated cyclopeptides and depsipeptides. In: Kirst H, Yeh WK, Zmijewski M (eds) Enzyme technologies for pharmaceutical and biotechnological applications. Dekker, New York, pp 471–497
Hawksworth DL (2001) The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycol Res 105:1422–1432
Hayashi H, Matsumoto H, Akiyama K (2004) New insecticidal compounds, communesins C, D, and E, from Penicillium expansum Link MK-57. Biosci Biotechnol Biochem 68:753–756
He J, Lion U, Sattler I, Gollmic FA, Grabley S, Cai J, Meiners M, Schünke H, Schaumann K, Dechert U, Kron M (2005) Diastereomeric quinolinone alkaloids from the marine-derived fungus Penicllium janczewskii. J Nat Prod 68:1397–1399
Houston DR, Shiomi K, Arai N, Omura S, Peter MG, Turberg A, Synstad B, Eijsink VG, van Aalten DMF (2002) High-resolution structures of a chitinase complex with natural product cyclopentapeptide inhibitors: mimicry of carbohydrate substrate. Proc Natl Acad Sci USA 99:9127–9132
Huang H, She Z, Lin Y, Vrijmoed LLP, Lin W (2007) Cyclic peptides from an endophytic fungus obtained from a Mangrove leaf (Kandelia candel). J Nat Prod 70:1696–1699
Isaka M, Kittakoop P, Kirtikara K, Hywel-Jones NI, Thebtaranonth Y (2005a) Bioactive substances from insect pathogenic fungi. Acc Chem Res 38:813–823
Isaka M, Palasarn S, Rachtawee P, Vimuttipong S, Kongsaeree P (2005b) Unique diketopiperazine dimers from the insect pathogenic fungus Verticillium hemipterigenum BCC 1449. Org Lett 7:2257–2260
Isaka M, Palasarn S, Kocharin K, Hywel-Jones NI (2007) Comparison of the bioactive secondary metabolites from the scale insect pathogens, anamorph Paecilomyces cinnamomeus, and teleomorph Torrubiella luteorostrata. J Antibiot 60:577–581
Ishiyama A, Otoguro K, Iwatsuki M, Namatame M, Nishihara A, Nonaka K, Kinoshita Y, Takahashi Y, Masuma R, shiomi K, Yamada H, Omura S (2009) In vitro and in vivo antitrypanasomal activities of three peptide antibiotics: leucinostatin A and B, alamethicin I and tsushimycin. J Antibiot 62:303–308
Jegorov A, Paizs B, Žabka M, Kuzma M, Havlièek V, Giannakopulos AE, Derrick PJ (2003) Profiling of cyclic hexadepsipeptides roseotoxins synthesised in vitro: a combined tandem mass spectrometry and quantum chemical study. Eur J Mass Spectrom 9:105–116
Jegorov A, Paizs B, Kuzma M, Zabka M, Landa Z, Sulc M, Barrow MP, Havlicek V (2004) Extraribosomal cyclic tetradepsipeptides beauverolides: profiling and modeling the fragmentation pathways. J Mass Spectrom 39:949–969
Kershaw M, Moorhouse ER, Bateman R; Reynolds SE, Charnley AK (1999) The role of destruxins in the pathogenicity of Metarhizium anisopliae for three species of insect. J Invert Pathol 74:213–223
Khachatourians GG, Qazi SS (2008) Entomopathogenic fungi: biochemistry and molecular biology. In: Brakhage AA, Zipfel PF (eds) Human and animal relationship, 2nd edn. Mycota VI. Springer, Berlin Heidelberg New York, pp 33–61
Köpcke B, Johansson M, Sterner O, Anke H (2002a) Biologically active secondary metabolites from the ascomycete A111-95. 1. Production, isolation and biological activities. J Antibiot 55:36–40
Köpcke B, Weber RWS, Anke H (2002b) Galiellalactone and its biogenetic precursors as chemotaxonomic markers of the Sacrosomataceae (Ascomyceta). Phytochemistry 60:709–714
Krasnoff SB, Reategui RF, Wagenaar MM, Gloer JB, Gibson DM (2005) Cicadapeptins I and II: new Aib-containing peptides from the entomopathogenic fungus Cordyceps heteropoda. J Nat Prod 68:50–55
Krasnoff SB, Keresztes I, Gillilan RE, Szebenyi DME, Donzelli BGG, Vhurchill ACL, Gibson DM (2007) Serinocyclins A and B, cyclic heptapeptides from Metarhizium anisopliae. J Nat Prod 70:1919–1924
Kumazawa S, Kanda M, Utagawa M, Chiba N, Ohtani H, Mikawa T (2003) MK7924, a novel metabolite with nematocidal activity from Coronophora gregaria. J Antibiot 56:652–654
Kusano M, Koshino H, Uzawa J, Fujioka S, Kawano T, Kimura Y (2000) Nematocidal alkaloids and related compounds produced by the fungus Penicllium cf. simplicissium. Biosci Biotechnol Biochem 64:2559–2568
Kuzma M, Jegorov A, Kacer P, Havlicek V (2001) Sequencing of new beauverolides by high-performance liquid chromatography and mass spectrometry. J Mass Spectrometry 36:1108–1115
Laser H, von Boberfeld WO, Wöhler K, Wolf D (2003) Effects of the botanical composition and weather conditions on mycotoxins in winter forage from grassland. Mycotoxicol Res 19:87–90
Leistner E, Steiner U (2009) Fungal origin of ergoline alkaloids present in dicotyledonous plants (Convolvulaceae) In: Anke T and Weber D (eds) Physiology and genetics. Mycota XV. Springer, Berlin Heidelberg New York, pp 197–208
Liermann JC, Kolshorn H, Antelo L, Hof C, Anke H, Opatz T (2009) Omphalotins E-I, five oxidatively modified nematicidal cyclopeptides from Omphalotus olearius. Eur J Org Chem 2009:1256–1262
Lira SP, Vita-Marques AM, Seleghim MHR, Bugni TS, LaBarbera DV, Sette LD, Sponchiado SRP, Ireland CM, Berlinck RGS (2006) New destruxins from the marine-derived fungus Beauveria felina. J Antibiot 59:553–563
López-Gresa MP, González MC, Ciavatta L, Ayala I, Moya P, Primo J; (2006) Insecticidal activity of paraherquamides, including paraherquamide H and paraherquamide I, two new alkaloids isolated from Penicillium cluniae. J Agric Food Chem 54:2921–2925
Matsuda D, Namatame I, Tomoda H, Kobayashi S, Zocher R, Kleinkauf H, Omura S (2004) New beauverolides produced by amino acid-supplemented fermentation of Beauveria sp. FO-6979. J Antibiot 57:1–9
Mayer A, Kilian M, Hoster B, Sterner O, Anke H (1999) In vitro and in vivo nematicidal activities of the cyclic dodecapeptide omphalotin A. Pest Sci 55:27–30
Meyer SLF, Huettel RN, Zhong X, Humber RA, Juba J, Nitao JK (2004) Activity of fungal culture filtrates against soybean cyst nematode and root-knot nematode egg hatch and juvenile motility. Nematology 6:23–32
Miyado S, Kawasaki H, Aoyagi K, Yaguchi T, Okada T, Sugiyama J (2000) Taxonomic position of the fungus producing the anthelmintic PF1022 based on the 18S rRNA gene base sequence. Nippon Kingakukai Kaiho 41:183–188
Mohanty SS, Prakash S (2008) Effects of culture media on larvicidal property of secondary metabolites of mosquito pathogenic fungus Chrysosporium lobatum (Moniliales: Moniliaceae) Acta Trop 109:50–54
Monma S, Sunazuka T, Nagai K, Arai T, Shiomi K, Matsui R, Mura S (2006) Verticilide: elucidation of absolute configuration and total synthesis. Org Lett 8:5601–5604
Muroi MN, Kaneko N, Suzuki K, Nishio T, Oku T, Sato T, Takatsuki A (1996) Efrapeptins block exocytic but not endocytic trafficking of proteins. Biochim Biophys Res Commun 227:800–809
Nagamitsu T, Takano D, Shiomi K, Ui H, Yamaguchi Y, Masuma R, Harigaya Y, Kuwajima I, Omura S (2003) Total synthesis of nafuredin-γ, a γ-lactone related to nafuredin with selective activity against NADH-fumarate reductase. Tetrahedron Lett 44:6441–6444
Nagaraj G, Uma MV, Shivayogi MS, Balaram H (2001) Antimalarial activities of peptide antibiotics isolated from fungi. Antimicrob Agents Chemother 45:145–149
Nakahara S, Kusano M, Fujioka S, Shimada A, Kimura Y (2004) Penipratynolene, a novel nematicide from Penicillium bilaiae Chalabuda. Biosci Biotechnol Biochem 68:257–259
Namatame I, Zomoda H, Ishibashi S, Omura S (2004) Antiatherogenic activity of fungal beauverolides, inhibitors of lipid droplet accumulation in macrophages. Proc Natl Acad Sci USA 101:737–742
Nilanonta C, Isaka M, Chanphen R, Thongorn N, Tanticharoen M, Thebtaranonth Y (2003) Unusual enniatins produced by the insect pathogenic fungus Verticillium hemipterigenum: isolation and studies on precursor-directed biosynthesis. Tetrahedron 59:1015–1020
Ohshiro T, Rudel LL, Omura S, Tomoda H (2007) Selectivity of microbial acyl-CoA:cholesterol acyltransferase inhibitors towards isoenzymes. J Antibiot 60:43–51
Omura S, Miyadera H, Ui H, Shiomi K, Yamaguchi Y, Masuma R, Nagamitsu T, Takano D, Sunazuka T, Harder A, Kölbl H, Namikoshi M, Miyoshi H, Sakamoto K, Kita K (2001) An anthelmintic compound, nafuredin, shows selelctive inhibition of complex I in helminth mitochondria. Proc Natl Acad Sci USA 98:60–62
Ondeyka JG, Dahl-Roshak AM, Tkacz JS, Zink DL, Zakson-Aiken M, shoop WL, Goetz MA, Singh SB (2002) Nodulisporic acid B, B1, and B2: A series of 1′-deoxy-nodulisporic acids from Nodulisporium sp. Bioorg Med Chem Lett 12:2941–2944
Ondeyka JG, Byrne K, Vesey D, Zink DL, Shoop WL, Goetz MA, Singh SB (2003) Nodulisporic acids C, C1, and C2: a series of D-ring-opened nodulisporic acids from the fungus Nodulisporium sp. J Nat Prod 66:121–124
Panaccione DC, Cipoletti JR, Sedlock AB, Blemings KP, Schradl CL, Machado C, Seidel GE (2006) Effects of ergot alkaloids on food preference and satiety in rabbits, as assessed with gene-knockout endophytes in perennial ryegrass (Lolium perenne). J Agric Food Chem 54:4582–4587
Park JO, Hargreaves JR, McConville EJ, Stirling GR, Ghisalberti EL, Sivasithamparam (2004) Production of leucinostatins and nematicidal activity of Australian isolates of Paecilomyces lilacinus (Thom) Samson. Lett Appl Microbiol 38:271–276
Pedras MSC, Zaharia LI, Ward DE (2002) The destruxins: synthesis, biosynthesis, biotransformation and biological activity. Phytochemistry 59:579–596
Rouxel T, Chupeau Y, Fritz R, Kollmann A, Bousquet J-F (1988) Biological effects of sirodesmin PL, a phytotoxin produced by Leptosphaeria maculans. Plant Sci 57:45–53
Saeger B, Schmitt-Wrede HP, Dehnhardt M, Benten WP, Krucken J, Harder A, Samson-Himmelstjerna G von, Wiegand H, Wunderlich F (2001) Latrophilin-like receptor from the parasitic nematode Haemonchus contortus as target for the anthelmintic depsipeptide PF1022A. FASEB J 15:1332–1334
Samson-Himmelstjerna G von, Harder A, Sangster NC, Coles GC (2005) Efficacy of two cyclooctadepsipeptides, PF022A and emodepside, against anthelmintic-resistant nematodes in sheep and cattle. Parasitology 130:343–347
Sarabia F, Chammaa S, Sánchez Ruiz A, Martín Ortiz L, López Herrera FJ (2004) Chemistry and biology of cyclic depsipeptides of medicinal and biological interest. Curr Med Chem 11:1309–1332
Sasaki T, Takagi M, Yaguchi T, Miyado S, Okada T, Koyama M (1992) A new anthelmintic cyclodepsipeptide, PF1022. J Antibiot 45:692–697
Schardl CL, Leuchtmann A, Spiering MJ (2004) Symbioses of grasses with seedborne fungal endophytes. Annu Rev Plant Biol 55:315–340
Scherkenbeck J, Jeschke P, Harder A (2002) PF1022A and related cyclodepsipeptides – a novel class of anthelmintics. Curr Top Med Chem 7:759–777
Schwarz M, Köpcke B, Weber RWS, Sterner O, Anke H (2004) 3-Hydroxypropionic acid a nematicidal principle of endophytic fungi. Phytochemistry 65:2239–2245
Seto Y, Takahasi K, Matsuura H, Kogami Y, Yada H, Yoshihara T, Nabeta K (2007) Novel cyclic peptide, epichlicin, from the endophytic fungus, Epichloe typhina. Biosci Biotechnol Biochem 71:1470–1475
Shiomi K, Ui H, Suzuki H, Hatano H, Nagamitsu T, Takano D, Miyadera H, Yamashita T, Kita K, Miyoshi H, Harder A, Tomoda H, Ōmura S (2005) A γ-lactone from nafuredin, nafuredin- γ, also inhibits helminth complex I. J Antibiot 58:50–55
Shoop WL, Gregory LM, Zaksonaiken M, Michael BF, Haines HW, Ondeyka JG, Meinke RT, Schmatz DM (2001) Systemic efficacy of nodulisporic acid against fleas on dogs. J Parasitol 87:419–423
Singh SB, Zink DL, Liesch JM, Mosley RT, Dombrowski AW, Bills GF, Darkin-Rattray SJ, Schmatz DM, Goetz MA (2002) Structure and chemistry of apicidins, a class of novel cyclic tetrapeptides without a terminal α-keto epoxide as inhibitors of histone deacetylase with potent antiprotozoal activities. J Org Chem 67:815–825
Singh SB, Ondeyka JG, Jayasuriya H, Zink DL, Ha SN, Dahl-Roshak A, Greene J, Kim JA, Smith MM, Shoop W, Tkacz JS (2004) Nodulisporic acids D-F: structure, biological activities, and biogenetic relationships. J Nat Prod 67:1496–1506
Skrobek A, Butt TM (2005) Toxicity testing of destruxins and crude extracts from the insect-pathogenic fungus Metarhizium anisopliae. FEMS Microbiol Lett 251:23–28
Smith AB, Cui H (2003) Indole-diterpene synthetic studies:total synthesis of (-)-21-isopentenylpaxilline. Helv Chim Acta 86:3908–3938
Supothina S, Isaka M, Kirtikara K, Tanticharoen M, Thebtaranonth Y. (2004) Enniatin production by the entomopathogenic fungus Verticillium hemipterigenum BCC 1449. J Antibiot 57:732–738
Tang CY, Chen YW, Jow GM, Chou CJ, Jeng CJ (2005) Beauvericin activates Ca2+−activated Cl− currents and induces cell deaths in Xenopus oocytes via influx of extracellular Ca2+. Chem Res Toxicol 18:825–833
Trost BM, Cramer N, Bernsmann H (2007) Concise total synthesis of (±)-marcfortine B. J Am Chem Soc 129:3086–3087
Uchida R, Imasato R, Yamaguchi Y, Masuma R, Shiomi K, Tomoda H, Omura S (2006a) Yaequinolones, new insecticidal antibiotics produced by Penicillium sp. FKI-2140. I. Taxonomy, fermentation, isolation and biological activities. J Antibiot 59:646–651
Uchida R, Imasato R, Tomoda H, Omura S (2006b) Yaequinolones, new insecticidal antibiotics produced by Penicillium sp. FKI-2140. II. Structural elucidations. J Antibiot 59:652–658
Vey A, Matha V, Dumas C (2002) Effects of the peptide mycotoxin destruxin E on insect haemocytes and on dynamics and efficiency of the multicellular immune reaction. J Invert Pathol 80:177–187
Whyte AC, Gloer JB, Wicklow DT, Dowd PF (1996) Sclerotiamide: a new member of the paraherquamide class with potent antiinsectan activity from the sclerotia of Aspergillus sclerotiorum. J Nat Prod 59:1093–1095
Williams RM (2002) Total synthesis and biosynthesis of the paraherquamides: an intriguing story of the biological Diels–Alder construction. Chem Pharm Bull 50:711–740
Williams RM, Cao J, Tsujishima H, Cox RJ (2003) Asymmetric, stereocontrolled total synthesis of paraherquamide A. J Am Chem Soc 125:12172–12178
Yasiu H, Hirai K, Yamamoto S, Takao K, Tadano K (2006) Total syntheses of (+)-1893B and its three diastereomers and evaluation of their biological activities. J Antibiot 59:456–463
Zimmermann G (2007a) Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Sci Technol 17:553–596
Zimmermann G (2007b) Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Sci Technol 17:879–920
Acknowledgements
I am grateful to Dr. L. Antelo for preparing the figures. The work in our laboratory was supported by Bayer AG, BASF SE, the State of Rhineland–Palatinate and the BMBF.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Anke, H. (2011). Insecticidal and Nematicidal Metabolites from Fungi. In: Hofrichter, M. (eds) Industrial Applications. The Mycota, vol 10. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-11458-8_7
Download citation
DOI: https://doi.org/10.1007/978-3-642-11458-8_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-11457-1
Online ISBN: 978-3-642-11458-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)