Abstract
This chapter describes the occurrence of cyclic peptides and cyclic depsipeptides within the kingdom Eumycota (true fungi), the diversity of structures and their chemical building blocks, their ecological roles and their different biological activities. Finally, it discusses the importance of cyclic peptides and depsipeptides as drugs and lead compounds for agricultural and pharmaceutical applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Cyclic peptides and depsipeptides are widely distributed in nature. They are found in plants (Gournelis et al. 1998; Tan and Zhou 2006), sponges and other lower sea animals (Bertram and Pattenden 2007), cyanobacteria (Welker and von Döhren 2006), bacteria and fungi alike and their bioactivities range from antimicrobial, insecticidal, nematicidal, antiviral, hepatotoxic, cytotoxic/cytostatic to immunosuppressive and other pharmacological properties (Kleinkauf and von Döhren 1997; Pomilio et al. 2006).
Some of the peptides and depsipeptides produced by fungi have gained entrance into the pharmaceutical market, like cyclosporins (Kürnsteiner et al. 2002), ergopeptides (Keller and Tudzynski 2002), penicillins (Demain and Elander 1999) and cephalosporins (Schmidt 2002), or are currently undergoing clinical trials, like the candines, promising antifungal drugs against aspergillosis and candidiasis (Denning 2002; Johnson and Perfect 2003; Pasqualotto and Denning 2008). Caspofungin derived from pneumocandin and micafungin derived from FR901379 are examples of those novel drugs targeting fungal cell wall synthesis, e.g. biosynthesis of 1,3-β-glucan (Odds et al. 2003; Butler 2004). For a recent review, see Hashimoto (2009). Emodepsin, a semi-synthetic depsipeptide, is used in veterinary medicine against helminths (von Samson-Himmelstjerna et al. 2005). The drug is derived from PF1022A, a metabolite of an endophytic fungus from Camellia japonica (Sasaki et al. 1992; Scherkenbeck et al. 2002). As these groups of compounds are well covered in the literature, they are not addressed here in detail.
The biosynthesis of cyclic peptides and depsipeptides has attracted the interest of biochemists since the mid1960s (Gevers et al. 1968). Today, the focus has shifted from enzymology to genetics, e.g. the biosynthetic genes and their regulation. Therefore Chap. 15 is dedicated to this topic, to which the reader is referred.
A special group of cyclopeptides are the diketopiperazines, which consist of two amino acids linked by two peptide bonds. In the related epipolythiodioxopiperazines the 6-ring is bridged by one to four sulfur atoms. The structural diversity of diketopiperazines (more than 100 different compounds are known from fungi; Buckingham 2008) is matched by their biological activities. Recently published reviews are available (Cole and Schweikert 2003; Gardiner et al. 2005). Interestingly, functions in the producing organisms have been detected for some of these compounds, e.g. gliotoxin and related compounds play a role as virulence factors in invasive aspergillosis (Sugui et al. 2007) and coprogens in host invasion of plant-pathogenic fungi (Oide et al. 2006; Hof et al. 2007). The reported biological activities of gliotoxin are very broad and diverse. Antibacterial, antifungal, antiviral, amoebicidal and immunosuppressive properties have been described (see below). Most of these activities are based on interactions with essential thiol groups in proteins (Waring and Beaver 1996). Iron chelators like dimerumic acid, rhodotorulic acid, coprogen and its derivatives are involved in iron uptake (Winkelmann and Drechsler 1997; Renshaw et al. 2002; Antelo et al. 2006), while other siderophores, e.g. the hexapeptides ferrichrome or ferricrocin, in addition to iron transport or storage functions act as virulence factors in some human and plant pathogens similar to coprogens (Howard 1999; Haas et al. 2008).
The group of peptaibiotics, a constantly growing family of linear α-aminobutyric acid (Aib)-containing linear peptides has been enlarged by a small group of cyclic peptides also containing Aib, now called cyclopeptaibiotics. Whereas the linear group comprises more than 800 compounds, only nine cyclic compounds have been reported to date. These are seven tetrapeptides structurally related to chlamydocin (Degenkolb et al. 2008) and the scytalidamides, two heptapeptides containing Aib residues (Tan et al. 2003).
2 Occurrence of Cyclic Peptides and Depsipeptides Within the Kingdom Eumycota (True Fungi)
2.1 Siderophores
The occurrence and distribution of siderophores among the taxonomic groups of fungi is very well covered by the reviews of Renshaw et al. (2002) and Haas et al. (2008). Zygomycetes very rarely produce cyclic peptide or depsipeptide siderophores. Up to now the hexapeptide ferrichrysin seems to be the only example. It is produced by Cunninghamella blakesleeana (Patil et al. 1995). The production of diketopiperazine and hexapeptide siderophores is common among asco- and basidiomycetes (Renshaw et al. 2002). The fact that members of some orders have not yet been reported to produce siderophores reflects a lack of investigation rather than presence. There are a few fungi, however, which do not produce siderophores: the ascomycetous yeasts Saccharomyces cerevisiae and Candida albicans or Geotrichum candidum and the basidiomycete Cryptococcus neoformans (teleomorph Filobasidiella; Howard 1999; Haas et al. 2008). The investigation of basidiomycetes is difficult because iron-free media, which upregulate the biosynthesis of siderophores, often hardly support mycelial growth, requiring incubation times of eight to ten weeks (Welzel et al. 2005). In contrast, modern analytical techniques like HPLC-MSn are sensitive enough to allow the detection and characterization of very small amounts (μg/l of culture). In addition, as more fungal genomes and NRPS genes and products become available, it is clear that siderophores and iron metabolism are important virulence determinants (Eichhorn et al. 2006; Oide et al. 2006; Haas et al. 2008).
It is remarkable that extracellular and intracellular siderophores are not identical and that the synthesis of intracellular siderophores is often not iron-dependent.
As an example, most Trichoderma species excrete coprogen-type siderophores and ferricrocin for the capture and transport of iron and use palmitoylcoprogen located within the mycelia as storage compound. In T. pseudokoningii and T. longibrachiatum however, palmitoylcoprogen was not detected, but these two species excrete fusigen-type siderophores in addition to coprogen and fericrocin (Anke et al. 1991). Magnaporthe grisea uses intracellular ferricrocin for iron storage and under iron deprivation excretes four coprogen derivatives (Hof et al. 2007). In other plant-pathogenic fungi like Fusarium graminearum, F. culmorum, F. pseudograminearum, Cochliobolus heterostrophus and Gibberella zeae ferricrocin has also been reported as intracellular siderophore (Oide et al. 2007; Tobiasen et al. 2007). The situation in the human pathogen Aspergillus fumigatus is similar. Ferricrocin is located in the mycelia, a hydroxylated derivative in the conidia and triacetylfusigen is excreted (Schrettl et al. 2007).
The structures of several iron-free siderophores, e.g. rhodotorulic acid, 2-N-methylcoprogen, palmitoylcoprogen, ferricrocin and ferrichrome are given in Fig. 13.1.
2.2 Diketopiperazines
Simple diketopiperazines may be detected in fermentations of many fungi. Sometimes it is difficult to decide whether these are degradation products of proteins and peptides or synthesized de novo (Prasad 1995). In the future, this problem might be solved by molecular genetics, since the presence of the relevant biosynthetic genes can be proof of de novo synthesis (Chap. 15). The recently demonstrated behavioral effects and occurrence in humans of cyclo(His-Pro) stimulated research on such compounds which are easily accessible by chemical synthesis. However, cyclo(His-Pro) has not yet been reported from fungi. This may be due to the fact that its bioactivities, e.g. inhibition of food intake and inhibition of prolactin secretion or modulation of pain perception (Prasad 1995) are not suited for a screening of microbial cultures. Usually these compounds are detected during the isolation of other metabolites and described as side-products. A recent example is l-alanyl-l-tryptophan anhydride isolated together with golmaenone, a radical scavenger compound, and neoechinulin from an marine Aspergillus species (Li et al. 2004). As in many other cases, the simple alkaloid is the biogenetic precursor of the other two compounds. With antimicrobial, cytotoxic, phytotoxic, insecticidal and other test systems which have been extensively used in screenings for bioactive natural products, simple diketopierazines are less frequently detected. One example is the fungistatic mactanamide from a marine Aspergillus species (Lorenz et al. 1998). Simple diketopiperazines have been described from hetero- and homobasidiomycetes, for example Ustilago cynodontis, Entoloma haastii and Stereum hirsutum (Turner and Aldridge 1983), ascomycetes like Rosellinia necatrix, Claviceps species, Eurotium and Emericella species, Leptosphaeria species including their anamorphs, Aspergillus, Phoma and Coniothyrium species (Turner and Aldridge 1983; Cole and Schweikert 2003; Blunt et al. 2006).
Aspergillus and Penicillium species are very prolific producers of cyclic dipeptide-derived mycotoxins like fumitremorgins, verruculogens or roquefortine C, while sporidesmins, mycotoxins that cause facial eczema in grazing sheep, are produced by Pithomyces chartarum (Betina 1989). From several Penicillium species, mycelianamide, one of the very “old” diketopiperazines, has been known since 1931. This compound was detected during early screenings after the discovery of penicillin G. The recently described sulfur-containing gliovictin was obtained from an endophytic Penicillium janczewskii (Gunatilaka 2006) and diketopiperazine-derived rostratins from a marine Exserohilium rostratum (Tan et al. 2004). To the long list of Penicillium species producing diketopiperazines, P. dipodomyis, P. nalgiovense, P. fellutanum and P. simplicissimum were recently added (Lewis 2002).
Examples for structures of simple and complex diketopiperazines are found in Fig. 13.2.
From cultures of a number of fungi producing cyclic depsipeptides, e.g. Beauveria bassiana, dipeptides composed of the amino acids occurring in the depsipeptides have been isolated. Other insect pathogens like Verticillium species and Metarhizium anisopliae as well as plant-pathogenic fungi, e.g. Colletotrichum gloeosporioides, Exserohilum holmi, Gliocladium deliquenscens, Alternaria and Trichoderma produce dipeptides. An unidentified endophyte from mangrove leaf produces two cyclic depsipeptides and three diketopiperazines (Huang et al. 2007). The role of the compounds, dipeptides and depsipeptides, in insect and plant-pathogenicity has not yet been completely elucidated. As molecular tools become more easily available, this question might be addressed or even answered in the near future, especially since the elucidation of the ecological function of secondary metabolites for the producers becomes more interesting (see below).
Epipolythiopiperazines with more than 60 members, gliotoxin being the most prominent, are widely distributed in nature. Their producers are mainly found among the ascomycete genera Aspergillus, Penicillium, Gliocladium, Verticillium, Chaetominum, Emericella, Acrostalagmus (syn. Verticillium), Pithomyces, Bionectria, Leptosphaeria, Hyalodendron, Trichoderma, Sirodesmium (syn. Coniosporium), Epicoccum, Arachniotus and Pseudallescheria (Turner and Aldridge 1983; Betina 1989; Takahashi et al. 1994; Gardiner at al. 2005; Li et al. 2006; Zheng et al. 2007). There is one report on the occurrence of an epipolythiopiperazine in lichens, e.g. Xanthoparmelia scabrosa (Ernst-Russell et al. 1999). As is true for many lichen metabolites, it may be also in this case the ascomycetous fungal partner which is responsible for the production of scabrosin. The production of epicorazine C by Stereum hirsutum, a basidiomycete, seems a bit questionable since related epicorazines are produced by Epicoccum nigrum and E. purpurascens (Kleinwachter et al. 2001). Overlaps between metabolites from basidiomycetes and ascomycetes are fairly rare but do occur occasionally. Other examples may be beauvericin and chlamydocin (see below). The structures of gliotoxin, epicorazines, scabrosin, vertihemiptellide A and other epipolythiopiperazines are given in Fig. 13.3.
2.3 Cyclic Peptides
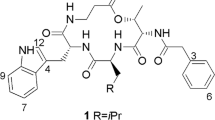
Cyclic peptides are mainly produced by ascomycetes and their anamorphs. Among cyclic peptides, the immunomodulating cyclosporins constitute the largest group with 46 members. The producing organisms are found mainly in the ascomycetous families Hypocreaceae and Clavivipitaceae and their anamorphs Tolypocladium inflatum, T. tundrense and T. terricola. In addition, three soil-borne insect pathogens, Neocosmospora vasinfecta, Acremonium luzulae, a Cyclindrotrichum species, Stachybotrys chartarum, Trichoderma viride, a Leptostroma anamorph of Hypoderma eucalyptii, Chaunopycnis alba and an unidentified mycelium sterilium have been reported to produce cyclosporins (Matha et al. 1992; Traber and Dreyfuss 1996). The structure of cyclosporin A is found in Fig. 13.4C. Figure. 13.4A shows examples of simpler cyclospeptides.
The malformins, a group of nine phytotoxic compounds, are only found within the Aspergillus niger group (Kobbe et al. 1977). Some authors classify the compounds as mycotoxins even so they are rarely found in food or feed stuff.
The antifungal echinocandins comprising different compounds (aculeacin A, echinocandin B, pneumocandins, mulundocandins, FR901379, WF11899A, B, C, FR227673, FR190293, etc.) have been reported from several Aspergilli, Coleophoma empetri, C. crateriformis, Chalara species, Tolypocladium parasiticum and Zalerion arboricola (Iwamoto et al. 1994b; Anke and Erkel 2002; Denning 2002; Kanasaki et al. 2006a, b, c,).
The structures of some of these compounds can be found in Fig. 13.5.
Producers of various cyclic peptides are found in many other families and genera, for example Diheterospora, Gliocladium, Cylindrocarpon, Clonostachys, Cochliobolus and Fusarium (Lewis 2002; Adachi et al. 2005; Weber et al. 2006; Degenkolb et al. 2008).
As endophytic fungi have recently come into focus as producers of bioactive natural compounds, it is not astonishing that also novel cyclic peptides have been reported from these fungi.
A pentapeptide was isolated from an unidentified endophyte from the seed of Avicinnia marina (Gunatilaka 2006), other cyclopeptides from endophytic Fusarium species (Shiono et al. 2007), Epichloe typhina (Seto et al. 2007) or endophyte “2221” from Castaniopsis fissa (Yin et al. 2005). More than 450 cyclic peptides are known from plants (Tan and Zhou 2006); some of these actually may be produced by endophytic fungi in planta.
In recent years, marine habitats have drawn much interest as ecological niches for producers of novel bioactive metabolites. The unguisins were isolated from a marine-derived strain of Emericella unguis (Malstrom 2002). Among cyclic peptides from obligate marine ascomycetes are the highly cytotoxic trapoxin A produced by Corollospora intermedia (Daferner 2000) or scytalidamides from a Scytalidium species from a marine alga (Tan et al. 2003). JM47, structurally related to HC-toxins and trapoxin, was isolated together with enniatin from a marine-derived Fusarium species (Jiang et al. 2002). Trapoxins are also known from terrestrial fungi, e.g. Helicoma ambiens, the anamorph of Thaxteriella pezicula (Itazaki et al. 1990) and structurally related metabolites have been described from the phytopathogenic Cyclindrocladium scorparium (teleomorph Calonectria morganii) and Cochliobolus carbonum (Degenkolb et al. 2008).
For structures see Fig. 13.6.
The only cyclopeptides, besides the siderophores, known from submerged cultures of basidiomycetes are the omphalotins from Omphalotus olearius (Büchel et al. 1998a,b), amanitins from Amanita exitialis (Zhang et al. 2005) and chlamydocins from a Peniophora strain isolated from soil (Tani et al. 2001). The chlamydocins are tetrapeptides with Aib and an unusual amino acid. Most of these are produced by ascomycetes, e.g. Diheterospora chlamyydosporia (Closse and Huguenin 1974) and V. coccosporum (Gupta et al. 1994). Interestingly, the omphalotins produced by a monokaryotic strain differ from those found in the dikaryotic parental strain (Liermann et al. 2009). However, all O. olearius strains irrespective of their geographical origin produce omphalotin derivatives (Anke et al., unpublished data). In fruiting bodies omphalotins could not be detected, contrary to Amanita exitialis carpophores which contained tenfold more α- and β-amanitin as compared to the slow growing mycelial cultures (Zhang et al. 2005). For recent surveys of Amanita toxins from fruiting bodies see Li and Oberlies (2005), Liu (2005) and Pomilio et al. (2006). Figure 13.4B shows the structures of omphalotins and α-amanitin.
2.4 Cyclic Depsipeptides
Most depsipeptides are metabolites from ascomycetes and their anamorphs. They are widespread in phytopathogens (e.g. Cochliobolus with anamorphs Helminthosporium and Bipolaris, Calonectria and its anamorph Cyclindrocladium, as well as Fusarium and Alternaria), insect pathogens (Aschersonia, Beauveria, Cordyceps, Diheterospora, Fusarium, Hirsutella, Isaria, Metharizium, Paecilomyces, Verticillium) and others (Zimmermann 2007a, b; Buckingham 2008). For a compilation of beauvericins and enniatins produced by Cordyceps species and their anamorphs as well as other insect pathogens see Isaka et al. (2005a, b). Figure 13.7 gives the structures of some cyclodepsipeptides.
Up to now the pteratides (Fig. 13.7C) are the only depsipeptides reported from basidiomycetes, namely from the fruiting bodies of a Pterula species (Chen et al. 2006). From zygomycetes none have been described. One report on the production of beauvericin by Laetiporus sulfureus (Badan et al. 1978) could not be confirmed by other groups. In our cultures from L. sulfureus from different locations we could only detect laetiporic acid and its derivatives (Davoli et al. 2005).
Since the review of Anke and Sterner (2002), additional producers of bioactive depsipeptides have been reported, for example marine-derived strains of Beauveria fellina (Lira et al. 2006), Verticillium sp. FKI-1033 (Monma et al. 2006), Aspergillus carneus (Capon et al. 2003), Torrubiella luteorostrata and its anamorph Paecilomyces cinnamomeus (both isolated from a scale insect; Isaka et al. 2007), Verticillium hemipterigenum (Supothina et al. 2004), an Aureobasidium species from the tropical rain forest (Boros et al. 2006), an unidentified endophytic fungus (Huang et al. 2007) and a soil-borne Phoma species (Aoyagi et al. 2007). Pseudodestruxins have been reported from Nigrosabulum globosum (Che et al. 2001) and reviews on destruxins and the producing organisms have been published by (Pedras et al. 2002) and Zimmermann (2007b).
The endophyte-producing PF1022A (and related anthelmintic cyclooctadepsipeptides) isolated from leaves of a camellia has been identified based on its 18S rRNA gene sequence as a member of the Xylariaceae close to Xylaria polymorpha and Rosellinia necatrix (Miyado et al. 2000).
One of the few aquatic fungi investigated for secondary metabolite production is Clavariopsis aquatica from which the antifungal clavariopsins A and B were isolated (Kaida et al. 2001).
Analogues of the lipopeptides with 1,3-β-glucan synthase inhibitory activity are the lipodepsipeptides FR901469 or LL15G256γ (see Fig. 13.5). The former is produced by an unidentified fungus, the latter (identical to arthrichitin from Arthrinum spaeospermum) by Hypoxylon oceanicum (Abbanat et al. 1998; Fujie et al. 2000).
3 Chemical and Biological Diversity of Cyclic Peptides and Depsipeptides
3.1 Diversity of Building Blocks
Cyclic peptides and depsipeptides constitute a class of natural compounds with an enormous structural diversity. This diversity is brought upon by the different building blocks in the ring: proteinogenic amino acids including their d-isomers, nonproteinogenic amino acids, branched or unbranched lipoamino acids and hydroxylated short-, medium- and long-chain fatty acids. The diversity of the building blocks can be deduced from Tables 13.1–13.3, which give a compilation of unusual building blocks (Table 13.1 unusual amino acids, Table 13.2 unusual fatty acids) and various modifications (Table 13.3).
3.2 Diversity of Structures
Additional variations are due to the different numbers of building blocks, their arrangement (e.g. sequence in the ring) and their linkage (e.g. amide and ester bonds). Some depsipetides like the enniatins, beauvericins, bassianolide or verticilide show a symmetric arrangement in the ring. The majority however are asymmetric, like the destruxins, beauverolides, isariins or Alternaria toxins (Fig. 13.7A,C).
Cyclic peptides including the cyclosporins are asymmetric, as are the echinocandins. The number of building blocks in cyclic peptides varies from two in the diketopiperazines, some of which are symmetric if composed of two residues of the same amino acid, to 12 in the omphalotins, which are at present the largest cyclopeptides known from fungi. In addition, the omphalotins are an example of modifications after ring closure. Omphalotins B, C and D are derived from omphalotin A by hydroxylation followed by acylation to the corresponding esters and formation of additional ring structures (Büchel et al. 1998).
Novel omphalotins were recently isolated from a monokaryotic strain. The elucidation of their structures was greatly hampered by their instability (Liermann et al. 2009). These omphalotins bear additional hydroxyl groups, thus bringing the number of known cyclic peptides from O. olearius to 11. A second hydroxylation at the tryptophan leads to a novel ring system (Fig. 13.4B). HPLC-MS spectra of enriched extracts indicate the presence of additional members of the group. The psychrophilins are nitropeptides with unusual structures (Fig. 13.4A). The compounds are produced by several psychrotolerant Penicillium species (Dalsgaard et al. 2004a, b; 2005). Cyclochlorotine, a mycotoxin from P. islandicum contains a dichloroprolyl residue (Betina 1989).
The depsipeptides start with four building blocks (angolide, beauverolides) up to 12 in the antibiotic FR901469 (a member of the 1,3-β-glucan synthase inhibitors; Fujie et al. 2001) and 13 in petriellin A (Lee et al. 1995). The latter contains β-phenyllactic acid, a building block not often found in cyclopeptides and -depsipeptides. Further modifications of cyclic peptides and depsipeptides include N-methylation, hydroxylations, acylation, isoprenylation and the introduction of sulfate-, nitro- chloro- or cyano- groups. These modifications can occur at the beginning of biosynthesis, like N-methylations, or after cyclization, e.g. C- and N-hydroxylations followed by an acylation (Glinski et al. 2001; Chap. 15). In many cases however it is not clear at which step the modifications occur. The low substrate specificity of the NRPS enzymes allows the incorporation of modified ring components. In fact, Zocher and his group have made use of this to produce novel enniatin derivatives in vitro (Feifel et al. 2007).
3.3 Diversity of Biological Activities
The structural diversity of diketopiperazines, cyclopeptides and -depsipeptides is matched by the diversity of their biological activities. To list all activities and compounds would be beyond of the scope of this chapter. An overview on biological activities of diketopiperazines is given by Martins and Carvalho (2007), cyclic depsipeptides and their biological activities are reviewed by (Sarabia et al. 2004), while insecticidal and other biological activities of destruxins, isariins, enniatins, and beauverolides are reviewed by Anke and Sterner (2002) and by Zimmermann (2007a, b). Some of the compounds exhibit rather selective activities like the antifungal, 1,3-β-glucan synthesis inhibitors (see below) whereas others like gliotoxin show a broad spectrum of activities. While the former (due to fewer side-effects) generally have a higher potential to be developed into drugs or pesticides, the latter might be of interest as biochemical tools or chemical building blocks. In the following, we attempt to give an overview on the different biological activities exhibited by fungal cyclopeptides and -depsipeptides.
Gliotoxin, already isolated in 1932, recently regained interest not only due to its immunosuppressive and apoptosis-inducing activities (Waring et al. 1988) but moreover due to its occurrence in the blood of aspergillosis patients and its effects on various human cells among them an inhibition of cell adherence in macrophages (Amitani et al. 1995; Kamei and Watanabe 2005). The plethora of biological activities is evident from the number of papers published on gliotoxin and related epipolythiodioxopiperazines (Waring and Beaver 1996; Hume et al. 2002; Gardiner et al. 2005).
The vertihemiptellides A and B and their S-methylated monomers exhibit antimycobacterial and cytotoxic effects (Isaka et al. 2005b). Sirodesmin PL produced by Leptosphaeria maculans has phytotoxic, antibacterial and insecticidal properties (Rouxel et al. 1988; Boudart 1989) and the leptosins inhibited the proliferation of P388 lymphocytic leukemia cells with an ED50 of 1.1–1.3 μg/ml (Takahashi et al. 1994).
The HC-toxins, host-specific toxins from Cochliobolus carbonum (anamorph Helminthosporium carbonum), are cyto- and phytotoxic and inhibitors of histone deacetylase (Taunton et al. 1996).
Structurally related tetrapeptides (Fig. 13.6) like apicidin from a Fusarium species (Darkin-Rattray et al. 1996; Singh et al. 2002), JM47 from a marine Fusarium species (Jiang et al. 2002), FR235222 from an Acremonium species (Mori et al. 2003) or the chlamydocins from Diheterospora chlamydosporia (Closse and Huguenin 1974) and Peniophora sp. (Tani et al. 2001) have been reported to exhibit antiprotozoal activity, to induce apoptosis, to have immunosuppressive effects or to retard plant growth (de Schepper et al. 2003).
Due to their toxic effects in animal and humans and their occurrence in food and feedstuff, fumitremorgins, verruculogens, roquefortins C and D, sporidesmins, chaetocin, cyclochlorotine and malformins were classified as mycotoxins (Betina 1989). For their different biological activities the reader is referred to the vast online literature on this group of fungal products.
Malformin C (Fig. 13.4), despite its antibacterial, plant-deforming and fibrinolytic activities, recently aroused some interest due to its inhibitory effects on bleomycin-induced G2 arrest, thus potentiating its DNA-damaging action, a mode of action that might be useful for the treatment of cancer (Hagimori et al. 2007).
Cyclosporins are not the only immunomodulating fungal metabolites. Many epipolythiodioxopiperazines, in addition to other biological activities, are immunosuppressants.
Sevastelins, cyclodepsipeptides with a lipophilic side-chain, from a Penicillium species blocked human T cell activation in vitro and showed low acute toxicity in mice (Morino et al. 1994). HUN-7293 acts as inhibitor of cytokine-induced expression of vascular cell adhesion molecule-1 on human endothelial cells (Hommel et al. 1996). It is structurally identical to pestahivin.
The depsipeptide aureobasidin A has an interesting mode of action, the inositol phosphoceramide synthase (IPS). The fungal enzyme is considered to be an attractive target for novel fungicides. Further development of aureobasidin A was hampered by its inhibitory effects on ABC transporters in yeasts and humans (Fostel and Lartey 2000). The pleofungins from a Phoma species showed antifungal activity towards Candida albicans, Cryptococcus neoformans and A. fumigatus with minimal inhibitory concentrations in the range of 1 μg/ml or lower (Yano et al. 2007). The compounds inhibited the A. fumigatus IPS with IC50 values of 1 ng/ml (Aoyagi et al. 2007).
Neoechinulin A has protective activity in PC12 cells against lethal effects of peroxynitrite and against 1-methyl-4-phenylpyridine, a neurotoxin capable of inducing neurodegeneration in humans (Kajimura et al. 2008). The cyclic tetrapeptide CJ-15,208 is a kappa opinoid receptor antagonist (Saito et al. 2002) and four depsipeptides were reported to be selective and competitive human tachykinin receptor antagonsits (Hedge et al. 2001).
Among nine beauverolides tested for acyl-CoA:cholesterol acyltransferase (ACAT) inhibitory activity in CHO-cells expressing ACAT1 or ACAT2, beauverolides I and III inhibited ACAT1 rather selectively, no antimicrobial or cytotoxic activities were detected and beauvericin was cytotoxic (Matsuda et al. 2004; Ohshiro et al. 2007). ACAT is discussed as a target for new antiatherosclerotic agents (Roth 1998; Namatame et al. 2004).
The outstanding anthelmintic activity of PF1022A combined with its mode of action, e.g. binding to the latrophilin-like receptor of Haemonchus contortus (Conder et al. 1995; Saeger et al. 2001) and low toxicity led to the development of emodepsin, a novel drug used in animal health.
Antiparasitic properties have been reported for cycloaspeptides A and D (Dalsgaard et al. 2004b). Verticilide, a cyclic depsipeptide isolated from the culture broth of Verticillium sp. FKI-1033, inhibits the binding of ryanodine to the receptor (RyR) and has insecticidal activity (Monma et al. 2006). Serinocyclin A isolated from M. anisopliae condia produced a sublethal locomotory defect in mosquito larvae (Krasnoff et al. 2007). Argifin and argadin, two cyclopentapeptides from a Gliocladium and a Clonostachys species, are potent inhibitors of chitinase B from Serratia marcescens (Houston et al. 2002). When injected into cockroach larvae, the moult was arrested. Besides cyclopeptides and -depsipeptides fungi also produce other peptides with insecticidal activities, recent examples are the neofrapeptins from Geotrichum candidum (Fredenhagen et al. 2006). Selective nematicidal properties have been reported only for the omphalotins with high inhibitory activity towards Meloidogyne incognita and low activity towards Caenorhabditis elegans (Mayer et al. 1997, 1999; Sterner et al. 1997). The nematicidal properties of the hydroxylated omphalotins are higher than those of the parent compound, but unfortunately they are not stable (Büchel et al. 1998a, Liermann et al. 2009).
Antiviral properties have been reported for sansalvamide A, a cyclodepsipeptide from a marine Fusarium, which inhibits viral topoisomerase-catalyzed DNA relaxation (Hwang et al. 1999).
The clavariopsins, cyclic depsipetides from Clavariopsis aquatica, show selective antifungal activity, bacteria are not affected and mice tolerate 100 mg/kg of clavariopsin A. As mode of action, an inhibition of cell components was proposed (Kaida et al. 2001). Glomosporin from a Glomospora species is a lipophilic depsipeptide with antifungal activity (Sato et al. 2000). Whether this compound also inhibits cell wall synthesis was not reported. Antifungal and cytotoxic activities were reported for petriellin A (Lee et al. 1995). Cytotoxic activities are exhibited by many cyclopeptides and -depesipeptides. The destruxins have been intensively investigated (Vey et al. 2002; Skrobek and Butt 2005). Psychrophilin D is weakly cytotoxic towards P388 mouse leukaemia cells with an IC50 value of 10 μg/ml (Dalsgaard et al. 2005), while the icosalides inhibit the replication of MDCK cells with LD50 of 5–10 μg/ml (Boros et al. 2006). The aspergillicins are weakly cytotoxic with LD99 of 25–50 μg/ml (Capon et al. 2003).
As inhibitors of 1,3-β-glucan synthesis have high potential as antimycotic drugs (Fostel and Lartey 2000), fungi have been intensively screened for the production of inhibitors of cell wall synthesis and cyclic peptides as well as cyclic depsipeptides have been found.
The antimycotic drugs already on the market (caspofungin, micafungin, anidulafungin) are derived from lipopeptides (Butler 2004; Morrison 2006). Their spectrum of activity is mainly restricted to Candida and Aspergillus species. Cryptococcus neoformans, Trichosporon and Fusarium species or Zygomycetes are not affected (Denning 2003), although the glucan synthase from C. neoformans is sensitive to echinocandins (Maligie and Selitrennikoff 2005).
4 Ecological Role of Cyclic Peptides and Depsipeptides
Many secondary metabolites play a crucial role for fungi in their natural habitats. Endophytic fungi of grasses belonging to the genera Neotyphodium/Epichloe confer protection from mammalian and insect herbivores, or enhanced resistance against nematodes and phytopathogenic fungi (Schardl et al. 2004; Panaccione et al. 2006). Some of these beneficial effects are due to NRPS products. Ergovaline has been identified among the fungal metabolites in the plant host. Malformins have been detected in onion scales after infection with A. niger (Curtis et al. 1974).
The role of siderophores in plant and human pathogens is currently elucidated by many research groups (for a review see Haas et al. 2008). Additional functions of siderophores for the producing organism are acquisition and storage of iron as well as regulation of asexual and sexual development and protection against oxidative stress (Einsendle et al. 2006; Hof et al. 2009). Nonproducing organisms like Saccharomyces cerevisiae are able to use, e.g. transport iron-siderophore complexes, thus the compounds might also play a role in fungus–fungus interactions.
In plant-pathogenic fungi cyclic peptides like HC-toxins in Cochliobolus carbonum, AM toxins in Alternaria alternata, sirodesmin PL in Leptosphaeria maculans (anamorph Phoma lingam) or enniatins in Fusarium species act as putative virulence factors. In some cases this has already been proven, when gene deletions result in apathogenic strains or strains with reduced virulence (Ahn and Walton 1998; Pedley and Walton 2001; Elliott et al. 2007). Likewise the insecticidal depsipetides of insect pathogens have the same function. Investigation on the role of destruxins in the pathogenicity of Metarhizium anisopliae against three species of insects revealed a direct relationship between the titer of destruxins produced by the strains in vitro and their destructive action (Kershaw et al. 1999). In the plant-pathogenic Alternaria brassicae, destruxin B is a host-specific toxin. In three Brassica species the degree of their sensitivity to destruxin B positively correlated with their degree of susceptibility (Pedras et al. 2002).
The function of shearamide A, an insecticidal cyclopeptide isolated from the ascostromata of Eupenicillium shearii (Belofsky et al. 1998) may be in protecting the fungus against insects, similar to ergopeptides in the sklerotia of Claviceps species (Chap. 9).
5 Conclusions
The capability to produce secondary metabolites derived from amino acids by NRPS is widespread among the higher fungi and not dependent on the ecological niches inhabited by them. There are no special habitats from which highly prolific secondary metabolite producers are isolated.
Cyclic peptides and -depsipeptides constitute an interesting class of secondary metabolites with great potential not only in medicine but also in agriculture. This can easily be grasped from the wide array of biological activities exhibited by these compounds. Their chemical diversity is enhanced by the possibility of producing an array of related compounds by precursor-supplemented fermentations of the correspondent fungus. This readily facilitates investigations on structure–activity relationships.
In agriculture, fungally derived pesticides offer ecological advantages and strains with enhanced production of bioactive compounds might be developed as biopesticides. For both agriculture and pharmacology bioactive natural compounds may lead to novel targets and serve as lead structures.
References
Abbanat D, Leighton M, Maiese W, Jones EBG, Pearce C, Greenstein M (1998) Cell wall active compounds produced by the marine fungus Hypoxylon oceanicum LL-15G56. J Antibiot 51:296–302
Adachi K, Kanoh K, Wisespong P, Nishijima M, Shizuri Y (2005) Clonostachysins A and B, new antidinoflagellate cyclic peptides from a marine-derived fungus. J Antibiot 58:145–150
Ahn JH, Walton JD (1998) Regulation of cyclic peptide biosynthesis and pathogenicity in Cochliobolus carbonum by TOXEP, a novel protein with a bZIP basic DNA-binding motif and four ankyrin repeats. Mol Gen Genet 260:462–469
Amitani R, Taylor G, Elezis EN, Liewellyn-Jones C, Mitchell J, Kuze F, Cole PJ, Wilson R (1995) Purification and characterization of factors produced by Aspergillus fumigatus which affect human ciliated respiratory epithelium. Infect Immun 63:3266–3271
Anke H, Sterner O (2002) Insecticidal and nematicidal metabolites from fungi. In: Osiewacz HD (ed) Industrial applications. Mycota X. Springer, Heidelberg, pp 109–127
Anke H, Kinn J, Bergquist KE, Sterner O (1991) Production of siderophores by strains of the genus Trichoderma: Isolation and characterization of the new lipophilic coprogen derivative, palmitoylcoprogen. Biol Metals 4:176–180
Anke T, Erkel O (2002) Non β-lactam antibiotics. In: Osiewacz HD (ed) Industrial applications. Mycota X. Springer, Heidelberg, pp 93–108
Antelo L, Hof C, Eisfeld K, Sterner O, Anke H (2006) Siderophores produced by Magnaporthe grisea in the presence and absence of iron. Z Naturforsch. 61c:461–464
Aoyagi A, Yano T, Kozuma S, Takatsu T (2007) Pleofungins, novel inositol phosphorylceramide synthase inhibitors, from Phoma sp. SANK 13899. J Antibiot 60:143–152
Arai N, Shiomi K, Iwai Y, Omura S (2000) Argifin, a new chitinase inhibitor, produced by Gliocladium sp. FTD-0668. II. Isolation, physico-chemical properties, and structure elucidation. J Antibiot 53:609–614
Badan SD, Ridley DD, Singh P (1978) Isolation of cyclodepsipeptides from plant pathogenic fungi. Aust J Chem 31:1397–1399
Belofsky GN, Gloer JB, Wicklow DT, Dowd PF (1998) Shearamide A: a new cyclic peptide from the ascostromata of Eupenicillium shearii. Tetrahedron Lett 39:5497–5500
Belofsky GN, Jensen PR, Fenical W (1999) Sansalvamide: a new cytotoxic cyclic depsipeptide produced by a marine fungus of the genus Fusarium. Tetrahedron Lett 40:2913–2916
Bertram A, Pattenden G (2007) Marine metabolites: metal binding and metal complexes of azole-based cyclic peptides of marine origin. Nat Prod Rep 24:18–30
Betina V (1989) Epipolythiopiperazine-3,6-diones. In: Mycotoxins, chemical, biological and evironmental aspects. Elsevier, Amsterdam, pp 388–405
Bills GF, Platas G, Peláez F, Masurekar P (1999) Reclassification of a pneumocandin-producing anamorph, Glarea lozoyensis gen. et sp. nov., previously identified as Zalerion arboricola. Mycol Res 103:179–192
Birch AJ, Massy-Westropp RA, Rickards RW (1956) Studies in relation to biosynthesis. Part VIII. The structure of mycelianamide. J Chem Soc 3717-3721
Blunt JW, Copp BR, Munro MHG, Northcote PT, Prinsep MR (2006) Marine natural products. Nat Prod Rep 23:26–78
Boros C, Smith CJ, Vasina Y, Che Y, Dix AB, Darveaux B, Pearce C (2006) Isolation and identification of the icosalides – cyclic peptolides with selective antibiotic and cytotoxic activities. J Antibiot 59:486–494
Boudart G (1989) Antibacterial activity of sirodesmin PL phytotoxin: application to the selection of phytoxin-deficient mutants. Appl Enivron Microbiol 55:1555–1559
Büchel E, Martini U, Mayer A, Anke H, Sterner O (1998a) Omphalotins B, C, and D, nematicidal cyclopeptides from Omphalotus olearius. Absolute configuaration of omphalotin A. Tetrahedron 54:5345–5352
Büchel E, Mayer A, Martini U, Anke H, Sterner O (1998b) Structure elucidation of omphalotin, a cyclic dodecapeptide with potent nematicidal activity from Omphalotus olearius. Pest Sci 54:309–311
Buckingham J (2008) (Ed) Dictionary of natural products on DVD, version 17.1. Chapman and Hall/CRC, Boca Raton
Butler MS (2004) The role of natural product chemistry in drug discovery. J Nat Prod 67:2141–2154
Capon RJ, Skene C, Stewart M, Ford J, O'Hair RAJ, Williams L, Lacey E, Gill JH, Heiland K, Friedel T (2003) Aspergillicins A-E: five novel depsipeptides from the marine-derived fungus Aspergillus carneus. Org Biomol Chem 1:1856–1862
Che Y, Swenson DC, Gloer JB, Koster B, Malloch D (2001) Pseudodestruxins A and B: new cycllic depsipeptides from the coprophilous fungus Nigrosabulum globosum. J Nat Prod 64:555–558
Chen CH, Lang G, Mitova MI, Murphy AC, Cole ALJ, Din LB, Blunt JW, Munro MHG (2006) Pteratides I-IV, new cyctotoxic cyclodepsipeptides from the Malaysian basidiomycete Pterula sp. J Org Chem 71:7947–7951
Closse A, Huguenin R (1974) Isolierung und Strukturaufklärung von Chlamydocin. Helv Chim Acta 57:533–545
Cole RJ, Schweikert MA (2003) Diketopiperazines. In: Handbook of secondary fungal metabolites, vol 1. Academic, Amsterdam, pp 145–244
Conder GA, Johnson SS, Nowakowski DS, Blake TE, Dutton FE, Nelson SJ, Thomas EM, Davis JP, Thompson DP (1995) Anthelmintic profile of the cyclodepsipeptide PF1022A in in vitro and in vivo models. J Antibiot 48:820–823
Curtis RW, Stevenson WR, Tuite J (1974) Malformin in Aspergillus niger-infected onion bulbs (Allium cepa). Appl Environ Microbiol 28:362–365
Daferner M (2000) Antibiotisch aktive Sekundärstoffe aus höheren marinen Pilzen. Dissertation, University of Kaiserslautern
Dalsgaard PW, Blunt JW, Munro MHG, Larsen TO, Christophersen C (2004a) Psychrophilin B and C: cyclic nitropeptides from the psychrotolerant fungus Penicillium rivulum. J Nat Prod 67:1950–1952
Dalsgaard PW, Larsen TO, Frydenvang K, Christophersen C (2004b) Psychrophilin A and cycloaspeptide D, novel cyclic peptides from the psychotolerant fungus Penicillium ribeum. J Nat Prod 67:878–881
Dalsgaard PW, Larsen TO, Christophersen C (2005) Bioactive cyclic peptides from the psychrotolerant fungus Penicillium algidum. J Antibiot 58:141–144
Darkin-Rattray SJ, Gurnett AM, Myers RW, Dulski PM, Crumley TM, Allocco JJ, Cannova C, Meinke PT, Colletti SL, Bednarel MA, Singh SB, Goetz MA, Dombrowski AW, Polishook ED, Schmatz DM (1996) Apicidin, a novel antiprotozoal agent that inhibits parasite histone deacetylase Proc Natl Acad Sci USA 93:13143–31147
Davoli P, Mucci A, Schenetti L, Weber RWS (2005) Laetiporic acids, a family of non-carotenoid polyene pigments from fruit-bodies and liquid cultures of Laetiporus sulphureus (Polyporales, Fungi). Phytochemistry 66:817–823
!de Schepper S, Bruwiere H, Verhulst T, Steller U, Andries L, Wouters W, Janicot M, Arts J, van Heusden J (2003) Inhibition of histone deacylases by chlamydocin induces apoptosis and proteasome-mediated degradation of survivin. J Pharmacol Exp Ther 304:881–888
Degenkolb T, Gams W, Brückner H (2008) Natural cyclopeptaibols and related cyclic tetrapeptides: structural diversity and future prospects. Chem Biodiver 5:693–706
Demain AL, Elander RP (1999) The beta-lactam antibiotics: past, present, and future. Antonie Van Leeuwenhoek 75:5–19
Denning DW (2002) Echinocandins: a new class of antifungals. J Antimicrob Chemother 49:889–891
Denning DW (2003) Echinocandin antifungal drugs. Lancet 362:1142–1151
Eichhorn H, Lessing F, Winterberg B, Schirawski J, Kamper J, Mueller P, Kahmann R (2006) A ferroxidation/permeation iron uptake system is required for virulence in Ustilago maydis. Plant Cell 18:3332–3345
Eickman N, Clardy J, Cole RJ, Kirksey JW (1975) The structure of fumitremorgin A. Tetrahedron Lett 16:1051–1054
Eisendle M, Schrettl M, Kragl C, Müller D, Illmer P, Haas H (2006) The intracellular siderophore ferricrocin is involved in iron storage, oxidative-stress resistance, germination, and sexual development in Aspergillus nidulans. Eukaryot Cell 5:1596–603
Elliott CE, Gardiner DM, Thoma G, Cozijnsen A, van de Wouw A, Howlett BJ (2007) Production of the toxin sirodesmin PL by Leptosphaeria maculans during infection of Brassica napus. Mol Plant Pathol 8:791–802
Ernst-Russell M, Chai CL, Hurne AM, Waring P, Hockless DCR, Elix JA (1999) Structure revision and cytotoxic activity ot the scabrosin esters, epithiopiperazinediones from the lichen Xanthoparmelia scabrosa. Aust J Chem 52:279–283
Feifel SC, Schmiederer T, Hornbogen T, Berg H, Süssmuth RD, Zocher R (2007) In vitro synthesis of new enniatins: probing the α-d-hydroxy carboxylic acid binding pocket of the multienzyme enniatin synthetase. ChemBioChem 8:1767–1770
Fostel JM, Lartey PA (2000) Emerging novel antifungal agents. Drug Discov Today 5:25–32
Fredenhagen A, Molleyres LP, Böhlendorf B, Laue G (2006) Structure determination of neofrapeptins A to N: peptides with insecticidal activity produced by the fungus Geotrichum candidum. J Antibiot 59:267–280
Fridrichsons J, Mathieson AMCL (1962) The structure of sporidesmin: causative agent of facial eczema in sheep. Tetrahedron Lett 3:1265–1268
Fujie A, Iwamoto T, Muramatsu H, Okudaira T, Nitta K, Nakanishi T, Sakamoto K, Hori Y, Hino M, Hashimoto S, Okuhara M (2000) FR901469, a novel antifungal antibiotic from an unidentified fungus No 11243. I. Taxonomy, fermentation, isolation, physico-chemical properties and biological properties. J Antibiot 53:912–919
Fujie A, Muramatsu H, Yoshimura S, Hashimoto M, Shigematsu N, Takase S (2001) FR901469, a novel antifungal antibiotic from an unidentified fungus No 112434. III. Structure determination. J Antibiot 54:588–594
Gardiner DM, Waring P, Howlett BJ (2005) The epipolythiodioxopiperazine (ETP) class of fungal toxins: distribution, mode of action, functions and biosynthesis. Microbiology 151:1021–1032
Gevers W, Kleinkauf H, Lipmann F (1968) The activation of amino acids for biosynthesis of gramicidin S. Proc Natl Acad Sci USA 63:1335–1342
Glinski M, Hornbogen T, Zocher R (2001) Enzymatic synthesis of fungal N-methylated cyclopeptides and depsipeptides. In: Kirst H, Yeh WK, Zmijewski M (eds) Enzyme technologies for pharmaceutical and biotechnological applications. Dekker, New York, pp 471–497
Gournelis DC, Laskaris GG, Verpoorte R (1998) Cyclopeptide alkaloids In: Herz W, Falk H, Kirby GW, Moore RE, Tamm Ch (eds) Fortschritte der Chemie organischer Naturstoffe, vol 75. Springer, Heidelberg, pp 1–179
Gross ML, McCrery D, Crow F, Tomer KB, Pope MR, Ciuffetti LM, Knoche HW, Daly JM, Dunkle DL (1982) The structure of the toxin from Helminthosporium carbonum. Tetrahedron Lett 51:5381–5384
Gunatilaka AAL (2006) Natural products from plant-associated microorganisms: distribution, structural diversity, bioactivity, and implications of their occurrence. J Nat Prod 69:509–526
Gupta S, Peiser G, Nakajima T, Hwang Y-S (1994) Characterization of a phytotoxic cyclotetrapeptide, a novel chlamydocin analogue, from Verticillium coccosporum. Tetrahedron Lett 35:6009–6012
Haas H, Eisendle M, Turgeon BG (2008) Siderophores in fungal physiology and virulence. Annu Rev Phytopathol 46:149–187
Hagimori K, Fukuda T, Hasegawa Y, Omura S, Tomoda H (2007) Fungal malformins inhibit bleomycin-induced G2 checkpoint in Jurkat cells. Biol Pharm Bull 30:1379–1383
Hashimoto S (2009) Micafungin: a sulfated echinocandin. J Antibiot 62:27–35
Hedge VR, Puar MS, Dai P, Pu H, Patel M, Anthes JC, Richard C, Terracciano J, Das PR, Gullo V (2001) A family of depsipeptide fungal metabolites, as selective and competitive human tachykinin receptor (NK2) antagonists: fermentation, isolation, physico-chemical properties, and biological activity. J Antibiot 54:125–135
Hof C, Eisfeld K, Welzel K, Antelo L, Foster AJ, Anke H (2007) Ferricrocin synthesis in Magnaporthe grisea and its role in pathogenicity. Mol Plant Pathol 8:163–172
Hof C, Eisfeld K, Antelo L, Foster AJ, Anke H (2009) Siderophore synthesis in Magnaporthe grisea is essential for vegetative growth, conidiation and resistance to oxidative stress. Fungal Genet Biol 46:321–332
Hommel U, Weber H-P, Oberer L, Naegeli HU, Oberhauser B, Foster CA (1996) The 3D-structure of a natural inhibitor of cell adhesion molecule expression. FEBS Lett 379:69–73
Houston DR, Shiomi K, Arai N, Omura S, Peter MG, Turberg A, Synstad B, Eijsink VG, van Aalten DMF (2002) High-resolution structures of a chitinase complex with natural product cyclopentapeptide inhibitors: Mimicry of carbohydrate substrate Proc Natl Acad Sci USA 99:9127–9132
Howard DH (1999) Acquisition, transport, and storage of iron by pathogenic fungi. Clin Microbiol Rev 12:394–404
Huang H, She Z, Lin Y, Vrijmoed LLP, Lin W (2007) Cyclic peptides from an endophytic fungus obtained from a Mangrove leaf (Kandelia candel). J Nat Prod 70:1696–1699
Hume AM, Chai CLL, Moermann K, Waring P (2002) Influx of calcium through a redox-sensitive plasma membrane channel in thymocytes causes early necrotic cell death induced by the epipolythiodioxopiperazine toxins. J Biol Chem 35:31631–31638
Hwang Y, Rowley D, Rhodes D, Gertsch J, Fenical W, Bushman F (1999) Mechanism of inhibition of a poxvirus topoisomerase by the marine natural product sansalvamide A. Mol Pharmacol 55:1049–1053
Isaka M, Kittakoop P, Kirtikara K, Hywel-Jones NI, Thebtaranonth Y (2005a) Bioactive substances from insect pathogenic fungi. Acc Chem Res 38:813–823
Isaka M, Palasarn S, Rachtawee P, Vimuttipong S, Kongsaeree P (2005b) Unique diketopiperazine dimers from the insect pathogenic fungus Verticillium hemipterigenum BCC 1449. Org Lett 7:2257–2260
Isaka M, Palasarn S, Kocharin K, Hywel-Jones NI (2007) Comparison of the bioactive secondary metabolites from the scale insect pathogens, anamorph Paecilomyces cinnamomeus, and teleomorph Torrubiella luteorostrata. J Antibiotics 60:577–581
Ishiyama D, Sato T, Honda R, Senda H, Konno H, Kanazawa S (2000) Glomosporin, a novel antifungal cyclic depsipeptide from Glomospora sp. II. Structure elucidation. J Antibiot 53:525–631
Itazaki H, Nagashima K, Sugita K, Yoshida H, Kawamura Y, Yashuda Y, Matsumoto K, Ishii K, Uotani N, Nakai H, Terui A, Yoshimatsu S, Ikenishi Y, Nakagawa Y (1990) Isolation and structural elucidation of new cyclotetrapeptides, trapoxins A and B, having detransformation. J Antibiot 43:1524–1532
Iwamoto T, Fujie A, Nitta K, Hashimoto S, Okuhara M, Kohsaka M (1994a) WF11899A, B and C, novel antifungal lipopeptides II. Biological properties. J Antibiot 45:1092–1097
Iwamoto T, Fujie A, Sakamota K, Tsurumi Y, Shigematsu N, Yamashita M, Hashimoto S, Okuhara M, Kohsaka M (1994b) WF11899A, B and C, novel antifungal lipopeptides I. Taxonomy, fermentation, isolation and physico-chemical properties. J Antibiot 47:1084–1091
Jiang Z, Barret MO, Boyd KG, Adams DR, Boid ASF, Burgess JG (2002) JM47, a cyclic tetrapeptide HC-toxin analogue from a marine Fusarium species. Phytochemistry 60:33–38
Johnson MD, Perfect JR (2003) Caspofungin: first approved agent in a new class of antifungals. Expert Opin Pharmacother 4:807–823
Kaida K, Fudou R, Kameyama T, Tubaki K, Suzuki Y, Ojika M, Sakagami Y (2001) New cyclic depsipeptide antibiotics, clavariopsins A and B, produced by an aquantic hyphomycete, Clavariopsis aquatica. J Antibiot 54:17–21
Kajimura Y, Aoki T, Kuramochi K, Kobayashi S, Sugawara F, Watanabe N, Arai T (2008) Neoechinulin A protects PC12 cells against MPP+-induced cytotoxicity. J Antibiot 61:330–333
Kamei K, Watanabe A (2005) Aspergillus mycotoxins and their effect on the host. Med Mycol 43[Suppl 1]:95–99
Kanasaki R, Abe F, Kobayashi M, Katsuoka M, Hashimoto M, Takase S, Tsurumi Y, Fujie A, Hino M, Hashimoto S, Hori Y (2006a) FR220897 and FR220899, novel antifungal lipopeptides from Coleophoma empetri No. 14573. J Antibiot 59:149–157
Kanasaki R, Kobayashi M, Fujine K, Sato I, Hashimoto M, Takase S, Tsurumi Y, Fujie A, Hino M, Hashimoto S (2006b) FR227673 and FR190293, novel antifungal lipopeptides from Chalara sp. No22210 and Tolypocladium parasiticum No 16616. J Antibiot 59:158–167
Kanasaki R, Sakamota K, Hashimoto M, Takase S, Tsurumi Y, Fujie A, Hino M, Hashimoto S, Hori Y (2006c) FR209602 and related compounds, novel antifungal lipopeptides from Coleophoma crateriformis No. 738. J Antibiot 59:137–144
Keller U, Tudzynski P (2002) Ergot alkaloids. In: Osiewacz HD (ed) Industrial applications. Mycota X. Springer, Heidelberg, pp 157–181
Keller-Juslen C, Kuhn M, Loosli HR, Petcher TJ, Weber HP, von Wartburg A (1976) Struktur des Cyclopeptid-Antibiotikums SL 7810 (= Echinocandin B) Tetrahedron Lett 17:4147–4150
Kershaw M, Moorhouse ER, Bateman R, Reynolds SE, Charnley AK (1999) The role of destruxins in the pathogenicity of Metarhizium anisopliae for three species of insect. J Invert Pathol 74:213–223
Kleinkauf H, von Döhren H (1997) Peptide antibiotics. In: Kleinkauf H, von Döhren H (eds) Products of secondary metabolism. Biotechnology, vol 7. VCH, Weinheim, pp 277–322
Kleinwachter P, Dahse HM, Luhmann U, Schlegel B, Dornberger K (2001) Epicorazine C, an antimicrobial metabolite from Stereum hirsutum HKI 0195. J Antibiot 54:521–525
Krasnoff SB, Keresztes I, Gillilan RE, Szebenyi DME, Donzelli BGG, Vhurchill ACL, Gibson DM (2007) Serinocyclins A and B, cyclic heptapeptides from Metarhizium anisopliae. J Nat Prod 70:1919–1924
Kobbe B, Cushman M, Wogan GN, Demain AL (1977) Production and antibacterial activity of malformin C, a toxic metabolite of Aspergillus niger. Appl Environ Microbiol 33:996–997
Kürnsteiner H, Zinner M, Kück U (2002) Immunosuppressants. In: Osiewacz HD (ed) Industrial applications. Mycota X. Springer, Heidelberg, pp 129–155
Lee KK, Gloer JB Scott JA, Malloch D (1995) Petriellin A: a novel antifungal depsipeptide from the coprophilous fungus Petriella sordida. J Org Chem 60:5384–5385
Lewis JR (2002) Amaryllidaceae, Sceletium, imidazole, oxazole, thiazole, peptide and miscellaneous alkaloids. Nat Prod Rep 19:223–258
Li C, Oberlies NH (2005) The most widely recognized mushroom: chemistry of the genus Amanita. Life Sci 78:532–538
Li X, Kim S-K, Nam KW, Kang JS, Choi HD, Son BW (2006) A new antibacterial dioxopiperazine alkaloid related to gliotoxin from a marine isolate of the fungus Pseudallescheria. J Antibiot 59:248–250
Li Y, Li X, Kim S-K, Kang JS, Choi HD, Rho JR, Son BW (2004) Golmaenone, a new diketopiperazine alkaloid from the marine-derived fungus Aspergillus sp. Chem Pharm Bull 52:375–376
Liermann JC, Kolshorn H, Antelo L, Hof C, Anke H, Opatz T (2009) Omphalotins E-I, oxidatively modified nematicidal cyclopeptides from Omphalotus olearius. Eur J Org Chem 2009:1256–1262
Lira SP, Vita-Marques AM, Seleghim MHR, Bugni TS, LaBarbera DV, Sette LD, Sponchiado SRP, Ireland CM, Berlinck RGS (2006) New destruxins from the marine-derived fungus Beauveria felina. J Antibiot 59:553–563
Liu J-K (2005) N-containing compounds of macromycetes. Chem Rev 105:2723–2744
Lorenz P, Jensen PR, Fenical W (1998) Mactanamide, a new fungistatic diketopiperazine produced by a marine Aspergillus sp. Nat Prod Lett 12:55–60
Maligie MA, Selitrennikoff CP (2005) Cryptococcus neoformans resistance to echinocandins: (1,3) β-glucan synthase activity is senitive to echinocandins. Antimicrob Agents Chemother 49:2851–2856
Malmstrom J, Ryager A, Anthoni U, Nielsen PH (2002) Unguisin C, a GABA-containing cyclic peptide from the fungus Emericella unguis. Phytochemistry 60:869–887
Martins MB, Carvalho I (2007) Diketopiperazines: biological activity and synthesis. Tetrahedron 64:9923–9932
Matha V, Jegorov A, Weiser J, Pillai JS (1992) The mosquitocidal activity of conidia of Tolypocladium tundrense and Tolypocladium terricola. Cytobios 69:163–170
Matsuda D, Namatame I, Tomoda H, Kobayashi S, Zocher R, Kleinkauf H, Omura S (2004) New beauverolides produced by amino acid-supplemented fermentation of Beauveria sp. FO-6979. J Antibiot 57:1–9
Mayer A, Sterner O, Anke H (1997) Omphalotin, a new cyclic peptide with potent nematicidal activity from Omphalotus olearius. 1. Fermentation and biological activity. Nat Prod Lett 10:25–33
Mayer A, Kilian M, Hoster B, Sterner O, Anke H (1999) In vitro and in vivo nematicidal activities of the cyclic dodecapeptide omphalotin A. Pest Sci 55:27–30
Miyado S, Kawasaki H, Aoyagi K, Yaguchi T, Okada T, Sugiyama J (2000) Taxonomic position of the fungus producing the anthelmintic PF1022 based on the 18S rRNA gene base sequence. Nippon Kinzoku Gakkai Kaiho 41:183–188
Mochizuki K, Ohmori K, Tamura H, Shizuri Y, Nishiyama S, Miyoshi E, Yamamura S (1993) The structures of bioactive cyclodepsipeptides, beauveriolides I and II, metabolites of entomopathogenic fungi Beauveria sp. Bull Chem Soc Jpn 66:3041–3046
Monma S, Sunazuka T, Nagai K, Arai T, Shiomi K, Matsui R, Mura S (2006) Verticilide: elucidation of absolute configuration and total synthesis. Org Lett 8:5601–5604
Mori H, Urano Y, Abe F, Furukawa S, Tsurumi Y, Sakamoto K, Hashimoto M, Takase S, Hino M, Fujii T (2003) FR235222, a fungal metabolite, is a novel immunosuppressant that inhibits mammalian histone deacetylase (HDAC) 1. Taxonomy, fermentation, isolation, and biological activities. J Antibiot 56:72–79
Morino T, Masuda A, Yamada M, Nishimoto Y, Nishikiori T, Saito S, Shimada (1994) Stevastelins, novel immunosuppresssants produced by Penicillium. J Antibiot 47:1341–1343
Morris SA, Schwartz RE, Sesin DF, Masurekar P, Hallada TC, Schmatz DM, Bartizal K, Hensens OD, Zink DL (1994) Pneumocandin D0, a new antifungal agent and potent inhibitor of Pneumocystis carinii. J Antibiot 47:755–764
Morrison VA (2006) Echinocandin antifungals: review and update. Expert Rev Anti Infect Ther 4:325–342
Namatame I, Zomoda H, Ishibashi S, Omura S (2004) Antiatherogenic activity of fungal beauverolides, inhibitors of lipid droplet accumulation in macrophages. Proc Natl Acad Sci USA 101:737–742
Nilanonta C, Isaka M, Chanphen R, Thong-orn N, Tanticharoen M, Thebtaranonth Y (2003) Unusual enniatins produced by the insect pathogenic fungus Verticillium hemipterigenum: isolation and studies on precursor-directed biosynthesis. Tetrahedron 59:1015–1020
Odds FC, Brown AJ, Gow NA (2003) Antifungal agents: mechanisms of action. Trends Microbiol 11:272–279
Oide S, Moeder W, Krasnoff S, Gibson D, Haas H, Yoshioka K, Turgeon BG (2006) NPS6, encoding a nonribosomal peptide synthetase involved in siderophore-mediated iron metabolism, is a conserved virulence determinant of plant pathogenic ascomycetes. Plant Cell 18:2836–2853
Oide S, Krasnoff SB, Gibson DM, Turgeon BG (2007) Intracellular siderophores are essential for ascomycete sexual development in heterothallic Cochliobolus heterostrophus and homothallic Gibberella zeae. Eukaryot Cell 6:1339–1353
Ohshiro T, Rudel LL, Omura S, Tomoda H (2007) Selectivity of microbial acyl-CoA:cholesterol acyltransferase inhibitors towards isoenzymes. J Antibiot 60:43–51
Ohyama T, Kurihara Y, Ono Y, Ishikawa T, Miyakoshi S, Hamano K, Arai M, Suzuki T, Igari H, Suzuki Y, Inukai M (2000) Arborcandins A, B, C, D, E, and F, novel 1,3-beta-glucan synthase inhibitors: production and biological activities. J Antibiot 53:1108–1116
Panaccione DC, Cipoletti JR, Sedlock AB, Blemings KP, Schradl CL, Machado C, Seidel GE (2006) Effects of ergot alkaloids on food preference and satiety in rabbits, as assessed with gene-knockout endophytes in perennial ryegrass (Lolium perenne). J. Agric Food Chem 54:4582–4587
Pasqualotto AC, Denning DW (2008) New and emerging treatments for fungal infections. J Antimicrob Chemother 61[Suppl 1]:i19–i30
Patil BB, Wakharkar RD, Chincholkar SB (1995) Siderophores of Cunninghamella blakesleeana NCIM 687. World J Microbiol Biotechnol 15:265–268
Pedley KF, Walton JD (2001) Regulation of cyclic peptide biosynthesis in a plant pathogenic fungus by a novel transcription factor. Proc Natl Acad Sci USA 98:14174–14179
Pedras MSC, Zaharia LI, Ward DE (2002) The destruxins: synthesis, biosynthesis, biotransformation, and biological activity. Phytochemistry 59:579–596
Pomilio AB, Battista ME, Vitale AA (2006) Naturally-occurring cycopeptides: structures and bioactivity. Curr Org Chem 10:2075–2121
Prasad C (1995) Bioactive cyclic peptides. Peptides 16:151–164
Rees NH, Penfold DJ, Rowe ME, Chowdhry BZ, Cole SCJ, Samuels RI, Turner DL (1996) NMR studies of the conformation of destruxin A in water and in acetonitrile. Magn Reson Chem 34:237–241
Renshaw JC, Robson GD, Trinci APJ, Wiebe MG, Livens FR, Collison DC, Taylor RJ (2002) Fungal siderophores: structures, functions and applications. Mycol Res 106:1123–1142
Roth BD (1998) ACAT inhibitors: evolution from cholesterol-absorption inhibitors to antiatherosclerotic agents. Drug Discov Today 3:19–25
Rouxel T, Chupeau Y, Fritz R, Kollmann A, Bousquet J-F (1988) Biological effects of sirodesmin PL, a phytotoxin produced by Leptosphaeria maculans. Plant Sci 57:45–53
Rüegger A, Kuhn M, Lichti H, Loosli HR, Huguenin R, Quiquerez C, von Wartburg A (1975) Cyclosporin A, ein immunsuppressiv wirksamer Peptidmetabolit aus Trichoderma polysporum (Link ex Pers.) Rifai. Helv Chim Acta 59:1075–1092
Saeger B, Schmitt-Wrede HP, Dehnhardt M, Benten WP, Krucken J, Harder A, Samson-Himmelstjerna von G, Wiegand H, Wunderlich F (2001) Latrophilin-like receptor from the parasitic nematode Haemonchus contortus as target for the anthelmintic depsipeptide PF1022A. FASEB J 15:1332–1334
Saito T, Hirai H, Kim Y-J, Kojima Y, Matsunaga Y, Nishida H, Sakakibara T, Suga O, Sujaku T, Kojima N (2002) CJ 15208, a novel kappa opioid receptor antagonist from a fungus, Ctenomyces serratus ATCC15502. J Antibiot 55:847–854
Samson-Himmelstjerna von G, Harder A, Sangster NC, Coles GC (2005) Efficacy of two cyclooctadepsipeptides, PF022A and emodepside, against anthelmintic-resistant nematodes in sheep and cattle. Parasitology 130:343–347
Sarabia F, Chammaa S, Sánchez Ruiz A, Martín Ortiz L, López Herrera FJ (2004) Chemistry and biology of cyclic depsipeptides of medicinal and biological interest. Curr Med Chem 11:1309–1332
Sasaki T, Takagi M, Yaguchi T, Miyado S, Okada T, Koyama M (1992) A new anthelmintic cyclodepsipeptide, PF1022. J Antibiot 45:692–697
Sato T, Ishiyama D, Honda R, Senda H, Konno H, Tokumasu S, Kanazawa S (2000) Glomosporin, a novel cyclic depsipeptide from Glomospora sp. I. Production, isolation, physico-chemical properties, and biological activities. J Antibiot 53:597–602
Schardl CL, Leuchtmann A, Spiering MJ (2004) Symbioses of grasses with seedborne fungal endophytes. Annu Rev Plant Biol 55:315–340
Scherkenbeck J, Jeschke P, Harder A (2002) PF1022A and related cyclodepsipeptides - a novel class of anthelmintics. Curr Topics Med Chem 7:759–777
Schmidt FR (2002) Beta-lactam antibiotics: aspects of manufacture and therapy. In: Osiewacz HD (ed) Industrial applications. Mycota X. Springer, Heidelberg, pp 69–91
Schrettl M, Bignell E, Kragl C, Sabiha Y, Loss O, Eisendle M, Wallner A, Arst HN Jr, Haynes K, Haas H (2007) Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog 3:1195–1207
Scott PM, Polonsky J, Merrien MA (1979) Configuration of the 3,12 double bond of roquefortine. J Agric Food Chem 27:201–202
Seto Y, Takahasi K, Matsuura H, Kogami Y, Yada H, Yoshihara T, Nabeta K (2007) Novel cyclic peptide, epichlicin, from the endophytic fungus, Epichloe typhina. Biosci Biotechnol Biochem 71:1470–1475
Shiono Y, Tschuchinari M, Shimanuki K, Miyajima T, Murayama T, Koseki T, Laatsch H, Funakoshi T, Takanami K, Suzuki K (2007) Fusaristatins A and B, two new cyclic lipopeptides from an endophytic Fusarium sp. J Antibiot 60:309–316
Singh SB, Zink DL, Liesch JM, Mosley RT, Dombrowski AW, Bills GF, Darkin-Rattray SJ, Schmatz DM, Goetz MA (2002) Structure and chemistry of apicidins, a class of novel cyclic tetrapeptides without a terminal α-keto epoxide as inhibitors of histone deacetylase with potent antiprotozoal activities. J Org Chem 67:815–825
Skrobek A, Butt TM (2005) Toxicity testing of destruxins and crude extracts from the insect-pathogenic fungus Metarhizium anisopliae. FEMS Microbiol Lett 251:23–28
Sterner O, Etzel W, Mayer A, Anke H (1997) Omphalotin, a new cyclic peptide with potent nematicidal activity from Omphalotus olearius. II. Isolation and structure determination. Nat Prod Lett 10: 33–38
Sugui JA, Pardo J, Chang YC, Zarember KA, Nardone G, Galvez EM, Müllbacher A, Gallin JI, Simon MM, Kwon-Chung KJ (2007) Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot Cell 6:1562–1569
Supothina S, Isaka M, Kirtikara K, Tanticharoen M, Thebtaranonth Y (2004) Enniatin production by the entomopathogenic fungus Verticillium hemipterigenum BCC 1449. J Antibiot 57:732–738
Takahashi C, Numata A, Matsumura E, Minoura K, Eto H, Shingu T, Ito T, Hasgawa T (1994) Leptosins I and J, cytotoxic substances produced by a Leptosphaeria sp. physico-chemical properties and structures. J Antibiot 47:1242–1249
Tan LT, Cheng XC, Jensen PR, Fenical W (2003) Scytalidamides A and B, new cytotoxic cyclic heptapeptides from a marine fungus of the genus Scytalidium. J Org Chem 68:8767–8773
Tan NH, Zhou J (2006) Plant cyclopeptides. Chem Rev 106:840–895
Tan RX, Jensen PR, Williams PG, Fenical W (2004) Isolation and structure assignments of rostratins A–D, cytotoxic disulfides produced by the marine-derived fungus Exserohilum rostratum. J Nat Prod 67:1374–1382
Tani H, Fujii Y, Nakajima H (2001) Chlamydocin analogues from the soil fungus Peniophora sp.: structures and plant growth-retardant activity. Phytochemistry 58:305–310
Taunton J, Hassig CA, Schreiber SL (1996) A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science 272:408–411
Tobiasen C, Aahman J, Ravnholt KS, Bjerrum MJ, Grell MN, Giese H (2007) Nonribosomal peptide synthetase (NPS) genes in Fusarium graminearum, F. culmorum and F. pseudograminearium and identification of NPS2 as the producer of ferricrocin. Curr Genet 51:43–58
Traber R, Dreyfuss MM (1996) Occurrence of cyclosporins and cyclosporin-like peptolides in fungi. J Indust Microbiol 17:397–401
Turner WB, Aldridge DC (1983) Diketopiperazines and related compounds. In: Fungal metabolites II. Academic, London, pp 405–423
Ueno T, Nakashima T, Hayashi Y, Fukami H (1975) Structures of AM-toxin I and II, host-specific phytotoxic metabolites produced by Alternaria mali. Agric Biol Chem 39:1115–1122
Vey A, Matha V, Dumas C (2002) Effects of the peptide mycotoxin destruxin E on insect haemocytes and on dynamics and efficiency of the multicellular immune reaction. J Invert Pathol 80:177–187
Waring P, Beaver J (1996) Gliotoxin and related epipolythiodioxopiperazines. Gen Pharmacol 27:1311–1316
Waring P, Eichner RD, Müllbacher A (1988) The chemistry and biology of the immunomodulating agent gliotoxin and related epipolythiodioxopiperazines. Med Res Rev 8:499–524
Weber D, Erosa G, Sterner O, Anke T (2006) Cyclindrocyclin A, a new cytotoxic cyclopeptide from Cylindrocarpon sp. J Antibiot 59:495–499
Welker M, von Döhren H (2006) Cyanobacterial peptides – nature's own combinatorial biosynthesis. FEMS Microbiol Rev 30:530–563
Welzel K, Eisfeld K, Antelo L, Anke T, Anke H (2005) Characterization of the ferrichrome A biosynthetic gene cluster in the homobasidiomycete Omphalotus olearius. FEMS Microbiol Lett 249: 157–163
Winkelmann W, Drechsel H (1997) Microbial siderophores. In: Kleinkauf H, von Döhren H (eds) Products of secondary metabolism. Biotechnology, vol 7. VCH, Weinheim, pp 199–246
Wolstenholme WA, Vining LC (1966) Determination of amino acid sequences in oligopeptides by mass spectrometry VIII. The structure of isariin. Tetrahedron Lett 7:2785–2791
Yano T, Aoyagi A, Kozuma S, Kawamura Y, Tanaka I, Suzuki Y, Takamatsu Y, Takatsu T, Inukai M (2007) Pleofungins, novel inositol phosphorylceramide synthase inhibitors, from Phoma sp. SANK 13899. J Antibiot 60:136–142
Yin WQ, Zou JM, She ZG, Vrijmoed LLP, Jones EBG, Lin YC (2005) Two cyclic peptides produced by the endophytic fungus 2221 from Castaniopsis fissa on the South China sea coast. Chin Chem Lett 16:219–222
Yoshioka H, Nakatsu K, Sato M, Tatsuno T (1973) The molecular structure of cyclochlorotine, a toxic chlorine-containing peptapentide. Chem Lett 12:1319–1322
Zhang P, Chen Z, Hu J, Wei B, Zhang Z, Hu W (2005) Production and characterization of amanitin toxins from a pure culture of Amanita exitialis. FEMS Microbiol Lett 252:223–228
Zheng CJ, Oark SH, Koshino H, Kim YH, Kim WG (2007) Verticillin G, a new antibacterial compound from Bionectria byssicola. J Antibiot 60:61–64
Zimmermann G (2007a) Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Sci Technol 17:553–596
Zimmermann G (2007b) Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Sci Technol 17:879–920
Acknowledgements.
Work in our Institute was supported by the State of Rhineland–Palatinate, BASF SE, Bayer AG, BMBF and the DFG.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Anke, H., Antelo, L. (2009). Cyclic Peptides and Depsipeptides from Fungi. In: Anke, T., Weber, D. (eds) Physiology and Genetics. The Mycota, vol 15. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-00286-1_13
Download citation
DOI: https://doi.org/10.1007/978-3-642-00286-1_13
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-00285-4
Online ISBN: 978-3-642-00286-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)