Abstract
Ergoline (i.e. ergot) alkaloids are a group of physiologically active natural products occurring in taxonomically unrelated fungal and plant taxa, Clavicipitaceae and Convolvulaceae. The disjointed occurrence of ergoline alkaloids seems to contradict the paradigm of chemotaxonomy that identical or at least structurally related natural products occur in taxonomically related organisms. This question has now been solved by the observation that some dicotyledonous plants belonging to the family Convolvulaceae (e.g. Ipomoea asarifolia, I. violacea and Turbina corymbosa carry epibiotic fungi. The fungi present on different plant species are not identical albeit taxonomically closely related clavicipitaceous fungi. Thus, the presence of ergoline alkaloids in dicotyledonous plants is not based on their capacity to synthesize ergoline alkaloids but rather on the ability to live in a symbiotic association with ergoline alkaloid producing fungi.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 The Ecological Role of Natural Products
Microorganisms and plants have one thing in common: both are equipped with a frequently elaborate biosynthetic machinery responsible for the formation of an almost unlimited variety of natural products. Typically, natural products – which are also called secondary metabolites – are characteristic of a limited number of microbial or plant taxa, e.g. an order, a family, a species or even a subspecies only. Many of the natural products exhibit physiological activities which is the basis for their use in medical applications (Clardy and Walsh 2004).
The high physiological activities of many natural products triggered a now historical dispute about the role of natural products in the producing organism. It was proposed that “the multiplicity of natural products is caused by random processes of mutations, i.e. it reflects the gambling of nature rather than a sophisticated strategy” (Mothes 1981; Mothes et al. 1985).
This hypothesis, however, neglects the possibility that mutations may turn out to be detrimental or advantageous to the mutated organism. In the former case a mutated organism may be eliminated, or in the latter case may benefit from an increased fitness and a better chance to survive in a certain ecological setting (Zenk 1967). Today the ecological role of natural products is well accepted in the scientific community (Eisner 2003; White Jr. et al. 2003; Harborne 2004).
Natural product research entered a new era when it was discovered that plants and fungi elaborated during evolution another way to acquire natural products: Not only may they be formed in biosynthetic processes by one particular organism itself, but a host organism may instead harbor a natural product-producing microorganism: A plant may be associated with a bacterium (Piel 2004; Strobel 2004; Gunatilaka 2006) or a fungus (Strobel 2004; Puri et al. 2005; Gunatilaka 2006), while a fungus may harbor a bacterium (Partida-Martinez and Hertweck 2005).
It is remarkable that three important and frequently used cytostatic compounds employed in today's tumor therapy apparently are synthesized by plant-associated microorganisms: Vincristine is produced by Fusarium oxysporum associated with Catharanthus roseus (Zhang et al. 2000; cited by Gunatilaka 2006), camptothecin, a lead compound in cancer research and therapy is produced by a fungal endophyte present in Nothapodytes foetida (Puri et al. 2005) and paclitaxel is believed to be formed by different fungi such as Taxomyces andreanae and different Taxus species, including T. brevifolia (Strobel et al. 2004; Leistner 2005). The latter case, however, may not yet be setteled because the genes in Taxomyces andreanae have not yet been reported whereas they are well known from the Taxus brevifolia plant (Croteau et al. 2006).
At present it is unclear whether these natural products and their biosynthetic machinery occur exclusively in the associated microorganism or in both the microorganism and the host plant. In such associations both organisms may be part of a symbiotum in which the associated microorganism benefits by receiving nutrients, protection, reproduction and dissemination, whereas the host takes advantage of physiologically active compounds which may promote plant growth, herbivore deterrence and/or increased fitness (Arnold et al. 2003; White Jr. et al. 2003; Saikkonen et al. 2004).
A point in case is the beneficial activity of ergoline alkaloids which are products of clavicipitaceous fungi (Clay and Schardl 2002; White Jr. et al. 2003; Schardl et al. 2006) colonizing grasses like Poaceae, Juncaceae and Cyperaceae.
In the current literature (including our own publications) two different adjectives for the fungi described herein are used: These are “clavicipitaceous” and “clavicipitalean”. The former term refers to the family Clavicipitaceae but the latter to the old order of Clavicipitales. Since the family Clavicipitaceae is now generally accepted to belong to the order Hypocreales (Sung et al. 2007) only the term “clavicipitaceous” should be used. We are grateful to Dr. Chris Schardl (Lexington, Kentucky, USA) for bringing this to our attention.
Ergoline alkaloids, however, are also present in higher dicotyledonous plants of the family Convolvulaceae (Hofmann 1961, 2006). This disjointed occurrence of a group of natural products in evolutionarily unrelated taxa (fungi, Convolvulaceae plants) seemed to contradict the generally accepted principle of chemotaxonomy that similar or even identical natural products are present in related taxa. It was therefore assumed that during evolution a horizontal transfer of genes responsible for ergoline alkaloid biosynthesis might have occurred from fungi to higher plants (Groeger and Floss 1998; Tudzynski et al. 2001; Clay and Schardl 2002). Alternatively it was discussed that ergoline alkaloid biosynthesis was repeatedly invented during evolution (Mothes et al. 1985). In a recent review in this series Keller and Tudzinsky (2002) dealt with the pharmacological aspects, biochemistry, genetics and biotechnology of ergoline alkaloids in fungi associated with Poaceae. We show in the present review that neither the horizontal transfer of genes encoding the ergoline alkaloid biosynthesis, nor the repeated invention of a rather complicated biosynthetic pathway took place during evolution, but rather that clavicipitaceous fungi not only live on different grasses but also colonize plant species of the dicotyledonous family of Convolvulaceae (Kucht et al. 2004; Steiner et al. 2006; Ahimsa-Mueller et al. 2007; Markert et al. 2008; Steiner et al. 2008). This indicates that ergoline alkaloids are components in a fungus/plant symbiotum characterized by mutual defense.
2 The Symbiosis Between Poaceae and Clavicipitaceous Fungi
A rather well investigated experimental system consists of clavicipitaceous fungi which colonize Juncaceae, Cyperaceae and Poaceae plants. In these symbiota ergoline alkaloids play an important role (Keller and Tudzynski 2002). The symbiotic fungi belong either to the tribe Clavicipeae or Balanseae within the family Clavicipitaceae (Bacon and Lyons 2005). The morphological associations of the fungi with grasses is either epicuticular, epibiotic or endophytic (Bacon and Lyons 2005). In epiphytic growth the fungal mycelium is concentrated on the surface of young leaves, buds, meristematic regions and reproductive structures (Clay and Schardl 2002). The association between fungi and their plant hosts is likely to be an example of host-symbiont codivergence (Schardl et al. 2008).
The fungus may be asexual belonging to the group of fungi imperfecti, show a sexual lifestyle or switch between sexual and asexual propagation. In the sexual lifestyle fungi parasitize a wide range of grasses where they form infections of single grass florets and replace the seed with individual sclerotia (Clay and Schardl 2002).
The asexual fungi are vertically transmitted through seeds. They have never been known to produce infectious spores and rely entirely on seed transmission. Especially the asexual fungi exhibit high host specificity. Most interestingly, sexual and asexual fungi may interact in parasexual processes contributing to a high diversity of fungal asexual endophytes (Tsai et al. 1994).
In general, grasses are poor producers of natural products that assist other plants in their long-term strategy to gain an ecological advantage. Grasses, however, have the ability to compensate for this deficiency by acquiring fungi notorious for their poisonous natural products. In some cases fungi can be considered the lifestock of grasses.
Fungi associated with plants may produce different classes of alkaloids among which toxic ergoline alkaloids are an important group (Schardl et al. 2004, 2007). The main ecological roles of ergoline alkaloids in nature are probably to protect the fungi from consumption by vertebrate and invertebrate animals (Schardl et al. 2006). Ergoline alkaloids benefit the fungus by protecting the health and productivity of the host (Schardl et al. 2006). Other benefits include growth of the plant, competitive abilities, resistence to drought (Malinowski and Belesky 2000), pests and fungal pathogens (Brem and Leuchtmann 2002; White Jr. et al. 2003). In some cases clavicipitaceous fungi are culturable in vitro (Keller and Tudzynski 2002). This helped to identify the fungus as the producer of ergoline alkaloids and revealed that the host plant is not the site of ergoline alkaloid biosynthesis.
It was therefore somewhat unexpected when Hofmann (1961, 2006) found that dicotyledonous plants belonging to the family Convolvulaceae contained ergoline alkaloids and that these alkaloids were responsible for the hallucinogenic properties enjoyed by Meso- and South American indians in religious ceremonies.
The idea that a fungus could be responsible for the alkaloid occurrence was discussed but no evidence for the presence of such a fungus was found (Hofmann 2006). This seemed to be in agreement with the notion that plant tissue cultures which are believed to be germ-free, i.e. devoid of any microbes, were reported to produce ergoline alkaloids (Dobberstein and Staba 1969; see below).
3 Epibiotic Clavicipitaceous Fungi Associated with Convolvulaceae
3.1 Identification of Clavicipitaceous Fungi
3.1.1 Microscopic and Electron Microscopic Characterization
The infestation of the clavicipitaceous fungi on members of the family Convolvulaceae is systemic. Evidence of systemic infection came from demonstrations that the fungi are seed transmitted, that surface-sterilized seeds grown in vitro and under germ-free conditions result in plantlets which are colonized exclusively by the respective clavicipitaceous fungi and that they are transmitted through vegetative propagation (Steiner et al. 2008). It is an unusual type of systemic infection in that there are no signs of penetration into the host tissue and growth on the host plant is superficial. Attempts made to visualize the fungus within the stem and leaf tissue, using methodologies commonly employed to detect endophytes in grasses (Bacon and White Jr. 1994), were not successful. Up to now the fungi have proved to be non-detectable using these procedures. Among the Clavicipitaceae, Atkinsonella hypoxylon, Balansia cyperi, B. pilulaeformis and Myriogenospora atramentosa are examples of epibiotic species that grow on the meristematic tissues of host plants (Luttrell and Bacon 1977; Rykard et al. 1985; Leuchtmann and Clay 1988, 1989; Clay and Frentz 1993). The clavicipitaceous fungi colonizing members of the Convolvulaceae inhabit an epibiotic niche and thus seem most comparable to the epibiotic members of the grass borne Clavicipitaceae. The mutualistic endophyte Neothyphodium typhinum also forms a stable external mycelial net on the leaves of the host plant (Moy et al. 2000). This suggested a possible alternative pathway of fungal dispersal and transmission to hosts, i.e. through epiphyllously produced conidia.
The clavicipitaceous fungi form colonies on the upper surfaces of young unfolded leaves which are visible to the naked eye, as shown for Turbina corymbosa (Fig. 9.1A) and Ipomoea asarifolia. They are also detectable by molecular biological techniques in seeds of I. violacea (Ahimsa-Müller et al. 2007). On T. corymbosa colony distribution mainly follows the veins of the leaves (Fig. 9.1A, B), in contrast to the distribution on I. asarifolia which is more random. These colonies differ in size and mycelium density, and depending on the developmental stage, the fungi produce synnemata-like structures. No stromata with perithecia and ascospores were detected in the mycelium mats. Maybe the environmental conditions are not suitable for the development of the sexual stage of the fungi, or they lost the ability to reproduce sexually, or the mating type is lacking. No traces of mycelium were detected on the lower side of the leaves. Visual inspection of leaf buds which were opened by manipulation showed that the fungus was well established as dense white mycelial layers on the adaxial leaf surfaces of both I. asarifolia and T. corymbosa plants (Fig. 9.1C) at this early stage of plant development. The mycelium is formed by tightly packed hyphae in the cavity between the leaf halves. Sections through colonized tissue revealed that the fungal mycelium is entirely superficial. The hyphae measured approximately 1.0–1.5 μm across, were hyaline, thin-walled and septated. Chlamydospore-like structures are produced. As indicated by the intense mycelium development, the space between the upper surfaces of folded leaves probably offer a refuge of protection to the fungus. As leaves expand and mature the hyphae are evident as isolated clumps only visible microscopically, often near or around peltate glandular trichomes, and the ends of the hyphae often appeared broken (Fig. 9.1D).
Colonization of Turbina corymbosa with the clavicipitaceous fungus (provisionally named TcorF01). A Colonies formed by white mycelium on the adaxial surface of a young unfolded leaf. Preferential development on the veins is visible with the naked eye. B Aggregated hyphae differentiating typical mycelium mats (mm) consisting of several layers which cover leaf areas with peltate glandular trichomes and are adhered to the cuticle. C Cross-section of a closed leaf bud showing that the fungus is well established on the adaxial leaf surfaces at this early stage of plant development. The mycelium is formed by tightly packed hyphae as a mycelium layer (ml, arrows) in the cavity between the halves of the leaf. D Close association of secretory cells (gsc) on the adaxial leaf surface with hyphae (hy) which often encircle the peltate glandular trichomes of the plants. E Cross-section of a peltate glandular trichome composed of basal cell (bc), stalk cell (sc) and secretory cells (gsc) showing the epiphytic development of mycelium embedded in a mucilage matrix concentrated on the cuticle over a subcuticular oil storage cavity. F Electron microscopic view of secretory cells with hyphae outside and inside of the subcuticular oil storage cavity (scc) bordered by the cuticle (c, arrow). No evidence for direct penetration of the plant cells is visible
The epibiotic fungi of I. asarifolia and T. corymbosa (Fig. 9.1D, E) are closely associated with secretory glands on the adaxial leaf surface, an anatomic feature which may be essential for ergoline alkaloid biosynthesis in the epibiotic fungus/plant association (Steiner et al. 2008). In cell cultures which harbor the fungus no ergoline alkaloids are synthesized and no secretory glands are developed.
Members of the Convolvulaceae like I. asarifolia and T. corymbosa (Fig. 9.1E) form peltate glandular trichomes, which consist of one basal cell, one stalk cell, up to eight glandular secretory cells and a subcuticular oil storage cavity that is derived from the cuticle of the secretory cells. Metabolites are released after rupture of the cuticle. As indicated by staining with the lipophilic dye Nile red these specialized structures contain essential oils (Kucht et al. 2004). The secretory glands and their specific metabolites may be the basis of a metabolic dialog between fungus and plant (Steiner et al. 2008). The fungi may feed on the volatile oil and derive precursors like terpenes for ergoline alkaloid biosynthesis from the oil. The fungi inhabit the epibiotic niche of glandular cells on the upper surface of leaves. This observation is supported by showing hyphae of the clavicipitaceous fungus on T. corymbosa both outside of the subcuticular oil storage cavity and inside of this compartment embedded in an electron-dense matrix (Fig. 9.1F). The localization of mycelium with the glandular cells ensures the close association between fungus and host tissues. A continuous maintenance of the symbiotic relationship requires that the fungus derives energy from the host plant. In clavicipitaceous epibiotic fungi, substrate utilization depends on the availability of organic material from the waxy cuticle covering the plant surface and exuded compounds, lipids, amino acids and vitamins. The main energy-yielding compounds are simple sugars that in the case of endophytic mycelia are derived from the apoplasm through intercellular fungal hyphae (White and Morgan-Jones 1996). In clavicipitaceous fungi present on I. asarifolia and T. corymbosa, superficial fungal hyphae have been observed with tip enlargements tightly adherent to the glandular cells as well as to the cuticle. Probably, mechanisms for a selective and efficient transfer of carbohydrates to the fungus could be present.
Physiological changes paralleled by morphological adaptations of the host have been described for some endophytic associations (Bacon and White Jr. 2000). In M. atramentosa, plant host changes in the epidermal cell size and shape suggest the activity of growth regulatory substances which are either produced by the fungus or secreted into the host or are produced by the host in response to the fungal symbiont (Bacon and White Jr. 2000).
The epiphytic proliferation of hyphae on the cuticle may be additionally enabled through degradation of the cuticular layers of the leaf surfaces. Previous ultrastructural studies of the host–fungus interfaces of the clavicipitaceous fungi on I. asarifolia and T. corymbosa revealed progressive cuticular disintegration. Substrate utilization studies have shown that epiphytic members of the Balansiaceae such as Atkinsonella hypoxylon, possess the capacity to colonize and degrade paraffin wax droplets (White Jr. et al. 1991). A. hypoxylon grows superficially on young leaves of grasses as an epiphyte, perhaps degrading wax in the cuticle to obtain nutrients for epiphytic growth (White Jr. et al. 1991). Leaves and inflorescence primordia within the stroma never develop a cuticular layer that would impede flow of nutrients and moisture to the fungus. Through these modifications of the host tissues, the endophyte removes the barriers which obstruct nutrient flow into the mycelium. Very similar to this situation, the cuticle covering the glandular cells of the Convolvulaceae appears thinner and therefore more permeable than the cuticle on epidermal cells.
Clavicipitaceous fungi have evolved to survive both as saprophytes, degrading organic material, and as biotrophs of plants, fungi, nematodes and insects. They are described to have become particularly successful as endophytes and epibionts of grasses. The associations between clavicipitaceous fungi and their hosts constitute unique biotrophic symbioses where the stages of physiological adaptation to the plant host may yield an understanding of how evolution among these fungi and their hosts has progressed (Schardl et al. 2008). The detection on Convolvulaceae of clavicipitaceous fungi able to synthesize ergoline alkaloids known to play a role in enhanced resistance to diseases, pests and tolerance to drought has shown that such associations have evolutionary value not only in grass hosts but also in dicots. The colonization of a unique plant niche, the clavicipitaceous fungi on Convolvulaceae, represents a novel finding among beneficial plant–fungus symbioses in non-graminaceous plants.
3.1.2 Phylogenetic Trees
The unusual colony-forming fungus (provisionally named IasaF13) on the leaf surface of I. asarifolia (white blooming) was found to belong to the family Clavicipitaceae. Proof, however, was not possible by conventional techniques because all attempts to cultivate the fungus on synthetic media usually supporting fungal growth were negative. This indicates that the leaf material contains factors or structures essential for growth of the fungus IasaF13. All experiments to characterize these fungi in terms of taxonomy are therefore based on molecular biological techniques (Steiner et al. 2006).
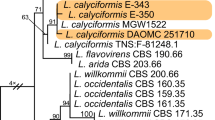
Construction of phylogenetic trees has been repeatedly and successfully employed in the systematic classification of grass-borne clavicipitaceous fungi (Spatafora and Blackwell 1993; Glenn et al. 1996; Kuldau et al. 1997; Reddy et al. 1998; Lewis et al. 2002; Bischoff and White Jr. 2005; Sung et al. 2007). Removal of fungal mycelium from the leaf surface of convolvulaceous plants was possible by ultrasonic treatment. After DNA from the fungus was extracted and sequenced phylogenetic trees were constructed from 18SrDNA and internal transcribed spacer. This showed that the fungus clustered with clavicipitaceous fungi. Confirmation was obtained by partial sequencing with phylogenetic analysis of the gene (partially) responsible for the committed step in ergoline alkaloid biosynthesis encoding the 4-[γ,γ-dimethylallyl]tryptophan synthase.
Essentially the same results were observed when the fungi associated with I. asarifolia (red variety), T. corymbosa , and I. violacea were investigated (Ahimsa-Mueller et al. 2007). This showed that our observations are not restricted to one single plant taxon (I. asarifolia, white blooming) and its associated fungus IasaF13 but are of a broader significance at least within the family Clavicipitaceae. The clavicipitaceous fungi present on representatives of the four convolvulaceous taxa turned out to be not identical, although closely related. In-depth investigation of the biosynthetic pathway leading to ergoline alkaloids in the fungus present on I. asarifolia (white blooming) provided further evidence for the clavicipitaceous nature of the fungus IasaF13 (Markert et al. 2008).
3.2 Fungicidal Treatment
Discovery of the fungus on the adaxial leaf surface was a surprise. The hypothesis that an associated fungus was responsible for the presence of alkaloids in Convolvulaceae had been tested but the fungus was not found for unexplained reasons (Hofmann 2006).
The presence of the clavicipitaceous fungus alone, however, was not evidence enough to postulate the fungal origin of ergoline alkaloids in the symbiotic association. Twelve endophytic fungi had been isolated from the I. asarifolia Roem. et Schult plant and an epibiotic fungus – provisionally named IasaF13 – was discovered. Not only was it possible that one of the fungi colonizing the plant (Steiner et al. 2006) would have been responsible for the occurrence of alkaloids in Convolvuaceae plants, it was also possible that the plant itself was the synthesizing organism. To clarify this situation I. asarifolia and T. corymbosa (L.) Raf (syn. Rivea corymbosa (L) Hall. f.) plants were treated with four different fungicides in a regime with two-week intervals. After 18 weeks, microscopic inspection and alkaloid analysis of plants belonging to both species revealed that systemic azole fungicides were most effective in the removal of both epibiotic fungus and ergoline alkaloids. Simultaneously, the volatile oil present in I. asarifolia was isolated by steam destillation, and as opposed to ergoline alkaloids, was found not to be removed during the fungicide treatment (Kucht et al. 2004). Thus, the removal of alkaloids is a specific process and the fungicide does not interfer with the supply of hemiterpenoid biosynthetic building stones which are precursors of both ergoline alkaloids (Groeger and Floss 1998; Keller and Tudzynski 2002) and terpenoid compounds. This result is a frequent observation: ergoline alkaloids and the epibiotic fungus always cooccur because they are both part of a functional entity (Ahimsa-Mueller et al. 2007).
3.3 Plant Growth Under Germ-Free Conditions
The notion that fungicides eliminate ergoline alkaloids from the plant is a clear indication that ergoline alkaloids in Convolvulaceae plants are of fungal origin. This observation is somewhat unusual because it had been reported that ergoline alkaloids are produced by plant cell cultures established from different Convolvulaceae plants (Dobberstein and Staba 1969). Plant cell cultures are usually germ-free; they should not contain any microbes and can therefore be used as a test system to probe the biosynthetic capacities of plant cells.
Numerous attempts, however, to reproduce this result (Dobberstein and Staba 1969) and to find a plant cell culture raised from Ipomoea asarifolia , Turbina corymbosa and I. violacea (L) (Convolvulaceae) showing ergoline alkaloid production were unsuccessful in our hands (Hussein 2004; Kucht et al. 2004). Indeed, thin-layer chromatography combined with vanUrk's spray reagent were used by Dobberstein and Staba (1969) to detect ergoline alkaloids, techniques which are of limited reliability in the identification of natural products (Jenett-Siems et al. 1994; Kucht et al. 2004).
Again, it was a surprise when we found that the epibiotic fungus lived together with the plant cells in the callus and cell suspension culture. Microscopic examination, single-strand conformation polymorphism (SSCP) and sequencing of the internal transcribed spacer revealed the presence in the cell culture of the epibiotic fungus (IasaF13) previously detected on the leaf surface of I. asarifolia. Fungi contaminating plant tissue cultures usually cause plant cells to react in a hypersensitive response.
This, however, was not observed in our cultures where the fungus coexisted asymptomatically and undetected by the naked eye in association with the plant cells. Other endophytic fungi which had been isolated from intact I. asarifolia plants were not detectable by SSCP within the callus and cell suspension culture (Steiner et al. 2006).
When a callus culture was subjected to a new hormone regime (the amount of benzylaminopurine was lowered from 2.0 mg/l to 0.01 mg/l) a plantlet regenerated from the callus. This plantlet was colonized by the fungus and contained ergoline alkaloids (Steiner et al. 2006, 2008).
These observations show also that an intact I. asarifolia plant colonized by the fungus IasaF13 is required for the successful synthesis of ergoline alkaloids and gives an idea about the extreme specificity in the interaction between the epibiotic fungus and the I. asarifolia plant (Steiner et al. 2008). It is in line with these conclusions that we were hitherto unable to grow the fungus IasaF13 in vitro (Steiner et al. 2006). Apparently the plant contains some kind of component essential for fungal growth. The specificity between the plant and its associated fungus is also evident from the fact that different plant taxa within the Convolvulaceae (I. asarifolia, I. violacea, T. corymbosa) are colonized by related but different clavicipitaceous fungi (Ahimsa-Mueller et al. 2007).
This raises the question as to how the specific interaction between the fungus and the host plant is brought about (Steiner et al. 2008). Interestingly, the fungus apparently has a very high affinity to the secretory glands on the adaxial leaf surface (Kucht et al. 2004).This seems to be unusual because essential oils may have an antifungal activity (Chang et al. 2008). It is conceivable that during evolution clavicipitaceous fungi were able to overcome this barrier and to take advantage of volatile oil components, using these compounds as mediators of specificity and even as substrates to feed upon.
The volatile oil of I. asarifolia consists of many minor but five major components, the latter of which are sesquiterpenes (Kucht et al. 2004). Sesquiterpenes play an important role in ecological interactions between plants and insects (Schnee et al. 2006; Gershenzon and Dudareva 2007). Our observations raise the question whether this class of terpenoids is also essential for the interaction between different Convolvulaceae species and their associated clavicipitaceous fungi.
3.4 Biosynthesis and Accumulation of Ergoline Alkaloids in the Fungus/Plant Symbiotum
Ergoline alkaloids are natural products of high physiological activity. They are likely to confer drought resistance, herbivore deterrence and fitness to the host plant (Malinowski and Belesky 2000; White Jr. et al. 2003; Bacon and Lyons 2005; Gershenzon and Dudareva 2007). This raises the question as to how this may be brought about when plant-associated clavicipitaceous fungi are the site of ergoline alkaloid biosynthesis. Indeed, Convolvulaceae plants do not seem to have the biosynthetic capacity to produce ergoline alkaloids: neither the genes nor the enzymic machinery were detectable in the shoots. The genetic material responsible for ergoline alkaloid biosynthesis was clearly found in the associated fungi present on I. asarifolia and T. corymbosa (Markert et al. 2008). The determinant step in ergoline alkaloid biosynthesis is the prenylation in the 4 position of tryptophan catalyzed by 4-[γ,γ-dimethylallyl] tryptophan synthase (DmaW; Groeger and Floss 1998; Keller and Tudzinski 2002). The encoding gene – which has different synonyms, i.e. dmaW or cpd1 (Schardl et al. 2006) or fgaPT2 (Unsöld and Li 2005) – is clearly present in the fungus and is part of a cluster in which the ergoline alkaloid genes are oriented. This is found in Claviceps but is different from Aspergillus and Neotyphodium species (Markert et al. 2008). A reverse genetics experiment showed that the fungus is also the site of transcription of the dmaW gene (Markert et al. 2008).
Initial attempts to detect ergoline alkloids in the fungal mycelium present on I. asarifolia and T. corymbosa failed although two different analytical approaches were used (Markert et al. 2008). When a sample of the mycelium found on T. corymbosa was directly placed into the injection port of a GC/MS system a trace of agroclavine was detectable and clearly identified by comparison with an authentic sample. No alkaloid was detectable when a mycelial sample from I. asarifolia was checked in the same way (W. Boland, personal communication). When the leaf material was analyzed for ergoline alkaloids after removal of the mycelium by ultrasonic treatment, alkaloids were qualitatively and quantitatively detected in the plant material, showing that the plant leaf material contained almost all alkaloids whereas the producing fungus provisionally named TcorF01 contained only a trace of agroclavine (Markert et al. 2008).
Thus, biosynthesis of alkaloids takes place in the mycelium; however, ergoline alkaloids accumulate in the host plant. We have to postulate a transport system that translocates ergoline alkaloids from the mycelium into the plant tissue. In an experimental system similar to the one discussed here transport was postulated to occur through the apparently intact cuticle (Smith et al. 1985).
3.5 Seed Transmittance of Epibiotic Fungi Colonizing Convolvulaceae
The genus Ipomoea comprises 600–700 pantropical species. Over half of them are concentrated in the Americas. The American species are mostly native but a few have been introduced (Austin and Huáman 1996). The classification of Ipomoea species is still under discussion (Amor-Prats and Harborne 1993; Austin and Huáman 1996). There may be multiple reasons for this: Some species are only endemic and the description of species is incomplete, especially those native to Brazil. Many have not been validly described or are even undiscovered. The genus Ipomoea consists of three subgenera, i.e. subgenus Eriospermum , subgenus Ipomoea and subgenus Quamoclit . Amor-Prats and Harborne (1993) attempted to find chemotaxonomic support for an infrageneric classification of the genus Ipomoea by analyzing seeds from 43 species for their ergoline alkaloid content. The alkaloid-bearing species fall, however, into each of the taxonomically defined subgenera.
Hence, there is no clear relationship between the distribution of alkaloids and the infrageneric classification of the genus Ipomoea. The whole problem is clouded by the inability to reproduce published analytical data in many cases. Consequently, Eich (2008) lists ergoline positive versus ergoline negative reports and Amor-Prats and Harborne (1993) believe that “the methods of analysis varied leading to some uncertainty”.
The reason for the inconsistent picture very likely depends on the (until recently unknown) presence of clavicipitaceous fungi on convolvulacous plants and within seeds (Kucht et al. 2004; Steiner et al. 2006; Ahimsa-Mueller et al. 2007) and the capacity of these fungi to synthesize ergoline alkaloids (see below).
A freshly harvested and surface-sterilized seed grown under germ-free conditions gives a plant colonized by the epibiotic clavicipitaceous fungus. This plant contains ergoline alkaloids. The epibiotic fungus is the only fungus that is detectable by SSCP on this particular plant. Such a fungus is detectable in seeds of I. asarifolia and I. violacea (Steiner et al. 2006; Ahimsa-Mueller et al. 2007). This shows that the fungus is seed-transmitted and points to the host specificity typical of asexual clavicipitaceous fungi (see above).
The viability of the seed-transmitted fungus very likely is limited and depends on seed age and storage (Schardl 1994) as well as moisture and storage temperature (Welty et al. 1987). An I. violacea plant devoid of ergoline alkaloids and derived from an alkaloid- and clavicipitaceous fungus-containing seed was recently described (Ahimsa-Mueller et al. 2007). In this particular case the viability of the seed exceeded the viability of the inhabiting fungus.
It follows that the presence in a convolvulaceous plant of ergoline alkaloids may be an unsuitable character for taxonomic classifications.
4 Additional Fungus/Plant Symbiota in Dicotyledonous Plants
An interesting association consisting of Ipomoea batatas (L.) Lam. (i.e. sweet potato; Convolvulaceae) and Fusarium lateritium Nees: Fr has also been reported. As described for our clavicipitaceous fungi (Sect. III) F. lateritium is primarily located between the halves of young unfolded leaves of the I. batatas plant (Hyun and Clark 1998). Yet there is another feature of this fungus/plant association which we also observed in our system (Sect. III): The fungus is associated on the phylloplane with pearl glands and is located around bases of trichomes (Clark 1992). The fungus apparently produces trichothecenes and protects the host plant against infection by pathogenic F. oxysporum f. sp. batatas (Wollenw.) W.C. Snyder & H.N. Hans. However, the associated F. lateritium may also be the cause of chlorotic leaf distortion (CLD) disease mediated by trichothecenes (Clark 1994). After light activation of trichotecenes during prolonged exposure of the plant to sunlight CLD occurs. Plants usually recover when cloudy weather prevails. Thus, the associated fungus may exert a beneficial and a detrimental effect on the host plant and in both cases trichothecenes are likely to be the causative agent.
Two new clavicipitaceous fungi belonging to a newly established genus (Hyperdermium) were isolated from an unidentified Asteraceae plant (genus Bernonia).The fungi were named H. bertonii (Speg.) J. White, R. Sullivan, G. Bills et N. Hywel-Jones and H. pulvinatum J. White, R. Sullivan, G. Bills et N. Hywel-Jones. As with the clavicipitaceous fungi described in Sect. III, the fungi are epibiotic. They belong to the subfamily Cordycipitoideae (Sullivan et al. 2000).
An entirely superficial mycelium was observed on a South American Asteraceae plant, Baccharis coridifolia D.C. The endophyte belongs to the Hypocreales, an order which accommodates also the family Clavicipitaceae. The fungus occurs not only epibiotically but also in meristematic tissue of leaf primordia. No reproductive structures were detectable. The plant is toxic and it was assumed that the epibiont is a trichothecene producer. Since this fungus and graminaceous Clavicipitaceae (Sect. II) are not closely related, colonizations (that must have occurred during evolution) were assumed to be distinct events (Bertoni et al. 1997).
The same conclusion was drawn for a Mentha piperita L. plant colonized by a pyrenomycete which is also associated with glandular trichomes (Mucciarelli et al. 2002), a striking observation which led to speculations about the possible function of the secretory glands and trichomes in the establishment of a symbiotic association: it may be that the glandular trichomes are entry gates for the fungus in its attempt to establish a molecular dialog with the host plant (Steiner et al. 2008).
Another interesting fungus/plant association has been described for locoweed plants belonging to the family Fabaceae. Astragalus molissimus, Oxytropis lambertii and O. sericea are collectively called locoweed and are colonized by endophytes which seem to be closely related to the genus Embellisia . Locoism, as observed in cattle intoxicated by locoweed plants, is a neurological disease resulting in a staggering walk and lack of muscular coordination. The causative agent seems to be the indolizidine alkaloid swainsonine. This alkaloid is also known to be a product of in vitro grown Rhizoctonia leguminicola cultures. Again there are observations that parallel those described in Sect. III:
-
1.
Some collections of host plants were devoid of natural products.
-
2.
The fungus and the alkaloid do not occur in the root system.
-
3.
The alkaloid exerts an ecological function in protecting the fungus against insects and animal feeding (Braun et al. 2003).
5 Conclusions
The data described in Sect. III solve a historical mystery and explain why ergoline alkaloids occur in disjointed taxa, clavicipitaceous fungi and convolvulaceous plants. They dispute the possibility that during evolution a horizontal transfer of genes responsible for the synthesis of ergoline alkaloids occurred from fungi to plants. They also show that there is no necessity to invoke a repeated invention of the ergoline alkaloid biosynthetic pathway during evolution. In fact genes present in IasaF13, TcorF01, Claviceps purpurea, C. fusiformis, Balansia obtecta, Neotyphodium coenophialum as well as Asperillus fumigatus involved in the biosynthesis of ergoline alkaloids share a high similarity (Markert et al. 2008). It is now evident that clavicipitaceous fungi do not only colonize Poaceae and related monocots but also Convolvulaceae. It is also clear that the association between fungus and convolvulaceous plant is asymptomatic and that a molecular dialog occurs between associated fungi and convolvulaceous plants, indicating that both are members of a symbiotum in which biosynthesis and accumulation of ergoline alkaloids is spatially separated and sequestered in different but associated organisms. One of the unsolved questions is whether there are also sexual forms of these vertically transmitted asexual clvicipitaceous fungi. It is also unknown how the plant-associated fungi spread within the plant. Despite repeated attempts to localize hyphea, spores or propagules within the host plants, endophytic structures of the fungi have remained undetected until now.
Some of these observations parallel those made with fungus/plant associations occurring in the plant families Asteraceae and Fabaceae and in the plant species I. batatas, as outlined in Sect. IV. In each case the associated fungus is the producer of poisonous natural products (trichothecenes, swainsonine) which may benefit and protect the host plant.
References
Ahimsa-Mueller MA, Markert A, Hellwig S, Knoop V, Steiner U, Drewke C, Leistner E (2007) Clavicipitaceous fungi associated with ergoline alkaloid-containing Convolvulaceae. J Nat Prod 70:1955–1960
Amor-Prats D, Harborne JB (1993) New sources of ergoline alkaloids within the genus Ipomoea. Biochem Syst Ecol 21:455–461
Arnold AE, Mejia LC, Kyllo D, Rojas EI, Maynard Z, Robbins N, Herre EA (2003) Fungal endophytes limit pathogen damage in a tropical tree. Proc Natl Acad Sci USA 100:15649–15654
Austin DF, Huáman Z (1996) A synopsis of Ipomoea (Convolvulaceae) in the Americas. Taxon 45:3–38
Bacon CW, Lyons P (2005) Ecological fitness factors for fungi within the Balansiae and Clavicipiteae. In: Dighton J, White JF Jr, Oudemans P (eds) The fungal community, its organisation and role in the ecosystem, 3rd edn. CRC Taylor and Francis, Boca Raton, pp 519–532
Bacon CW, White JF Jr (1994) Stains, media, and procedures for analyzing endophytes. In: Bacon CW, White JF Jr (eds) Biotechnology of endophytic fungi of grasses. CRC, Boca Raton, pp 47–58
Bacon CW, White JF Jr (2000) Microbial endophytes. Dekker, New York, pp 341–388
Bertoni MD, Romero N, Reddy PV, White JF Jr (1997) A hypocralean epibiont on meristems of Baccharis coridifolia. Mycologia 89:375–382
Bischoff JF, White JF Jr (2005) Evolutionary development of the Clavicipitaceae. In: Dighton J, White JF Jr, Oudemans P (eds) The fungal community – its organisation and role in the ecosystems, 3rd edn. CRC Taylor and Francis, Boca Raton, pp 505–518
Braun U, Romero J, Liddell C, Creamer R (2003) Production of swainsonine by fungal endophytes of locoweed. Mycol Res 107:980–988
Brem D, Leuchtmann A (2002) Intraspecific competition of endophyte infected vs uninfected plants in two woodland grass species. Oikos 96:281–290
Chang H-T, Cheng Y-H, Wu C-L, Chang S-T, Chang T-T, Su Y-C (2008) Antifungal activity of essential oil and its constituents from Calocedrus macrolepis var. formosana Florin leaf against plant pathogenic fungi. Bioresour Technol 99:6266–6270
Clardy J, Walsh C (2004) Lessons from natural molecules. Nature 432:829–837
Clark CA (1992) Histological evidence that Fusarium lateritium is an exopathogen on sweetpotato with chlorotic leaf distortion. Phytopathology 82:656–663
Clark CA (1994) The chlorotic leaf distortion pathogen, Fusarium lateritium, cross protects sweetpotato against Fusarium wilt caused by Fusarium oxysporum f.sp.batatas. Biol Control 4:59–66
Clay K, Frentz IC (1993) Balansia pilulaeformis, an epiphytic species. Mycologia 85:527–534
Clay K, Schardl C (2002) Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am Nat 160:S99–S127
Croteau R, Ketchum REB, Long RM, Kaspera R, Wildung MR (2006) Taxol biosynthesis and molecular genetics. Phytochem Rev 5:75–97
Dobberstein RH, Staba EJ (1969) Ipomoea, Rivea and Argyreia tissue cultures: influence of various chemical factors on indole alkaloid production and growth. Lloydia 32:141–177
Eich E (2008) Solanaceae and Convolvulaceae: secondary metabolites – biosynthesis, chemotaxonomy, biological and economic significance (a handbook). Springer, Heidelberg
Eisner T (2003) For love of insects. Harvard University Press, Cambridge, Mass.
Gershenzon J, Dudareva N (2007) The function of terpene natural products in the natural world. Nat Chem Biol 3:408–414
Glenn AE, Bacon CW, Price R, Hanlin RT (1996) Molecular phylogeny of Acremonium and its taxonomic implications. Mycologia 88:369–383
Groeger D, Floss HG (1998) Biochemistry of ergot alkaloids – achievements and challenges. In: Cordell GA (ed) The alkaloids: chemistry and biology, vol 50. Academic, New York, pp 171–218
Gunatilaka AAL (2006) Natural products from plant-associated microorganisms: distribution, structural diversity, bioactivity, and implications of their occurrence. J Nat Prod 69:509–526
Harborne JB (2004) Introduction to ecological biochemistry, 4th edn, Academic, London
Hofmann A (1961) Die Wirkstoffe der mexikanischen Zauberdroge “Ololuiqui”. Planta Med 9:354–367
Hofmann A (2006) LSD – mein Sorgenkind, die Entdeckung einer Wunderdroge. Deutscher Taschenbuchverlag, Munich
Hussein YHA (2004) Biochemical analysis of Convolvulaceae plant tissue cultures for the presence of ergoline alkaloids. Dissertation, Zagazig University
Hyun J-W, Clark CA (1998) Analysis of Fusarium lateritium using RAPD and rDNA RFLP techniques. Mycol Res 102:1259–1264
Jenett-Siems K, Kaloga M, Eich E (1994) Ergobalansine/ergobalansinine, a proline-free peptide type alkaloid of the fungal genus Balansia is a constituent of Ipomoea piurensis. J Nat Prod 57:1304–1306
Keller U, Tudzynski P (2002) Ergot alkaloids. In: Osiewacz HD (ed) The Mycota, industrial applications, vol X. Springer, Heidelberg, pp 157–181
Kucht S, Gross J, Hussein Y, Grothe T, Keller U, Basar S, Koenig WA, Steiner U, Leistner E (2004) Elimination of ergoline alkaloids following treatment of Ipomoea asarifolia (Convolvulaceae) with fungicides. Planta 219:619–625
Kuldau GA, Liu JS, White JF Jr, Siegel MR, Schardl CL (1997) Molecular systematics of Clavicipitaceae supporting monophyly of genus Epichloë and form genus Ephelis. Mycologia 89:431–441
Leistner E (2005) Die Biologie der Taxane. Pharm Unserer Zeit 34:98–103
Leuchtmann A, Clay K (1988) Atkinsonella hypoxylon and Balansia cyperi, epiphytic members of the Balansiae. Mycologia 80:192–199
Leuchtmann A, Clay K (1989) Morphological, cultural and mating studies on Atkinsonella, including A. texensis. Mycologia 81:692–701
Lewis EA, Bills GF, Heredia G, Reyes M, Arias RM, White JF Jr (2002) A new species of endophytic balansia from Veracruz, Mexico. Mycologia 94:1066–1070
Luttrell ES, Bacon CW (1977) Classification of Myriogenospora in the Clacicipitaceae. Can J Bot 55:2090–2097
Malinowski DP, Belesky DP (2000) Adaptations of endophyte-infected cool-season grasses to environmental stresses. Crop Sci 40:923–940
Markert A, Steffan N, Ploss K, Hellwig S, Steiner U, Drewke C, Li S-M, Boland W, Leistner E (2008) Biosynthesis and accumulation of ergoline alkaloids in a mutualistic association between Ipomoea asarifolia (Convolvulaceae) and a clavicipitalean fungus. Plant Physiol 147:296–305
Mothes K (1981) The problem of chemical convergence in secondary metabolism. Sci Scientists 1981:323–326
Mothes K, Schütte HR, Luckner M (1985) Biochemistry of alkaloids. VEB, Berlin
Moy M, Belanger F, Duncan R, Freehoff A, Leary C, Meyer W, Sullivan R, White JF Jr (2000) Identification of epiphyllous mycelial nets on leaves of grasses infected by clavicipitaceous endophytes. Symbiosis 28:291–302
Mucciarelli M, Scannerini S, Bertea CM, Maffei M (2002) An ascomycetous endophyte isolated from Mentha piperita L.: biological features and molecular studies. Mycologia 94:28–39
Partida-Martinez LP, Hertweck C (2005) Pathogenic fungus harbours endosymbiotic bacteria for toxin production. Nature 437:884–888
Piel J (2004) Metabolites from symbiotic bacteria. Nat Prod Rep 21:519–538
Puri SC, Verma V, Amna T, Qazi GN, Spiteller M (2005) An endophytic fungus from Nothapodytes foetida that produces camptothecin. J Nat Prod 68:1717–1719
Reddy PV, Bergen MS, Patel R, White JF Jr (1998) An examination of molecular phylogeny and morphology of the grass endophyte Balansia claviceps and similar species. Mycologia 90:108–117
Rykard DM, Bacon CW, Luttrell ES (1985) Host relations of Myriogenospora atramentosa and Balansia epichloë (Clavicipitaceae). Phytopathology 75:950–956
Saikkonen K, Wäli P, Helander M, Feath SH (2004) Evolution of endophyte-plant symbiosis. Trends Plant Sci 9:275–280
Schardl CL (1994) Molecular and genetic methodologies and transformation of grass endophytes. In: Bacon CW, White JF Jr (eds) Biotechnology of endophytic fungi of grasses. CRC Taylor and Francis, Boca Raton, pp 151–166
Schardl CL, Craven KD, Speakman S, Stromberg A, Lindstrom A, Yoshida R ( 2008) A novel test for host-symbiotum codivergence indicates ancient origin of fungal endophytes in grasses. Syst Biol 57:483–498
Schardl CL, Grossman RB, Nagabhyru P, Faulkner JR, Mallik UP (2007) Loline alkaloids: currencies of mutualism. Phytochemistry 68:980–996
Schardl CL, Leuchtmann A, Spiering MJ (2004) Symbiosis of grasses with seedborne fungal endophytes. Annu Rev Plant Biol 55:315–340
Schardl CL, Panaccione DG, Tudzynski P (2006) Ergot alkaloids - biology and molecular biology. In: Cordell GA (ed) The alkaloids: chemistry and biology, vol 63. Academic, New York, pp 45–86
Schnee C, Köllner TG, Held M, Turlings TCJ, Gershenzon J, Degenhardt J (2006) The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Natl Acad Sci USA 103:1129–1134
Smith KT, Bacon CW, Luttrell ES (1985) Reciprocal translocation of carbohydrates between host and fungus in Bahiagrass infected with Myriogenospora atramentosa. Phytopathology 75:407–411
Spatafora JW, Blackwell M (1993) Molecular systematics of unitunicate perithecial ascomycetes: the Clavicipitales–Hypocreales connection. Mycologia 85:912–922
Steiner U, Ahimsa-Mueller MA, Markert A, Kucht S, Gross J, Kauf N, Kuzma M, Zych M, Lamshoeft M, Furmanowa M, Knoop V, Drewke C, Leistner E (2006) Molecular characterisation of a seed transmitted clavicipitaceous fungus occurring on dicotyledonous plants (Convolvulaceae). Planta 224:533–544
Steiner U, Hellwig S, Leistner E (2008) Specificity in the interaction between an epibiotic clavicipitalean fungus and its convolvulaceous host in a fungus/plant symbiotum. Plant Signal Behav 3:704–706
Strobel G, Daisy B, Castillo U, Harper J (2004) Natural products from endophytic microorganisms. J Nat Prod 67:257–268
Sullivan RF, Bills GF, Hywel-Jones NL, White JF Jr (2000) Hyperdermium: a new clavicipitalean genus for some tropical epibionts of dicotyledonous plants. Mycologia 92:908–918
Sung GH, Sung JM, Hywel-Jones NL, Spatafora JW (2007) A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): Identification of localized incongruence using a combinational bootstrap approach. Mol Phyl Evol 44:1204–1223
Tsai H-F, Liu J-S, Staben C, Christensen MJ, Latch CM, Siegel MR, Schardl CL (1994) Evolutionary diversification of fungal endophytes of tall fescue grass by hybridisation with Epichloë species. Proc Natl Acad Sci USA 91:2542–2546
Tudzynski P, Correia T, Keller U (2001) Biotechnology and genetics of ergot alkaloids. Appl Microbiol Biotechnol 57:593–605
Unsöld IA, Li S-M (2005) Overproduction, purification and characterization of FgaPT2, a dimethylallyltryptophan synthase from Aspergillus fumigatus. Microbiology 151:1499–1505
Welty RE, Azevedo MD, Cooper TM (1987) Influence of moisture content, temperature, and length of storage on seed germination and survival of endophytic fungi in seeds of tall fescue and perennial ryegrass. Phytopathology 77:893–900
White JF Jr, Morgan-Jones G (1996) Morphological and physiological adaptations of Balansieae and trends in the evolution of grass endophytes. In: Redlin SC, Carris LM (eds) Endophytic fungi in grasses and woody plants, APS, St. Paul, pp 133–154
White JF Jr, Bacon CW, Hinton DM (1991) Substrate utilization in selected Acremonium, Atkinosella, and Balansia species. Mycologia 83:601–610
White JF Jr, Bacon CW, Hywel-Jones NL, Spatafora JW (2003) Clavicipitalean fungi, evolutionary biology, chemistry, biocontrol, and cultural impacts. Mycology series, vol 19, Dekker, New York
Zenk MH (1967) Biochemie und Physiologie sekundärer Pflanzenstoffe. Ber Dtsch Bot Ges 80:573–591
Zhang L, Guo B, Li H, Zeng S, Shao H, Gu S, Wei R (2000) Zhongcaoyao 31:805–807
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Additional information
Dedicated to Prof. Dr. Detlef Gröger on the occasion of his 80th birthday
Rights and permissions
Copyright information
© 2009 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Leistner, E., Steiner, U. (2009). Fungal Origin of Ergoline Alkaloids Present in Dicotyledonous Plants (Convolvulaceae). In: Anke, T., Weber, D. (eds) Physiology and Genetics. The Mycota, vol 15. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-00286-1_9
Download citation
DOI: https://doi.org/10.1007/978-3-642-00286-1_9
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-00285-4
Online ISBN: 978-3-642-00286-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)