Abstract
Neurons form transient functionally specialized assemblies by coordinating their activity within networks. Assembly activity is important for coding and information processing in the brain; oscillations are assumed to entrain and provide temporal structure to this. Recent work from different laboratories has uncovered cell type-specific activity patterns during network oscillations, indicating that the cells may differentially contribute to the generation of oscillation and thereby the coordination of cell assemblies. The purpose of this chapter is to summarize recent findings from these works in in vitro preparations highlighting the importance of different neuronal activity patterns of hippocampal principal cells and different subtypes of interneurons. Special attention will be paid to the role of the firing properties of hippocampal interneurons on the network oscillatory activity at the theta and gamma frequency range.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Overview
Neurons form transient functionally specialized assemblies by coordinating their activity within networks. Assembly activity is important for coding and information processing in the brain; oscillations are assumed to entrain and provide temporal structure to this. Recent work from different laboratories has uncovered cell type-specific activity patterns during network oscillations, indicating that the cells may differentially contribute to the generation of oscillation and thereby the coordination of cell assemblies. The purpose of this chapter is to summarize recent findings from these works in in vitro preparations highlighting the importance of different neuronal activity patterns of hippocampal principal cells and different subtypes of interneurons. Special attention will be paid to the role of the firing properties of hippocampal interneurons on the network oscillatory activity at the theta and gamma frequency range. Models based on these ideas are found in Kopell et al. chapter of this book.
In Vitro Models of Network Oscillations
Hippocampal Population Activity Patterns In Vivo and In Vitro
Hippocampal networks show rhythmic oscillations in various frequency ranges in a behavior-dependent manner (Singer 1999; Buzsáki and Draguhn 2004). In the freely moving rat, three types of hippocampal oscillatory activity have been observed (Leung et al. 1982), which are broadly termed theta (4–12 Hz)-, gamma (30–90 Hz)-, and sharp-wave ripples (100–300 Hz). Theta and gamma frequency rhythms are observed in the rat during exploration and rapid eye movement sleep (Fig. 1A). The frequency range of both rhythms is described differently in different studies. These two rhythms often coexist but can also occur separately [Fig. 1A, for review see Whittington and Traub (2003)]. Gamma and theta rhythms also occur throughout the neocortex in vivo and have been proposed to constitute a fundamental mechanism underlying cognitive tasks such as feature recognition, associative learning, and content- and context-sensitive processing of sensory information. In addition, intermittent population bursts, sharp-wave-associated field ripples, are present in the CA3-CA1-subiculum-entorhinal cortex axis during awake immobility, consummatory behaviors, and slow-wave sleep (Fig. 1B, Vanderwolf 1969; Buzsáki et al. 1983; Bland 1986; Chrobak and Buzsáki 1996, Csicsvari et al. 1999).
Hippocampal network oscillations in vivo (A, B) and in vitro (C–E). (A) Theta- and gamma-related modulation of the field in the dentate gyrus (hilar region) during exploratory walking. (B) Sharp-wave-associated field ripples in CA1 area during slow-wave sleep. Upper traces, wideband recording, lower traces, band bath (40–150 Hz, A; 150–250 Hz, B) filtered gamma and ripple activity. (C) Metabotropic glutamate receptor activation under conditions of reduced AMPA receptor activation generates in CA1 area theta population activity. (D) Kainate receptor activation induces network oscillations at the gamma frequency range in CA3 area. (E) Spontaneously occurring sharp-wave-associated ripple oscillation in CA1 area in vitro. Upper trace, wideband recording, lower trace, ripple band-pass (140–320 Hz) filtered activity. Panels are adapted from (A) Bragin et al. (1995), (B) Csicsvari et al. (1999), (c) Gillies et al. (2002), (D) Gloveli et al. (2005b) and (E) Both et al. (2008)
Various in vitro models have been developed to gain insight into the cellular and synaptic mechanisms of theta, gamma, and ripple oscillations (Fig. 1C–E). In vitro models of network oscillations, such as the carbachol (Fisahn et al. 1998; Buhl et al. 1998), the kainate (Buhl et al. 1998), the metabotropic glutamate receptor activation (Gilles et al. 2002), and the tetanically induced (Whittington et al. 1997) gamma activity models, reproduce salient features of oscillatory activity in slice preparations maintained in “interface” slice chamber. It has been shown that using an intact hippocampus, and very high flow rates, intrinsic theta and gamma oscillations are maintained without the need for pharmacological intervention (Goutagny et al. 2009). To determine activity pattern of individual neurons, sharp microelectrode or blind whole-cell patch-clamp recordings have been obtained from principal cells or putative interneurons. In addition, an in vivo model, the juxtacellular recording technique, was developed to conjointly record action potential series from single neurons and the extracellular field potential during different forms of network activity in anesthetized animals (Pinault 1996; Klausberger et al. 2003, 2004; see also chapter by Tukker in this book). These in vitro and in vivo methods have some clear advantages in studying network activity. However, the sparse distribution of interneurons makes them unlikely targets for these blind approaches. Therefore, these investigations are very inefficient in mapping neuronal activity patterns. Whole-cell patch-clamp recordings using infrared differential contrast videomicroscopy (Dodt and Zieglgänsberger 1994) have greatly facilitated selection and recordings from interneuron. However, this approach has been hampered by the difficulty of generating population activity in the submerged-type slice chambers. Technical modification of the pharmacological paradigms: brief pressure ejection of kainate (Gloveli et al. 2005a, b) or bath application of kainate (Dugladze et al. 2007, 2012; Zarnadze et al. 2016) and carbachol (Hájos et al. 2004) permitted the reproduction of the network oscillatory activity in submerged slices. The increased use of fluorescent protein expressing reporter lines (i.e., GFP, YFP, td-Tomato, etc.) under the genetic promotion of different unique markers of interneurons and pyramidal cells (PCs) alike (Giepmans et al. 2006) has enabled targeted whole-cell recording from neurochemically defined cells. Furthermore, the use of increasingly more rapid genetically encoded receptors, opsins, and calcium sensors (DREADD, channelrhodopsin, GCaMP6F) has allowed the manipulation of cellular activity to more comprehensively assess their function in the context of network oscillations. Using these approaches, it is possible to record from visually identified pyramidal cells and interneurons during gamma and theta frequency network oscillation in vitro.
Cell Types Involved in Rhythms
Morphological and physiological properties discriminate hippocampal PCs from inhibitory interneurons. In addition, further distinctions exist within both PCs and interneurons. It is reasonable to postulate that hippocampal neurons with different structural features are also likely to have different functions in the network.
Pyramidal Cells
Despite the morphological similarities (pyramid-shaped somata, apical and basal dendritic trees), PCs in CA1 and CA3 areas display some important differences such as the existence of excitatory recurrent collaterals. The latter is considered to be the hallmark of the CA3 but not the CA1 area. PCs of the CA3 area themselves are not homogeneous. Whereas most axon collaterals of the CA3a and CA3b neurons give rise to extensive recurrent collaterals that are confined to the CA3 region, PCs in CA3c subregion are mostly projection cells, with most of their axon collaterals terminating in the ipsi- and contralateral CA1 regions (Li et al. 1994; Wittner et al. 2007). It was hypothesized (Csicsvari et al. 2003) that intrahippocampal gamma oscillations emerge in the recurrent collateral-rich CA3a,b subregions; their activity recruits CA3c subregion, which, in turn, entrains CA1 cells. A further level of complication is added when one considers the less well-studied CA2 PCs, which receive strong theta-modulated input from the supramammillary region (Pan and McNaughton 2002), and they themselves are more preferentially excited by entorhinal cortex inputs than CA3 or CA1 PCs (Chevaleyre and Siegelbaum 2010). While the role of CA2 PCs in the control of oscillatory patterning remains unclear, they may contribute significantly to the timing of theta oscillations.
Interneuron Types
In contrast to glutamatergic principal cells, GABAergic interneurons of the hippocampus exhibit substantial diversity. In the CA1 area, for instance, at least 21 classes of interneurons were described (for review see Klausberger and Somogyi (2008) [see Vida chapter of this book]). In contrast to principal cells, the vast majority of interneurons have locally restricted axons and lack spines. Interneurons can be broadly classified into several classes on the basis of different criteria, such as action potential firing properties, somato-dendritic architecture and axonal ramification pattern, neurochemical content, voltage and ligand-gated conductances as well as plastic changes in excitatory synaptic transmission [for reviews see Freund and Buzsáki (1996), McBain and Fisahn (2001), and Klausberger and Somogyi (2008)]. Functionally, at least four main GABAergic cell classes coexist in hippocampal networks: (1) perisomatic inhibitory neurons, (2) dendritic inhibitory interneurons, (3) GABAergic cells specifically innervating other inhibitory interneurons, and (4) projection interneurons which cross hippocampal subfields (Miles et al. 1996; Klausberger and Somogyi, 2008; Booker and Vida 2018). The most striking morpho-functional dichotomy in the population of cortical interneurons is the targeting of the dendritic versus the perisomatic domain of principal cells. Dendritic inhibition is likely to control the efficacy and plasticity of excitatory synaptic inputs of principal cells, whereas perisomatic inhibition is ideally suited to control output and the generation of action potentials and can synchronize the firing of large groups of principal cells (Cobb et al. 1995; Miles et al. 1996; Freund and Buzsáki 1996). Further distinctions exist within the same classes of interneurons. Thus, different types of perisomatic targeting parvalbumin (PV)-expressing interneurons innervate distinct subcellular domains of principal cells. Axo-axonic cells (AACs) innervate exclusively the axon initial segment of PCs; in contrast basket cells (BCs) innervate the somata and proximal apical dendrites. In addition, two distinct populations of basket cells – PV-expressing and cholecystokinin (CCK)-expressing interneurons – could be defined on the basis of their neurochemical content [see Vida chapter of this book]. Dendrite-targeting interneurons could be further subdivided into distal (such as oriens lacunosum-moleculare, O-LM, or radiatum lacunosum-moleculare, R-LM, cells) and proximal dendrite-targeting cells (such as bistratified or trilaminar cells). Interneurons belonging to distinct classes defined by their axonal target domain on the PC have clearly different intrinsic, synaptic, and firing properties.
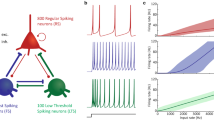
As an example of this diversity, Fig. 2 illustrates the morphology and the physiological properties of two types of interneurons: non-fast-spiking distal dendrite-targeting O-LM cells, which present one of the best studied interneuron classes in the hippocampus, and fast-spiking proximal dendrite-targeting trilaminar cells. These cells differ in their morphology, neurochemical marker contents, and intrinsic membrane properties (see Fig. 2A, B). Clear differences were also detected in spontaneous EPSCs properties between these two subtypes of interneurons – with slower kinetics in O-LM than those in trilaminar interneurons (Fig. 2B). Furthermore, while the excitatory input displayed a late-persistent firing in O-LM cells, fast-spiking trilaminar interneurons displayed an onset-transient firing in response to stimulation of CA1 axons in the alveus (Fig. 2B; Pouille and Scanziani 2004).
Properties of distal (O-LM) and proximal (trilaminar) dendrite-targeting interneurons. (A) Morphology of O-LM and trilaminar cells. Somata and dendrites are drawn in red; axons are in black. The somata of O-LM cells are located in stratum oriens and have mainly horizontally running dendrites. The main axon of these cells crosses strata pyramidale and radiatum and branches in stratum lacunosum-moleculare. O-LM cells innervate the distal dendrites of PCs which are co-aligned with the entorhinal input (Sik et al. 1995; Maccaferri et al. 2000). (A), inset, O-LM cells are immunopositive for the metabotropic glutamate receptor (mGluR1α) and the neuropeptide somatostatin (SOM, Tukker et al. 2007) and express low levels of calcium-binding protein PV (Maccaferri et al. 2000; Klausberger et al. 2003). The trilaminar cells have similar horizontally distributed dendrites in stratum oriens but are clearly different from O-LM cells in respect of axonal arborization (Sik et al. 1995). Sub, subiculum; str.or, stratum oriens; str.pyr., stratum pyramidale; str. rad., stratum radiatum; str. l-m., stratum lacunosum-moleculare. B Intrinsic, intrinsic membrane (i) and firing properties (ii) of O-LM and trilaminar cells during hyperpolarizing and depolarizing current injection. O-LM cells demonstrate clear “sag” potential and non-fast-spiking pattern in marked contrast to trilaminar cells showing no “sag” and fast spiking character upon hyperpolarizing and depolarizing pulses. (B) Synaptic (i) – spontaneous EPSC (sEPSC) in O-LM and trilaminar interneurons. Forty individual traces are black and superimposed averaged currents are red. (B) Synaptic (ii), Cell-attached responses from an O-LM and trilaminar cells. Current deflections indicate action potential firing in response to the stimulation of CA1 axons in the alveus (indicated by the vertical arrows). (Panels A and B, Intrinsic (i,ii) and Synaptic (i), are from Gloveli et al. 2005a; panels B Synaptic (ii) are from Pouille and Scanziani (2004); Insert (A, O-LM) is from Tukker et al. (2007))
These differences in morphological and electrophysiological properties of interneurons indicate that they are likely to have specific roles in the network. In fact, analysis of their spike timing during the oscillations suggests that a division of labor exist among interneurons subtypes involved in hippocampal network oscillations (see sections “Firing Patterns in Gamma Oscillations” and “Firing Patterns in Theta Oscillations”).
Gamma Oscillations
Two forms of local network gamma frequency oscillations can be induced in vitro in hippocampal slices (Table 1). Transient forms of gamma frequency oscillations (lasting for a few seconds or minutes) can be evoked in vitro by tetanic stimulation (Whittington et al. 1997) or through pressure ejection of glutamate (Pöschel et al. 2002) and high molarities of locally applied kainate (Gloveli et al. 2005a, b; Craig and McBain 2015) or potassium (LeBeau et al. 2002; Towers et al. 2002) (Table 1).
Another model of gamma frequency oscillations is known as “persistent gamma” (lasting for hours). This kind of oscillation can be induced in the hippocampal CA3 area in vitro by bath application of agonists of muscarinic acetylcholine (mAChR) (Fisahn et al. 1998; Fellous and Sejnowski 2000; Shimono et al. 2000; Fisahn et al. 2002) and kainite receptors (KAR) (Fisahn et al. 2004; Gloveli et al. 2005a, b; Dugladze et al. 2012; Zarnadze et al. 2016) (Table 1). mAChR agonist (carbachol)- and kainate-induced fast network oscillations provide a useful model to explore the mechanisms underlying physiological gamma frequency oscillations for the following reasons. The hippocampus receives a dense cholinergic projection from the medial septum/diagonal band of Broca, which plays an important role in the generation of hippocampal network activity (Leung 1985). In addition, kainate receptors are expressed by both principal cells and interneurons of the hippocampus (Cossart et al. 1998; Frerking et al. 1998; for review see Lerma (2003)). These oscillations in vitro share many of the features of intrahippocampal gamma oscillations in vivo, including the firing of pyramidal neurons at low frequencies (<5 Hz) phase-locked to the oscillation and the generation of oscillations in CA3 and their subsequent propagating to CA1 (Fisahn et al. 1998; Gloveli et al. 2005a). Finally, in vivo and in vitro cholinergically induced oscillations have similar current source density profiles, and the gamma phase relationship between PCs and perisomatic-innervating interneurons is comparable (Csicsvari et al. 2003; Mann et al. 2005; Oren et al. 2006).
Both persistent and transient gamma oscillations can be evoked in different hippocampal areas, including CA3, CA1, and DG (Table 1, Towers et al. 2002; Pöschel et al. 2002; Gloveli et al. 2005a). However, there are regional differences in frequency and power of the oscillations, suggesting the existence of different rhythm-generating networks in the hippocampus. In line with this suggestion, both persistent and transient forms of kainate-induced gamma oscillations demonstrate faster gamma frequency oscillations in isolated CA1 area than those in CA3 area (N. Maziashvili and T. Gloveli, unpublished observation, Middleton et al. 2008; Craig and McBain 2015). However, gamma oscillations in the same area (e.g., the CA3 area) induced by different pharmacological drugs (carbachol and DHPG) also show significant differences in their properties (the peak frequencies, maximal power, and spectral width, Table 1, Pálhalmi et al. 2004), suggesting involvement of different network mechanisms, such as the recruitment of distinct types of interneurons. In addition, the gamma oscillations evoked under different conditions differ in their dependence on excitation and inhibition (Table 1). Thus, one form of transient oscillation, “interneuronal network gamma” (ING) (Whittington et al. 1995), is based on mutual inhibition between the interneurons [for computational models, see Wang and Buzsáki (1996), White et al. (1998), and Vida et al. (2006)], whereas “pyramidal-interneuronal network gamma” (PING) (Whittington et al. 1997) is based on reciprocal interneuron-PC interaction. Furthermore, fast gamma oscillations in CA1 induced by kainate puff application are independent of CA1 PC firing, as evidenced by optogenetic silencing of them, further reinforcing the idea that local interneurons may be a key determinant of this form of gamma oscillation (Craig and McBain 2015). It seems likely that all of these forms are relevant in vivo, possibly reflecting region and state dependence of mechanisms underlying hippocampal gamma oscillations.
Firing Patterns in Gamma Oscillations
A key requirement for the generation of network oscillations is rhythmic and synchronized activity of large sets of neurons. An important step in understanding the role of hippocampal neurons in network oscillations is to examine their spike patterns during these oscillations.
Principal Cells
Analysis of firing properties of electrophysiologically and morphologically identified PCs in CA3 area has been performed in vitro for KAR (Gloveli et al. 2005a, b) and mAChR (Fisahn et al. 1998; Hájos et al. 2004) agonist-induced gamma frequency oscillations. Both KAR and mAChR activation (by kainate and carbachol, respectively) revealed low frequency, <5 Hz, firing of PCs (Table 2, Fisahn et al. 1998; Hájos et al. 2004; Gloveli et al. 2005a). These results are in agreement with in vivo observations demonstrating similar low-frequency firing of PCs (Csicsvari et al. 2003). Moreover, PC firing is phase-locked to the field oscillations (Table 2). In carbachol-induced gamma oscillations, PCs fired action potentials around the negative peak of the field recorded in the PC layer (Fig. 3A, D, Hájos et al. 2004). Both in vivo and in vitro observations suggest that during gamma frequency oscillations, PCs of CA3 area drive local interneurons in a feedback manner (Fisahn et al. 1998; Csicsvari et al. 2003; Pálhalmi et al. 2004; Hájos etal. 2004). If PC-interneuron interactions generate gamma oscillations, the firing of PCs should precede interneuron discharge so that PC can recruit interneuron activity in the next gamma cycle (Oren et al. 2006). Consistent with this suggestion, interneuron responses were indeed preceded by PC firing (Fig. 3D, Hájos et al. 2004).
Morphological and firing properties of hippocampal neurons during pharmacologically induced gamma oscillations. Reconstructions of representative biocytin-filled pyramidal (Ai), PV-positive basket (Bi) and trilaminar (Ci) cells. The soma and dendrites are drawn in red, whereas the axons are in black. CA3, CA3 area; str. or., stratum oriens; str. pyr., stratum pyramidale; str. rad., stratum radiatum; str. l.-m., stratum lacunosum-moleculare. During kainate-induced field oscillatory activity (Aii; Bii), PCs fire sporadically (Aiii), whereas basket cells discharged with single spikes interrupted by irregularly occurring doublets of action potentials, phase-locked to the field gamma activity (Biii). Trilaminar cell produced spike doublets (Ciii) on every gamma cycle (Cii). (D) (left), Time sequence of firing of different neuron types during carbachol-induced oscillatory cycle (top trace). PCs fired at the negative peak of the oscillation followed by the interneurons. Gaussian functions were fitted to the spike time distribution for each type of neuron, and the average mean and SD were used to represent each cell class as a Gaussian function. (D) (right), Schematic diagram of the connectivity among phase-coupled neuron types in the CA3 hippocampal circuitry taking part in the gamma oscillation. (Panels A–C are adapted from Gloveli et al. (2005a); D is adapted from Hájos et al. (2004))
During in vitro gamma frequency oscillations induced by kainate, the interneurons receive a high-frequency barrage of compound EPSPs, modulated at gamma frequency, which are temporally correlated with extracellular population activity (Gloveli et al. 2005a). Since the slice is de-afferented, it is likely that the action potential-dependent excitatory events are mediated by local excitatory input from neighboring pyramidal neurons. Given the relatively low PC somatic spike rate with respect to the frequency of EPSPs invading interneurons, the question remains as to how PCs generate these rhythmic burst of events and reliably discharge interneurons. Interneurons may receive a rhythmic barrage of gamma frequency EPSPs, for the following reasons. First, in the active network, multiple PCs are likely to fire on any given oscillatory cycle. Due to the convergence of numerous PC axons onto a single postsynaptic interneuron it follows that each interneuron is also likely to receive multiple unitary excitatory inputs on each successive gamma wave. Second, there are suggestions that activity in PC axons may orthodromically excite interneurons, without PC somata necessarily firing (Traub et al. 2003). Computational models of carbachol (Traub et al. 2000)- and kainate-induced gamma oscillations (Fisahn et al. 2004) emphasize the importance of ectopic axonal action potentials for the generation of hippocampal gamma oscillations. The coexistence of phasic, high-frequency oscillations in principal cell axon populations and field potential gamma frequency oscillations was demonstrated in kainate model (Traub et al. 2003).
Interneuron Types
During gamma frequency oscillation in vivo and in vitro, the different classes of interneurons fire action potentials at different times and inhibit distinct subcellular domains of PCs (Figs. 2, 3, 4). During pharmacologically induced gamma frequency oscillations in vitro, perisomatic-targeting PV-expressing basket cells generate a predominantly gamma frequency output (Fig. 3B, Gloveli et al. 2005a; Hájos et al. 2004). Moreover, the firing of perisomatic basket cells is tightly coupled to the oscillation (Fig. 3B, D). The anatomical and physiological properties make these neurons ideally suited for generating local gamma rhythms, and indeed selective inhibition of PV basket cells’ output synapses abolished carbachol-induced gamma oscillations in CA1 (Gulyás et al. 2010). Meanwhile, in kainate-induced gamma oscillations, there was no correlation of putative PV basket cells with the gamma oscillation (Craig and McBain 2015), suggesting that different interneuron mechanisms underlie the different paradigms employed. In contrast, spiking of other PV-expressing perisomatic-targeting interneurons, axo-axonic cells was found to be only moderately coupled to the field gamma in anesthetized animals (Tukker et al. 2007), and their GABA release is not critical for the generation of carbachol-induced gamma field oscillations (Gulyás et al. 2010). However, inhibition mediated by axo-axonic cell can separate axonal and somatic activity, maintaining the functional polarization of PCs during gamma oscillations by preventing action potential spread across the axon initial segment (Dugladze et al. 2012).
Morphological and firing properties of O-LM interneurons. (A) Neurolucida reconstructed of biocytin-filled O-LM cell in area CA3 from transverse, longitudinal, and coronal slices. The soma and dendrites are drawn in red, whereas the axon is in green. Note different axonal ramification pattern in stratum lacunosum-moleculare in different slice preparation. Hippocampal layers are depicted schematically. CA3, CA3 area; str. or., stratum oriens; str. pyr., stratum pyramidale; str. rad., stratum radiatum; str. l.-m., stratum lacunosum-moleculare. (B) Typical example of extracellular field potential (fp) and concomitant current clamp (−60 mV) recordings in an O-LM cell after induction of oscillatory activity with kainate in different slices. (C) Corresponding power spectra (60-s epoch) from field (black) and current clamp (red) recordings. (D–F), O-LM firing in vivo is specifically associated with different types of brain state and network activity. Filtered extracellular network oscillations (top) and extracellularly recorded action potentials (bottom). Note that the O-LM cell firing is not phase-coupled to gamma cycle but fired rhythmically on the trough of theta oscillations and was silent during sharp-wave-associated ripples. Calibrations: (D) 0.1 mV (upper trace); 0.2 mV (lower trace) and 0.1 s; E,F, 0.3 mV (lower traces), 0.2 mV (upper theta trace), 0.05 mV (upper sharp-wave trace), and 300 ms (theta), 50 ms (sharp wave). (Adapted from panels A to C from Gloveli et al. (2005a, b), Dugladze et al. (2007), and Tort et al. (2007); panels D to F from Klausberger et al. (2003); and panel F from Tukker et al. (2007))
There is no in vitro data available on the activity of identified CCK-expressing basket cells. However, recordings from central ganglionic eminence (CGE)-derived basket cells, which are mostly CCK-expressing, showed minimal spiking in response to kainate-evoked gamma oscillations, with a mean firing probability of 0.069 ± 0.027 (Craig and McBain 2015), much lower than for PV-expressing BCs. In vivo results confirm that, in contrast to PV-expressing BCs, these interneurons fire earlier than PCs and out of phase with PV-expressing interneurons, during the gamma oscillations in anesthetized animals (Tukker et al. 2007). Therefore, CCK-expressing basket cells are likely to interfere with gamma synchronicity (Freund and Katona 2007; Galarreta et al. 2008).
While PV-expressing perisomatic inhibitory interneurons are thought to play a major role in gamma oscillations (Hájos et al. 2004; Gloveli et al. 2005a), other classes of fast-spiking interneurons, such as bistratified and trilaminar cells, may also be important for this rhythm (Gloveli et al. 2005a). Bistratified cells were so named because the axonal arbor is found in two strata: oriens and radiatum (Buhl et al. 1994). In addition to PCs, they also innervate interneurons including basket cells (Halasy et al. 1996). During the gamma oscillations in vitro, bistratified cells discharge at high frequency, phase-locked to the field gamma (Gloveli et al. 2005a; Hájos et al. 2004; Tukker et al. 2007). Therefore, they are also likely to be involved in the generation of the gamma oscillatory activity. Interestingly, the most prominent interneuron output seen during pharmacologically induced gamma oscillations in vitro was associated with trilaminar interneurons (Gloveli et al. 2005a; Hájos et al. 2004; Craig and McBain 2015). These fast-spiking cells project to three dendritic layers of CA3 and CA1 areas, with axons densely innervating strata oriens, pyramidale and radiatum. Additionally, axon collaterals of CA3 trilaminar cells were seen projecting to area CA1 and into the subiculum and possibly to other brain areas as well (Somogyi and Klausberger 2005). These cells generated highly regular, short latency spike doublets (Fig. 3C, Gloveli et al. 2005a). Their axonal arborization indicates that these interneurons innervate somatic and dendritic compartments of PCs locally as well as distant regions. Thus, via these cells, gamma rhythms generated locally in area CA3 could be efficiently transmitted to distal sites “downstream” in the hippocampal processing pathway.
Interneuron located in the stratum radiatum (with both the dendrites and axonal arborization localized in the stratum radiatum) have the lowest firing rate among all dendrite-targeting interneurons with weak coupling to the gamma oscillations in vitro (Table 2, Hájos et al. 2004). Although R-LM cells (with dendritic tree in stratum radiatum and axon restricted to stratum lacunosum-moleculare) fire at higher frequency than other radiatum cells, they also do not show significant phase-related firing (Hájos et al. 2004).
Stratum oriens O-LM cells, as described above (Fig. 2), are the archetypal feedback inhibitory interneuron and are more commonly associated with oscillations at lower frequencies (i.e., theta frequency; see below). However, they can also show preferential firing during in vitro generation of gamma oscillations (Chittajallu et al. 2013). Two populations of O-LM cells are observed following kainate puff-induced gamma activity, those which derive from the medial ganglionic eminence, which had a mean firing probability of 0.158 ± 0.029 during each gamma cycle, much higher than CGE-derived 5-HT3a containing O-LM cells which had a firing probability of 0.033 ± 0.008 for each cycle of gamma (Chittajallu et al. 2013). This serves to demonstrate that while both subtypes of O-LM cell are morphologically similar and express somatostatin, they are differentially recruited to local network activity.
Interestingly, projection interneurons, such as back-projecting CA1 interneurons, are very strongly modulated to gamma oscillations (Craig and McBain 2015), suggesting that these cells may serve to coordinate PC firing at these frequencies across subregions of the hippocampus.
Theta Oscillations
A prominent network pattern in the hippocampus of all mammals studied to date, including humans (Arnolds et al. 1980; Tesche and Karhu 2000), is a slow oscillation in the theta frequency band (4–12 Hz). Theta oscillations are most consistently present during various types of locomotor activities (Vanderwolf 1969) and rapid eye movement (REM) sleep (Jouvet 1969). In general, theta waves are absent in the immobile animal (Bland 1986; for review see Buzsáki (2002)). To explain the generation of these oscillations, various external pacemakers have been proposed [for review see Buzsáki (2002)]. One classical hypothesis is that cholinergic excitation from the septum and the diagonal band of Broca activates inhibitory interneurons, which in turn induce rhythmic IPSPs on the soma of depolarized PCs (Petsche et al. 1962). Indeed, this was elegantly demonstrated in organotypic hippocampal slice cultures, where the medial septum was cultured alongside, leading to the emergence of spontaneous theta oscillations (Fischer et al. 1999). Alternatively, the entorhinal cortex may entrain hippocampal areas at theta frequency. In rodents, hippocampal theta activity has maximal power in the CA1 region, and the synaptic currents underlying these oscillations are mainly generated by the entorhinal input [for review see Buzsáki (2002)]. However, recent in vitro experimental data and computational analysis indicate that theta activity can be generated intrinsically in the CA1 (Gillies et al. 2002; Rotstein et al. 2005) and CA3 (Gloveli et al. 2005b) areas, or the hippocampus as a whole (Goutagny et al. 2009). In fact, Cobb et al. (1995) demonstrated that individual GABAergic interneurons can effectively phase subthreshold membrane potential oscillations and spontaneous firing in PCs at theta frequencies. Alternating inhibition and post-inhibitory “rebound” activation underlies the entrainment of PCs (Cobb et al. 1995). Intrinsic GABAergic mechanisms are thus sufficient to generate theta activity in cortical networks. Somewhat unexpectedly, the entrainment of local PV and SOM interneurons to theta oscillation is entirely dependent on CA1 recurrent collaterals, with little involvement of CA3 inputs (Huh et al. 2016.).
Various in vitro models of the theta oscillatory activity have been developed, based on either mAChR (Konopacki et al. 1992; Fisahn et al. 1998), metabotropic glutamatergic (mGluR) (Gillies et al. 2002), or kainate receptor activation (Gloveli et al. 2005a, b). Coactivation of mGluRs and metabotropic cholinergic receptors has also been reported to generate robust theta frequency oscillations in the hippocampus in vitro (Cobb et al. 2000).
Metabotropic GluR activation generates prominent, inhibition-based, atropine-resistant theta population oscillations under conditions of reduced AMPA receptor activation in the hippocampal CA1 area (Gillies et al. 2002). This field oscillation was independent of muscarinic cholinergic receptor drive but strongly dependent on NMDA receptor and GABAA receptor activity (Table 1, Gillies et al. 2002). The mechanism of generation of theta frequency population activity in this model appeared to involve intrinsic theta frequency membrane potential oscillations in a subset of stratum oriens interneurons. The blockade of AMPA receptors was a critical requirement of the experimental conditions needed to see this population theta activity. Many of the properties of theta frequency oscillations in this reduced model match those seen in area CA1 in vivo (Gillies et al. 2002). In particular, the resulting population theta rhythm resembled atropine-resistant theta oscillations recorded in vivo (Buzsáki et al. 1986) and may be generated by a subset of stratum oriens interneurons displaying intrinsic membrane potential oscillations at theta frequency (Gillies et al. 2002). In addition, the coherent theta oscillations may come from the interaction of other GABAergic interneurons with the O-LM cells.
In the CA3 area, theta oscillations can be induced by application of kainate. A necessary prerequisite to ensure precisely synchronized theta activity was specific orientation of the slices: theta frequency population activity was detected predominantly in longitudinal hippocampal slice preparation (Gloveli et al. 2005b). These data demonstrate that theta activity can be generated intrinsically both in the CA1 and the CA3 areas of the hippocampus. In an intact hippocampus, theta activity is capable of spreading in the CA3 -> CA1 direction but also in the alternative direction, dependent on subiculum inputs (Jackson et al. 2014), which contradicts elements of the classic intrinsic theta generation. While there are several differences between these models, a common feature is their dependence on GABAergic inhibition (Table 1).
Firing Patterns in Theta Oscillations
Principal Cells
In a model of atropine-resistant theta oscillations following mGluR activation, with AMPA receptor activation blocked, PC somatic firing was seen in only few cells recorded but could be elicited with injection of tonic depolarizing current (0.1–0.2 nA). In these conditions, PCs fired one spike per field theta (~7 Hz) cycle during the trough of the field oscillation (Gillies et al. 2002). Consistent with this finding, pyramidal neurons in the CA1 region showed subthreshold resonance and firing preference at theta frequencies (range 2–7 Hz) (Pike et al. 2000). Recent data has shown that CA1 PC spiking is sparse during intrinsically generated theta oscillations, and its timing is highly dependent on local inhibition (Huh et al. 2016)
Interneuron Types
Ample evidence supports the critical involvement of hippocampal interneurons in theta oscillations. The best documented is involvement of stratum oriens distal dendrite-targeting O-LM interneurons (Fig. 2, Fig. 4A) in generation of theta rhythm. This cell type was found to participate in hippocampal theta activity both in vivo (Buzsáki 2002; Klausberger et al. 2003) and in vitro (Pike et al. 2000; Gillies et al. 2002; Hájos et al. 2004; Gloveli et al. 2005a, b, Huh et al. 2016), with a small subpopulation that are not phase-locked to theta (Huh et al. 2016). In particular, involvement of O-LM cells was investigated in vitro in kainate- and mAChR-mediated network oscillatory activity. In kainate-induced oscillations, O-LM cells fired in the theta frequency range during both theta and gamma population activity (Fig. 4B, Gloveli et al. 2005b). O-LM cells show prominent membrane potential oscillations in the theta frequency range (Maccaferri and McBain 1996). By contrast, hippocampal fast-spiking cells preferentially resonate in the gamma range (Pike et al. 2000). Furthermore, O-LM cells have longer membrane time constants than the gamma-preferring interneurons and a considerably longer afterhyperpolarization (AHP). Changes in AHP profiles in interneurons have been shown to have dramatic effects on firing patterns (e.g., see Savić et al. (2001)). Thus, O-LM cells and gamma-preferring interneurons discharge at different frequencies and participate preferentially in theta or gamma activity, respectively (Gloveli et al. 2005a). The theta frequency discharge of O-LM interneurons (Fig. 4B, C, Gloveli et al. 2005b) will provide a robust theta frequency rhythmic inhibitory output to the apical dendrites of PCs.
There is a growing body of evidence that PV-expressing basket cells are critical for the generation and maintenance of theta oscillations in vitro (Korotkova et al. 2010; Amilhon et al. 2015; Huh et al. 2016). Indeed, PV basket cells show very strong phase-locking to the peak of theta oscillations, with a spike probability of one per theta cycle (Huh et al. 2016). Indeed, the specific timing of intrinsic theta oscillations are controlled by PV-expressing interneurons, with increasing activity through optogenetic stimulation resulting in increased theta oscillation frequencies (Amilhon et al. 2015).
The dendrite domains of principal cells are innervated by other dendritic inhibitory interneurons, whose involvement in the hippocampal oscillations has not been addressed in vitro. This includes, for example, recently described interneuron type in CA1 area in anesthetized animal, Ivy cells, expressing neuropeptide Y (NPY) and the neuronal nitric oxide (NO). The soma of these cells is located in stratum pyramidale, and axonal collaterals innervate two strata: oriens and radiatum. Ivy cells discharge at low frequency during theta as well as gamma and ripple oscillations in anesthetized animals (Fuentealba et al. 2008). Another GABAergic interneuron type, neurogliaform cell that shares many similarities with Ivy cells, such as dense axonal fields, low-frequency discharge, and slow synaptic transmission (Vida et al. 1998; Price et al. 2005; Szabadics et al. 2007), is located in stratum lacunosum-moleculare and innervates the apical dendritic tuft of CA1 PCs co-aligned with the entorhinal input (Price et al. 2005). This cell type provides both fast GABAA receptor-mediated and slow GABAB receptor-mediated (Price et al. 2005; Szabadics et al. 2007) inhibition and therefore represents a potential candidate to be involved in both theta and gamma frequency oscillations. However, there is very little information about the activity pattern of this interneuron type and their role in network oscillations.
Nested Theta and Gamma Oscillations
Theta and gamma oscillations often occur simultaneously and show interaction. Amplitude of gamma oscillations is modulated with the phase of the theta rhythm. In addition, the frequencies of the two oscillations are also correlated, providing additional evidence of their interrelated function (Bragin et al. 1995). The coordinated nature of the two rhythms, and the observation that gamma power is stronger during theta-associated behavior (Leung et al. 1982; Bragin et al. 1995), implies that the neuronal generators of the two rhythms interact (and may be also overlap). This nested activity pattern is hypothesized to play a critical role in memory encoding and retrieval (Lisman and Idiart 1995; Lisman 2005).
Combined anatomical and physiological studies have provided evidence that in vitro gamma and theta rhythms are supported by neuronal circuits arranged orthogonally along the transverse and longitudinal axes, respectively (Table 1, Gloveli et al. 2005b). In hippocampal coronal slice preparation with intermediate orientations (between the transverse and longitudinal axis) both theta and gamma population rhythms were manifested (Fig. 4B and C, Gloveli et al. 2005b). The reason for that is a differential preservation of rhythm-generating microcircuits in transverse, longitudinal, and coronal slice preparation. Analysis of the three-dimensional axonal arborization patterns of different hippocampal CA3 interneurons recorded in transverse slices show that PV-expressing perisomatic targeting interneurons, along with trilaminar and bistratified cells, show a clear tendency to arborize widely within the transverse plane (Gloveli et al. 2005a, b). Indeed, mutual inhibition between PV-expressing interneurons produces strong nesting of gamma oscillations to theta cycles (Wulff et al. 2009), likely due to the high theta phase discharge of PV interneurons themselves (Huh et al. 2016). In contrast, distal dendrite-targeting O-LM cells arborized most extensively in the longitudinal plane forming two or three clusters in this direction (see Fig. 4A and Gloveli et al. (2005b)). In longitudinal slices, the preservation of these projections facilitated the generation of theta rhythms (Fig. 4B, C) with robust coherence over large distances (Gloveli et al. 2005b; Tort et al. 2007). Thus, orthogonal arrangement of rhythm-generating microcircuits alongside the longitudinal and transverse axis, and distinct firing patterns of certain classes of interneurons during the theta and gamma frequency oscillations (Gloveli et al. 2005b; Tort et al. 2007), enables the hippocampus to produce different (solely or combined) population activity.
Sharp-Wave Ripple Activity
The high-frequency oscillations termed ripples (100–300 Hz) are typically associated with sharp-wave activity (Buzsáki et al. 1992; Wilson and McNaughton 1994; O’Neill et al. 2006). The hippocampal sharp-wave ripple (SWR) complex is thought to play an important role in synaptic plasticity and the transfer of new memory trace from the hippocampus to the neocortex (Buzsáki 1989).
The mechanisms of these fast oscillatory patterns in the hippocampus and neocortex are not fully understood (Buzsáki et al. 1992; Ylinen et al. 1995; Draguhn et al. 1998). Both in vivo and in vitro studies suggest that SWRs arise in the recurrent collateral system of the CA3 area (similar to gamma oscillations), propagate toward CA1, and leave the hippocampal formation via the subiculum and the EC (Chrobak and Buzsáki 1996; Csicsvari et al. 2000; Maier et al. 2003, Both et al. 2008). During this state, the hippocampus seems to be less controlled by input from the EC; rather, it generates output signals itself (Chrobak and Buzsáki 1996). Data from the rodent hippocampus showed that GABAergic interneurons, in particular, parvalbumin (PV)-expressing fast-spiking basket cells, play a crucial role in SWR generation in vivo (Schlingloff et al. 2014; Stark et al. 2014).
SWR complex can be induced in vitro by electrical stimulation, pharmacologically, or can occur spontaneously with similar properties to the events seen in vivo (Maier et al. 2003; Nimmrich et al. 2005; Behrens et al. 2005; Hájos et al. 2009; HájosEller et al. 2015; Zarnadze et al. 2016). Several local network mechanisms underlying these patterns have been identified within CA1, including strong inhibition of nonparticipating PCs during SWR (Ylinen et al. 1995; Maier et al. 2003) and electrical coupling of CA1 PCs (Draguhn et al. 1998; Schmitz et al. 2001; Nimmrich et al. 2005). Concomitant extracellular and intracellular recordings of SWR complexes show that PCs display EPSP-IPSP sequences, IPSP-EPSP sequences, and prominent IPSPs, but never isolated EPSPs (Behrens et al. 2005). These results suggest that inhibitory inputs are strong during the development of ripple complexes. Consistent with this finding, fast-spiking basket and bistratified interneurons strongly increase their firing rate during ripple oscillations in vivo (Ylinen et al. 1995; Klausberger et al. 2004). Another fast-spiking cell type, axo-axonic interneurons, fires before the ripple episode but is silenced during and after (Klausberger et al. 2003). In contrast, non-fast-spiking O-LM cell firing is suppressed during ripples in vivo (Klausberger et al. 2003), however ~50% of O-LM cells show phase-locking to the end of ripples in vitro (Pangalos et al. 2013). This suggests that although they do not fire during ripples, they may contribute to the subsequent refractory period. Gap junctions also seem to be important for ripples, since the blockade of gap junctions with carbenoxolone attenuated ripple occurrence (Behrens et al. 2005, LeBeau et al. 2003). Interestingly, SWR can be induced with stimulation protocols known to induce LTP, a model of learning and memory, suggesting that this pattern is associated with changes in functional connectivity (Behrens et al. 2005).
The Interdependence of Gamma Oscillations and Sharp-Wave Ripples
Gamma oscillations and SWRs, involved in memory encoding (Jutras and Buffalo 2010) and consolidation (Buzsáki 1989; Girardeau et al. 2009; Jadhav et al. 2012), respectively, appear to be interlinked in the course of memory processing. These two rhythms reflect two “competing,” mutually exclusive network states in vitro (Eller et al. 2015; Zarnadze et al. 2016, Fig. 5): spontaneously occurring SWRs disappear shortly after onset of gamma rhythms induced by bath application of kainic acid (KA, 400 nM) and reappear within a few minutes after KA washout. However, transient slow gamma synchrony may synergistically interact with SWRs and promote hippocampal memory replay (Carr et al. 2012). Thus the two network patterns are not fully independent. Indeed, in an in vitro model, it was found that plastic changes initiated in the network by means of persistent gamma activity altered the subsequent SWR pattern (Fig. 5). Comparison of SWRs before the oscillatory gamma episode with post-gamma SWRs (p-SWR) reveals a significantly increased p-SWR area. In good agreement with this data, gamma oscillations induced by bath application of carbachol (20 μM), an alternative drug to trigger persistent gamma oscillations based on a different network mechanisms (Fisahn et al. 1998, 2002; Hájos et al. 2004), also result in a significant increase in SWR area (Zarnadze et al. 2016). This indicates that gamma activity itself, and not the pharmacological agent, is responsible for network alteration. Moreover, the intervening gamma episode also has an enhancing effect on subsequent gamma oscillations (Fig. 5E and F). Together, these results demonstrate that a gamma frequency episode significantly affects subsequent network activities. These effects are independent of the pharmacological agent used for the induction, but correlate with the presence and the power of gamma oscillations, highlighting the general potential of gamma rhythms to alter network activity. This form of plasticity is impaired by mGluR5 and NMDAR antagonists (Fig. 5D) suggesting that in the hippocampal area, CA3 gamma frequency oscillations influence the subsequent network activity through mGluR5- and NMDAR-dependent mechanisms.
Gamma oscillations promote long-lasting alterations in the network activity. (A) SWRs recorded in the stratum pyramidale of the CA3 region occurred spontaneously (left), disappeared shortly after bath application of KA (middle), and reappeared with a significantly higher amplitude after KA washout (right, p-SWR). Note the persistent gamma network oscillation after KA washout. (B) Example of the wavelet transform (color-coded power spectral density) for three consecutive highlighted SWRs (white trace) before (SWR) and after (p-SWR) intermediate gamma oscillations. (C) Gamma oscillation induces a significant SWR area increase. (D) Gamma oscillation-induced SWR area increase is significantly reduced by administration of AP5 (gray open bar), MPEP (black open bar), and MPEP+AP5 (black bar with gray filling). (E) Brief “weak” field gamma episodes were induced by bath application of 50 nM KA (left, top). After this test period, “conventional” gamma frequency oscillations were induced by 400 nM KA application (left middle), followed by KA washout achieving a complete cessation of oscillatory gamma activity. In a third step, the network behavior was tested again with the same low KA concentration as applied in the first step (left, bottom). The spectral analysis of the 1st and 2nd “weak” gamma oscillation reveals a strengthening effect of the intervening “conventional” gamma episode. (F), Summary bar charts of peak power (left) and frequency (right) obtained before (1st “weak” gamma) and after “conventional” gamma (2nd “weak” gamma). (Panels A–F are adapted from Zarnadze et al. (2016))
In parallel to this facilitating network effect, the excitability of CA3 PCs following gamma rhythms is also enhanced. In contrast to this excitatory neurons, the excitability of two types of perisomatic targeting inhibitory interneurons, PV-expressing and CCK-expressing basket cells, displayed opposing effects (Zarnadze et al. 2016). In particular, fast-spiking PV-expressing cells, mediating rapid inhibition and contributing to the precise timing of neuronal synchronization and emergence of network oscillation (Cobb et al. 1995; Gloveli et al. 2005a; Sohal et al. 2009; Schlingloff et al. 2014), exhibit enhanced activation, while regular firing CCK-expressing cells, mediating slower inhibition (Hefft and Jonas 2005; Daw et al. 2009), show reduced excitability (Zarnadze et al. 2016). In this cell type-specific, differential plasticity of the two major GABAergic interneuron types, in turn, may underlie enhanced network excitability and thus promote synaptic plasticity. These results also emphasize the pivotal role of network gamma oscillations as a tool to investigate synaptic plasticity in the network.
Cellular, Synaptic, and Axonal Mechanisms Involved in Oscillations
Intrinsic Properties
The voltage-gated ion channels strongly contribute to PC excitability. These channels influence intrinsic properties of the neuron, such as the action potential threshold, spike AHP and afterdepolarization (ADP), and action potential firing mode. Na+ and A-type K+ channels are expressed in both CA1 and CA3 PCs, whereas hyperpolarization-activated cation channels (HCN channels) are expressed in CA1 PCs but are almost absent from CA3 PCs (for review see Spruston (2008)). The HCN channels in PCs have important influences also on synaptic integration. Deactivation of these channels reduces EPSP duration and results in a slight hyperpolarization following EPSPs (Magee 1999). Conversely, activation of HCN channels reduces IPSP duration and produces a slight depolarization following the IPSP (Williams and Stuart 2003; for review see Spruston (2008)). This interaction of HCN channels and synaptic conductance may represent elementary mechanisms for rhythmogenesis at the cellular and subcellular levels.
Another feature relevant for the firing pattern of PCs in the hippocampus is their ability to generate subthreshold membrane potential oscillations (MPOs) in the theta frequency range and their resonance properties (Leung and Yu 1998; Pike et al. 2000). These properties of hippocampal PCs are likely to contribute to theta activity (Leung and Yu 1998). In hippocampal PCs the electrical resonance at theta frequencies is generated by M-current, h-current, and persistent Na+ current (Hu et al. 2002).
How the different firing patterns of certain GABAergic interneurons are generated remains largely unknown. Intrinsic membrane properties of these cells may be important for hippocampal network oscillations. For instance, O-LM cells have a longer membrane time constant and a considerably longer (five- to tenfold slower) AHP than the gamma-preferring interneurons (Gloveli et al. 2005a), restricting their firing to low, theta frequencies (see Savić et al. (2001)). In addition, O-LM cells show prominent slow subthreshold membrane potential oscillations and the resonance properties in the theta frequency range (Maccaferri and McBain 1996; Pike et al. 2000).
Hyperpolarization-activated cationic currents (Ih) and IA currents which have been detected in hippocampal interneurons may not only influence the intrinsic and firing properties but also their synchronization. Ih currents are activated at voltages close to rest (Gu et al. 2005). Different subunit composition (HCN1-4) that is coexpressed in hippocampal GABAergic interneurons (Notomi and Shigemoto 2004) influences not only the kinetics but also the voltage dependency of Ih activation [see Chen et al. (2002)]. Besides O-LM and other types of non-fast-spiking interneurons, Ih channels are expressed in the somato-dendritic region, axon, and presynaptic elements of fast-spiking basket cell in the hippocampus (Aponte et al. 2006). In contrast, hippocampal lacunosum-moleculare and radiatum interneurons display subthreshold MPOs generated by an interplay of Na+ and 4-AP-sensitive A-type K+ currents, independent of Ih currents and muscarine-sensitive K+ currents, IM (Bourdeau et al. 2007).
Synaptic Properties
The properties of excitatory events discriminate hippocampal principal cells from inhibitory neurons (Miles 1990; Jonas et al. 1993; Geiger et al. 1997; Toth et al. 2000). It appears that excitatory synapses onto interneurons not only tend to have a larger number of AMPA receptors (Nusser et al. 1998), thereby increasing the quantal amplitude, but the postsynaptic receptors also appear to have a different molecular composition (Geiger et al. 1995), which, in turn, endows them with faster kinetics (Geiger et al. 1997). Further discrimination in the properties of excitatory input was found between different interneuron types. Different classes of hippocampal interneurons with distinct axonal ramification patterns and efferent target profiles show clear differences in both amplitude and kinetics of EPSCs/Ps during gamma frequency network oscillations (Gloveli et al. 2005a). For instance, the amplitudes of excitatory drive are considerably larger in fast-spiking BCs and trilaminar cells than in O-LM cells (Fig. 2B), suggesting the intensity of synaptic drive may play a role in generating their different outputs (Gloveli et al. 2005a).
Similar to the kinetics of excitatory postsynaptic currents at PC-BC, unitary inhibitory postsynaptic currents at BC-BC synapses demonstrated very fast kinetics (Bartos et al. 2001, 2002). In addition to IPSCs with fast kinetic properties (GABAA,fast) mediated by perisomatic synapses, IPSCs with slowly rising and decaying kinetic (GABAA,slow) mediated by dendritic synapses were also detected in CA1 area (Banks et al. 2000). Interplay of CA1 interneurons, mediating GABAA,slow and GABAA,fast may contribute to theta and gamma rhythms occurring separately or as a nested gamma/theta rhythm (Banks et al. 2000). Furthermore, GABAB receptors contribute to the maintenance and modulation of gamma oscillations, as evidenced by the selective agonist acting at presynaptic receptors strengthening gamma power, while activation of pre- and postsynaptic receptors reduces gamma power (Dugladze et al. 2013). Theta oscillations are strongly inhibited by presynaptic GABAB receptor activation (Booker et al., unpublished observations). Further, it has been shown through modeling of PV BC networks that postsynaptic GABAB receptor activity on those interneurons has the potential to give rise to theta/gamma nesting (Booker et al. 2013). Finally, activation of GABAB receptors strongly suppresses the occurrence of sharp-wave ripples in CA3 (Hollnagel et al. 2014).
Thus, different intrinsic membrane properties together with different kinetics of excitatory and inhibitory inputs govern the specific roles of hippocampal cells in shaping distinct network oscillatory activity.
Axonal Properties
In central neurons, action potentials (APs) are generated close to the soma, at the axon initial segment (AIS), and propagate along the axon to downstream neurons. The impact of the PCs in network oscillations is typically evaluated from intrasomatic recordings. However, PC axonal activity may arise independently under certain conditions and itself undergo activity-dependent modifications which in turn may affect the network properties and activity. In line with this, a novel form of activity-dependent plasticity in a subclass of cortical interneurons was recently reported (Sheffield et al. 2011). During persistent firing (at beta/gamma frequencies of 20–40 Hz), APs are generated in the distal axon and persists for tens of seconds to minutes. The increased frequency of presynaptic firing as result of altered intrinsic properties of axons may enhance the reliability of signal transmission and affect the network properties [see Ganguly et al. (2000)]. Many different types of sodium and potassium channels, as well as calcium transients and hyperpolarization-activated inward currents have been described in axons [for review see Debanne et al. (2011) and Ruiz and Kullmann (2013)]. The complex time and voltage dependence resulting from the properties of ion channels can lead to activity-dependent changes in resting membrane properties, such as a membrane potential, lowering thereby the threshold of ectopic (axonal) spike initiation. Importantly, the changes in AP threshold were shown to be dependent on local protein synthesis and the long-term changes in axon excitability can occur in response to relatively brief changes in activity [see Bucher and Goaillard (2011)]. In addition, a growing number of studies show that the axon can express receptors to glutamate, GABA, acetylcholine, or biogenic amines, changing the relative contribution of some channels to axonal excitability [for review see Debanne et al. (2011), Bucher and Goaillard (2011), and Ruiz and Kullmann (2013)].
Recently developed techniques that allowed to record simultaneously from axons and soma of single PCs (Dugladze et al. 2012; Sasaki et al. 2012) revealed highly unexpected properties of the axon of CA3 PCs during gamma frequency oscillations (Dugladze et al. 2012, Fig. 6). In particular, it was found that (1) under physiological condition AP are initiated also in the distal part of CA3 PCs, (2) the frequency of APs in axons recorded >600 μm from the soma was four- to fivefold higher, and (3) dual somatic and axonal cell-attached recordings from individual cells demonstrate that distal axonal spikes achieve the proximal axons but fail to invade the soma (Dugladze et al. 2012).
High-frequency discharge of the axon but not the soma of hippocampal CA3 PCs during gamma frequency oscillations in vitro. (A) Scheme of dual somatic and axonal recording configuration. APs evoked in whole-cell configuration by brief depolarizing current injection into the soma (800 pA, inset) reliably induced ACs in the axon, confirming that recordings are made from two compartments of the same cell. (B) Dual somatic and axonal cell-attached recoding directly demonstrate that high-frequency axonal spikes (“axon”) fail to invade the soma (“soma”) during gamma frequency oscillations (“LFP”). (C) Summary plot shows the highly significant difference in the discharge frequency observed at the soma and proximal axon in dual recordings (n = 9 cells) during network oscillations. (Panels A–C are adapted from Dugladze et al. (2012))
These findings have wider implication for the understanding of signal processing in the hippocampus. First, they provide direct and unequivocal evidence that APs could be initiated independently in axonal and somato-dendritic compartments of PCs during network oscillations. Second, the axonal spikes are generated at high frequency in the active network, and these axonal properties may participate in experimentally observed high-frequency excitatory input to both PCs and interneurons in the active network (Gloveli et al. 2005a). Finally, hippocampal principal cells require an additional mechanism to efficiently control the backpropagation of AP to the somato-dendritic compartments. Indeed, it was found that inhibition at the AIS caused by PV-positive axo-axonic cells underlies the suppression of ectopic AP invasion to the parent PC soma. In particular, shunting inhibition rather than membrane potential hyperpolarization prevents the backpropagation of ectopic APs across the AIS (Dugladze et al. 2012).
Neuromodulatory Control of In Vitro Oscillations
Sources of neuromodulators in the brain are the four aminergic systems: the dopaminergic, histaminergic, serotonergic, and noradrenergic systems. All four of the associated modulators (dopamine, histamine, serotonin, and noradrenalin) are released from small groups of neurons, which have projection patterns to most of the brain, including the hippocampus. Effects of these neuromodulators have been tested in vitro on theta and gamma oscillatory activity.
The hippocampus receives dopaminergic input from the ventral tegmental area. Activation of D1-like dopamine receptors strongly depresses cholinergic gamma oscillations in area CA3 of rat hippocampus, and this effect is most likely mediated via impairment of interneurons involved in generation and maintenance of the carbachol-induced network rhythm (Weiss et al. 2003). Conversely, D4 receptor activation strongly enhances gamma oscillation power (Andersson et al. 2012).
Histamine 3 (H3) receptors seems likely to play an important role in regulation of hippocampal theta oscillation. Systemic administration of the H3 receptor antagonists (ciproxifan and thioperamide) enhances the power of spontaneous theta in anesthetized rats. Since H3 receptors are located at axon terminals of histamine-containing neurons and function as autoreceptors (Arrang et al. 1983), their blockade could enhance histamine release and subsequently promote hippocampal theta oscillation. Regulation of hippocampal theta oscillations by H3 receptors may represent one of the probable mechanisms involved in histamine-induced modulation of higher brain functions, such as attention and learning (Hajós et al. 2008).
Serotonergic neurons of the midbrain raphe have been implicated in the control of affective and cognitive functions and in modulating the neural activities of networks across the sleep–wake cycle. The midbrain raphe nuclei form a strong serotonergic projection to the hippocampus. Recent in vivo finding suggests that a subpopulation of raphe neurons discharged action potentials that were phase-locked to the hippocampal theta rhythm (Kocsis et al. 2006). Hippocampal PCs and interneurons show different expression of metabotropic 5-HT receptor subtypes, such as 5-HT1A, 5-HT1B, and 5-HT2 (Ropert and Guy 1991; Schmitz et al. 1995; Shen and Andrade 1998), which may result in a differential modulation of intrinsic and synaptic properties of these cells in response to serotonin [for review see Schmitz et al. (1998)]. Serotonin input may influence the hippocampal network also via ionotropic 5-HT3 receptors, which are expressed by several classes of GABAergic interneurons (Tecott et al. 1993; Ropert and Guy 1991; Morales et al. 1998). These cells include CCK-containing basket cells, an interneuron type that has been proposed to hamper the gamma rhythm (see section “Firing Patterns in Gamma Oscillations,” Freund and Katona 2007; Galarreta et al. 2008), as well as calbindin- and calretinin-containing GABAergic cells (Morales and Bloom 1997). In contrast, serotonergic fibers do not contact the PV-containing GABAergic basket cells, which are responsible for some gamma frequency oscillations (see section “Firing Patterns in Gamma Oscillations”). Therefore, the rhythmic serotonergic input may modulate, but not drive, hippocampal network oscillations at gamma frequency range.
The brain noradrenergic (NE) neurons, located in the pontine nucleus of locus coeruleus (LC), are presumed to play a role in regulation of the circadian sleep-wake cycle and alertness (Aston-Jones and Cohen 2005). Several experimental findings suggest involvement of this neuromodulators on the hippocampal network activity. Local injection of glutamate in the LC results in multiple actions on the hippocampus, which include an increase in theta rhythm (Brown et al. 2005). Activation of LC-NE neurons by local application of a cholinergic agonist (bethanechol) induces theta oscillation of MS/DB neurons and theta-wave oscillation of hippocampal EEG in anesthetized rats (Berridge and Foote 1991). Furthermore, the selective NE reuptake inhibitor reboxetine modulates hippocampal theta activity in a state-dependent manner, i.e., can either increase or decrease theta amplitude depending on the behavioral state of the animal (Kocsis et al. 2007).
Pharmacological agents which are used with in vitro models of oscillation, such as KAR, mGluR, and mAChR agonist, also have a direct modulatory effect on hippocampal neurons in a manner that is remarkably cell specific. Various types of interneurons express KARs (Cossart et al. 1998, 2002; Frerking et al. 1998; Mulle et al. 2000; Lerma 2003). Kinetics of KA-mediated EPSCs is slower than that of AMPAR (Frerking et al. 1998; Cossart et al. 2002), which could enable these two receptor types to generate oscillations with different dominating frequencies (Frerking and Ohliger-Frerking 2002). Consistent with this, O-LM interneurons, which receive a large input mediated by KARs (Cossart et al. 2002), show postsynaptic KAR-mediated action potential firing at 10 Hz during theta stimulations, in contrast to perisomatic, bistratified, or septum/back-projecting cells (Goldin et al. 2007). Activation of mGluRs in the hippocampus has a range of effects (Anwyl 1999) which include decreases in Im and IAHP currents. Therefore, activation of these receptors increases the excitability of hippocampal cells. mGluRs subtypes are differentially expressed in specific hippocampal interneurons resulting in their different responsiveness to agonists. Thus, O-LM cells express a large number of group I mGluRs and are very sensitive to agonists, in marked contrast to other stratum oriens interneurons, including basket cells that express only small number of this receptor subtype and are less sensitive (van Hooft et al. 2000). Also mAChR agonists may act on different GABAergic inhibitory interneurons that possess the muscarinic receptors (Pitler and Alger 1992). Activation of these receptors may increase the excitability of interneuronal activity. In particular, muscarinic receptor agonist-carbachol blocks several potassium conductances, including IAHP and IM in a concentration-dependent manner (Madison et al. 1987). This depolarizes the PCs, unmasking subthreshold membrane potential oscillations in the theta frequency range (Leung and Yim 1986; Fellous and Sejnowski 2000). In addition, muscarinic receptor activation consistently enhanced firing frequency and produced large, sustained ADPs of O-LM but not other stratum oriens interneurons (Lawrence et al. 2006).
In summary, the effects of state-dependent activation of different neuromodulators can be markedly different on hippocampal network activity and depend on the expression and distribution of receptors across the cellular components of the network.
Oscillations in Disease
Schizophrenia
A number of studies have shown changes in gamma frequency EEG activity in schizophrenia. Reduced gamma activity was found in stimulus-dependent responses in the auditory and visual cortices of schizophrenic patients [for review see Kehrer et al. (2008)], and there is also evidence for a change in neuronal synchrony during high-frequency oscillations (Spencer et al. 2004). Interestingly, the amounts of RNA and immunoreactivity for PV are reduced in postmortem tissue from the frontal cortex and the hippocampus pointing to a reduction in perisomatic inhibitory interneuron population (Zhang and Reynolds 2002). The loss of these interneurons could directly explain the observed changes in gamma oscillations (Lewis et al. 2005; Vierling-Claassen et al. 2008). Although most clinical studies have found reductions in gamma band activity in schizophrenic patients [e.g., Slewa-Younan et al. (2001)], there appears to be a symptom-specific pattern in the alterations in gamma activity indicating that increases in amplitude and power are associated with positive symptoms, particularly hallucinations and reality distortions, whereas negative symptoms such as psychomotoric deficits are linked to decreased gamma activity (Baldeweg et al. 1998; Bucci et al. 2007). Similar to clinical observations, in in vitro NMDA-hypofunction models of schizophrenia, both increased (Kehrer et al. 2007; Pinault 2008) and decreased gamma activities (Cunningham et al. 2006) have been demonstrated [for review see Kehrer et al. (2008) and Roopun et al. (2008)]. Recently it been shown that the acute effects of NMDA-hypofunction result in increased gamma but decreased theta, while chronic administration of NMDA receptor inhibitors results in both decreased gamma and theta oscillation power (Kittelberger et al. 2012).
Alterations of cortical interneurons in schizophrenia, especially parvalbumin- and somatostatin-containing interneurons, are well documented. These alterations are likely to have significant effects on the network oscillatory activity and therefore on cognitive processes (Gonzalez-Burgos and Lewis 2008; Morris et al. 2008). The axo-axonic subclass of GABAergic interneurons containing the calcium-binding protein parvalbumin have attracted the most scrutiny in studies of schizophrenia (Howard Behrens et al. 2007; Sakai et al. 2008; Wang et al. 2008). Although the altered network activities were reported in vivo and in vitro [see, e.g., Cunningham et al. (2006); Behrens et al. (2007); Braun et al. (2007); Gonzalez-Burgos and Lewis (2008), and Spencer (2008)], further investigation needs to be undertaken to address possible model- and region-specific alterations in the gamma network oscillatory activity in different animal models of schizophrenia. Establishing the contingencies of increased versus decreased gamma band activity is of high importance since aberrant network oscillatory activity may underlie the cognitive decline observed in schizophrenic patients and offer vital clues to the relationship between positive and negative symptoms in schizophrenia at a network levels (Cho et al. 2006; Bucci et al. 2007; Ford et al. 2007).
Mesial Temporal Lobe Epilepsy (mTLE)
Epileptic seizures are less frequent in conditions during which theta frequency occurs (e.g., wakefulness or REM sleep, Montplaisir et al. 1987), and thus the theta rhythm appears to indicate a hippocampal functional state in which generation of seizures is hindered (Colom et al. 2006).
In epileptic mice, the power of low-frequency theta oscillations has been reported to be reduced (Arabadzisz et al. 2005; Dugladze et al. 2007). Apart from suppression of the theta oscillatory activity, as a potential anticonvulsant factor (Colom et al. 2006), a strong enhancement of the gamma activity (Dugladze et al. 2007) may underlie emergence of epileptiform activity. Observations in humans support this scenario: spatially localized increase in the power of gamma frequency oscillations have been observed preceding seizures in human TLE patients (Fisher et al. 1992). In addition, gamma oscillatory activity and increases in firing rate of the interneuronal network have been suggested as mechanisms of seizure occurrence in patients with drug-resistant TLE (Bragin et al. 2007). Consistent with this suggestion, intracellular recordings in kainate model of mTLE reveal an increased firing frequency of both PCs and dendrite-inhibiting interneurons in the ventral hippocampal CA3 area of epileptic mice (Dugladze et al. 2007). The involvement of the perisomatic targeting interneurons remains, however, to be investigated. In fact, recent evidence suggests that GABAB receptor-mediated presynaptic control of the inhibitory output of parvalbumin- and CCK-expressing basket cells are differentially affected (Dugladze et al. 2013). A cell type-specific upregulation of presynaptic GABAB receptor expression and consequently enhanced presynaptic inhibition in CCK basket cells promotes aberrant high-frequency oscillations and hyperexcitability in hippocampal networks of chronic epileptic mice (Dugladze et al. 2013).
The epileptic tissue may also generate a specific rhythm, transient high-frequency oscillations (HFOs) in the 200–600 Hz frequency band known as fast ripple (Bragin et al. 1999). In fact, fast ripples may represent a specific marker for the area of the brain in which seizures begin [Bragin et al. 2002; for review see Jacobs et al. (2012) and Frauscher et al. (2017)]. The frequency of these oscillations is about twice as fast as the maximum rate at which most neurons in the hippocampus can fire action potentials. This fact raises the question how these oscillations are generated. Analysis of the firing properties of hippocampal neurons during HFOs in vitro low-Mg2+ model of epileptiform activity showed that the PCs fired at the rising phase of the highest frequency portion of the field oscillation. In addition, distal dendrite-targeting interneurons (R-LM cells) fired at the start of the epileptiform bursts (on average 140 Hz) but stopped firing before its end (Spampanato and Mody 2007). However, neither the principal cells nor the distal dendrite-targeting interneurons (R-LM and O-LM cell) fired action potentials at high frequencies (200–600 Hz) seen in the field oscillations (Spampanato and Mody 2007). Another study (Foffani et al. 2007) suggested that these synchronous population oscillations could be generated as a consequence of the out-of-phase activities of two independent oscillators, each operating at half the frequency of the ensemble. In line with this suggestion, it was found that hippocampal PCs fired short bursts of action potentials at frequencies up to 300 Hz (Kandel and Spencer 1961), and some interneurons could sustain frequencies of 400 Hz (Foffani et al. 2007). It is currently hypothesized that the temporary uncontrolled firing of principal cells due to a brief functional collapse of perisomatic inhibition generates fast ripples (Gulyás and Freund 2015).
Thus, numerous studies suggest that alterations in inhibition-based network oscillations may underlie the pathophysiology in schizophrenia and mTLE, which are associated with impaired information processes.
Perspectives
The spatiotemporal patterns of activity during network oscillations would ideally be explored in vivo (Bragin et al. 1995; Penttonen et al. 1998; Csicsvari et al. 2003; Buzsáki et al. 2003). However, it is also reasonable to use relevant in vitro models to test hypotheses for the basic mechanisms involved. The replication of an endogenous brain pattern in vitro allows for the investigation of a number of important cellular and synaptic mechanisms that are difficult or even impossible to explore in vivo. In addition, using transgenic, fluorescent EGFP-expressing mice under the control of different gene promoters (Oliva et al. 2000; Meyer et al. 2002) enables the identification and selection of different interneurons in the acute slice preparation. The firing properties of single cells in the active network, and the contribution of excitation and inhibition to the generation of network oscillatory activity, can be systematically examined in different models of in vitro oscillations (Table 1), which may reflect region and state dependence of mechanisms underlying network oscillations in vivo. Although there are some differences between the data obtained from in vitro and in vivo observation, these observations show substantial homology. The development of new transgenic methods for activating, inactivating, and labeling neurons and synapses (Marek and Davis 2003; Polleux 2005) has led to important new insights and will certainly further facilitate progress in this area. Furthermore, several in vitro methods may help to overcome the limitations in vivo. These include simultaneous patch-clamp recording from several cells in a brain slice (Miles and Poncer 1996; Markram et al. 1997; Peng et al. 2017), recording of sufficiently large numbers of cells at once using optical methods, and stimulation/suppression of different cellular compartments using uncaging of different substance or optically activated channels (Callaway 2002; Boyden et al. 2005; Deisseroth et al. 2006; Zhang et al. 2007; Deisseroth and Hegemann 2017).
References
Amilhon B, Huh CY, Manseau F, Ducharme G, Nichol H, Adamantidis A, Williams S (2015) Parvalbumin interneurons of hippocampus tune population activity at theta frequency. Neuron 5:1277–1289
Andersson RH, Johnston A, Herman PA, Winzer-Serhan UH, Karavanova I, Vullhorst D, Fisahn A, Buonanno A (2012) Neuregulin and dopamine modulation of hippocampal gamma oscillations is dependent on dopamine D4 receptors. Proc Natl Acad Sci U S A 109:13118–13123
Anwyl R (1999) Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Rev 29:83–120
Aponte Y, Lien CC, Reisinger E, Jonas P (2006) Hyperpolarization-activated cation channels in fast-spiking interneurons of rat hippocampus. J Physiol 574:229–243
Arabadzisz D, Antal K, Parpan F, Emri Z, Fritschy JM (2005) Epileptogenesis and chronic seizures in a mouse model of temporal lobe epilepsy are associated with distinct EEG patterns and selective neurochemical alterations in the contralateral hippocampus. Exp Neurol 194:76–90
Arnolds DE, Lopes da Silva FH, Aitink JW, Kamp A, Boeijinga P (1980) The spectral properties of hippocampal EEG related to behaviour in man. Electroencephalogr Clin Neurophysiol 50:324–328
Arrang JM, Garbarg M, Schwartz JC (1983) Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature (Lond) 302:832–837
Aston-Jones G, Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28:403–450
Baldeweg T, Spence S, Hirsch SR, Gruzelier J (1998) Gamma-band electroencephalographic oscillations in a patient with somatic hallucinations. Lancet 352:620–621
Banks MI, White JA, Pearce RA (2000) Interactions between distinct GABA(A) circuits in hippocampus. Neuron 25:449–457
Bartos M, Vida I, Frotscher M, Geiger JR, Jonas P (2001) Rapid signaling at inhibitory synapses in a dentate gyrus interneuron network. J Neurosci 21:2687–2698
Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger JR, Jonas P (2002) Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Natl Acad Sci U S A 99:13222–13227
Behrens CJ, van den Boom LP, de Hoz L, Friedman A, Heinemann U (2005) Induction of sharp wave-ripple complexes in vitro and reorganization of hippocampal networks. Nat Neurosci 8:1560–1567
Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL (2007) Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science 318:1645–1647
Berridge CW, Foote SL (1991) Effects of locus coeruleus activation on electroencephalographic activity in neocortex and hippocampus. J Neurosci 11:3135–3145
Bland BH (1986) Physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol 26:1–54
Booker SA, Vida I (2018) Morphological diversity and connectivity of hippocampal interneurons. Cell Tissue Res 373:619–641
Booker SA, Gross A, Althof D, Shigemoto R, Bettler B, Frotscher M, Hearing M, Wickman K, Watanabe M, Kulik Á, Vida I (2013) Differential GABAB-receptor-mediated effects in perisomatic-and dendrite-targeting parvalbumin interneurons. J Neurosci 18:7961–7974
Both M, Bähner F, von Bohlen und Halbach O, Draguhn A (2008) Propagation of specific network patterns through the mouse hippocampus. Hippocampus 18:899–908
Bourdeau ML, Morin F, Laurent CE, Azzi M, Lacaille JC (2007) Kv4.3-mediated A-type K+ currents underlie rhythmic activity in hippocampal interneurons. J Neurosci 27:1942–1953
Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K (2005) Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8:1263–1268
Bragin A, Jandó G, Nádasdy Z, Hetke J, Wise K, Buzsáki G (1995) Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci 15:47–60
Bragin A, Engel J Jr, Wilson CL, Fried I, Mathern GW (1999) Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid--treated rats with chronic seizures. Epilepsia 40:127–137
Bragin A, Mody I, Wilson CL, Engel J Jr (2002) Local generation of fast ripples in epileptic brain. J Neurosci 22:2012–2021
Bragin A, Claeys P, Vonck K, Van Roost D, Wilson C, Boon P, Engel J Jr (2007) Analysis of initial slow waves (ISWs) at the seizure onset in patients with drug resistant temporal lobe epilepsy. Epilepsia 48:1883–1894
Braun I, Genius J, Grunze H, Bender A, Möller HJ, Rujescu D (2007) Alterations of hippocampal and prefrontal GABAergic interneurons in an animal model of psychosis induced by NMDA receptor antagonism. Schizophr Res 97:254–263
Brown RA, Walling SG, Milway JS, Harley CW (2005) Locus ceruleus activation suppresses feedforward interneurons and reduces β-γ electroencephalogram frequencies while it enhances theta frequencies in rat dentate gyrus. J Neurosci 25:1985–1991
Bucci P, Mucci A, Merlotti E, Volpe U, Galderisi S (2007) Induced gamma activity and event-related coherence in schizophrenia. Clin EEG Neurosci 38:96–104
Bucher D, Goaillard JM (2011) Beyond faithful conduction: short-term dynamics, neuromodulation, and long-term regulation of spike propagation in the axon. Prog Neurobiol 94:307–346
Buhl EH, Halasy K, Somogyi P (1994) Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature 368:823–828
Buhl EH, Tamás G, Fisahn A (1998) Cholinergic activation and tonic excitation induce persistent gamma oscillations in mouse somatosensory cortex in vitro. J Physiol 513:117–126
Buzsáki G (1989) Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience 31:551–570
Buzsáki G (2002) Theta oscillations in the hippocampus. Neuron 33:325–340
Buzsáki G, Buhl DL, Harris KD, Csicsvari J, Czéh B, Morozov A (2003) Hippocampal network patterns of activity in the mouse. Neuroscience 116:201–211
Buzsáki G, Draguhn A (2004) Neuronal oscillations in cortical networks. Science 304:1926–1929
Buzsáki G, Leung LW, Vanderwolf CH (1983) Cellular bases of hippocampal EEG in the behaving rat. Brain Res 287:139–171
Buzsáki G, Czopf J, Kondakor J, Kellenyi L (1986) Laminar distribution of hippocampal rhythmic slow activity (RSA) in the behaving rat; current source density analysis, effects of urethane and atropine. Brain Res 365:125–137
Buzsáki G, Horvath Z, Urioste R, Hetke J, Wise K (1992) High-frequency network oscillation in the hippocampus. Science 256:1025–1027
Callaway EM (2002) Cell type specificity of local cortical connections. J Neurocytol 31:231–237
Carr MF, Karlsson MP, Frank LM (2012) Transient slow gamma synchrony underlies hippocampal memory replay. Neuron 75:700–713
Chen K, Aradi I, Santhakumar V, Soltesz I (2002) H-channels in epilepsy: new targets for seizure control? Trends Pharmacol Sci 23:552–557
Chevaleyre V, Siegelbaum SA (2010) Strong CA2 pyramidal neuron synapses define a powerful disynaptic cortico-hippocampal loop. Neuron 66:560–572
Chittajallu R, Craig MT, McFarland A, Yuan X, Gerfen S, Tricoire L, Erkkila B, Barron SC, Lopez CM, Liang BJ, Jeffries BW, Pelkey KA, McBain CJ (2013) Dual origins of functionally distinct O-LM interneurons revealed by differential 5-HT 3A R expression. Nat Neurosci 11:1598–1607
Cho RY, Konecky RO, Carter CS (2006) Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A 103:19878–19883
Chrobak JJ, Buzsáki G (1996) High-frequency oscillations in the output networks of the hippocampal-entorhinal axis of the freely behaving rat. J Neurosci 16:3056–3066
Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P (1995) Synchronisation of neuronal activity in hippocampus by individual GABAergic interneurons. Nature 378:75–78
Cobb SR, Butlers DO, Davies CH (2000) Coincident activation of mGluRs and MachRs imposes theta frequency patterning on synchronised network activity in the hippocampal CA3 region. Neuropharmacology 23:1933–1942
Colom LV, Garcia-Hernandez A, Castaneda MT, Perez-Cordova MG, Garrido-Sanabria ER (2006) Septo-hippocampal networks in chronically epileptic rats: potential antiepileptic effects of theta rhythm generation. J Neurophysiol 95:3645–3653
Cossart R, Esclapez M, Hirsch JC, Bernard C, Ben-Ari Y (1998) GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nat Neurosci 1:470–478
Cossart R, Epsztein J, Tyzio R, Becq H, Hirsch J, Ben-Ari Y, Crépel V (2002) Quantal release of glutamate generates pure kainate and mixed AMPA/kainate EPSCs in hippocampal neurons. Neuron 35:147–159
Craig MT, McBain CJ (2015) Fast gamma oscillations are generated intrinsically in CA1 without the involvement of fast-spiking basket cells. J Neurosci 35:3616–3624
Csicsvari J, Hirase H, Czurko´ A, Mamiya A, Buzsáki G (1999) Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J Neurosci 19:274–287
Csicsvari J, Hirase H, Mamiya A, Buzsáki G (2000) Ensemble patterns of hippocampal CA3-CA1 neurons during sharp wave-associated population events. Neuron 28:585–594
Csicsvari J, Jamieson B, Wise KD, Buzsáki G (2003) Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron 37:311–322
Cunningham MO, Hunt J, Middleton S, FE LB, Gillies MJ, Davies CH, Maycox PR, Whittington MA, Racca C (2006) Region-specific reduction in entorhinal gamma oscillations and parvalbumin-immunoreactive neurons in animal models of psychiatric illness. J Neurosci 26:2767–2776
Daw MI, Tricoire L, Erdelyi F, Daw MI, Tricoire L, Erdelyi F, Szabo G, McBain CJ (2009) Asynchronous transmitter release from cholecystokinin-containing inhibitory interneurons is widespread and target-cell independent. J Neurosci 29:11112–11122
Debanne D, Campanac E, Bialowas A, Carlier E, Alcaraz G (2011) Axon physiology. Physiol Rev 91:555–602
Deisseroth K, Hegemann P (2017) The form and function of channelrhodopsin. Science 357:eaan5544
Deisseroth K, Feng G, Majewska AK, Miesenböck G, Ting A, Schnitzer MJ (2006) Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci 26:10380–10386
Dodt HU, Zieglgänsberger W (1994) Infrared videomicroscopy: a new look at neuronal structure and function. Trends Neurosci 17:453–458
Draguhn A, Traub RD, Schmitz D, Jefferys JG (1998) Electrical coupling underlies high-frequency oscillations in the hippocampus in vitro. Nature 394:189–192
Dugladze T, Vida I, Tort AB, Gross A, Otahal J, Heinemann U, Kopell NJ, Gloveli T (2007) Impaired hippocampal rhythmogenesis in a mouse model of mesial temporal lobe epilepsy. Proc Natl Acad Sci U S A 104:17530–17535
Dugladze T, Schmitz D, Whittington MA, Vida I, Gloveli T (2012) Segregation of axonal and somatic activity during fast network oscillations. Science 336:1458–1461
Dugladze T, Maziashvili N, Börgers C, Gurgenidze S, Häussler U, Winkelmann A, Haas CA, Meier JC, Vida I, Kopell NJ (2013) GABAB autoreceptor-mediated cell type-specific reduction of inhibition in epileptic mice. Proc Natl Acad Sci U S A 110(37):15073–15078
Eller J, Zarnadze S, Bäuerle P, Dugladze T, Gloveli T (2015) Cell type-specific separation of subicular principal neurons during network activities. PLoS One 10(4):e0123636
Fellous JM, Sejnowski TJ (2000) Cholinergic induction of oscillations in the hippocampal slice in the slow bands (0.5–2 Hz), theta (5–12 Hz), and gamma (35–70 Hz). Hippocampus 10:187–197
Ferguson KA, Huh CY, Amilhon B, Williams S, Skinner FK (2013) Experimentally constrained CA1 fast-firing parvalbumin-positive interneuron network models exhibit sharp transitions into coherent high frequency rhythms. Front Comput Neurosci 7:144
Fisahn A, Pike FG, Buhl EH, Paulsen O (1998) Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature 394:186–189
Fisahn A, Yamada M, Duttaroy A, Gan JW, Deng CX, McBain CJ, Wess J (2002) Muscarinic induction of hippocampal gamma oscillations requires coupling of the M1 receptor to two mixed cation currents. Neuron 33:615–624
Fisahn A, Contractor A, Traub RD, Buhl EH, Heinemann SF, McBain CJ (2004) Distinct roles for the kainate receptor subunits GluR5 and GluR6 in kainate-induced hippocampal gamma oscillations. J Neurosci 24:9658–9668
Fischer Y, Gähwiler BH, Thompson SM (1999) Activation of intrinsic hippocampal theta oscillations by acetylcholine in rat septo-hippocampal cocultures. J Physiol 519:405–413
Fisher RS, Webber WR, Lesser RP, Arroyo S, Uematsu S (1992) High-frequency EEG activity at the start of seizures. J Clin Neurophysiol 9:441–448
Foffani G, Uzcategui YG, Gal B, Menendez de la Prida L (2007) Reduced spike-timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron 55:930–941
Ford JM, Krystal JH, Mathalon DH (2007) Neural synchrony in schizophrenia: from networks to new treatments. Schizophr Bull 33:848–852
Frauscher B, Bartolomei F, Kobayashi K, Cimbalnik J, van ‘t Klooster MA, Rampp S, Otsubo H, Höller Y, Wu JY, Asano E, Engel J Jr, Kahane P, Jacobs J, Gotman J (2017) High-frequency oscillations: The state of clinical research. Epilepsia 58:1316–1329
Frerking M, Ohliger-Frerking P (2002) AMPA receptors and kainate receptors encode different features of afferent activity. J Neurosci 22:7434–7443
Frerking M, Malenka RC, Nicoll RA (1998) Synaptic activation of kainate receptors on hippocampal interneurons. Nat Neurosci 1:479–486
Freund TF, Buzsáki G (1996) Interneurons of the hippocampus. Hippocampus 6:347–470
Freund TF, Katona I (2007) Perisomatic inhibition. Neuron 56:33–42
Fuentealba P, Begum R, Capogna M, Jinno S, Márton LF, Csicsvari J, Thomson A, Somogyi P, Klausberger T (2008) Ivy cells: a population of nitric-oxide-producing, slow-spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron 57:917–929
Galarreta M, Erdélyi F, Szabó G, Hestrin S (2008) Cannabinoid sensitivity and synaptic properties of 2 GABAergic networks in the neocortex. Cereb Cortex 18:2296–2305
Ganguly K, Kiss L, Poo M (2000) Enhancement of presynaptic neuronal excitability by correlated presynaptic and postsynaptic spiking. Nat Neurosci 3:1018–1026
Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H (1995) Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 15:193–204
Geiger JR, Lübke J, Roth A, Frotscher M, Jonas P (1997) Submillisecond AMPA receptor-mediated signaling at a principal neuron-interneuron synapse. Neuron 18:1009–1023
Giepmans BN, Adams SR, Ellisman MH, Tsien RY (2006) The fluorescent toolbox for assessing protein location and function. Science 312:217–224
Gillies MJ, Traub RD, LeBeau FE, Davies CH, Gloveli T, Buhl EH, Whittington MA (2002) A model of atropine-resistant theta oscillations in rat hippocampal area CA1. J Physiol (Lond) 543:779–793
Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB (2009) Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci 12:1222–1223
Gloveli T, Dugladze T, Saha S, Monyer H, Heinemann U, Traub RD, Whittington MA, Buhl EH (2005a) Differential involvement of oriens/pyramidale interneurones in hippocampal network oscillations in vitro. J Physiol 562:131–147
Gloveli T, Dugladze T, Rotstein HG, Traub RD, Monyer H, Heinemann U, Whittington MA, Kopell NJ (2005b) Orthogonal arrangement of rhythm-generating microcircuits in the hippocampus. Proc Natl Acad Sci U S A 102:13295–13300
Goldin M, Epsztein J, Jorquera I, Represa A, Ben-Ari Y, Crépel V, Cossart R (2007) Synaptic kainate receptors tune oriens-lacunosum moleculare interneurons to operate at theta frequency. J Neurosci 27:9560–9572
Gonzalez-Burgos G, Lewis DA (2008) GABA Neurons and the Mechanisms of Network Oscillations: Implications for Understanding Cortical Dysfunction in Schizophrenia. Schizophr Bull 34:944–961
Goutagny R, Jackson J, Williams S (2009) Self-generated theta oscillations in the hippocampus. Nat Neurosci 12:1491–1493
Gu N, Vervaeke K, Hu H, Storm JF (2005) Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J Physiol 566:689–715
Gulyás AI, Freund TT (2015) Generation of physiological and pathological highfrequency oscillations: the role of perisomatic inhibition in sharp-wave rippleand interictal spike generation. Curr Opin Neurobiol 31:26–32
Gulyás AI, Szabó GG, Ulbert I, Holderith N, Monyer H, Erdélyi F, Szabó G, Freund TF, Hájos N (2010) Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci 30:15134–15145
Hájos N, Pálhalmi J, Mann EO, Németh B, Paulsen O, Freund TF (2004) Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci 24:9127–9137
Hajós M, Siok CJ, Hoffmann WE, Li S, Kocsis B (2008) Modulation of hippocampal theta oscillation by histamine H3 receptors. J Pharmacol Exp Ther 324:391–398
Hájos N, Ellender TJ, Zemankovics R, Mann EO, Exley R, Cragg SJ, Freund TF, Paulsen O (2009) Maintaining network activity in submerged hippocampal slices: importance of oxygen supply. Eur J Neurosci 29:319–327
Halasy K, Buhl EH, Lörinczi Z, Tamás G, Somogyi P (1996) Synaptic target selectivity and input of GABAergic basket and bistratified interneurons in the CA1 area of the rat hippocampus. Hippocampus 6:306–329
Hefft S, Jonas P (2005) Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci 8:1319–1328
Hollnagel JO, Maslarova A, Haq RU, Heinemann U (2014) GABAB receptor dependent modulation of sharp wave-ripple complexes in the rat hippocampus in vitro. Neurosci Lett 574:15–20
Hu H, Vervaeke K, Storm JF (2002) Two forms of electrical resonance at theta frequencies, generated by M-current, h-current and persistent Na+ current in rat hippocampal pyramidal cells. J Physiol 545:783–805
Huh CY, Amilhon B, Ferguson KA, Manseau F, Torres-Platas SG, Peach JP, Scodras S, Mechawar N, Skinner FK, Williams S (2016) Excitatory inputs determine phase-locking strength and spike-timing of CA1 stratum oriens/alveus parvalbumin and somatostatin interneurons during intrinsically generated hippocampal theta rhythm. J Neurosci 36:6605–6622
Jackson J, Amilhon B, Goutagny R, Bott J-B, Manseau F, Kortleven C, Bressler SL, Williams S (2014) Reversal of theta rhythm flow through intact hippocampal circuits. Nat Neurosci 17:1362–1370
Jacobs J, Staba R, Asano E, Otsubo H, Wu JY, Zijlmans M, Mohamed I, Kahane P, Dubeau F, Navarro V, Gotman J (2012) High-frequency oscillations (HFOs) in clinical epilepsy. Prog Neurobiol 98:302–315
Jadhav SP, Kemere C, German PW, Frank LM (2012) Awake hippocampal sharp-wave ripples support spatial memory. Science 336:1454–1458
Jonas P, Major G, Sakmann B (1993) Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J Physiol 472:615–663
Jouvet M (1969) Biogenic amines and the states of sleep. Science 163:32–41
Jutras MJ, Buffalo EA (2010) Synchronous neural activity and memory formation. Curr Opin Neurobiol 20:150–155
Kandel ER, Spencer WA (1961) Electrophysiology of hippocampal neurons. II. After-potentials and repetitive firing. J Neurophysiol 24:243–259
Kehrer C, Dugladze T, Maziashvili N, Wójtowicz A, Schmitz D, Heinemann U, Gloveli T (2007) Increased inhibitory input to CA1 pyramidal cells alters hippocampal gamma frequency oscillations in the MK-801 model of acute psychosis. Neurobiol Dis 25:545–552
Kehrer C, Maziashvili N, Dugladze T, Gloveli T (2008) Altered excitatory-inhibitory balance in the NMDA-hypofunction model of schizophrenia. Front Mol Neurosci 1:6
Kittelberger K, Hur EE, Sazegar S, Keshavan V, Kocsis B (2012) Comparison of the effects of acute and chronic administration of ketamine on hippocampal oscillations: relevance for the NMDA receptor hypofunction model of schizophrenia. Brain Struct Funct 217:395–409
Klausberger T, Somogyi P (2008) Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321:53–57
Klausberger T, Magill PJ, Márton LF, Roberts JD, Cobden PM, Buzsáki G, Somogyi P (2003) Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature 421:844–848
Klausberger T, Márton LF, Baude A, Roberts JD, Magill PJ, Somogyi P (2004) Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat Neurosci 7:41–47
Kocsis B, Varga V, Dahan L, Sik A (2006) Serotonergic neuron diversity: identification of raphe neurons with discharges time-locked to the hippocampal theta rhythm. Proc Natl Acad Sci U S A 103:1059–1064
Kocsis B, Li S, Hajos M (2007) Behavior-dependent modulation of hippocampal EEG activity by the selective norepinephrine reuptake inhibitor reboxetine in rats. Hippocampus 17:627–633
Konopacki J, Gołebiewski H, Eckersdorf B (1992) Carbachol-induced rhythmic slow activity (theta) in cat hippocampal formation slices. Brain Res 578:13–16
Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H (2010) NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron 68:557–569
Lawrence JJ, Statland JM, Grinspan ZM, McBain CJ (2006) Cell type-specific dependence of muscarinic signalling in mouse hippocampal stratum oriens interneurones. J Physiol 570:595–610
LeBeau FEN, Towers SK, Traub RD, Whittington MA, Buhl EH (2002) Fast network oscillations induced by potassium transients in the rat hippocampus in vitro. J Physiol 542:167–179
LeBeau FE, Traub RD, Monyer H, Whittington MA, Buhl EH (2003) The role of electrical signaling via gap junctions in the generation of fast network oscillations. Brain Res Bull 62:3–13
Lerma J (2003) Roles and rules of kainate receptors in synaptic transmission. Nat Rev Neurosci 4:481–495
Leung LW (1985) Spectral analysis of hippocampal EEG in the freely moving rat effects of centrally active drugs and relations to evoked potentials. Electroencephalogr Clin Neurophysiol 60:65–77
Leung LS, Yim CY (1986) Intracellular records of theta rhythm in hippocampal CA1 cells of the rat. Brain Res 367:323–327
Leung LS, Yu HW (1998) Theta-frequency resonance in hippocampal CA1 neurons in vitro demonstrated by sinusoidal current injection. J Neurophysiol 79:1592–1596
Leung LS, Lopes da Silva F, Wadman WJ (1982) Spectral characteristics of the hippocampal EEG in the freely moving rat. Clin Neurophysiol 54:203–219
Lewis DA, Hashimoto T, Volk DW (2005) Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6:312–324
Li XG, Somogyi P, Ylinen A, Buzsáki G (1994) The hippocampal CA3 network: an in vivo intracellular labeling study. J Comp Neurol 339:181–208
Lisman JE (2005) The theta/gamma discrete phase code occurring during the hippocampal phase precession may be a more general brain coding scheme. Hippocampus 15:913–922
Lisman JE, Idiart MAP (1995) Storage of 7+- 2 short term memories in oscillatory subcycles. Science 267:1512–1515
Maccaferri G, McBain CJ (1996) The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. J Physiol 497:119–130
Maccaferri G, Roberts JD, Szucs P, Cottingham CA, Somogyi P (2000) Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. J Physiol 524:91–116
Madison DV, Lancaster B, Nicoll RA (1987) Voltage clamp analysis of cholinergic action in the hippocampus. J Neurosci 7:733–741
Magee JC (1999) Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci 18:7613–7624
Maier N, Nimmrich V, Draguhn A (2003) Cellular and network mechanisms underlying spontaneous sharp wave-ripple complexes in mouse hippocampal slices. J Physiol 550:873–887
Mann EO, Suckling JM, Hájos N, Greenfield SA, Paulsen O (2005) Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron 45:105–117
Marek KW, Davis GW (2003) Controlling the active properties of excitable cells. Curr Opin Neurobiol 13:607–611
Markram H, Lubke J, Frotscher M, Roth A, Sakmann B (1997) Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J Physiol 500:409–440
McBain CJ, Fisahn A (2001) Interneurons unbound. Nat Rev Neurosci 2:11–23
Meyer AH, Katona I, Blatow M, Rozov A, Monyer H (2002) In vivo labeling of parvalbumin-positive interneurons and analysis of electrical coupling in identified neurons. J Neurosci 22:7055–7064
Middleton S, Jalics J, Kispersky T, Lebeau FE, Roopun AK, Kopell NJ, Whittington MA, Cunningham MO (2008) NMDA receptor-dependent switching between different gamma rhythm-generating microcircuits in entorhinal cortex. Proc Natl Acad Sci U S A 105:18572–18577
Miles R (1990) Synaptic excitation of inhibitory cells by single CA3 hippocampal pyramidal cells of the guinea-pig in vitro. J Physiol 428:61–77
Miles R, Poncer JC (1996) Paired recordings from neurones. Curr Opin Neurobiol 6:387–394
Miles R, Tóth K, Gulyás AI, Hájos N, Freund TF (1996) Differences between somatic and dendritic inhibition in the hippocampus. Neuron 16:815–823
Montplaisir J, Laverdiere M, Saint-Hilaire JM, Rouleau I (1987) Nocturnal sleep recording in partial epilepsy: a study with depth electrodes. J Clin Neurophysiol 4:383–388
Morales M, Bloom FE (1997) The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J Neurosci 17:3157–3167
Morales M, Battenberg E, Bloom F (1998) Distribution of neurons expressing immunoreactivity for the 5HT3 receptor subtype in the rat brain and spinal cord. J Comp Neurol 402:385–401
Morris HM, Hashimoto T, Lewis DA (2008) Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex 18:1575–1587
Mulle C, Sailer A, Swanson GT, Brana C, O’Gorman S, Bettler B, Heinemann SF (2000) Subunit composition of kainate receptors in hippocampal interneurons. Neuron 28:475–484
Nimmrich V, Maier N, Schmitz D, Draguhn A (2005) Induced sharp wave-ripple complexes in the absence of synaptic inhibition in mouse hippocampal slices. J Physiol 563:663–670
Notomi T, Shigemoto R (2004) Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol 471:241–276
Nusser Z, Lujan R, Laube G, Roberts JD, Molnar E, Somogyi P (1998) Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron 21:545–559
Oliva AA Jr, Jiang M, Lam T, Smith KL, Swann JW (2000) Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci 20:3354–3368
O’Neill J, Senior T, Csicsvari J (2006) Place-selective firing of CA1 pyramidal cells during sharp wave/ripple network patterns in exploratory behavior. Neuron 49:143–155
Oren I, Mann EO, Paulsen O, Hájos N (2006) Synaptic currents in anatomically identified CA3 neurons during hippocampal gamma oscillations in vitro. J Neurosci 26:9923–9934
Pálhalmi J, Paulsen O, Freund TF, Hájos N (2004) Distinct properties of carbachol- and DHPG-induced network oscillations in hippocampal slices. Neuropharmacology 47:381–289
Pan WX, McNaughton N (2002) The role of the medial supramammillary nucleus in the control of hippocampal theta activity and behaviour in rats. Eur J Neurosci 16:1797–1809
Pangalos M, Donoso JR, Winterer J, Zivkovic AR, Kempter R, Maier N, Schmitz D (2013) Recruitment of oriens-lacunosum-moleculare interneurons during hippocampal ripples. Proc Natl Acad Sci U S A 110:4398–4403
Paré D, Collins DR (2000) Neuronal correlates of fear in the lateral amygdala: multiple extracellular recordings in conscious cats. J Neurosci 20:2701–2710
Peng Y, Barreda Tomás FJ, Klisch C, Vida I, Geiger JRP (2017) Layer-Specific Organization of Local Excitatory and Inhibitory Synaptic Connectivity in the Rat Presubiculum. Cereb Cortex 27:2435–2452
Penttonen M, Kamondi A, Acsády L, Buzsáki G (1998) Gamma frequency oscillation in the hippocampus of the rat: intracellular analysis in vivo. Eur J Neurosci 10:718–728
Petsche H, Stumpf C, Gogolák G (1962) The significance of the rabbit’s septum as a relay station between midbrain and the hippocampus: I. The control of hippocampus arousal activity by the septum cells. Electroencephalogr Clin Neurophysiol 14:202–211
Pike FG, Goddard RS, Suckling JM, Ganter P, Kasthuri N, Paulsen O (2000) Distinct frequency preferences of different types of rat hippocampal neurones in response to oscillatory input currents. J Physiol 529:205–213
Pinault D (1996) A novel single-cell staining procedure performed in vivo under electrophysiological control: Morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or neurobiotin. J Neurosci Methods 65:113–136
Pinault D (2008) N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry 63:730–735
Pitler TA, Alger BE (1992) Cholinergic excitation of GABAergic interneurons in the rat hippocampal slice. J Physiol 450:127–142
Polleux F (2005) Genetic mechanisms specifying cortical connectivity: let’s make some projections together. Neuron 46:395–400
Pöschel B, Draguhn A, Heinemann U (2002) Glutamate-induced gamma oscillations in the dentate gyrus of rat hippocampal slices. Brain Res 938:22–28
Pouille F, Scanziani M (2004) Routing of spike series by dynamic circuits in the hippocampus. Nature 429:717–723
Price CJ, Cauli B, Kovacs ER, Kulik A, Lambolez B, Shigemoto R, Capogna M (2005) Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area. J Neurosci 25:6775–6786
Roopun AK, Cunningham MO, Racca C, Alter K, Traub RD, Whittington MA (2008) Region-specific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia. Schizophr Bull 34:962–973
Ropert N, Guy N (1991) Serotonin facilitates GABAergic transmission in the CA1 region of rat hippocampus in vitro. J Physiol (Lond) 441:121–136
Rotstein HG, Pervouchine DD, Acker CD, Gillies MJ, White JA, Buhl EH, Whittington MA, Kopell N (2005) Slow and fast inhibition and an H-current interact to create a theta rhythm in a model of CA1 interneuron network. J Neurophysiol 94:1509–1518
Ruiz AJ, Kullmann DM (2013) Ionotropic receptors at hippocampal mossy fibers: roles in axonal excitability, synaptic transmission, and plasticity. Front Neural Circ 6:1–12
Sakai T, Oshima A, Nozaki Y, Ida I, Haga C, Akiyama H, Nakazato Y, Mikuni M (2008) Changes in density of calcium-binding-protein-immunoreactive GABAergic neurons in prefrontal cortex in schizophrenia and bipolar disorder. Neuropathology 28:143–150
Sasaki T, Matsuki N, Ikegaya Y (2012) Targeted axon-attached recording with fluorescent patch-clamp pipettes in brain slices. Nat Protoc 7:1228–1234
Savić N, Pedarzani P, Sciancalepore M (2001) Medium afterhyperpolarization and firing pattern modulation in interneurons of stratum radiatum in the CA3 hippocampal region. J Neurophysiol 85:1986–1997
Schlingloff D, Káli S, Freund TF, Hájos N, Gulyás AI (2014) Mechanisms of sharp wave initiation and ripple generation. J Neurosci. 34:11385–11398
Schmitz D, Empson RM, Heinemann U (1995) Serotonin reduces inhibition via 5-HT1A receptors in area CA1 of rat hippocampal slices in vitro. J Neurosci 15:7217–7225
Schmitz D, Gloveli T, Empson RM, Heinemann U (1998) Comparison of the effects of serotonin in the hippocampus and the entorhinal cortex. Mol Neurobiol 17:59–72
Schmitz D, Schuchmann S, Fisahn A, Draguhn A, Buhl EH, Petrasch-Parwez E, Dermietzel R, Heinemann U, Traub RD (2001) Axo-axonal coupling. A novel mechanism for ultrafast neuronal communication. Neuron 31:831–840
Sheffield ME, Best TK, Mensh BD, Kath WL, Spruston N (2011) Slow integration leads to persistent action potential firing in distal axons of coupled interneurons. Nat Neurosci 14:200–207
Shen RY, Andrade R (1998) 5-Hydroxytryptamine2 receptor facilitates GABAergic neurotransmission in rat hippocampus. J Pharmacol Exp Ther 285:805–812
Shimono K, Brucher F, Granger R, Lynch G, Taketani M (2000) Origins and distribution of cholinergically induced beta rhythms in hippocampal slices. J Neurosci 20:8462–8473
Sik A, Penttonen M, Ylinen A, Buzsáki G (1995) Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci 15:6651–6665
Singer W (1999) Neuronal synchrony: a versatile code for the definition of relations? Neuron 24:49–65
Slewa-Younan S, Gordon E, Williams L, Haig AR, Goldberg E (2001) Sex differences, gamma activity and schizophrenia. Int J Neurosci 107:131–144
Sohal VS, Zhang F, Yizhar O, Deisseroth K (2009) Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459:698–702
Somogyi P, Klausberger T (2005) Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol (Lond) 562:9–26
Spampanato J, Mody I (2007) Spike timing of lacunosom-moleculare targeting interneurons and CA3 pyramidal cells during high-frequency network oscillations in vitro. J Neurophysiol 98:96–104
Spencer KM (2008) Visual gamma oscillations in schizophrenia: implications for understanding neural circuitry abnormalities. Clin EEG Neurosci 39:65–68
Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW (2004) Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A 101:17288–11793
Spruston N (2008) Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci 9:206–221
Stark E, Roux L, Eichler R, Senzai Y, Royer S, Buzsáki G (2014) Pyramidal cell-interneuron interactions underlie hippocampal ripple oscillations. Neuron 83:467–480
Szabadics J, Tamas G, Soltesz I (2007) Different transmitter transients underlie presynaptic cell type specificity of GABAA, slow and GABAA, fast. Proc Natl Acad Sci U S A 104:14831–14836
Szabo G, McBain CJ (2009) Asynchronous transmitter release from cholecystokinin-containing inhibitory interneurons is widespread and target-cell independent. J Neurosci 29:11112–11122
Tecott LH, Maricq AV, Julius D (1993) Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proc Natl Acad Sci U S A 90:1430–1434
Tesche CD, Karhu J (2000) Theta oscillations index human hippocampal activation during a working memory task. Proc Natl Acad Sci USA 97:919–924
Tort AB, Rotstein HG, Dugladze T, Gloveli T, Kopell NJ (2007) On the formation of gamma-coherent cell assemblies by oriens lacunosum-moleculare interneurons in the hippocampus. Proc Natl Acad Sci U S A 104:13490–13495
Toth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ (2000) Differential mechanisms of transmission at three types of mossy fiber synapse. J Neurosci 20:8279–8289
Towers SK, LeBeau FE, Gloveli T, Traub RD, Whittington MA, Buhl EH (2002) Fast network oscillations in the rat dentate gyrus in vitro. J Neurophysiol 87:1165–1168
Traub RD, Bibbig A, Fisahn A, LeBeau FE, Whittington MA, Buhl EH (2000) A model of gamma-frequency network oscillations induced in the rat CA3 region by carbachol in vitro. Eur J Neurosci 12:4093–4106
Traub RD, Cunningham MO, Gloveli T, LeBeau FE, Bibbig A, Buhl EH, Whittington MA (2003) GABA-enhanced collective behavior in neuronal axons underlies persistent gamma-frequency oscillations. Proc Natl Acad Sci U S A 100:11047–11052
Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T (2007) Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci 27:8184–8189
van Hooft JA, Giuffrida R, Blatow M, Monyer H (2000) Differential expression of group I metabotropic glutamate receptors in functionally distinct hippocampal interneurons. J Neurosci 20:3544–3551
Vanderwolf CH (1969) Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol 26:407–418
Vida I, Halasy K, Szinyei C, Somogyi P, Buhl EH (1998) Unitary IPSPs evoked by interneurons at the stratum radiatum-stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro. J Physiol 506:755–773
Vida I, Bartos M, Jonas P (2006) Shunting inhibition improves robustness of gamma oscillations in hippocampal interneuron networks by homogenizing firing rates. Neuron 49:107–117
Vierling-Claassen D, Siekmeier P, Stufflebeam S, Kopell N (2008) Modeling GABA alterations in schizophrenia: a link between impaired inhibition and altered gamma and beta range auditory entrainment. J Neurophysiol 99:2656–2671
Wang X-J, Buzsáki G (1996) Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci 16:6402–6413
Wang CZ, Yang SF, Xia Y, Johnson KM (2008) Postnatal Phencyclidine Administration Selectively Reduces Adult Cortical Parvalbumin-Containing Interneurons. Neuropsychopharmacology 33:2442–2455
Weiss T, Veh RW, Heinemann U (2003) Dopamine depresses cholinergic oscillatory network activity in rat hippocampus. Eur J Neurosci 18:2573–2580
White JA, Chow CC, Ritt J, Soto-Treviño C, Kopell N (1998) Synchronization and oscillatory dynamics in heterogeneous, mutually inhibited neurons. J Comput Neurosci 5:5–16
Whittington MA, Traub RD (2003) Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci 26:676–682
Whittington MA, Traub RD, Jefferys JGR (1995) Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 373:612–615
Whittington MA, Stanford IM, Colling SB, Jefferys JG, Traub RD (1997) Spatiotemporal patterns of gamma frequency oscillations tetanically induced in the rat hippocampal slice. J Physiol 502:591–607
Williams SR, Stuart GJ (2003) Voltage- and site-dependent control of the somatic impact of dendritic IPSPs. J Neurosci 23:7358–7367
Wilson MA, McNaughton BL (1994) Reactivation of hippocampal ensemble memories during sleep. Science 265:676–679
Wittner L, Henze DA, Záborszky L, Buzsáki G (2007) Three-dimensional reconstruction of the axon arbor of a CA3 pyramidal cell recorded and filled in vivo. Brain Struct Funct 212:75–83
Wulff P, Ponomarenko AA, Bartos M, Korotkova TM, Fuchs EC, Bähner F, Both M, Tort AB, Kopell NJ, Wisden W, Monyer H (2009) Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc Natl Acad Sci U S A 106:3561–3566
Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, Buzsáki G (1995) Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: Network and intracellular mechanisms. J Neurosci 15:30–46
Zarnadze S, Bäuerle P, Santos-Torres J, Böhm C, Schmitz D, Geiger JR, Dugladze T, Gloveli T (2016) Cell-specific synaptic plasticity induced by network oscillations. Elife 5:pii: e14912
Zhang ZJ, Reynolds GP (2002) A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res 55:1–10
Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K (2007) Multimodal fast optical interrogation of neural circuitry. Nature 446:633–639
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gloveli, T., Booker, S.A., Kopell, N., Dugladze, T. (2018). Cell Type-Specific Activity During Hippocampal Network Oscillations In Vitro. In: Cutsuridis, V., Graham, B., Cobb, S., Vida, I. (eds) Hippocampal Microcircuits. Springer Series in Computational Neuroscience. Springer, Cham. https://doi.org/10.1007/978-3-319-99103-0_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-99103-0_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99102-3
Online ISBN: 978-3-319-99103-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)