Abstract
Complex intracranial aneurysms are rare cerebrovascular lesions that pose significant management challenges. Their microsurgical interventions often require complex reconstruction or bypass procedures to achieve the goal of aneurysmal obliteration and vascular preservation.
This chapter summarizes contemporary microsurgical techniques and literature on complex intracranial aneurysms using clip reconstruction. The illustrations were mainly based on literature reported in recent years involving complex clip reconstruction for anterior and posterior cerebral circulation aneurysms.
A variety of complex clip reconstruction techniques have been used successfully in the treatment of complex aneurysms in both anterior and posterior cerebral circulation. These methods involve the use of multiple standard clips, fenestrated clips, or complex-shaped clips to achieve the goal of aneurysmal obliteration and arterial branch and perforator branch preservation. In cases where multiple clip applications are necessary, modifications of classical approaches such as Drake’s tandem clipping or Sugita tandem clipping method have led to increased versatility of microsurgical approach in treating complex intracranial aneurysms.
Microsurgical intervention using complex clip reconstructions represents an effective and versatile approach for selected complex intracranial aneurysms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Complex intracranial aneurysms
- Clip reconstruction
- Multi-clip reconstruction
- Fenestrated clip
- Middle cerebral artery aneurysm
- Anterior circulation aneurysm
Complex intracranial aneurysms (CIA) are rare cerebrovascular lesions that pose significant neurosurgical challenges [1]. CIAs are aneurysms with one or more of the following features: (1) giant size, (2) difficult access, (3) complicated aneurysmal wall structure, and (4) involvement with arterial trunks or branches [2]. Their presentations can vary as to the shape, size, location, orientation, thrombosis, neck calcification, and the relationships with the parent arteries and perforators, all of which play critical roles in clinical decision-making.
Complex clip reconstructions are microsurgical interventions that employ multiple clips, including straight, fenestrated, and complex-shaped clips to achieve the goal of aneurysmal obliteration and vascular flow preservation. They are versatile and efficient microsurgical tools for selected patients with complex intracranial aneurysms. In this chapter, recent developments in complex clip reconstruction are discussed focusing on the complex aneurysms in the anterior and posterior cerebral circulation. In addition, two cases are included to demonstrate the utility of complex clip reconstruction in the treatment of CIAs.
Clip Reconstructions for Middle Cerebral Artery (MCA) and Internal Carotid Artery (ICA) Aneurysms
Middle cerebral artery (MCA) aneurysms represent 33% of all intracranial aneurysms [3]. MCA aneurysms can present as CIA with large size, involvement of neural and vascular structures, and complex wall structures. These aneurysms are difficult to clip precisely by a single clip without compromise or kinking of the parent or perforator vessels. To overcome this problem, Babbu et al. reported a “multi-clip” method to achieve a precise and complete clipping [4]. In this multi-clip method, the first clip is placed in the distal part of the aneurysm sac using a standard clip. Then a second miniclip or a fenestrated clip is positioned to “jump” the blades of the first clip for accurate and precise clipping of the aneurysmal sac remnant [4]. In an aneurysm that has a perforator branch at the neck of an aneurysm, Drake’s tandem clipping technique can be utilized. In this technique, a first clip is placed slightly away from the perforator, and the second clip is applied over the first to catch the remnant of the sac in a tandem fashion [4].
MCA aneurysms often have wide necks that splay the bifurcation. Clatterbuck et al. [5] reported an orthogonal interlocking tandem clipping technique to completely obliterate an aneurysm and simultaneously “reconstruct” the MCA bifurcation. In a case with a bilobed aneurysm at the MCA bifurcation [5], the authors placed the first clip, which was a long curved clip, across the fundus along the long axis of the M1 segment. Subsequently, a fenestrated straight clip was set at a right angle to the first clip, incorporating it into the fenestration [5]. In another case of a giant aneurysm at the MCA bifurcation, the aneurysm was clipped first with several long straight clips placed across the fundus along the long axis of the M1 segment, followed by two fenestrated clips placed at right angles to the first clip to complete the clip reconstruction [5]. Postoperative angiogram confirmed the complete obliteration of the aneurysms [5].
Occasionally, MCA aneurysms can present as fusiform aneurysms. In a recent report, a new fenestrated Yasargil T-bar clip was used to treat the fusiform M1 aneurysm [6]. These clips have T-shaped blades as opposed to traditional straight edges. They also have fenestrations with variable sizes and blade angles [6]. In a case of fusiform aneurysm of the M1 segment, the fenestration of the clip was placed around the M1 segment, while the blades were placed along the lateral wall of the M1 segment to reconstruct the vessel wall [6]. This method reconstructed posteromedial wall and preserved the origin of the lenticulostriate arteries. The obliteration of aneurysm was confirmed using postoperative angiogram [6]. This new clip allowed successful reconstruction of the M1 segment with a single clip application.
Non-branching segment of internal carotid artery (ICA) can sometimes give rise to complex aneurysm such as blister-like aneurysms. These are rare but dangerous vascular lesions, representing 1% of all intracranial aneurysms [7]. Blister-like aneurysms represent great challenges for clinical management. Surgical treatments of these aneurysms included direct clipping, direct micro-suturing, and wrapping or endovascular treatment [7, 8]. Kantelhardt et al. recently reported a combined method of suture and clipping for the reconstruction of a ruptured blister-like aneurysm [8]. In this method, the aneurysm is first trapped by temporary clips. Then the vessel wall is adapted by microsutures. Clips are then applied over the sutures, which now serve to prevent dislocation of the clips. This clip and microsuture combination technique is faster than watertight microsutures. More importantly, it also facilitates precise approximation of the edges of the vascular defect and allows consecutive safe clip placement for aneurysm obliteration and vascular reconstruction [8]. Indeed, although various surgical techniques have been described to treat blister-like aneurysms of the supraclinoid ICA, in our experience, excluding the diseased segment of the artery with trapping and bypass is superior and safer than direct clipping or clip-wrapping technique [9].
Clip Reconstruction of Anterior Communicating Artery (ACOMA) and Anterior Cerebral Artery (ACA) Aneurysms
AcomA aneurysms often present in a complex manner. They may have broad A1–A2 junction and are located near the origins of the recurrent artery of Heubner and AcomA perforators. These aneurysms are difficult to reconstruct with a single clip due to the complex wall structure of the aneurysms and potential compromise of the branch arteries and perforators [10].
Straight fenestrated clips can be placed in a successive array to create tunnels that reconstruct origins of branching vessels at the neck of complex aneurysms [11]. This technique has been used to treat AcomA or other anterior circulation aneurysms. In a report by Yang and Lawton, three variations of fenestrated tubes were conceived, namely, the antegrade fenestration tubes, the retrograde fenestration tubes, and the aneurysm dome fenestration tubes [11]. In an illustrative case, the antegrade fenestration tube, built with stacked straight fenestrated clips with an open fenestration tube, was used to clip an AcomA aneurysm with a broad A1–A2 junction where an antegrade fenestration tube was created to transmit the ipsilateral A2, the recurrent artery of Heubner, and the perforators [11]. In retrograde fenestration tubes, a closed fenestration tube was built with stacked straight fenestrated clips and a non-fenestrated clip at the end and was used to reverse the direction of blood flow into the efferent artery exiting from the base of the tube. This technique was used to treat an ICA bifurcation aneurysm that had efferent ACA and MCA originating from the base of an aneurysm [11]. In the dome fenestration tube, the stacked straight fenestrated clips are placed perpendicular to the aneurysm neck rather than parallel to the neck [11]. The dome fenestrated tube was used successfully to reconstruct a supraclinoid ICA aneurysm where the fenestration tube was used to encircle the thickened aneurysm sac [11].
Similar to complex AcomA aneurysms, giant distal anterior cerebral artery (ACA) aneurysms are also rare anterior circulating aneurysms that represent great challenges for neurovascular surgeons. They may have complex arterial branches at the aneurysmal neck and deep location in the skull base. Various techniques have been used to treat these aneurysms, including endovascular interventions, surgical trapping, clip reconstruction, and bypass techniques. In a recent report, a direct clip reconstruction of a 5 cm giant partially thrombosed aneurysm of the distal ACA was described [12]. In this report, microdissection was carried out after thromboendarterectomy to expose the ipsilateral and contralateral A1 and A2 and the aneurysm. The neck of the aneurysm was reconstructed using temporary clips under pentobarbital burst suppression. A postoperative angiography revealed obliteration of the aneurysm and filling of the entire pericallosal artery. The patient experienced a short hospital stay and made an excellent recovery [12].
Clip Reconstruction for Paraclinoid Aneurysms
Paraclinoid aneurysms are thought to be among the most difficult aneurysms to be treated by microsurgery due to the complexity of their vascular anatomy, frequently large and irregular size, proximity to obstructing parts of the medial sphenoid wing, and direct proximity to the optical apparatus [13]. However, some progress has been made in the microsurgical treatment of these aneurysms. In a recent report, Seifert et al. reported their experience with exclusive intradural exposure and clip reconstruction in complex paraclinoid aneurysms [14]. Their reconstruction approach was used in treating a paraclinoid aneurysm where the aneurysmal dome was below the carotid artery. This anatomy allowed microsurgical approach using fenestrated clips under temporary occlusion of the extracranial carotid artery and application of the suction decompression technique [14]. The principles of the clipping techniques essentially follow the Sugita tandem clipping method where fenestrated and angled clips are placed one after the other, locking themselves in place [14]. In an operative video atlas manuscript, Liu reported a similar approach where a large complex ventral paraclinoid carotid artery aneurysm was first decompressed with an initial trapping and direct suction decompression strategy followed by various fenestrated clip reconstructions of the internal carotid artery (ICA) via a modified orbitozygomatic approach [15]. In a case series by Xu et al., microsurgical treatment of large and giant paraclinoid aneurysms were performed using a combination of endovascular and open microsurgical approaches [16]. In direct microsurgical treatment, tandem right-angled fenestrated clips were placed across the carotid artery, and one or two additional long angled or curved clips were added to reinforce the aneurysm neck occlusion [16].
Clip Reconstruction of Posterior Circulation Aneurysms
Posterior circulation aneurysms , representing only 10–15% of all intracranial aneurysms, are ambitious targets for microsurgical interventions [17]. The contributing factors include deep location of the aneurysms, obstructing bony prominences, and the presence of nearby critical structures, such as brain stem perforators and lower cranial nerves. Although most posterior circulation aneurysms are treated with endovascular approaches, open surgical approaches have been utilized to treat selected aneurysms that are not suitable for endovascular treatment [17].
Recent literature has provided ideas that make open microsurgical treatment an option for selected posterior circulation aneurysms. In a recent Neurosurgical Focus video, microsurgical clipping with hypothermic circulatory arrest has been used to a treat a giant vertebrobasilar junction aneurysm after initially failed coil occlusion of the right vertebral artery [18]. This method has been rarely used and provided insights into developing new strategies for microsurgical treatment of posterior circulation aneurysm.
Modifications of existing surgical approaches have also been developed to improve the microsurgical treatment of posterior circulation aneurysms. Gross et al. have discussed the application of petrosal approaches to posterior circulation aneurysms, especially basilar aneurysms [19]. In the anterior petrosal approach, low-lying basilar apex and upper basilar trunk aneurysms are exposed via a subtemporal-transtentorial approach with added traction on the temporal lobe. In addition, an extradural petrous apicectomy was included to provide an additional 1–1.5 cm exposure of the basilar artery [19]. In the posterior petrosal approach, a presigmoid retrolabyrinthine exposure was employed in addition to a temporooccipital craniotomy and mobilization of a skeletonized sigmoid sinus after dividing the superior petrosal sinus and tentorium [19]. This approach, however, has been associated with a relatively high rate of CSK leak [19]. In a report by McLaughlin and Martin, a modified technique for tentorial incision and reflection that optimizes the exposure of the aneurysm was introduced. The key steps for this approach include the critical dissection of the trochlear nerve from its dural canal up to the entrance in the cavernous sinus and extension of the tentorial incision up to the Meckel cave. This technique results in a significantly increased visibility and maneuverability for basilar aneurysms by increasing the rostrocaudal and anterolateral exposure [17].
Exposure to posterior inferior cerebellar artery (PICA) aneurysms is usually opened between vagus, accessory, and hypoglossal nerves for safe clipping [20]. In a recent report, three anatomical triangles and their relationships with PICA aneurysms are defined [20], namely, the vagoaccesory triangle, suprahypoglossal triangle, and infrahypoglossal triangle. The vagoaccesory triangle is defined by CN X superiorly, CN XI laterally, and medulla medially. It can be further divided by CN XII into the suprahypoglossal triangle and infrahypoglossal triangle. These triangles provided anatomical framework to improve the access and exposure of the PICA aneurysms. For example, PICA aneurysms originating distally on the VA near the vertebrobasilar junction can be accessed via the suprahypoglossal triangle, whereas PICA aneurysms originate distally on the VA can be found within the vagoaccessory triangle [20].
Case Presentations
Case 1: Giant Left MCA Aneurysm
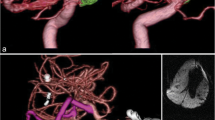
A right-handed 71-year-old female with a history of DM and HTN presents with two episodes of roughly 1 min of the inability to speak. Imaging studies, including head CT, MRI, and diagnostic angiogram, were performed and demonstrated a giant left MCA bifurcation aneurysm measuring 3.2 × 2.0 × 1.4 cm with a large thrombus and a dysplastic neck (Fig. 9.1). The patient elected to have surgery to treat her aneurysm. A left pterional craniotomy was performed to access the giant MCA aneurysm for this case. Immediately after opening the dura, the aneurysm was evident, and the Sylvian fissure was dissected free. After careful dissection circumferentially around the aneurysm, the M1 segment in the ICA was identified to obtain proximal control with temporary clips. A thromboendarterectomy was then performed during which there was brisk bleeding at the center of the aneurysm, and it was controlled with temporary clips. Most of the dome of the aneurysm was removed. Further dissection and aneurysm wall resection were performed to make this giant base easier for clipping. In the end, two clips were placed across the neck of the aneurysm, including a large straight clip and a large fenestrated clip perpendicular to the other clip. This combination was sufficient to prevent the aneurysm from filling. There was a small residual base left on purpose to ensure flow from the M1 and to all of the distal MCA branches. Two additional fenestrated clips were placed below the large stray clip to eliminate a little bit more of the residual neck, and the transected portion of the aneurysm dome was oversewn in a simple running fashion to enforce the clipping. An ICG video angiography confirmed that there was a good flow. An intraoperative angiography confirmed the obliteration of the aneurysm (Fig. 9.2). The postoperative course was uneventful. The patient recovered well from her surgery and experienced no neurological symptoms at 3-year follow-up.
Preoperative imaging studies for a giant left MAC aneurysm in case 1. (a) Non-contrast axial-view computerized tomography (CT) of the head demonstrating a well-circumscribed, partially calcified mass in the frontoparietal area of the brain causing a 7 mm midline shift. (b) T2-weighted axial-view magnetic resonance imaging (MRI) of the head revealing a well-circumscribed hypointense heterogeneous mass in the left frontoparietal area of the brain. (c) T1-weighted, post-contrast, coronal-view MRI demonstrating a central hyperintense, contrast-filling mass representing the living part of the aneurysm. (d) CT angiogram demonstrating a giant, partially thrombosed, left M1/M2 bifurcation aneurysm in sagittal view. (e) 3D CT angiogram reveals a giant MCA bifurcation aneurysm. (f) Digital subtraction angiogram (DSA) of the left internal carotid artery (ICA) with AP projection demonstrating a giant, left M1/M2 bifurcation aneurysm. (g) Lateral projection of DSA demonstrating a giant, left M1/M2 bifurcation aneurysm
Postoperative imaging studies for a giant left MCA aneurysm in case 1. (a) Postoperative AP projection of DSA with left carotid injection, demonstrating no residual filling. (b) Postoperative lateral DSA revealing no residual filling. (c) Postoperative oblique DSA demonstrating the patent cerebral vasculature with no aneurysmal filling
Case 2: Large Left ICA Aneurysm
A 62-year-old man reports word-finding difficulty, confusion, seizure, and subsequent aphasia for a couple of months. A CT scan was performed and illustrated a large frontotemporal mass measuring approximately 8 cm in the greatest dimension. This was followed with an MRI scan and an angiogram, which showed a large left ICA bifurcation aneurysm that had a significant amount of thrombosis with filling (Fig. 9.3). The patient elected to undergo surgical treatment. A left-sided orbitozygomatic skull base approach was performed for this case. Upon opening the dura, the aneurysmal mass was apparent on the aperture of the Sylvian fissure. After careful dissection, a small segment of the supraclinoid ICA was isolated for proximal control. The aneurysmal thromboendarterectomy was performed, and the aneurysm was mobilized. It was noted that the MCA bifurcation, the M1 segment, the anterior choroidal artery, and the lenticulostriate arteries were adherent to the aneurysmal wall, which were dissected free subsequently and protected. Before temporary clips were placed on the A1, M1, and supraclinoid ICA, fresh arterial bleeding was experienced and was controlled with suction. Under temporary clipping, thrombectomy was continued with thromboendarterectomy and incision of a redundant aneurysmal wall. Ten aneurysm clips, including straight and bayonet clips, were stacked to reconstruct the aneurysm (Fig. 9.4). Indocyanine green video angiography confirmed the patency of all cerebral vessels. Postoperative angiogram confirmed the obliteration of the aneurysm (Fig. 9.4). The patient did well postoperatively and had no neurological symptoms at 1-year follow-up after the surgery [18].
Preoperative imaging studies for a large left ICA aneurysm in case 2 (Cikla et al. [18]). (a) Non-contrast, axial CT of the head revealing a left frontotemporal, well-circumscribed heterogeneous, 8 cm mass causing 6 mm midline shift. The lesion harbors some centrolateral, strongly hyperdense areas representing calcification. (b) FLAIR MRI revealing perilesional edema, mass effect to the thalamus, and anterior limb of the internal capsule. (c) Non-contrast, T1-weighted axial magnetic resonance images revealing a round, well-circumscribed left frontotemporal lesion which is hyperintense to the brain parenchyma with posterior heterogeneity. The lesion is 8 cm in maximal diameter with 6 mm of midline shift from left to right. (d) T2-weighted coronal magnetic resonance images of the head revealing a left frontotemporal, hypointense mass lesion with peripheral hyperintensities causing approximately 6 mm midline shift due to mass affect. (e) T1-weighted, post-contrast, sagittal magnetic resonance images revealing an 8 × 8 cm, hyperintense mass lesion harboring heterogenous areas. (f) FLAIR MRI reveals perilesional edema with mass effect to the brain stem. (g) Preoperative oblique DSA with left ICA injection demonstrates a giant carotid bifurcation aneurysm. (h) Preoperative lateral DSA of the left ICA demonstrates the carotid bifurcation aneurysm
Postoperative imaging studies for a large left ICA aneurysm in case 2 (Cikla et al. [18]). (a and c) Postoperative, 3D CTA reveals successful clipping of the aneurysm without any residual filling. (b and d) Postoperative, AP-DSA confirms total obliteration of the aneurysm. (e) Postoperative, DSA with lateral projection reveals no residual filling. (f and g) Postoperative AP-DSAs confirm the patency of cerebral blood flow after multiple clipping
Summary
Complex aneurysms are those with a wide neck, unusual branches, and efferent arteries that sometimes require sophisticated clipping techniques to achieve the goal of aneurysmal obliteration and parent and branch artery preservation. Complex clip reconstruction has proven to be useful in the microsurgical intervention for these aneurysms with careful preoperative surgical planning and clip construction. Whereas anterior cerebral circulation aneurysms have better defined surgical approaches and clipping techniques, posterior circulation aneurysms require modification of existing approaches to achieve proper exposure of aneurysms for surgical clipping. Application of microsurgical techniques combined with novel clip construct and new surgical approaches have increased the versatility and success of microsurgical treatment for complex intracranial aneurysms.
References
Cantore G, Santoro A, Guidetti G, Delfinis CP, Colonnese C, Passacantilli E. Surgical treatment of giant intracranial aneurysms: current viewpoint. Neurosurgery. 2008;63(279–89):289–90.
Andaluz N, Zuccarello M. Treatment strategies for complex intracranial aneurysms: review of a 12-year experience at the University of Cincinnati. Skull Base. 2011;21:233–42.
Diaz FG, Guthikonda M, Guyot L, Velardo B, Gordon V. Surgical management of complex middle cerebral artery aneurysms. Neurol Med Chir. 1998;38:50–7.
Babbu D, Sano H, Kato Y, Arabi O, Karagiozov K, Yoneda M, Imizu S, Watanabe S, Oda J, Kanno T. The “multi clip” method in unruptured complex middle cerebral artery aneurysms-a case series. Minim Invasive Neurosurg. 2006;49:331–4.
Clatterbuck RE, Galler RM, Tamargo RJ, Chalif DJ. Orthogonal interlocking tandem clipping technique for the reconstruction of complex middle cerebral artery aneurysms. Neurosurgery. 2006;59:ONS347–51; 351–2.
Baskaya MK, Uluc K. Application of a new fenestrated clip (Yasargil T-bar clip) for the treatment of fusiform M1 aneurysm: case illustration and technical report. Neurosurgery. 2012;70:339–42.
Ishikawa T, Nakamura N, Houkin K, Nomura M. Pathological consideration of a “blister-like” aneurysm at the superior wall of the internal carotid artery: case report. Neurosurgery. 1997;40:403–6.
Kantelhardt SR, Archavlis E, Giese A. Combined suture and clipping for the reconstruction of a ruptured blister-like aneurysm. Acta Neurochir. 2016;158:1907–11.
Cıkla U, Baggott C, Başkaya MK. How I do it: treatment of blood blister-like aneurysms of the supraclinoid internal carotid artery by extracranial-to-intracranial bypass and trapping. Acta Neurochir. 2014;156:2071–7.
Solomon RA. Anterior communicating artery aneurysms. Neurosurgery. 2001;48:119–23.
Yang I, Lawton MT. Clipping of complex aneurysms with fenestration tubes: application and assessment of three types of clip techniques. Neurosurgery. 2008;62(371–8):378–9.
Cikla U, Yilmaz T, Li Y, Baskaya MK. Clip reconstruction of giant distal anterior cerebral artery aneurysm: 3-dimensional operative video. Neurosurgery. 2015;11(Suppl 3):463.
Heros RC, Nelson PB, Ojemann RG, Crowell RM, DeBrun G. Large and giant paraclinoid aneurysms: surgical techniques, complications, and results. Neurosurgery. 1983;12:153–63.
Seifert V, Güresir E, Vatter H. Exclusively intradural exposure and clip reconstruction in complex paraclinoid aneurysms. Acta Neurochir. 2011;153:2103–9.
Liu JK. Direct suction decompression and fenestrated clip reconstruction of complex paraclinoid carotid artery aneurysm: operative video and nuances of skull base technique. Neurosurg Focus. 2015;39(Video Suppl 1):V4.
Xu B, Sun Z, Romani R, Jiang J, Wu C, Zhou D, Yu X, Hernesniemi J, Li B. Microsurgical management of large and giant paraclinoid aneurysms. World Neurosurg. 2010;73:137–46.
McLaughlin N, Martin NA. Extended subtemporal transtentorial approach to the anterior incisural space and upper clival region: experience with posterior circulation aneurysms. Neurosurgery. 2014;10(Suppl 1):15–23. discussion 23–4
Cikla U, Uluc K, Baskaya MK. Microsurgical clipping of a giant vertebrobasilar junction aneurysm under hypothermic circulatory arrest. Neurosurg Focus. 2015;39(Video Suppl 1):V13.
Gross BA, Tavanaiepour D, Du R, Al-Mefty O, Dunn IF. Petrosal approaches to posterior circulation aneurysms. Neurosurg Focus. 2012;33:E9.
Rodríguez-Hernández A, Lawton MT. Anatomical triangles defining surgical routes to posterior inferior cerebellar artery aneurysms: clinical article. J Neurosurg. 2011;114:1088–94.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wei, Z., Cikla, U., Seckin, H., Baskaya, M.K. (2019). Microsurgical Treatment of Complex Intracranial Aneurysms. In: Spiotta, A., Turner, R., Chaudry, M., Turk, A. (eds) Management of Cerebrovascular Disorders. Springer, Cham. https://doi.org/10.1007/978-3-319-99016-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-99016-3_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99015-6
Online ISBN: 978-3-319-99016-3
eBook Packages: MedicineMedicine (R0)