Abstract
In the case of elective clipping of unruptured aneurysms, the surgeon has the luxuries of time and careful planning, which he or she should leverage fully to ensure a fully optimized case. Prior to entering the operating theater, it is important to reassess the patient’s history, specifically reviewing their history for any prior subarachnoid hemorrhage (SAH) or cranial trauma, cranial surgery, endovascular therapy – both neurosurgical and otherwise – and medications, including antiplatelets, anticoagulation, and antiepileptics. Next, review all imaging modalities obtained in the workup of their lesion, including catheter-based angiogram, computed tomography angiography, and magnetic resonance angiography. These images should be reviewed with careful attention paid to any vascular variants or anomalies, aneurysm dome projection and configuration, distance from the Sylvian fissure to the aneurysm when applicable, presence of multiple aneurysms, estimating the skull thickness, anterior clinoid process (ACP) anatomy, frontal sinus size and configuration, and cortical veins. Finally, the patient should be asked for any changes in their medical history since their last office visit, and a brief physical examination should be performed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Microsurgical clipping of unruptured aneurysms
- Unruptured aneurysms
- Aneurysms
- Unruptured
- Craniotomy for anterior and posterior circulation aneurysms
Technical Considerations
In the case of elective clipping of unruptured aneurysms, the surgeon has the luxuries of time and careful planning, which he or she should leverage fully to ensure a fully optimized case. Prior to entering the operating theater, it is important to reassess the patient’s history, specifically reviewing their history for any prior subarachnoid hemorrhage (SAH) or cranial trauma, cranial surgery, endovascular therapy – both neurosurgical and otherwise – and medications, including antiplatelets, anticoagulation, and antiepileptics. Next, review all imaging modalities obtained in the workup of their lesion, including catheter-based angiogram, computed tomography angiography, and magnetic resonance angiography. These images should be reviewed with careful attention paid to any vascular variants or anomalies, aneurysm dome projection and configuration, distance from the Sylvian fissure to the aneurysm when applicable, presence of multiple aneurysms, estimating the skull thickness, anterior clinoid process (ACP) anatomy, frontal sinus size and configuration, and cortical veins. Finally, the patient should be asked for any changes in their medical history since their last office visit, and a brief physical examination should be performed.
The operating room staff should, ideally, be part of a specialized operative team comfortable with nuances, tools, and terminology associated with aneurysm management consisting of the surgeon, surgical assistance, neuro-anesthesiologist, and scrub and circulating nurses. When operating in a hybrid operating theater with biplane endovascular capabilities, two radiologic technicians should also be present [1]. An anesthesia plan should be briefly reviewed, including alerting the neuro-anesthesiologist of the need for burst suppression during temporary clip placement and determining which agents to use, such as propofol, etomidate, or pentobarbital [2]. The operating table should be appropriate for the planned procedure and any possible additional procedures (i.e., radiolucent for endovascular use if necessary) but at a minimum should allow position changing throughout the case, such as head-of-bed alterations, Trendelenburg, reverse Trendelenburg, and bed tilting. An arterial line and Foley catheter should be placed after induction of anesthesia. Both groin regions should be shaved, prepped, and draped, if the operating room has endovascular capabilities. If an intraoperative angiogram is anticipated, a femoral arterial sheath can be placed at this time. If indocyanine green is to be used, a microscope with near-infrared indocyanine green video angiography must be available [3]. If intraoperative monitoring, such as continuous electroencephalography and motor or somatosensory evoked potentials, is to be used, it can be placed at this time. The operative microscope should be inspected and balanced, the interpupillary distance of the eye pieces adjusted for the surgeon, and a beam splitter set to the assistant’s eye piece. The surgeon should decide if he plans to operate in a standing position or sitting position. If sitting, the operating stool height and armrests should be adjusted by the surgeon. The surgeon should inspect and review the microsurgical tools with the scrub nurse prior to beginning the case. Standard instruments include retractors, microinstruments, and aneurysm clips [4]. The microinstruments should include microdissectors and probes, straight and curved microscissors, and sharp dissectors, including an arachnoid knife, diamond knife, and beaver blade. An irrigating bipolar cautery avoids the need for continuous irrigation during surgical dissection and minimizes tissue adherence to the bipolar tips. Microsuction tips should be smooth and have pressure-regulating holes to provide low-pressure suction when dissecting around arteries and veins. A high-speed drill with a perforator bit and craniotome for fashioning the craniotomy, as well as a matchstick or diamond burr for sphenoid wing, ACP, or other skull base drilling, should be available. A micro-Doppler can be invaluable for assessing patency of arteries adjacent to the clip construct.
Once the patient is deeply anesthetized, the patient’s head should be secured in a rigid head holder such as a Mayfield-Keys or Sugita, which should be radiolucent if endovascular procedures might be performed. Pin sites should not obstruct the planned surgical incision or possible extensions of the incision. The pins should be placed in a “headband” distribution, above the temporalis muscle to minimize risk of hematoma formation or pin slippage. If neuronavigation is to be used, as is often considered in cases of pericallosal or distal MCA segment aneurysms, it should be set up and the patient’s preoperative scan registered to the patient. If the self-retaining retractor system attaches to the head holder, adequate positioning of the bar and space for attaching the system should be ensured.
A strategy for brain relaxation should be considered and discussed with the neuro-anesthesiologist at this time. Mannitol is often suitable for elective operations. If the patient has central venous access, 3% normal saline can also be considered. A pCO2 goal should be discussed with the anesthesiologist, as well as any anticipated adjustments to the goal (i.e., when opening the dura). A lumbar drain or external ventricular drain can be placed preoperatively, while the patient is under general anesthesia. A ventriculostomy can also be performed intraoperatively via Paine’s point, or alternatively a subarachnoid cistern (i.e., carotid cistern or lamina terminalis) can be opened early in the operation for rapid CSF drainage [5]. Additional bony removal, such as further drilling of the sphenoid wing or an orbitozygomatic (OZ) osteotomy, should be considered prior to extensive retraction of the brain. Two units of typed and crossed blood should be in the room or on call and immediately available at the start on the case.
An appropriate incision should be planned, with every attempt made to limit the incision to behind the hairline for cosmesis. Hair should be clipped, rather than shaved. The patient’s eyes should be protected with watertight protective closure using an adhesive barrier such as a small Tegaderm (3 M, St Paul, Minn) dressing. The patient’s external auditory meatus should be protected from blood or preoperative skin preparation agents with a small plug of petroleum gauze.
During the case, the surgeon should be positioned at the patient’s head with their assistant to the left and the scrub nurse to the right. Adjustments should be made to the position based on the location of the lesion, patient positioning, and intraoperative adjustments of the bed.
Craniotomy for Anterior and Posterior Circulation Aneurysm
Pterional Craniotomy
The work horse for anterior circulation aneurysms is the pterional craniotomy with or without an OZ osteotomy. A pterional craniotomy will provide access to the internal carotid artery (ICA), anterior cerebral artery (ACA), middle cerebral artery (MCA), and distal basilar artery (BA).
Positioning
Patient is placed supine on the operating table. An ipsilateral shoulder cushion or bolster is placed to aid in neck positioning in patients with unfavorable body habitus. The head is placed with 20° extension and rotated 15°–30° contralateral to the lesion. These maneuvers will result in the malar eminence being elevated to the highest point in the surgical field and allow gravity to assist in frontal and temporal lobe retraction. The surgical field from a top-down viewpoint will provide an unobstructed view of the sphenoid wing and ACP.
Skin Incision and Scalp Dissection
The incision should begin 0.5–1 centimeter (cm) anterior to the tragus and curve anteriorly ending in the midline behind the hairline. An incision 1 cm anterior to the tragus avoids the main trunk of the superficial temporal artery and facial nerve twigs. There are several techniques for dissecting the temporalis muscle, including interfascial dissection and submuscular dissection [6]. The goal of all techniques is ultimately to protect the facial nerve and optimize exposure. The fastest and safest method is to incise the temporalis muscle at the zygomatic arch and continue along the same path as the skin incision, ending 1 cm below the superior temporal line. The dissection is then continued anteriorly, leaving a small cuff of muscle and fascia to allow for reattachment of the temporalis at the end of the case. Fishhook retractors are used to retract the scalp and muscle inferiorly in order to clearly expose the pterion.
Craniotomy
The craniotomy can be completed with a minimum of two burr holes, but as many as three or more burr holes can be used, depending on how easily the dura is stripped from the inner table of the skull. The first burr hole is placed very low on the temporal squama immediately above the zygoma and the second at the “keyhole” or pterion. Optional additional burr holes may be created at the superoanterior and superoposterior limits of the craniotomy. After the dura is stripped, the craniotome is used to fashion a bone flap by proceeding from the posterior margin of the temporal squama burr hole superiorly along the temporalis incision, curving anteromedially to the supraorbital notch. Then craniotome is taken laterally along the floor of the anterior cranial fossa to the pterional burr hole. Care must be taken when stripping the dura along the anterior aspect of the craniotomy, as the dura thins and risk of inadvertent durotomy is highest here. The craniotome is then used to carry a cut from the temporal squama burr hole superoanteriorly, until resistance is encountered at the sphenoid ridge. A cut is then made inferiorly from the pterional burr hole, again until resistance from the sphenoid ridge is met. The skull flap can then be removed by fracturing it at the sphenoid ridge. The next step is to drill down the lesser wing of the sphenoid medially until encountering the superior orbital fissure. If the frontal sinus is opened during the craniotomy, a vascularized flap of pericranium can be laid over the opening. The squamosal portion of the temporal bone is then drilled down to the floor of the middle cranial fossa.
Durotomy
The middle meningeal artery should be coagulated with bipolar cautery, and any bleeding from the bone can be controlled with bone wax. The dura should then be opened in a C shape, extending from the floor of the middle cranial fossa to the floor of the anterior cranial fossa. The dura should be retracted inferiorly with tacking sutures.
Orbitozygomatic Osteotomy
The OZ osteotomy can be added to a standard pterional craniotomy in order to improve access. An OZ osteotomy should be considered when approaching basilar apex aneurysms or any challenging anterior circulation aneurysms.
The positioning, skin incision, and durotomy are the same as the standard pterional craniotomy described above.
Osteotomy
Following the craniotomy and durotomy described for the pterional craniotomy above, the OZ osteotomy may be undertaken. A malleable ribbon retractor can be used to protect the frontal lobe and orbital contents during the osteotomy. A reciprocating saw should be used to make the cuts. The first cut is made lateral to the supraorbital notch in a posterior direction for approximately 2.5 cm and then turned laterally across the orbital roof, ending toward the inferior orbital fissure. The next cut is made through the root of the zygoma, anterior to the temporomandibular joint. The temporalis muscle is then dissected free from the bone, and it is removed in one piece. Steps of muscle dissection and osteotomy cuts of the OZ in details can be reviewed in a previously published paper [7].
Far-Lateral Craniotomy
A far-lateral suboccipital-transcondylar craniotomy offers a versatile approach to the vertebral artery (VA) and proximal posterior inferior cerebellar artery (PICA) segments.
Positioning
The patient is placed in three-quarter prone position with the side ipsilateral to the aneurysm placed up. The head should be flexed with a fingerbreadth left between the chin and the chest, in order to maintain patency of venous drainage from the skull. The head is rotated 45 degrees contralaterally and then flexed 30 degrees contralaterally toward the opposite shoulder. When completed, the ipsilateral mastoid process will be elevated to the highest point of the surgical field. The goal of these maneuvers is to place the clivus vertical to the ground. The ipsilateral shoulder is then gently taped inferiorly in order to increase space between the neck and shoulder for surgeon maneuverability.
Incision
A hockey stick incision is made, beginning at the mastoid tip and curving to the inion at the midline and then inferior to the spinous process at C4. The midline dissection is carried down along the avascular, midline nuchal ligament to the C2 spinous process. It is then carried laterally 1 cm below the superior nuchal line, leaving a muscular-facial cuff for reattaching the muscle at the end of the case. The muscle flap is then retracted inferolaterally. The VA is identified and protected as it travels medially from the C1 transverse foramen to the sulcus arteriosus of the posterior arch of C1.
Craniotomy
A curette is used to strip the dura from the posterior margin of the foramen magnum, and the craniotome is used to fashion a craniotomy. The bone cut begins at the foramen magnum and is taken superiorly to the transverse sinus. It is then curved laterally to the sigmoid sinus and finally taken inferiorly, ending at the foramen magnum. If the patient has adherent dura and a burr hole is required, it can be placed at the asterion, junction of the transverse and sigmoid sinus, in the superolateral aspect of the craniotomy. The posterior arch of C1 is then removed with the craniotome. Two cuts are required, with the first cut medial to the sulcus arteriosus and the second cut in the midline of the posterior arch. Next, the lateral foramen magnum and posterior one-third to half of the occipital condyle are removed with a diamond bit in a high-speed drill. Significant venous bleeding from the condylar emissary vein is encountered as the condyle is drilled down, but it is easily controlled with bone wax and should not preclude further drilling of the condyle if less than the desired amount has yet to be removed. Care must be taken to avoid removing greater than two-thirds of the condyle, as this may compromise stability of the occipital cervical junction requiring operative fixation [8].
Durotomy
A dural incision is made beginning from the inferior midline portion of the C1 laminectomy in a superior direction across the circular sinus. Cuts are then made from the superior and inferior extents of the vertical dural incision, toward the lateral extent of the craniotomy. Dural tacking sutures are then used to retract the dural flap laterally.
Unique Considerations for Aneurysms of the Anterior Communicating Artery Complex
The anterior communicating artery (AcoA) is the connection between the right and left anterior hemisphere circulations, constituting the anterior-most segment of the circle of Willis. It bridges the two A1 segments of the ACA, is superior to the optic chiasm, and lies approximately 12.7 mm medial to the bifurcation of the internal cerebral artery [9]. The AcoA is, on average, between 2 and 3 mm in length [9]. There are many named arterial branches surrounding the AcoA complex, which warrant strong consideration when planning and executing a surgical strategy. These include the bilateral A1 segments, A2 segments, recurrent artery of Heubner, AcoA perforators, orbitofrontal branches, and frontopolar segments. In addition, there are on average 6.4 branches arising from the superior surface of the A1 segment, which project into the anterior perforated substance [10]. Surgical team should be aware of the presence of the median artery of callosum, which is a large single trunk of AcoA perforator, or so-called A2.
When evaluating AcoA aneurysms, careful assessment of the adjacent vasculature is important. Asymmetry of the A1 segment is an anatomic variant that contributes to the formation of aneurysms [9, 11]. While found in 10% of the general population, this phenotype is seen in approximately 80% of patients with AcoA aneurysms [11, 12]. Angiographically occult A1 segments are often encountered, but true aplasia of an A1 segment is very rare; in patients with suspected A1 segment aplasia, an A1 segment is often identified intraoperatively [13, 14]. AcoA complex aneurysms typically lend themselves to a standard pterional craniotomy, but in the case of large or giant aneurysms, an OZ osteotomy or bifrontal craniotomy may be required. In cases of symmetric A1 segments, a right-sided craniotomy is preferred to avoid retraction of the language-dominant hemisphere. However, in cases of hypoplastic A1 segments, an approach ipsilateral to the dominant A1 is preferred to allow for proximal arterial control, early visualization of the aneurysm neck, and aneurysm dome avoidance.
AcoA aneurysms typically arise from two sections of the anterior communicating artery complex: the junction of the A1 and A2 sections or the anterior communicating artery proper.
The projection of the aneurysm dome can vary in three-dimensional space and for simplification can be divided into predominantly superior-, inferior-, anterior-, and posterior-projecting. Each projection carries important operative considerations. Superior-projecting aneurysms are the most commonly encountered and are associated with asymmetric A1 segments [11]. These aneurysms will project into the interhemispheric fissure, blocking visualization of the contralateral A2 segment. Inferior-projecting aneurysms hinder visualization of the contralateral A1 segment. Anterior-projecting aneurysms block the A1–A2 junction and obscure visualization of the contralateral optic nerve. Posterior-projecting aneurysms can block visualization of the contralateral A2 segment, and the aneurysm neck is closely associated with AcoA perforators. Head rotation can be used to optimize approach to aneurysms projecting in various planes. Superior- and anterior-projecting aneurysms are optimally visualized with 30 degrees of head rotation, while posterior-projecting aneurysms are best seen with 15 degrees and inferior-projecting aneurysms with 45 degrees of head rotation [15] (Case 1, Fig. 7.1; Case 2, Fig. 7.2).
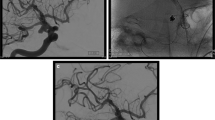
Case 1. A 76-year-old right-handed man with a history of multiple sclerosis was found to have a large anterior communicating artery (AcoA) aneurysm on head MRI done for evaluation of multiple sclerosis status. After uncomplicated microsurgical clipping, the patient was discharged home without any neurological deficit. (a–c) Preoperative, anteroposterior (AP) (a), lateral (b), and 3D reconstruction angiograms (c) show a complex large AcoA aneurysm with superior projection. (d–e) Postoperative lateral (d) and AP (e) angiograms confirm total obliteration of the aneurysm and preservation of the parent arteries. (f–h) Postoperative AP (f) and oblique (g, h) non-subtracted bone-window angiograms demonstrate the positions of a long straight and two small Sugita clips
Case 2. A 65-year-old woman with a new onset of seizure was found to have three cerebral aneurysms during workup. After uncomplicated microsurgical clipping, the patient was discharged home without any neurological deficit. (a) Preoperative coronal brain MRI shows partially thrombosed giant middle cerebral artery (MCA) bifurcation aneurysm (red arrow). (b) Preoperative oblique angiogram demonstrates an AcoA aneurysm (black arrow), the giant partially thrombosed right MCA bifurcation aneurysm (red arrow), and a right M2 aneurysm (blue arrow). (c) Preoperative 3D reconstruction angiogram of the AcoA aneurysm (black arrow), the right giant MCA (red arrow), and the right M2 bifurcation aneurysms (blue arrow). (d) Postoperative 3D reconstruction (d), postoperative AP (e), and oblique (f) angiograms show complete obliteration of aneurysms with multiple clips
Unique Considerations for Posterior Communicating Aneurysms
The posterior communicating artery (PcoA) is the connection between the anterior and posterior intracranial circulation. It arises from the posteromedial communicating segment of the ICA within the carotid cistern. The origin of the PcoA is immediately proximal to the anterior choroidal artery (AChorA), which must be carefully protected during operative interventions. The PcoA courses posteromedially over the oculomotor nerve to connect with the second segment of the posterior cerebral artery (PCA), within the interpeduncular cistern. During embryologic development, the PcoA initially supplies blood to the PCA, but in the majority of people, the basilar artery eventually supplants the PcoA in this role. When this fails to occur, as is the case in approximately 8–20% of the population, fetal posterior circulation is retained, resulting in a PcoA that courses posterolaterally, either dorsal or lateral to the oculomotor nerve [13, 16, 17].
There are a variable number – approximately eight – of anterior thalamo-perforating arteries arising from the superior surface of the PcoA; these supply the thalamus, hypothalamus, subthalamus, and internal capsule. The perforators variably supply, in decreasing frequency, the anterior floor of the third ventricle, posterior perforated substance, optic tract, pituitary stalk, and optic chiasm [14]. The premammillary, or thalamotuberal, branch is the largest, often arising in the middle of the PcoA. This branch courses anteriorly to the mammillary bodies to enter the floor of the third ventricle, supplying the posterior hypothalamus, ventral thalamus, and posterior limb of the internal capsule [14, 18].
Caution must be taken when evaluating the PcoA, because its caliber is highly variable and does not correlate to the number or size of essential anterior thalamoperforators arising from it [19]. To further complicate matters, evaluation of anterior thalamoperforators is often difficult during catheter angiograms, as the MCA candelabra often obscures their opacification. The anterior thalamoperforators arise within the anterior half of the PcoA in 50%, the posterior half of the PcoA in 20%, and equally the anterior and posterior halves in 25% of the population [14]. If the PcoA should be sacrificed during surgery, it is imperative to sacrifice it as closely as possible to the junction with the second segment of the PCA artery. Additionally, the anterior thalamoperforators should be excluded from the clip construct.
PcoA aneurysms represent approximately 26% of all ICA aneurysms [20]. The clinical presentation of these aneurysms is most commonly either SAH or, unique to PcoA aneurysms, a pupil-involved oculomotor palsy (ONP). Approximately 20% of PcoA aneurysms present with ONP. Conversely, 80% of patients with pupil-involving third nerve palsies will have a PcoA aneurysm. As such, an isolated pupil-involving third nerve palsy should be considered secondary to a PcoA until proven otherwise. A third nerve palsy, whether in the context of a ruptured or unruptured aneurysm, is often seen with posterior- and inferior-projecting aneurysms. Aneurysms presenting with third nerve palsies often improve following occlusion of the aneurysm, with chance of recovery likely related to time from onset. The choice to clip or coil PcoA aneurysms presenting with third nerve palsies can present a conundrum for treating physicians. Several meta-analyses indicate significant, superior recovery of oculomotor nerve palsies in patients with ruptured PcoA aneurysm treated with surgical clip ligation of the aneurysm. However, recovery rates of oculomotor palsies associated with unruptured aneurysms treated with clip ligation versus endovascular coiling are not significantly different [21, 22].
The approach of PcoA aneurysm projections presents unique intraoperative challenges. When infratentorial and projecting posterolaterally, these aneurysms increase the risk for ONP or adherence to the oculomotor nerve. Dissecting the aneurysm from the oculomotor nerve is unnecessary, as traction may result in a permanent deficit and the decrease in pulsations from a treated aneurysm likely is the major contributor to resolution of ONP seen in treated, unruptured aneurysms. These aneurysms may also be supratentorial and project superolaterally. In this case, they may become adherent to the temporal lobe. In the setting of a rupture, this could result in a temporal lobe intracerebral hemorrhage or temporal horn intraventricular hemorrhage. Alternatively, the aneurysm may adhere to the tentorial dura, in which case patients may present with a subdural hematoma in the event of rupture [14]. When projecting anterolaterally, the aneurysm may obscure the origin of the PcoA during operative dissection.
The majority of PcoA aneurysms should be accessible through a pterional craniotomy. In some giant or large PcoA aneurysms or in the presence of very short and lateral course of supraclinoid ICA, ACP removal is indicated. We prefer extradural drilling of the ACP in such cases (Case 3, Fig. 7.3a–e). The origins of the PcoA, the anterior thalamoperforators, and the AChorA should be identified, with the latter two often displaced medially by large PcoA aneurysms. If the aneurysm is adherent to the temporal lobe, care should be taken during dissection to ensure the dome is not avulsed. A simple straight or gently curved clip is generally sufficient for occluding these aneurysms.
Case 3. (a) A 64-year-old male presented with seizures. Brain MRI shows a giant thrombosed aneurysm of the fetal PcoA. Axial T2 (a, b) and coronal T1 (c, d) MR images demonstrate the detailed location of the aneurysm and the mass effect to the temporal and frontal lobes of the brain. (b) Preoperative DSA (a, b, c, d) revealed multilobulated PcoA aneurysm. (c) Intraoperative pictures via right cranio-orbital approach show PcoA (yellow arrow) and the aneurysm (blue A) (a, b). After temporary clipping (c), further dissection was achieved, and thrombosed part of the aneurysm was started to be cut (d). A combination of sharp (d) and blunt dissection was done under temporary clipping to reveal the detailed anatomy of the aneurysm, the surrounding brain, and the cerebral vasculature. (d) Intraoperative image shows the primer incision (yellow arrow) to the thrombosed part of the aneurysm (a). The thrombus inside the aneurysm was dissected out for the elimination of the mass effect (b). After removal of the thrombosed portion, the vascular anatomy around the aneurysm was able to be seen better (c). The intraoperative picture shows the final view after total removal of the thrombus and aneurysm wall around it (d). (e) Intraoperative picture shows preservation of the PcoA (yellow arrow) after clipping (a). The flow of the PcoA was confirmed with a micro-Doppler ultrasound (b). Postoperative DSA images show total obliteration of the aneurysm with preservation of the cerebral vasculature (c, d)
Unique Considerations for Anterior Choroidal Artery Aneurysms
The AChorA arises from the communicating segment of the ICA 2–5 mm distal to the origin of the posterior communicating artery [11, 23, 24]. The AChorA is a small vessel ranging from 0.5 to 2 mm in diameter [11, 24, 25]. The majority of AChorA arise as a single trunk [11, 24]. The two segments of the AChorA are the cisternal segment, which encompasses the origin to the choroidal fissure, and the plexal segment, which encompasses the branches passing through the choroidal fissure to supply the choroid plexus of the temporal horn [11, 24, 25]. The cisternal segment of the AChorA projects posterolaterally from the ICA, coursing through the carotid cistern before turning posteromedially into the crural cistern. From there, it continues to the ambient cistern prior to entering the choroidal fissure. Between 2 and 18 perforators arise along the course of the cisternal segment of the AChorA, including at the origin of the AChorA [26]. These perforators variably supply many structures, leading to a spectrum of presentations of infarcts, ranging from asymptomatic to severely disabling strokes. The most consistent deficit is contralateral hemiplegia secondary to infarction of the pyramidal tract in the posterior limb of the internal capsule [27]. Other possible sequelae of infarcts include contralateral hemisensory loss (due to involvement of the ventral posterolateral nucleus of the thalamus or thalamocortical sensory fibers) or homonymous hemianopia (from a lateral geniculate body or geniculocalcarine tract infarction) [27].
Aneurysms of the AChorA are infrequent, occurring as 2–5% of all intracranial aneurysms [26]. AChorA aneurysms arise at the posterolateral wall of the communicating segment of the internal carotid artery and project laterally along the course of the AChorA. Lehecka et al. described four possible variations of AChorA aneurysms in relation to the parent vessel [26]. The first is the aneurysm arising anterolateral to the AChorA; the second is the aneurysm arising superolateral to the AChorA; the third is the aneurysm arising posterolateral to the AChorA; and the fourth is the aneurysm arising between a duplicated AChorA [26]. Given the small size of the AChorA and the many important perforators arising from it, there is little room for error in clipping, with rates of permanent AChorA syndrome ranging from 5.3% to 15.7% in surgical series [28, 29].
A standard pterional craniotomy is often adequate for approaching these aneurysms. The head should be turned contralaterally, approximately 20°, tilted laterally, and kept neutral in an anterior-posterior direction [26, 30]. The goal of these maneuvers is to position the Sylvian fissure vertically in order to facilitate exposure of the distal ICA. One must avoid turning the head too far contralaterally, which would cause the temporal lobe to block visualization of the aneurysm. As these aneurysms are often intimately involved with the medial temporal lobe, it is important to avoid undue traction on the lobe given the risk of aneurysm rupture in cases where the aneurysm is adherent to the medial temporal lobe. A wide Sylvian dissection from the limen insula to the ICA bifurcation and entire length of the supraclinoid ICA is crucial to have full control of the proximal and distal neck of the aneurysm. Once the final clip has been placed, it is essential to confirm patency of the AChorA with either microvascular Doppler, noninvasive indocyanine green angiography, or catheter angiography.
Unique Considerations for Ophthalmic Artery Aneurysms
The ophthalmic segment of the ICA extends from the cavernous sinus roof to the origin of the PcoA. The dura originating from the superomedial ACP forms the distal dural ring, the site at which the ICA enters the subarachnoid space. This ring of dura, which is thick laterally and thins medially, acts as an anchor between the ICA and adjacent structures. The ophthalmic artery projects medially from the superomedial surface of the ophthalmic segment of the ICA immediately distal to the distal dural ring. In approximately 8% of the population, the ophthalmic artery will arise from the intracavernous carotid artery [13]. The artery projects medially beneath the optic nerve; it enters the optic canal inferolateral to the optic nerve. Distal to the origin of the ophthalmic artery, the superior hypophyseal artery (SHA) arises posteromedially and is the only other named branch arising from the ophthalmic segment of the ICA. The SHA ranges from a single branch to as many as five small branches [14]. No perforators from this segment supply the brain parenchyma, but some may supply the optic nerve or chiasm. Ophthalmic artery aneurysms most often arise just distal to the origin of the ophthalmic artery and project superiorly or superomedially. Aneurysms originating further along the ophthalmic ICA segment, incorporating the SHA or perforating branches, are considered SHA aneurysms. Ophthalmic artery aneurysms have a profound female predominance, with females representing more than 80% of patients with this particular aneurysm [31]. In addition, they have a tendency toward multiplicity, with 45% of patients harboring multiple aneurysms [31].
Surgery of ophthalmic artery aneurysms, as well as other supraclinoid ICA aneurysms, necessitates a thorough anatomic understanding of the ACP. The ACP is the posterior continuation of the lesser wing of the sphenoid bone, forming the roof of the superior orbital fissure and the lateral wall of the optic canal. The falciform ligament is the dura from the ACP to the planum sphenoidale and can form a sharp edge, which may compress the optic nerve. The ACP often blocks intraoperative visualization of the ophthalmic artery origin and should be removed to provide adequate exposure of the proximal ICA for adequate proximal control.
Understanding the relationship of the ophthalmic artery to the optic nerve is important for understanding clinical presentations and surgical approaches to these aneurysms. The ophthalmic artery often runs inferolaterally to the optic nerve in the optic canal. As such, ophthalmic artery aneurysms are associated with visual symptoms in approximately 30% of patients. With aneurysms of 1 cm or more, this is often manifested as an ipsilateral, monocular nasal visual field deficit [31, 32]. Initially, an aneurysm may compress the inferolateral optic nerve and cause a superior nasal quadrantanopia. With larger aneurysms, the superolateral optic nerve is displaced superomedially into the falciform ligament, causing an inferior nasal quadrantanopia. Surgical series report variable degrees of vision improvement following surgical clip ligation of ophthalmic artery aneurysms [18].
An ipsilateral pterional craniotomy with unroofing of the optic canal and removal of the ACP is the most appropriate approach for the majority of these aneurysms. When bilateral, it is possible to address contralateral ophthalmic ICA segment aneurysms from a single approach. With bilateral ophthalmic segment aneurysms, it is advised to approach the symptomatic or larger aneurysm from an ipsilateral craniotomy. When positioning for ophthalmic artery aneurysms, the anterior cranial fossa should be vertically oriented to optimize the operative view of the clinoidal triangle. When the ACP is likely to be drilled or aneurysms are large and/or complex, it is prudent to have the neck prepped for access to the cervical ICA for proximal control. After the initial pterional craniotomy is completed, the posterior orbital roof and the superior and medial surfaces of the superior orbital fissure are removed extradurally. This is followed by removal of the ACP. For unruptured aneurysms, either an intradural or extradural resection can be chosen. However, in ruptured aneurysms an intradural removal of the ACP might be chosen to provide early visualization and access to the aneurysm in the case of an intraoperative rupture. After removing the ACP, cavernous sinus bleeding is often encountered from the cavernous cave (the space between the proximal and distal dural rings housing the clinoidal segment of the ICA), which can be controlled with packing with a hemostatic agent or injecting fibrin glue. Often the optic nerve must be manipulated during aneurysm dissection, but it is important to section the falciform ligament prior to mobilizing the optic nerve, as compression against the falciform ligament may cause or exacerbate visual field deficits. A straight or side-angled clip is often appropriate for clipping ophthalmic artery aneurysms, but a fenestrated, angled clip is typically necessary for SHA aneurysms, given ICA perforators’ intimate involvement with these aneurysms (Case 4, Fig. 7.4).
Case 4. A 49-year-old female, with a complaint of continuous headache after a major car accident, was found to have an ophthalmic artery aneurysm on her workup. After uncomplicated microsurgical clipping performed via pterional craniotomy with extradural drilling of the ACP and optic roof, and sectioning the entire distal dural ring, the patient was discharged home without any neurological deficit. (a–d) Preoperative, anteroposterior (AP) (a), lateral (b), oblique (c), and 3D reconstruction angiograms (d) show a right complex ophthalmic bilobed aneurysm with involving clinoidal extradural ICA. (e, f) Postoperative AP (e) and lateral (f) angiograms reveal total obliteration of the aneurysm and preservation of the parent arteries. (g, h) Postoperative AP bone-window DSA (g) and 3D reconstruction angiogram (h) reveal successful clipping of the aneurysm and the positions of the multiple aneurysm clips
Unique Considerations for MCA Aneurysms
The MCA is the larger terminal branch of the ICA. It projects laterally, consisting of four segments. These are the M1 (sphenoidal), the M2 (insular), the M3 (opercular), and the M4 (cortical) segments [33]. The sphenoidal segment extends laterally from the bifurcation of the ICA, parallel to the sphenoid ridge, to the first genu of the MCA at the limen insula. This segment often contains the initial division of the MCA. The insular segment extends from the limen insula to the turn of the branches at the circular sulcus. The opercular segment extends from the circular sulcus through the operculum until reaching the convexity. The cortical segment is composed of the branches on the hemispheric surface. Lenticulostriate arteries arise from the posterior aspect of the M1 and M2 segments and enter the anterior perforated substance to supply the internal capsule and parts of the corpus striatum [14].
In more than 80% of patients with MCA aneurysms, the lesion arises at the initial division of the MCA trunk. However, in 12% of patients, they will arise from the more proximal portion of the M1 segment [14]. Mycotic and traumatic aneurysms are more commonly located along the most distal MCA segments. MCA bifurcation aneurysms project laterally in 45%, inferiorly in 38%, superiorly in 15%, and medially in 2% [34].
MCA aneurysms presented with rupture in 90% of patients in one case series, but as with other aneurysms, they can present with focal neurologic deficits due to mass effect when they are large or giant in size [34].
MCA bifurcation aneurysms are best approached through a pterional craniotomy. When positioning for these aneurysms, the head should be elevated above the heart to aid in venous drainage, in slight extension for gravity-assisted frontal lobe retraction, and turned 20°–30° to the contralateral side. Rotation beyond 30 degrees risks shortening the angle between the operative view and the M1 course, causing it to appear shorter and causing the aneurysm dome to obscure the aneurysmal neck [14]. Modifications to the craniotomy for MCA bifurcation aneurysms include the following: ensuring the bone flap is flush with the anterior cranial fossa and medial enough to allow a subfrontal view of the M1 segment of the MCA, as well as removing the lateral sphenoid wing until entrance of the meningo-orbital artery. When bony removal is flush with the anterior cranial fossa, less frontal lobe retraction is necessary for viewing the MCA bifurcation. Following the craniotomy and dural opening, the Sylvian fissure must be separated for access to the proximal MCA segments. The Sylvian fissure may be opened in a medial to lateral or lateral to medial direction. A medial to lateral opening of the Sylvian fissure allows visualization of the terminal ICA and proximal M1 segment distal to the aneurysm, affording access to vessels for proximal control prior to encountering the aneurysm. A medial transsylvian approach is the safest approach to the majority of lateral- and superior-projecting MCA aneurysms. A lateral to medial opening of the Sylvian fissure is often faster, requiring less dissection. However, the aneurysm dome is encountered before the aneurysm neck is seen and access to vasculature for proximal control is not possible prior to exposing the aneurysm. A lateral transsylvian approach is quite useful for anterior-projecting aneurysms, which can obscure the proximal MCA exposure and may have domes adherent to the sphenoid ridge. A lateral transsylvian approach can also be used for inferior-projecting aneurysms, as the dome will be encountered following exposure of the aneurysm neck and proximal MCA segment. When dividing the Sylvian fissure, the arachnoid should be sharply dissected along the frontal aspect of the Sylvian fissure in order to preserve the inferior draining connections from the Sylvian veins to the sphenoparietal sinus. Veins bridging the frontal lobe and Sylvian fissure can be safely divided, but the superficial and middle cerebral veins must be preserved. If brain relaxation measures have caused the Sylvian fissure to become difficult to identify, the cortical MCA segments can be traced medially to where they emerge from the fissure. A straight or gently curved clip placed parallel to the MCA divisions can be used in MCA bifurcation aneurysms (Case 2, Fig. 7.2).
Unique Considerations for PICA Aneurysms
The PICA originates from the VA. Many muscular and segmental arterial branches arise from the VA, but the two largest branches are PICA and the anterior spinal artery, which arises distal to PICA. The origin of PICA arises from the posterior or lateral wall of the VA, approximately 13–16 mm proximal to the origin of the BA [35]. In 57% of patients, PICA appears above the level of the foramen magnum [35, 36]. In 18% of patients, PICA originates below the level of the foramen magnum, and the remainder of patients’ PICA arises at the level of the foramen magnum [35, 36]. PICA is absent or hypoplastic in up to 20% of patients. PICA is often described as having five segments [35, 37,38,39]. The segments are the anterior medullary, lateral medullary, tonsillomedullary, telovelotonsillar, and cortical. The anterior medullary segment begins at the origin and extends along the anterior face of the medulla to the inferior olivary prominence. The lateral medullary segment extends from the inferior olivary prominence to the descending half of the caudal loop, corresponding to the origins of cranial nerves (CN) IX, X, and XI. The tonsillomedullary segment begins as the ascending half of the caudal loop and extends to the superior surface of the cerebellar tonsils. The telovelotonsillar segment forms the cranial loop of PICA, with a choroidal branch arising at the apex. Medullary perforators from PICA most often arise from the first three segments of PICA.
In patients with saccular PICA aneurysms, the defect arises from the PICA origin in nearly two-thirds of patients and from the distal PICA segments in one-third of patients [39]. Aneurysms arising from the PICA origin most often project superiorly, and those arising from more distal segments often occur at vessel bends along the caudal or cranial loops and project in the direction of blood flow. PICA aneurysms can also be fusiform in nature, arising along any portion of the vessel.
The most common presentation of PICA aneurysms is SAH, seen in two-thirds of patients. Focal deficits, while rare, have been described, related to mass effect from large aneurysms.
Aneurysms arising from the proximal segments of PICA can be addressed most often from a far-lateral-transcondylar approach, which provides excellent access to the VA for proximal control. Aneurysms arising from the cortical segment of PICA can often be addressed from a midline suboccipital craniotomy. A straight clip placed perpendicular to the VA can be used for these aneurysms if the origin of PICA can be clearly visualized and excluded from the clip blades. Often it is necessary to utilize a tandem clip strategy when the PICA origin is at the base of a superior-projecting aneurysm. In the case of tandem clip construct, a fenestrated straight clip is placed along the base of the aneurysm with the fenestration encircling the PICA followed by a straight clip above the portion of the aneurysm excluded by the fenestration [13]. In cases of PICA aneurysms arising from the fourth or fifth segments, the vessel may be sacrificed if necessary with only minor consequences, as the brainstem perforators arise proximally, from the first three segments (Case 5, Fig. 7.5) .
Case 5. A 61-year-old female with a known right-sided 2 cm vestibular schwannoma was found to have a 7 mm right posterior cerebellar artery (PICA) aneurysm. After uncomplicated microsurgical clipping, the patient was discharged home without any neurological deficit. (a, b) Preoperative 3D reconstruction angiogram shows a fusiform aneurysm of the proximal right PICA (arrow). (c) Postoperative intravenous digital subtraction angiogram (DSA) reveals total obliteration of the aneurysm and preservation of the parent arteries after successful microsurgical clipping
Unique Considerations for Basilar Aneurysms
The BA is formed from the union of the two vertebral arteries at the pontomedullary sulcus. It travels through the prepontine cistern in a straight configuration in 25–50% of patients and an S shape in the remainder [40]. The average length of the BA is 32 mm [41]. Fenestrations of the BA are seen in 1–5% of patients and are frequently associated with aneurysms [42]. The anterior inferior cerebellar arteries arise from the BA in the proximal portion of the BA. Along its course, the BA gives rise to an average of three paired sets of brainstem perforators: the paramedian, short circumflex, and long circumflex. It terminates in the interpeduncular cistern, bifurcating into the PCAs immediately above the origin of the paired superior cerebellar arteries. The BA terminates at the level of the posterior clinoid process in approximately 50%, above the process in 30%, and below it in 20% [35]. Surrounding the basilar bifurcation laterally are the third nerve and uncus on both sides, anteriorly the dorsum sella and upper clivus, posteriorly the mesencephalon, and superiorly the floor of the third ventricle.
The basilar bifurcation is the most common location for basilar aneurysms. Basilar bifurcation aneurysms have been associated with higher rupture risks in several series, a finding which argues for treatment of these aneurysms [43,44,45]. Around 80% of basilar bifurcation aneurysms present with hemorrhage, but when large, they may present uniquely with oculomotor nerve palsies, focal deficits from brainstem compression, or even hydrocephalus from compression of the third ventricle and cerebral aqueduct.
Many surgical approaches can be considered with basilar bifurcation aneurysms, depending on the aneurysm size, projection, and location relative to the clivus and posterior clinoid process. A right-sided approach is most often chosen to avoid retraction of the dominant hemisphere. However, in cases of established deficits, the side corresponding to the deficit should be used for the approach. Aneurysms less than 1 cm from the top of the posterior clinoid, which represent the majority of these lesions, can be approached with a pterional craniotomy combined with an OZ osteotomy. Aneurysms greater than 1 cm below the posterior clinoid are often obscured by the posterior clinoid. They may be more easily accessed via a subtemporal craniotomy, though this approach is not without shortcomings, as it requires significant retraction of the temporal lobe and does not provide reliable visualization of the contralateral P1 segment of the PCA. It is essential to preserve all brainstem perforators throughout the dissection, as disruption of even a small perforator may leave the patient with profound deficits. Recently, a transcavernous approach has gained wide acceptance for access to basilar bifurcation aneurysms. This consists of extradural drilling of the ACP and skeletonizing the oculomotor nerve. This is the approach of choice in our practice for almost all of the basilar bifurcation aneurysms. In cases with a low basilar bifurcation, posterior clinoid drilling is necessary for proximal control. A straight or gently curved clip applied parallel to the PCA is often acceptable for clipping basilar bifurcation aneurysms.
References
Murayama Y, Saguchi T, Ishibashi T, et al. Endovascular operating suite: future directions for treating neurovascular disease. J Neurosurg. 2006;104:925–30.
Randell T, Niemela M, Kytta J, et al. Principles of neuroanesthesia in aneurysmal subarachnoid hemorrhage: the Helsinki experience. Surg Neurol. 2006;66:382–8. discussion 8.
Roessler K, Krawagna M, Dorfler A, Buchfelder M, Ganslandt O. Essentials in intraoperative indocyanine green videoangiography assessment for intracranial aneurysm surgery: conclusions from 295 consecutively clipped aneurysms and review of the literature. Neurosurg Focus. 2014;36:E7.
Hernesniemi J, Niemela M, Karatas A, et al. Some collected principles of microneurosurgery: simple and fast, while preserving normal anatomy: a review. Surg Neurol. 2005;64:195–200.
Paine JT, Batjer HH, Samson D. Intraoperative ventricular puncture. Neurosurgery. 1988;22:1107–9.
Poblete T, Jiang X, Komune N, Matsushima K, Rhoton AL Jr. Preservation of the nerves to the frontalis muscle during pterional craniotomy. J Neurosurg. 2015;122:1274–82.
Seckin H, Avci E, Uluc K, Niemann D, Baskaya MK. The work horse of skull base surgery: orbitozygomatic approach. Technique, modifications, and applications. Neurosurg Focus. 2008;25:E4.
Rhoton AL Jr. The far-lateral approach and its transcondylar, supracondylar, and paracondylar extensions. Neurosurgery. 2000;47:S195–209.
Perlmutter D, Rhoton AL Jr. Microsurgical anatomy of the anterior cerebral-anterior communicating-recurrent artery complex. J Neurosurg. 1976;45:259–72.
Rosner SS, Rhoton AL Jr, Ono M, Barry M. Microsurgical anatomy of the anterior perforating arteries. J Neurosurg. 1984;61:468–85.
Yaşargil MG. Microneurosurgery. Stuttgart/New York: Georg Thieme Verlag/Thieme Medical Publishers; 1987.
Kirgis HD, Fisher WL, Llewellyn RC, Peebles EM. Aneurysms of the anterior communicating artery and gross anomalies of the circle of Willis. J Neurosurg. 1966;25:73–8.
Lawton MT. Seven aneurysms: tenets and techniques for clipping. New York: Thieme; 2011.
Le Roux PD, Winn HR, Newell DW. Management of cerebral aneurysms. Philadelphia: Saunders; 2004.
Ozdemir M, Comert A, Ugur HC, Kahilogullari G, Tubbs RS, Egemen N. Anterior communicating artery aneurysm surgery: which is the most appropriate head position? J Craniofac Surg. 2014;25:2205–8.
Ture U, Yasargil MG, Al-Mefty O, Yasargil DC. Arteries of the insula. J Neurosurg. 2000;92:676–87.
Gibo H, Lenkey C, Rhoton AL Jr. Microsurgical anatomy of the supraclinoid portion of the internal carotid artery. J Neurosurg. 1981;55:560–74.
Spetzler RF, Kalani Y, Nakaji P. Neurovascular surgery. New York: Theime; 2015.
Vincentelli F, Caruso G, Grisoli F, Rabehanta P, Andriamamonjy C, Gouaze A. Microsurgical anatomy of the cisternal course of the perforating branches of the posterior communicating artery. Neurosurgery. 1990;26:824–31.
Sahs AL, Perret G, Locksley HB, Nishioka H, Skultety FM. Preliminary remarks on subarachnoid hemorrhage. J Neurosurg. 1966;24:782–8.
Zheng F, Dong Y, Xia P, et al. Is clipping better than coiling in the treatment of patients with oculomotor nerve palsies induced by posterior communicating artery aneurysms? A systematic review and meta-analysis. Clin Neurol Neurosurg. 2017;153:20–6.
Gaberel T, Borha A, di Palma C, Emery E. Clipping versus coiling in the management of posterior communicating artery aneurysms with third nerve palsy: a systematic review and meta-analysis. World Neurosurg. 2016;87:498–506. e4
Fujii K, Lenkey C, Rhoton AL Jr. Microsurgical anatomy of the choroidal arteries: lateral and third ventricles. J Neurosurg. 1980;52:165–88.
Rhoton AL Jr, Fujii K, Fradd B. Microsurgical anatomy of the anterior choroidal artery. Surg Neurol. 1979;12:171–87.
Erdem A, Yasargil G, Roth P. Microsurgical anatomy of the hippocampal arteries. J Neurosurg. 1993;79:256–65.
Lehecka M, Dashti R, Laakso A, et al. Microneurosurgical management of anterior choroid artery aneurysms. World Neurosurg. 2010;73:486–99.
Leys D, Mounier-Vehier F, Lavenu I, Rondepierre P, Pruvo JP. Anterior choroidal artery territory infarcts. Study of presumed mechanisms. Stroke. 1994;25:837–42.
Lee YS, Park J. Anterior choroidal artery aneurysm surgery: ischemic complications and clinical outcomes revisited. J Korean Neurosurg Soc. 2013;54:86–92.
Bohnstedt BN, Kemp WJ 3rd, Li Y, et al. Surgical treatment of 127 anterior choroidal artery aneurysms: a cohort study of resultant ischemic complications. Neurosurgery. 2013;73:933–9. discussion 9-40.
Heros RC. Microneurosurgical management of anterior choroidal artery aneurysms. World Neurosurg. 2010;73:459–60.
Day AL. Aneurysms of the ophthalmic segment. A clinical and anatomical analysis. J Neurosurg. 1990;72:677–91.
Date I, Asari S, Ohmoto T. Cerebral aneurysms causing visual symptoms: their features and surgical outcome. Clin Neurol Neurosurg. 1998;100:259–67.
Gibo H, Carver CC, Rhoton AL Jr, Lenkey C, Mitchell RJ. Microsurgical anatomy of the middle cerebral artery. J Neurosurg. 1981;54:151–69.
Rinne J, Hernesniemi J, Niskanen M, Vapalahti M. Analysis of 561 patients with 690 middle cerebral artery aneurysms: anatomic and clinical features as correlated to management outcome. Neurosurgery. 1996;38:2–11.
Huber P, Bosse G. Cerebral angiography. New York: Thieme-Stratton; 1982.
Bambakidis NC, Dickman CA, Spetzler RF, Sonntag VKH. Surgery of the craniovertebral junction [DVD included]. New York/Stuttgart: Thieme; 2013.
Lister JR, Rhoton AL Jr, Matsushima T, Peace DA. Microsurgical anatomy of the posterior inferior cerebellar artery. Neurosurgery. 1982;10:170–99.
Rhoton AL Jr. Anatomy of saccular aneurysms. Surg Neurol. 1980;14:59–66.
Hudgins RJ, Day AL, Quisling RG, Rhoton AL Jr, Sypert GW, Garcia-Bengochea F. Aneurysms of the posterior inferior cerebellar artery. A clinical and anatomical analysis. J Neurosurg. 1983;58:381–7.
Lang J. Skull base and related structures: atlas of clinical anatomy. Stuttgart: Schattauer; 2001.
Saeki N, Rhoton AL Jr. Microsurgical anatomy of the upper basilar artery and the posterior circle of Willis. J Neurosurg. 1977;46:563–78.
Campos J, Fox AJ, Vinuela F, et al. Saccular aneurysms in basilar artery fenestration. AJNR Am J Neuroradiol. 1987;8:233–6.
International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms – risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339:1725–33.
Ishibashi T, Murayama Y, Urashima M, et al. Unruptured intracranial aneurysms: incidence of rupture and risk factors. Stroke. 2009;40:313–6.
Wermer MJ, van der Schaaf IC, Algra A, Rinkel GJ. Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: an updated meta-analysis. Stroke. 2007;38:1404–10.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Towner, J., Miranpuri, A.S., Cikla, U., Baskaya, M.K. (2019). Microsurgical Clipping of Unruptured Aneurysms: The Basics. In: Spiotta, A., Turner, R., Chaudry, M., Turk, A. (eds) Management of Cerebrovascular Disorders. Springer, Cham. https://doi.org/10.1007/978-3-319-99016-3_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-99016-3_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99015-6
Online ISBN: 978-3-319-99016-3
eBook Packages: MedicineMedicine (R0)