Abstract
Purpose of review
Cerebral aneurysms are commonly diagnosed incidentally with non-invasive neuro-imaging modalities (i.e., brain MRA and/or head CTA). The first decision to be made in the management of patients with unruptured cerebral aneurysms is to determine if the aneurysm should undergo treatment as any intervention carries a risk of morbidity and mortality.
Recent findings
The multiple risk factors that are associated with increased risk of aneurysm rupture should be evaluated (size, shape, and location of aneurysm; history of hypertension and cigarette smoking and family history of cerebral aneurysms). With the advent and rapid evolution of less traumatic neuro-endovascular surgery techniques in the past two decades, many more patients are undergoing treatment of cerebral aneurysms. The neuro-endovascular surgeon has multiple options for the treatment of aneurysms including coiling, with or without balloon/stent assistance, and flow diversion. A number of intrasaccular devices for the neuro-endovascular treatment of cerebral aneurysms are being evaluated. The percentage of patients with cerebral aneurysms treated with craniotomy and clip ligation is decreasing. This is controversial as it has direct impact in neurosurgical training and the aneurysms that are usually recommended for microsurgical clipping are the ones with challenging anatomy that cannot be treated safely with endovascular approaches.
Summary
The best outcomes are achieved with management by experienced, high-volume practitioners at specialized cerebrovascular treatment centers that consist of individuals with dedicated training in neuro-endovascular surgery as well as individuals trained in open cerebrovascular neurosurgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The clinical decision-making involved with managing patients who harbor unruptured intracranial aneurysms (UIA) is complex. There are no universally accepted protocols regarding who and when treatment is warranted. Therapeutic decisions are best made in a collaborative, multidisciplinary setting with the involvement of a cerebrovascular team. This team often involves a neurosurgeon, a neuro-endovascular surgeon, and a vascular neurologist. Patient education regarding the pathogenesis of intracranial aneurysms and ultimately their desires regarding intervention are also critical elements that drive treatment recommendations as well. Here, we review the current diagnostic and therapeutic considerations for the management of unruptured cerebral aneurysms. Conservative management, endovascular therapy, and microsurgical treatments are discussed in detail (Table 1).
Clinical presentation and diagnostic imaging
Intracranial aneurysms have a prevalence of about 2–3% in the general population. They commonly develop at sites of vessel branching where wall shear stress is high. Histologically, cerebral aneurysms have lost the internal elastic lamina of the vessel wall which separates the intima from the concentric smooth muscle layer called the media. Loss of elastic fibers promotes outpouching of the remaining vessels layers.
The annual rate of rupture is specific to the aneurysm location, size and configuration, and other patient factors. These patient factors that modulate both the development and ultimately the rupture risk of aneurysms include medical comorbidities such as hypertension, toxic habits including smoking, and whether or not there is a family history of cerebral aneurysms. Syndromes associated with the development of intracranial aneurysms include autosomal dominant polycystic kidney disease, Ehlers-Danlos syndrome type IV, and Marfan syndrome. Overall, an average aneurysm rupture rate of 1–2% is often quoted.
Most unruptured intracranial aneurysms are found incidentally. This often occurs during diagnostic imaging workup for unrelated pathology including most commonly headaches. Neurovascular imaging including magnetic resonance (MRA) and computed tomography (CTA) angiography are favored during initial evaluation. If a decision has been made to consider treatment, catheter cerebral angiography is utilized to provide additional anatomic details and to delineate the possible therapeutic interventions.
Conservative management and indications for intervention
The treatment strategy of UIAs is based on several aspects: risk factors for aneurysm growth and rupture and the therapeutic options offered by the experienced vascular team to achieve long-term definitive aneurysmal occlusion (Table 2). Earlier research demonstrated a variance in the natural history of UIAs; however, rupture was influenced by the size, location, and shape of the aneurysm [1, 2]. An important strategy to predict the 5-year aneurysm rupture risk is the PHASES score based on patient (population, hypertension, age, earlier SAH) and aneurysm characteristics (size, site) [3]. Since higher PHASES scores were also shown to be associated with an increased risk of aneurysmal growth, this can be used as a surrogate marker for aneurysm rupture [4•, 5•]. Recently, the ELAPSS system was developed, adding shape of the aneurysm to the PHASES item scores, which more securely estimates the actual risk of growth than the PHASES score [4•]. It produces a risk stratification which has to be assessed in respect to the complication risk of the proposed intervention. Furthermore, the decision for conservative or interventional management can be supported by using the UIA treatment score (UIATS) which relies more on best practice among cerebrovascular specialists than on published data [6]. Unfortunately, a first attempt to perform a randomized controlled trial comparing conservative treatment with coiling (TEAM) did not succeed due to poor recruitment [7]. Guidelines by the American Heart Association (AHA) and the European Stroke Organization recommend treatment of hypertension and counseling of patients regarding the importance of smoking cessation, since both appear to increase the risk of UIA formation, growth, and rupture [8, 9]. The AHA guidelines advise to treat patients with documented aneurysm enlargement or in patients with a positive family history in the absence of prohibitive comorbidities [8]. Furthermore, risk factors like heavy alcohol use and familial subarachnoid hemorrhage (SAH; two or more first-degree relatives) should be taken into account in estimating the risk of rupture [10]. All of the above-mentioned considerations should be extensively discussed with the patient and their family, since it concerns preventative treatment that poses possible serious complications.
In the case of conservative management, imaging follow-up with MRA or CTA should be performed since aneurysm growth of greater than 1 mm in any direction is associated with increased risk of rupture [8, 10, 11]. The frequency and duration of radiographic follow-up are variable. The first follow-up image after 6 and 12 months followed by subsequent yearly or every other year seems to be reasonable, although evidence is lacking [8, 12].
Endovascular treatment
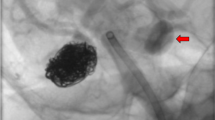
The history of endovascular therapy for cerebral vascular lesions began with the development of cerebral angiography in the 1920s by Egas Moniz. However, coil embolization of cerebral aneurysms would not become a reality until about seven decades later with FDA approval of Guglielmi detachable coils. In 1998, data on endovascular embolization of basilar tip aneurysms as an alternative to surgical clipping in patients with high-risk surgical procedures was published which further ushered in the endovascular era of aneurysm treatment [13]. In the years following, endovascular treatment (EVT) gained a prominent role in the treatment of UIAs and showed promising clinical and radiological results. Less traumatic procedures with faster recovery have accelerated the experience and technological advances in EVT of cerebral aneurysms, compared to microsurgical techniques, in the past two decades (Fig. 1).
a Digital subtraction angiogram (DSA) of right internal carotid artery (ICA) in oblique projection demonstrated a wide necked aneurysm at the origin of the large right posterior communicating artery that supplied the right posterior cerebral artery territory in a patient that presented with subarachnoid hemorrhage. b Unsubtracted image demonstrated the coil mass inside the aneurysm and dual microcatheter technique that was required due to the shape of the aneurysm and the size of the neck associated with the parent artery. Of note, there is evidence of an external ventricular drain. c DSA of the right ICA in oblique projection demonstrated occlusion of the treated aneurysm using coils and preservation of the parent artery.
The first review on EVT in UIAs included 30 studies that showed in 1379 patients a case-fatality rate of 0.6% with a permanent morbidity rate of 7% [14]. Recurrent bleeding rate was 0.9% per year in incomplete coil treatments. The authors also showed a positive timeline trend indicating that technological innovations and treatment regimens are effective in decreasing the morbidity rate of the years. A review in 2010 showed that EVT was considered a relatively safe procedure, with an annual bleeding risk of 0.2% (although limited by short clinical follow-up time) with a retreatment percentage of 9.1%, however emphasized the importance of randomized controlled trials to determine the efficacy and safety of EVT in UIAs since the lack of good-quality studies [15]. A subsequent review performed by the same authors demonstrated a higher safety of coiling with respect to liquid embolic agents and flow diversions [16]. The risk of intervention was also investigated in the ISUIA trial [1]. The authors reported an overall morbidity and mortality rate in UIA of 9.8%. A recent study showed an annual rupture rate of 0.09% over 20 years of follow-up [17]. In 2008, the first prospective study on immediate clinical outcome in patients with UIA treated by EVT (ATENA) was published [18]. Morbidity and mortality rates were 1.7% and 1.4%, respectively, of the cases; EVT failed in 4.3% and technical adverse events occurred in 15.4% of the patients. Future research is mandatory to determine the annual bleeding risk, complication risk, and the amount of procedures performed after recanalization in UIAs and proposed clinical guidelines in applying EVT in UIAs.
High recurrence rate after coil embolization especially in large and giant aneurysms as well as in those that have a wide neck [19] has generated a drive for technical improvements for endovascular devices in order to improve long-term outcomes. Techniques such as balloon-assisted coiling were introduced to improve the density of coil packing—which correlates with improved long-term aneurysm occlusion—and reduce the incidence of coil prolapse into the parent vessel [20, 21]. However, balloon-assisted coiling did not always result in a stable coil mass. Thus, a permanent stent was developed for deployment across the aneurysmal neck [22]. Multiple stent configurations have been developed, especially for bifurcation aneurysms. Since the introduction of the intracranial stents, complex constructs such as Y-stenting and X-stenting, where multiple stents were deployed with concurrent use of coils, have been applied for complex aneurysm necks [23]. Additional devices for bifurcation aneurysms are currently under development and research [24,25,26].
Flow diverter devices were introduced more recently. These minimally porous endovascular devices with high metal wall coverage were developed in order to achieve reduction of in-flow into the aneurysmal sac. They induce intrasaccular thrombosis and provide a scaffolding for endothelial overgrowth with neointima formation over the device struts across the aneurysmal neck with complete anatomical definitive cure [27]. The pipeline for uncoilable or failed aneurysms study (PUFS) which was the first prospective trial of pipeline endovascular device (PED) treatment for large and giant aneurysms lead to FDA approval of the PED for these aneurysms located on the proximal intracranial carotid artery [28]. Follow-up studies on the cohort that participated in the PUFS trial demonstrated long-term occlusion with improvement in occlusion rates and with no additional risk of rupture or any new neurological complications [29, 30]. Currently, neuro-endovascular surgeons continue to push the envelope using flow diverters for complex aneurysms in distal locations, the posterior circulation, and at vessel bifurcations [31,32,33,34].
Microsurgical treatment
History
Unlike endovascular therapy, microsurgical aneurysm treatments including clip ligation has been developed and technically refined for more than a century. Victor Horsley utilized Hunterian ligation of the internal carotid artery to treat an unruptured cerebral aneurysm in 1885. Normal Dott muscle wrapped a ruptured anterior circulation aneurysm in 1931 and is credited which being the first surgeon to directly attack an intracranial aneurysm. He subsequently pioneered aneurysm neck suture ligation which has since fallen out of favor. Walter Dandy was the first surgeon to utilize aneurysm clip ligation of a cerebral aneurysm to effect permanent obliteration. In 1937, Dandy applied a silver clip across the neck of an unruptured posterior communicating artery aneurysm in a patient that presented with a third nerve palsy. The modern era of microsurgical aneurysm treatment was ushered in by Gazi Yasargil who pioneered the use of the operating microscope for neurosurgical procedures in the 1970s.
General principles and surgical strategies
The nuances of microsurgical adjuncts and technique for aneurysm occlusion are beyond the scope of this article but general principles include immediate and permanent obliteration of blood flow across the aneurysm neck, achieving brain relaxation, utilizing cerebral protection, minimizing brain compression, and avoiding cerebral ischemia. To achieve brain relaxation, a variety of strategies are utilized including initiation of moderate hyperventilation, CSF drainage by wide opening of the basal cisterns and Sylvain fissure, utilization of osmotic diuretics such as mannitol, and dexamethasone administration. These techniques also reduce and, in many cases, eliminate the need for brain retraction. Cerebral protection during microsurgery can be achieved by initiating mild hypothermia to 34 °C and by pharmacologically promoting cerebral burst suppression. Cerebral ischemia is avoided by all the aforementioned strategies as well as meticulous operative technique, wide skull base exposure to reduce the need for retraction, and avoidance of prolonged parent vessel occlusion.
There are a number of strategies that a neurosurgeon may microsurgically utilize to obliterate flow into a cerebral aneurysm or otherwise alter the natural history of an aneurysm including direct neck clipping, flow modification using parent vessel ligation and/or cerebral bypass, aneurysm trapping, and a variety of wrapping techniques. Whenever possible, direct clipping is favored as it immediately and permanently excludes flow into the aneurysm and thus essentially eliminates any risk of future hemorrhage. Flow modification such as Hunterian ligation of a parent vessel with or without bypass does not immediately eliminate flow into the aneurysm but can, over time, promote aneurysm involution due to its promotion of blood stasis within the aneurysm sac. Aneurysm trapping is achieved by both proximal and distal clip occlusion of the parent vessel to eliminate flow into the region of the aneurysm. Trapping may or may not require adjunctive bypass to ensure that no downstream ischemia occurs. Aneurysm wrapping or clip-wrapping with various materials including cotton, muslin, Gore-Tex, and others is a second-line strategy for aneurysms that cannot be treated directly. These wrapping techniques promote fibrosis of the aneurysm wall and in some cases (clip-wrapping) directly buttress the vessel wall and thus indirectly reduce the risk of wall perforation and rupture.
Conclusion
Unruptured intracranial aneurysms are complex lesions that require a dedicated team of specialists to manage appropriately. Management does not follow strict protocols and each patient and aneurysm is considered a unique entity. Conservative, endovascular therapy and microsurgical occlusion should be discussed by the multidisciplinary cerebrovascular team for each case. Sometimes a combined endovascular and microsurgical approach is necessary for obliteration of a cerebral aneurysm. The best outcomes are achieved with management by experienced, high-volume practitioners at specialized cerebrovascular treatment centers.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown RD, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–10.
UCAS Japan Investigators, Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366:2474–82.
Greving JP, Wermer MJH, Brown RD, Morita A, Juvela S, Yonekura M, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 2014;13:59–66.
• Backes D, Rinkel GJE, Greving JP, Velthuis BK, Murayama Y, Takao H, et al. ELAPSS score for prediction of risk of growth of unruptured intracranial aneurysms. Neurology. 2017;88:1600–6 The ELAPSS score helps the practitioners to determine the risk of natural history of cerebral aneurysms.
• Backes D, Rinkel GJE, Laban KG, Algra A, Vergouwen MDI. Patient- and aneurysm-specific risk factors for intracranial aneurysm growth: a systematic review and meta-analysis. Stroke. 2016;47:951–7 Close follow up of untreated incidental aneurysms is important as growth observed on follow up examination increases the risk of hemorrhage 12X when compared to aneurysms that don’t exhibit growth.
Etminan N, Brown RD, Beseoglu K, Juvela S, Raymond J, Morita A, et al. The unruptured intracranial aneurysm treatment score: a multidisciplinary consensus. Neurology. 2015;85:881–9.
Raymond J, Darsaut TE, Molyneux AJ, TEAM collaborative Group. A trial on unruptured intracranial aneurysms (the TEAM trial): results, lessons from a failure and the necessity for clinical care trials. Trials. 2011;12:64.
Thompson BG, Brown RD, Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES, et al. Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2368–400.
Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G, et al. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 2013;35:93–112.
Etminan N, Rinkel GJ. Unruptured intracranial aneurysms: development, rupture and preventive management. Nat Rev Neurol. 2016;12:699–713.
Turan N, Heider RA, Roy AK, Miller BA, Mullins ME, Barrow DL, et al. Current perspectives in imaging modalities for the assessment of unruptured intracranial aneurysms: a comparative analysis and review. World Neurosurg. 2018;113:280–92.
Etminan N, Rinkel GJE. Cerebral aneurysms: Cerebral aneurysm guidelines—more guidance needed. Nat Rev Neurol. 2015;11:490–1.
Eskridge JM, Song JK. Endovascular embolization of 150 basilar tip aneurysms with Guglielmi detachable coils: results of the Food and Drug Administration multicenter clinical trial. J Neurosurg. 1998;89:81–6.
Lanterna LA, Tredici G, Dimitrov BD, Biroli F. Treatment of unruptured cerebral aneurysms by embolization with guglielmi detachable coils: case-fatality, morbidity, and effectiveness in preventing bleeding--a systematic review of the literature. Neurosurgery. 2004;55:767–75 discussion 775-778.
Naggara ON, White PM, Guilbert F, Roy D, Weill A, Raymond J. Endovascular treatment of intracranial unruptured aneurysms: systematic review and meta-analysis of the literature on safety and efficacy. Radiology. 2010;256:887–97.
Naggara ON, Lecler A, Oppenheim C, Meder J-F, Raymond J. Endovascular treatment of intracranial unruptured aneurysms: a systematic review of the literature on safety with emphasis on subgroup analyses. Radiology. 2012;263:828–35.
Koyanagi M, Ishii A, Imamura H, Satow T, Yoshida K, Hasegawa H, et al. Long-term outcomes of coil embolization of unruptured intracranial aneurysms. J Neurosurg. 2018:1–7.
Pierot L, Spelle L, Vitry F, ATENA Investigators. Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke. 2008;39:2497–504.
Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398–403.
Mascitelli JR, Moyle H, Oermann EK, Polykarpou MF, Patel AA, Doshi AH, et al. An update to the Raymond-Roy Occlusion Classification of intracranial aneurysms treated with coil embolization. J Neurointerv Surg. 2015;7:496–502.
Shapiro M, Babb J, Becske T, Nelson PK. Safety and efficacy of adjunctive balloon remodeling during endovascular treatment of intracranial aneurysms: a literature review. AJNR Am J Neuroradiol. 2008;29:1777–81.
Fiorella D, Albuquerque FC, Han P, McDougall CG. Preliminary experience using the Neuroform stent for the treatment of cerebral aneurysms. Neurosurgery. 2004;54:6–16 discussion 16-17.
Bartolini B, Blanc R, Pistocchi S, Redjem H, Piotin M. “Y” and “X” stent-assisted coiling of complex and wide-neck intracranial bifurcation aneurysms. AJNR Am J Neuroradiol. 2014;35:2153–8.
Chiu AH, De Vries J, O’Kelly CJ, Riina H, McDougall I, Tippett J, et al. The second-generation eCLIPs Endovascular Clip System: initial experience. J Neurosurg. 2018;128:482–9.
Gory B, Aguilar-Pérez M, Pomero E, Turjman F, Weber W, Fischer S, et al. One-year angiographic results after pCONus stent-assisted coiling of 40 wide-neck middle cerebral artery aneurysms. Neurosurgery. 2017;80:925–33.
Spiotta AM, Chaudry MI, Turner RD, Turk AS, Derdeyn CP, Mocco J, et al. An update on the adjunctive neurovascular support of wide-neck aneurysm embolization and reconstruction trial: 1-year safety and angiographic results. AJNR Am J Neuroradiol. 2018;39:848–51.
Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007;38:2346–52.
Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology. 2013;267:858–68.
Becske T, Brinjikji W, Potts MB, Kallmes DF, Shapiro M, Moran CJ, et al. Long-term clinical and angiographic outcomes following pipeline embolization device treatment of complex internal carotid artery aneurysms: five-year results of the pipeline for uncoilable or failed aneurysms trial. Neurosurgery. 2017;80:40–8.
Becske T, Potts MB, Shapiro M, Kallmes DF, Brinjikji W, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: 3-year follow-up results. J Neurosurg. 2017;127:81–8.
Dmytriw AA, Adeeb N, Kumar A, Griessenauer CJ, Phan K, Ogilvy CS, et al. Flow diversion for the treatment of basilar apex aneurysms. Neurosurgery. 2018;0:1–8.
Yavuz K, Geyik S, Saatci I, Cekirge HS. Endovascular treatment of middle cerebral artery aneurysms with flow modification with the use of the pipeline embolization device. AJNR Am J Neuroradiol. 2014;35:529–35.
Nossek E, Chalif DJ, Chakraborty S, Setton A. Modifying flow in the ICA bifurcation: pipeline deployment from the supraclinoid ICA extending into the M1 segment-clinical and anatomic results. AJNR Am J Neuroradiol. 2014;35:2125–9.
Nossek E, Zumofen DW, Setton A, Potts MB, Raz E, Shapiro M, et al. Treatment of distal anterior cerebral artery aneurysms with the pipeline embolization device. J Clin Neurosci. 2017;35:133–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Vascular Disease
Rights and permissions
About this article
Cite this article
Ellis, J.A., Nossek, E., Kronenburg, A. et al. Intracranial Aneurysm: Diagnostic Monitoring, Current Interventional Practices, and Advances. Curr Treat Options Cardio Med 20, 94 (2018). https://doi.org/10.1007/s11936-018-0695-y
Published:
DOI: https://doi.org/10.1007/s11936-018-0695-y