Abstract
Degenerative cervical myelopathy (DCM) and radiculopathy refer to a host of age-related disorders that can inflict ongoing spinal cord and/or nerve root injury in the cervical spine, causing substantial disability. This term incorporates spondylosis, disc herniation, facet arthropathy, spondylolisthesis, and ligamentous degeneration. To diagnose DCM and radiculopathy, the clinician must rely on the clinical exam as well as advanced radiographic modalities that demonstrate compromise of the neural elements. Conventional MRI has been the imaging modality of choice to confirm the diagnosis of DCM and radiculopathy. However, several studies have noted that signal intensity changes observed via conventional MRI do not convey structural changes within the spinal cord parenchyma. Moreover, findings may not convincingly correspond with disease severity or surgical outcomes in DCM. As such, new advanced MRI techniques have been studied to improve the understanding, diagnosis, and treatment of DCM.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Radiographic modalities
- DCM
- X-ray
- CT
- CT myelography
- MRI
- Upright/dynamic MRI
- Diffusion tensor imaging (DTI)
- Magnetization transfer
- Myelin water fraction
- MR spectroscopy
- Functional MRI
-
1.
Degenerative cervical myelopathy (DCM) and radiculopathy refer to a host of age-related disorders that can inflict ongoing spinal cord and/or nerve root injury in the cervical spine, causing substantial disability. Consequently, accurate and timely assessment of these disorders is paramount, which may require advanced radiographic modalities for diagnostic and therapeutic purposes.
-
2.
Conventional magnetic resonance imaging (MRI) has been the radiographic modality of choice for the diagnosis and treatment of DCM and radiculopathy.
-
3.
Unfortunately, conventional MRI findings do not convey reliable data regarding the health status of the spinal cord parenchyma. As such, these findings are not predictive of neurological status or treatment outcomes.
-
4.
Advanced MRI techniques—namely, diffusion tensor imaging (DTI), magnetization transfer (MT), myelin water fraction (MWF), magnetic resonance spectroscopy (MRS), and functional MRI (fMRI)—may help elucidate details regarding the microscopic structure and functional composition of the spinal cord parenchyma.
Introduction
Degenerative cervical myelopathy (DCM) and radiculopathy refer to a host of age-related disorders that can inflict ongoing spinal cord and/or nerve root injury in the cervical spine, causing substantial disability [1, 2]. This term incorporates spondylosis, disc herniation, facet arthropathy, spondylolisthesis, and ligamentous degeneration (hypertrophy, calcification, or ossification) [1,2,3]. In due course, these age-related degenerative changes constrict the spinal canal and/or foramen, compressing spinal cord parenchyma and/or nerve roots. Persistent compression of the spinal cord and nerve roots leads to anatomic distortion (flattening and/or widening), vascular compromise, and pathophysiological consequences (viz., endothelial cell loss, disruption of vascularization, compromise of the blood-spinal cord barrier, neuroinflammation, and apoptosis) [4] that result in lasting features of spinal cord damage (cystic cavitation, gliosis, central gray and white matter degeneration, Wallerian degeneration of posterior columns and posterolateral tracts, and anterior horn cell loss) [5].
To diagnose DCM and radiculopathy, the clinician must rely on the clinical exam as well as advanced radiographic modalities that demonstrate compromise of the neural elements [6]. Conventional MRI has been the imaging modality of choice to confirm the diagnosis of DCM and radiculopathy. However, several studies have noted that signal intensity changes observed via conventional MRI do not convey structural changes within the spinal cord parenchyma [7]. Moreover, findings may not convincingly correspond with disease severity or surgical outcomes in DCM [7, 8]. As such, new advanced MRI techniques have been studied to improve the understanding, diagnosis, and treatment of DCM [3].
Imaging Modalities
X-Rays
Plain radiographs are generally the initial imaging modality for assessment of DCM and radiculopathy. To obtain an image, a generator directs a beam of X-rays toward an object, which absorbs a variable quantity of X-rays based on its density and composition. X-rays that pass through the object are captured by a detector, which provides an image. Though limited when compared to sophisticated techniques, such as CT and MRI, plain lateral radiographs are typically obtained with the patient in the upright position and provide valuable information regarding sagittal alignment, distinguishing normal lordosis from a pathologic kyphosis, and define the extent of disc disease [1].
Accumulating evidence imply that sagittal alignment might be a contributor to disease severity in patients with DCM ; indeed, close to 12% DCM patients can possess spondylolisthesis/subluxation [3], where dynamic motion can cause sporadic spinal cord compromise. Moreover, extent of cervical kyphosis or lordosis can alter the decision between an anterior versus posterior surgical approach. In addition, if movement-dependent instability is suspected, dynamic flexion and extension radiographs can be obtained.
Dynamic lateral X-rays (such as flexion and extension films) may also be used to detect cervical instability. From cervical trauma studies [9, 10], concerns for cervical instability include (1) a translational displacement ≥ 3.5 mm and (2) angulation between adjacent vertebra ≥ 11 degrees. On the other hand, limited studies exist regarding instability associated with degenerative spondylosis; White et al. [11] evaluated this condition, where concerns for instability were a translational displacement ≥ 2 mm; overall, the authors concluded that ~1% exhibited spondylolisthesis only on dynamic films and 3% exhibited change in spondylolisthesis. Dynamic films may have limited information if patient’s mobility is compromised by neck pain.
CT
A computed tomography (CT) scan incorporates numerous X-ray images obtained from various angles to yield cross-sectional depictions of a scanned object. Moreover, modern scanners offer multiplanar reconstruction, such as orthogonal planes (coronal and sagittal), that can enhance visualization of the spinal column. This permits better assessment of the intervertebral disc spaces, which can be difficult to visualize on axial images, and of the relative relationship of the vertebral bodies, since multiple spinal levels can be studied simultaneously.
CT imaging can visualize bony anatomy effectively. This is particularly significant for assessment of calcified pathology, such as OPLL . CT has been regarded as the noninvasive modality of choice to assess calcified pathology, since a strong relationship exists between tissue density and extent of calcification/ossification. The correlation is not exact, as tissue density can be influenced by other compositions (i.e., hemosiderin content in hemorrhages). Ideally, the gold standard for diagnosis of OPLL is histological confirmation. However, no studies have formally addressed the accuracy of CT for the diagnosis of OPLL , correlating radiographic findings to histological confirmation. For other calcified pathology, such as hemorrhagic brain tumors [12], gallstones [13, 14], and atherosclerotic plaques [15], CT may not be sensitive for certain patterns.
Nevertheless, the modality remains the preferred method to assess ossification (i.e., OPLL). Compared to CT, MRI has much lower sensitivity for cervical OPLL ; large studies have shown that only 33–44% of ossified pathology could be recognized on sagittal T1 sequences and 44–57% on sagittal T2 sequences; axial T1 and T2 sequences were more sensitive (up to 91%) [16,17,18]. Moreover, a recent study involving 41 patients yielded an overall MR sensitivity of 49% [18].
CT Myelography
With CT myelography (CTM), a CT scan is obtained once radiographic contrast has been introduced into the CSF space. The contrast helps to visualize the spinal cord and nerve roots. This imaging modality is an alternative option if MRI is contraindicated (i.e., due to body habitus, metallic foreign bodies, stimulators/batteries/pacemakers, or severe claustrophobia) [19] or compromised due to the previous insertion of spinal instrumentation. The procedure however is associated with more risk, including spinal headaches associated with a dural puncture, and greater radiation exposure. Moreover, given a theoretical danger of seizures, patients are asked to hold certain medications pre- and post-myelogram, (such as phenothiazines, MAO inhibitors, antidepressants, and antipsychotics).
For most parameters (characterization of facet joint disease, lateral recess disease, spinal canal stenosis, cord size, and neural foraminal stenosis), the agreement between the interpretation of CTM compared to MRI remains modest at best [20]. CTM tends to stress more severe findings with respect to spinal canal stenosis and neural foraminal stenosis [21]. CTM can help outline bony anatomy and the nature of the pathology (i.e., OPLL). Unfortunately, depiction of soft issue pathology (including disc herniation and spinal cord edema) can be lacking; CTM fails to depict intrinsic spinal cord pathology, and if the pathology completely obliterates the CSF space, visualization distal to the block may be hampered.
MRI
With an MR scanner, a strong magnet applies an external magnetic field that orients hydrogen atoms either in a “north” or “south” direction. Pulses of radio waves can provide enough energy where the atoms “spin” the other way; once the pulse is removed, these atoms will return to their original position, releasing energy that is captured and converted into an image. By adjusting the parameters of the pulse sequence, different types of tissues can be discerned based on the relaxation properties of the hydrogen atoms. Consequently, MRI can depict impressive images of the spinal cord, nerve roots, intervertebral disc, ligament, and cerebrospinal fluid. Compared to CTM, MRI is noninvasive; moreover, the modality can visualize intrinsic spinal cord pathologies and can reveal findings distal to a complete myelographic obstruction. However, MRI can be hampered by a larger section thickness and ongoing artifacts from CSF dynamics [21].
For conventional MRI, the scan is performed with the patient supine and enclosed in a long, narrow tube (close configuration). Contraindications include large body habitus, implanted metal (foreign bodies, stimulators/batteries/pacemakers), or severe claustrophobia. An open-configuration scanner reduces claustrophobia and permits the imaging of obese patients; however, imaging quality may be diminished due to a theoretically greater inhomogeneity of the magnetic field [22].
Upright/Dynamic MRI
Supine imaging can be misleading. Approximately 20–30% of individuals who have disc protrusions or disc ruptures discovered through MRI are completely asymptomatic [23]. Moreover, symptoms may be alleviated in the supine position but exacerbate in an upright/flexion/extension position. Via cadaveric studies, nerve root compression has been associated with decreased foramen width and area [24]; moreover, flexion increases foramen width while extension decreases foramen width [25].
Upright/dynamic MRI in the cervical spine has sparsely been reported in the literature [23, 26,27,28,29,30,31]. The technique permits imaging of patients with relative contraindications (obesity, claustrophobia, severe spinal kyphosis, severe congestive heart failure, or severe chronic obstructive pulmonary disease) [23]. The technique provides reasonable resolution without artifact [32]. On the other hand, the scan time is lengthened, which increases the risk for image degradation with patient movements [23, 33].
With upright/dynamic MRI, there is a potential to diagnose occult stenosis, disc protrusion, or instability [23]. Patients can be scanned in a position that elicits symptoms [23]. Employing MRI in asymptomatic patients, Muhle et al. [34] noted that flexion significantly increased the foramen height, width, and cross-sectional area (CSA), while extension significantly decreased these parameters. In a group of symptomatic patients, Muhle et al. [35] concluded that exacerbated pain was related to a decrease in foramen size and to nerve root motion, often associated with extension and axial rotation to the side of the pain. Through a qualitative assessment of symptomatic patients, Ferreiro et al. [36] found that 4 of 31 diagnosed posterior disc herniations were only discovered in the upright-sitting position. However, focal posterior disc herniations were seen to comparatively enlarge in size for 21 patients but reduce in size for 5 patients.
MRI: Imaging Modality of Choice for DCM Patients
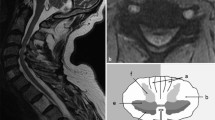
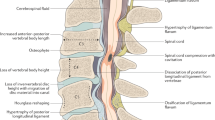
The introduction of MRI in the mid-1980s offered clinicians high-resolution anatomic images to facilitate clinical decision-making [8]. Conventional T1 and T2 sequences (along axial and sagittal planes) are typically employed for detailed visualization of the cervical spine anatomy. The normal cervical spinal cord travels through the lordotic spinal column, bounded anteriorly by vertebral bodies (VBs) and posterior longitudinal ligament (PLL), posteriorly by ligamentum flavum (LF) and lamina, and laterally by pedicles (Fig. 9.1). Kato et al. [37] described the normal morphology, age-related changes, and abnormal findings on MRI in 1211 asymptomatic patients to establish mean values for the cervical spinal canal, dural tube, and spinal cord. Spinal canal size is dependent on many factors, including spinal level, age (decreasing size with increasing age) [6, 37], gender [38], and underlying pathology (congenital stenosis or degenerative changes) [37].
With conventional MRI, degenerative changes can be visualized along the spinal column, neural foramen, and spinal cord. Spondylosis, defined as multilevel disc and bone changes, can be seen in up to 89.7% in a recent large prospective cohort study regarding DCM [3]. On both T1 and T2 sequences, the nucleus pulposus tends to be hyperintense relative to the surrounding annulus [6]. With age, the nucleus pulposus can exhibit (1) loss of T2 hyperintensity, whereby the boundaries between the nucleus pulposus and annulus become obscured; (2) loss of height, associated with the collapse of the intervertebral disc space and potential auto-fusion of adjacent VBs; and (3) herniation, which can be associated with a compromised annulus, with extrusion of disc material into the spinal canal [6, 39].
With perpetual static and dynamic stresses, VBs can exhibit morphologic alterations (such as increased anteroposterior dimensions and formation of osteophytes that border degenerated discs) [1]. Disc herniation into VB (Schmorl’s nodes) can also be seen in the cervical spine. As described by Modic et al. [40], signal intensity variation within the VBs and their end plates on MRI imply bone marrow changes associated with degenerative disc disease. Modic changes (MCs) have been classified into three types: (1) low intensity on T1W and high intensity on T2W (reflects disruption of end plates and inflammation), (2) high intensity on both T1W and T2W (reflects yellow marrow replacement), and (3) low intensity in both T1W and T2W (reflects sclerotic changes). The cervical segments with MCs were significantly more likely to have disc degeneration and spinal canal stenosis [41, 42]. The C5/C6 and C6/C7 levels are most affected [41]. Some studies have suggested that MCs denote cervical instability at the same level [43], while others found that such levels exhibit less angular motion, implying loss of mobility [42].
Finally, ligamentous pathology may be evident on MRI. Anterior compression of the cord can occur through hypertrophy (HPLL) or ossification (OPLL) of the posterior longitudinal ligament. Unfortunately, conventional MRI does not convey a clear delineation between the PLL and disc material, as both appear hypointense on T1 and T2 sequences [6]. Some sources have observed that HPLL appears isointense or mildly hyperintense to paravertebral muscles on T1, while OPLL and osteophytes look hypointense to paravertebral muscles [6, 44]. With the absence of significant spondylosis and/or with the presence of multilevel anterior compression, OPLL is suggested [6]. The pathology can be observed in up to 10.5% DCM patients [3]. CT remains the modality of choice for diagnosis of OPLL [18]. The presence of OPLL can alter surgical approach [1]. Similarly, posterior compression of the spinal cord can occur through either hypertrophy or ossification of the LF, which can be evident in up to 56.8% [3].
Diagnosis of DCM requires evidence of spinal cord compression with associated clinical signs of myelopathy [6]. Degenerative changes eventually constrict the spinal canal and compress the spinal cord parenchyma. Spinal cord compression has been quantified through various definitions. The compression ratio (CR) [45, 46], the ratio between the anteroposterior diameter and the transverse diameter, and the method of maximum spinal cord compression (MSCC) [47] have been utilized frequently. Both have limitations, as both do not account for lateral compression, while MSCC neglects variation of spinal cord size along the spinal column [6]. The C5/C6 level is the most common site of MSCC , followed by C4/C5 and C3/C4 [3]. Overall, spinal cord compression is a sensitive feature of myelopathy, but it can also present in approximately 5.3–13.3% of asymptomatic patients [37, 39, 48]. Moreover, spinal cord re-expansion (or lack thereof) may be associated with surgical outcomes [49, 50].
As the extent of spinal cord damage increases, the water content increases; this affects changes to tissue relaxation, which equates to changes on T1 and T2 sequences. With more water content, tissue exhibits hypointensity on T1 and hyperintensity on T2. Overall, as T2 is more sensitive to variation in water content than T1, changes on T2 are frequently observed prior to changes on T1. DCM patients frequently demonstrate T2 hyperintensity and, less commonly, T1 hypointensity, at the level of compression [6]. Both features are helpful diagnostic findings [6]. In particular, T2 hyperintensity can be present in up to 85% of patients with DCM [6]. Moreover, the extent of T2 hyperintensity (including size of signal change, relative intensity of signal change, and pattern of signal change) has been linked to clinical impairment in DCM patients [51]. On the other hand, T1 hypointensity, which can indicate cavitation and disruption of fiber tracts, may be a specific feature for poorer baseline neurological function and worse surgical results [52,53,54]. Snake-eye appearance, where symmetric hyperintensity of the spinal cord is observed on axial T2 sequences, is a rare finding. Through autopsy studies, this imaging feature has been associated with cystic necrosis due to mechanical compression and venous infarction, corresponding to damage of gray matter and neuronal loss in the anterior horn [55]. Not surprisingly, snake-eye appearance has been considered a poor prognostic factor for surgical outcome [55].
Limitations of Conventional MRI
Despite the utility of MRI in the assessment of DCM patients, findings can be nonspecific and do not reveal data pertaining to pathophysiology at the microscopic level. Degenerative changes on MRI can be seen in asymptomatic patients. In particular, evidence of disc degeneration (annular tears and/or disc bulge/protrusions/herniations) can be observed in up to 36.7 to 89% of patients [37, 39, 48]. Cervical OPLL can also be observed in asymptomatic patients. Several demographic factors have been associated with the pathology. National background has a profound role, as up to 3.6% patients exhibit radiographic evidence of OPLL in the Japanese population; this remains markedly higher than other Asian countries and non-Asian countries [18]. Less demonstrated, older age and male gender appear to be risk factors for disease development as well [1]. Overall, spinal cord compression can occur in up to 5.3% to 13.3% of asymptomatic patients [37, 48]; furthermore, up to 2.3% and 3.1% display T2 signal change and cord deformity, respectively [37, 39]. Per a recent systematic review by Wilson et al. [56], for patients without clinical myelopathy, but evident canal stenosis and cord compromise due to degenerative changes, 8% will exhibit clinical myelopathy at 12-month follow-up, and 23% will do so at 44-month follow-up; surprisingly, the lack of T2 hyperintensity was associated with early progression to myelopathy, while the presence of T2 hyperintensity was associated with late progressive myelopathy [56]. Risk of early progression to symptomatic myelopathy (≤12 months) was predicted by the presence of clinically symptomatic radiculopathy and abnormal SEP and MEP [57].
On the other hand, MRI findings noted in DCM patients can exhibit inadequate correlates with clinical status. Studies have observed that T2 signal changes can be delayed manifestations and may forecast worse prognosis despite surgical intervention [58]. To complicate matters, not all T2 signal changes are the equivalent; there are two broad characterizations based on the level of signal intensity and pattern of signal change [3, 51]. In addition, although T1 hypointensity is considered a specific finding of poorer baseline neurological function and worse postoperative recovery, the imaging feature is less common compared to T2 hyperintensity and can be tougher to observe as consistently as T2 hyperintensity [52,53,54, 59]. A recent systematic review [8] assessed the role of MRI characteristics to direct treatment (surgery versus conservative management) and to predict postsurgical outcomes. There are three MRI features that may be considered negative predictors of postsurgical outcomes (number of high-signal intensity segments, combined T1/T2 signal change, and signal intensity ratio) [8].
Overall, the current literature offers weak recommendations based on low strength of evidence: (1) MRI can help with the diagnosis of DCM , but physicians should depend on clinical history and examination to assess progression and severity of DCM ; (2) T2 signal may be a helpful prognostic factor but should be used in combination with other features (such as signal intensity on T1 or compression ratios) [8]. These conclusions underlie the variable tolerance of spinal cord compression in different patients, where minimal degenerative changes/“mild” stenosis can be observed on conventional MRI despite prominent clinical cervical myelopathy and vice versa.
In addition, the diagnosis of DCM depends on the assessment of spinal canal stenosis in the setting of degenerative changes. Definitive guidelines for the assessment of this feature remain deficient. Spinal canal stenosis can occur through acquired degenerative disease or congenital stenosis. Unfortunately, no clear quantitative criteria have been established to differentiate these two etiologies [6]. Recently, authors have defined an “occupation rate” of the spinal cord within the dural tube (the ratio between the sagittal diameter of the spinal cord parenchyma to the sagittal diameter of the neural tube on T2 sequences), where ≥70% threshold was used to establish a working diagnosis of congenital stenosis [3]. With this demarcation, roughly 8.4% DCM patients can exhibit congenital stenosis [3]. Unfortunately, this definition requires further studies for validation [3].
Advanced MRI Techniques
Over the last decade, MRI has continued to evolve. These novel imaging protocols have significantly improved our ability to gather information pertaining to the microstructure and intrinsic functional properties of the spinal cord. This information may prove very useful in predicting operative outcome, overcoming limitations observed with conventional MRI. The following is a brief description of some of these novel modalities.
Diffusion Tensor Imaging (DTI)
DTI aims to analyze the presence, strength, and directionality of water particles—properties that can be distorted in DCM [60]. In particular, findings can implicate degeneration of white matter tracts [61,62,63,64,65]. Various metrics have been introduced, including fractional anisotropy (FA), apparent diffusion coefficient, mean diffusivity, tractography, and fiber tractography ratio [60]. FA, which ranges from 0 for isotropic diffusion [same in all directions] to 1 for anisotropic [all in 1 direction], has gained the most traction [6]. A recent systematic review delineated nine studies, where Level 3 evidence suggests that DTI is associated with preoperative severity and postsurgical outcomes in DCM patients and may be a good adjunct to assess those that may benefit from surgery [60].
Magnetization Transfer (MT)
MT offers a measure of myelin quantity [3, 66]; values can implicate the extent of demyelination [67]. The protocol employs a pre-pulse that evaluates the relative chemical and magnetization exchange between protons bound to lipid macromolecules and those neighboring water protons [3]. The feature is expressed as a ratio between scans with and without the pre-pulse or between the spinal cord and cerebrospinal fluid [3]. The method has been predominantly evaluated in multiple sclerosis [3, 67]. Nevertheless, recent preliminary data suggest that DCM patients have reduced MT ratio compared to healthy patients; moreover, MT ratio may correlate with mJOA scores [65].
Myelin Water Fraction (MWF)
MWF, also a modality to assess demyelination, is designed to measure myelin by taking advantage of T2 relaxation of water compartmentalized within different tissue (white matter, gray matter, and CSF) [3, 6]. The method has been studied in multiple sclerosis , but limited studies have focused on DCM [3, 66, 68].
MR Spectroscopy (MRS)
MRS offers a measure of different molecules within a single voxel, namely, N-acetyl aspartate, myoinositol, choline, creatine, and lactate [66]. Two studies [69, 70] discovered that the N-acetyl aspartate/creatine ratio was significantly reduced in DCM patients compared to healthy subjects; however, when Holly et al. [69] included mJOA scores, there was no significant correlation with N-acetyl aspartate/creatine ratio. On the other hand, Salamon et al. [71] showed that choline/N-acetyl aspartate ratio was increased in DCM patients compared to healthy subjects and that the ratio significantly correlated mJOA scores.
Functional MRI (fMRI)
fMRI attempts to correlate changes in neurological function (via motor tasks or sensory stimuli) with either neurovascular coupling (where fluctuations in function parallel fluctuations in local blood flow) or signal enhancement by extravascular protons (where fluctuation in intracellular and extracellular volumes may correspond with neural activity) [3]. The method has been studied in multiple sclerosis and chronic spinal cord injury but has not been pursued in DCM [3]. For multiple sclerosis , cervical cord demonstrated increased number of active voxels, increased mean signal intensity change in active voxels, and increased distribution of activation outside expected ipsilateral dorsal horns [66]. For chronic spinal cord injury, fMRI demonstrated increased bilateral activation compared to healthy controls [66].
Case Presentations
-
A.
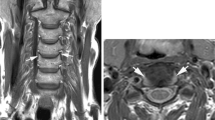
Significant stenosis/T2 signal with no myelopathy: 71-year-old male who presented with bilateral hand numbness in the first to third digits, where workup included MRI C spine and electromyography. The former demonstrated severe stenosis at C3/C4; the latter was consistent with bilateral carpal tunnel syndrome. He underwent carpal tunnel releases, with improvement of symptoms (Fig. 9.2).
-
B.
Significant stenosis/T2 signal and clinical myelopathy: 72-year-old male who presented with progressive weakness in the hands/arms/legs, now requiring wheelchair for mobilization. MRI C spine demonstrated multilevel cervical stenosis (Fig. 9.3).
-
C.
Minimal stenosis/T2 signal and clinical myelopathy: 44-year-old female who presented with frequent falls, hand dexterity issues, and bilateral Hoffman’s signs. MRI C spine demonstrated C5/C6 canal stenosis. Though there was slight CSF signal posterior to the spinal cord, there was a concern for dynamic spinal cord damage with the pronounced herniated disc and the loss of cervical lordosis. After an ACDF C5/C6, she noted improvement with balance and with fine motor skills (Fig. 9.4).
71-year-old male who presented with bilateral hand numbness in the first to third digits, where workup included MRI C spine and electromyography. The former demonstrated severe stenosis at C3/C4 (arrow); the latter was consistent with bilateral carpal tunnel syndrome. He underwent carpal tunnel releases, with improvement of symptoms
44-year-old female who presented with frequent falls, hand dexterity issues, and bilateral Hoffman’s signs. MRI C spine demonstrated C5/C6 canal stenosis (arrow). Though there was slight CSF signal posterior to the spinal cord, there was a concern for dynamic spinal cord damage with the pronounced herniated disc and the loss of cervical lordosis. After an ACDF C5/C6, she noted improvement with balance and with fine motor skills
Conclusions
Degenerative cervical myelopathy and radiculopathy comprise a host of age-related disorders which can cause significant damage to the spinal cord and/or nerve root. For the past 30 years, conventional magnetic resonance imaging has been the primary imaging protocol for the assessment of DCM. Unfortunately, imaging findings can be nonspecific and do not convey data regarding the health status of the spinal cord parenchyma. Advanced MRI techniques may help elucidate details regarding the microscopic structure and functional composition of the spinal cord parenchyma.
Key Recommendations
-
1.
Conventional MRI is a useful imaging tool for diagnosis of DCM, but it has significant limitations.
-
2.
Radiographs and CT imaging continue to have supplementary roles in the management of DCM.
-
3.
Clinical judgment remains a key component to assess disease severity and surgical prognosis.
-
4.
Emerging advanced MRI techniques, specifically DTI, can potentially offer more details regarding the health status of the spinal cord with respect to DCM.
-
5.
Extensive prospective studies that correlate advanced MRI data to clinical examination and outcome need to be completed before the techniques can become clinically relevant.
-
6.
Advanced MRI techniques will likely be a valuable adjunct to surgical decision-making and prognosis in the future for patients with DCM.
References
Tetreault L, Goldstein CL, Arnold P, et al. Degenerative cervical myelopathy: a spectrum of related disorders affecting the aging spine. Neurosurgery. 2015;77(Suppl 4):S51–67.
Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine. 2015;40:E675–93.
Nouri A, Martin A, Tetreault L, et al. MRI analysis of the combined prospectively collected AOSpine North America and International Data: the prevalence and spectrum of pathologies in a global cohort of patients with degenerative cervical myelopathy. Spine. 2017;42:1058–67.
Karadimas SK, Erwin WM, Ely CG, Dettori JR, Fehlings MG. Pathophysiology and natural history of cervical spondylotic myelopathy. Spine. 2013;38:S21–36.
Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy. Neuroscientist. 2012;19:409–21.
Nouri A, Martin AR, Mikulis D, Fehlings MG. Magnetic resonance imaging assessment of degenerative cervical myelopathy: a review of structural changes and measurement techniques. Neurosurg Focus. 2016;40:E5.
Wada E, Ohmura M, Yonenobu K. Intramedullary changes of the spinal cord in cervical spondylotic myelopathy. Spine. 1995;20:2226–32.
Tetreault LA, Dettori JR, Wilson JR, et al. Systematic review of magnetic resonance imaging characteristics that affect treatment decision making and predict clinical outcome in patients with cervical spondylotic myelopathy. Spine. 2013;38:S89–110.
Nasir S, Mahmud R, Hussain M, Min D, Shuang-ming S. Flexion/extension cervical spine views in blunt cervical trauma. Chin J Traumatol. 2012;15:166–9.
Khan SN, Erickson G, Sena MJ, Gupta MC. Use of flexion and extension radiographs of the cervical spine to rule out acute instability in patients with negative computed tomography scans. J Orthop Trauma. 2011;25:51–6.
White AP, Biswas D, Smart LR, Haims A, Grauer JN. Utility of flexion-extension radiographs in evaluating the degenerative cervical spine. Spine. 2007;32:975–9.
Berberat J, Grobholz R, Boxheimer L, Rogers S, Remonda L, Roelcke U. Differentiation between calcification and hemorrhage in brain tumors using susceptibility-weighted imaging: a pilot study. AJR Am J Roentgenol. 2014;202:847–50.
Sarva RP, Farivar S, Fromm H, Poller W. Study of the sensitivity and specificity of computerized tomography in the detection of calcified gallstones which appears radiolucent by conventional roentgenography. Gastrointest Radiol. 1981;6:165–7.
Middleton WD, Thorsen MK, Lawson TL, Foley WD. False-positive CT diagnosis of gallstones due to thickening of the gallbladder wall. AJR Am J Roentgenol. 1987;149:941–4.
Pham PH, Rao DS, Vasunilashorn F, Fishbein MC, Goldin JG. Computed tomography calcium quantification as a measure of atherosclerotic plaque morphology and stability. Investig Radiol. 2006;41:674–80.
Otake S, Matsuo M, Nishizawa S, Sano A, Kuroda Y. Ossification of the posterior longitudinal ligament: MR evaluation. AJNR Am J Neuroradiol. 1992;13:1059–67; discussion 1068–1070.
Yamashita Y, Takahashi M, Matsuno Y, et al. Spinal cord compression due to ossification of ligaments: MR imaging. Radiology. 1990;175:843–8.
Wong J, Leung O, Yuen M. Questionable adequacy of magnetic resonance for the detection of ossification of the posterior longitudinal ligament of the cervical spine. Hong Kong J Radiol. 2011;14:78–83.
Houser OW, Onofrio BM, Miller GM, Folger WN, Smith PL. Cervical spondylotic stenosis and myelopathy: evaluation with computed tomographic myelography. Mayo Clin Proc. 1994;69:557–63.
Shafaie FF, Wippold FJ 2nd, Gado M, Pilgram TK, Riew KD. Comparison of computed tomography myelography and magnetic resonance imaging in the evaluation of cervical spondylotic myelopathy and radiculopathy. Spine. 1999;24:1781–5.
Song KJ, Choi BW, Kim GH, Kim JR. Clinical usefulness of CT-myelogram comparing with the MRI in degenerative cervical spinal disorders: is CTM still useful for primary diagnostic tool? J Spinal Disord Tech. 2009;22:353–7.
Enders J, Rief M, Zimmermann E, et al. High-field open versus short-bore magnetic resonance imaging of the spine: a randomized controlled comparison of image quality. PLoS One. 2014;8:e83427.
Gilbert JW, Wheeler GR, Lingreen RA, Johnson RR. Open stand-up MRI: a new instrument for positional neuroimaging. J Spinal Disord Tech. 2006;19:151–4.
Sohn HM, You JW, Lee JY. The relationship between disc degeneration and morphologic changes in the intervertebral foramen of the cervical spine: a cadaveric MRI and CT study. J Korean Med Sci. 2004;19:101–6.
Yoo JU, Zou D, Edwards WT, Bayley J, Yuan HA. Effect of cervical spine motion on the neuroforaminal dimensions of human cervical spine. Spine. 1992;17:1131–6.
Hughes TB Jr, Richman JD, Rothfus WE. Diagnosis of Os odontoideum using kinematic magnetic resonance imaging. A case report. Spine. 1999;24:715–8.
Muhle C, Weinert D, Falliner A, et al. Dynamic changes of the spinal canal in patients with cervical spondylosis at flexion and extension using magnetic resonance imaging. Investig Radiol. 1998;33:444–9.
Muhle C, Wiskirchen J, Weinert D, et al. Biomechanical aspects of the subarachnoid space and cervical cord in healthy individuals examined with kinematic magnetic resonance imaging. Spine. 1998;23:556–67.
Vives MJ, Harris C, Reiter MF, Drzala M. Use of stand-up magnetic resonance imaging for evaluation of a cervicothoracic injury in a patient with ankylosing spondylitis. Spine J. 2008;8:678–82.
Suzuki F, Fukami T, Tsuji A, Takagi K, Matsuda M. Discrepancies of MRI findings between recumbent and upright positions in atlantoaxial lesion. Report of two cases. Eur Spine J. 2008;17(Suppl 2):S304–7.
Gilbert JW, Wheeler GR, Lingreen RA, et al. Imaging in the position that causes pain. Surg Neurol. 2008;69:463–5; discussion 465.
Jinkins JR, Dworkin JS, Damadian RV. Upright, weight-bearing, dynamic-kinetic MRI of the spine: initial results. Eur Radiol. 2005;15:1815–25.
Janssen M, Nabih A, Moussa W, Kawchuk GN, Carey JP. Evaluation of diagnosis techniques used for spinal injury related back pain. Pain Res Treat. 2011;2011:478798.
Muhle C, Resnick D, Ahn JM, Sudmeyer M, Heller M. In vivo changes in the neuroforaminal size at flexion-extension and axial rotation of the cervical spine in healthy persons examined using kinematic magnetic resonance imaging. Spine. 2001;26:E287–93.
Muhle C, Bischoff L, Weinert D, et al. Exacerbated pain in cervical radiculopathy at axial rotation, flexion, extension, and coupled motions of the cervical spine: evaluation by kinematic magnetic resonance imaging. Investig Radiol. 1998;33:279–88.
Ferreiro Perez A, Garcia Isidro M, Ayerbe E, Castedo J, Jinkins JR. Evaluation of intervertebral disc herniation and hypermobile intersegmental instability in symptomatic adult patients undergoing recumbent and upright MRI of the cervical or lumbosacral spines. Eur J Radiol. 2007;62:444–8.
Kato F, Yukawa Y, Suda K, Yamagata M, Ueta T. Normal morphology, age-related changes and abnormal findings of the cervical spine. Part II: magnetic resonance imaging of over 1,200 asymptomatic subjects. Eur Spine J. 2012;21:1499–507.
Ulbrich EJ, Schraner C, Boesch C, et al. Normative MR cervical spinal canal dimensions. Radiology. 2013;271:172–82.
Matsumoto M, Fujimura Y, Suzuki N, et al. MRI of cervical intervertebral discs in asymptomatic subjects. J Bone Joint Surg. 1998;80:19–24.
Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–9.
Mann E, Peterson CK, Hodler J. Degenerative marrow (modic) changes on cervical spine magnetic resonance imaging scans: prevalence, inter- and intra-examiner reliability and link to disc herniation. Spine. 2011;36:1081–5.
Hayashi T, Daubs MD, Suzuki A, Phan K, Shiba K, Wang JC. Effect of Modic changes on spinal canal stenosis and segmental motion in cervical spine. Eur Spine J. 2014;23:1737–42.
Tong T, Gao XD, Li J, et al. Do modic changes affect cervical sagittal alignment and motion in symptomatic patients? Eur Spine J. 2017;26:1945–52.
Inamasu J, Guiot BH, Sachs DC. Ossification of the posterior longitudinal ligament: an update on its biology, epidemiology, and natural history. Neurosurgery. 2006;58:1027–39; discussion 1027–1039.
Fujiwara K, Yonenobu K, Hiroshima K, Ebara S, Yamashita K, Ono K. Morphometry of the cervical spinal cord and its relation to pathology in cases with compression myelopathy. Spine. 1988;13:1212–6.
Okada Y, Ikata T, Yamada H, Sakamoto R, Katoh S. Magnetic resonance imaging study on the results of surgery for cervical compression myelopathy. Spine. 1993;18:2024–9.
Fehlings MG, Rao SC, Tator CH, et al. The optimal radiologic method for assessing spinal canal compromise and cord compression in patients with cervical spinal cord injury. Part II: results of a multicenter study. Spine. 1999;24:605–13.
Ernst CW, Stadnik TW, Peeters E, Breucq C, Osteaux MJ. Prevalence of annular tears and disc herniations on MR images of the cervical spine in symptom free volunteers. Eur J Radiol. 2005;55:409–14.
Mastronardi L, Elsawaf A, Roperto R, et al. Prognostic relevance of the postoperative evolution of intramedullary spinal cord changes in signal intensity on magnetic resonance imaging after anterior decompression for cervical spondylotic myelopathy. J Neurosurg Spine. 2007;7:615–22.
Arvin B, Kalsi-Ryan S, Karpova A, et al. Postoperative magnetic resonance imaging can predict neurological recovery after surgery for cervical spondylotic myelopathy: a prospective study with blinded assessments. Neurosurgery. 2011;69:362–8.
Vedantam A, Rajshekhar V. Does the type of T2-weighted hyperintensity influence surgical outcome in patients with cervical spondylotic myelopathy? A review. Eur spine J. 2013;22:96–106.
Alafifi T, Kern R, Fehlings M. Clinical and MRI predictors of outcome after surgical intervention for cervical spondylotic myelopathy. J Neuroimaging. 2007;17:315–22.
Arvin B, Kalsi-Ryan S, Mercier D, Furlan JC, Massicotte EM, Fehlings MG. Preoperative magnetic resonance imaging is associated with baseline neurological status and can predict postoperative recovery in patients with cervical spondylotic myelopathy. Spine. 2013;38:1170–6.
Morio Y, Teshima R, Nagashima H, Nawata K, Yamasaki D, Nanjo Y. Correlation between operative outcomes of cervical compression myelopathy and mri of the spinal cord. Spine. 2001;26:1238–45.
Mizuno J, Nakagawa H, Inoue T, Hashizume Y. Clinicopathological study of “snake-eye appearance” in compressive myelopathy of the cervical spinal cord. J Neurosurg Spine. 2003;99:162–8.
Wilson JR, Barry S, Fischer DJ, et al. Frequency, timing, and predictors of neurological dysfunction in the nonmyelopathic patient with cervical spinal cord compression, canal stenosis, and/or ossification of the posterior longitudinal ligament. Spine. 2013;38:S37–54.
Bednarik J, Kadanka Z, Dusek L, et al. Presymptomatic spondylotic cervical myelopathy: an updated predictive model. Eur Spine J. 2008;17:421–31.
Suri A, Chabbra RPS, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3:33–45.
Nouri A, Tetreault L, Zamorano JJ, et al. Role of magnetic resonance imaging in predicting surgical outcome in patients with cervical spondylotic myelopathy. Spine. 2015;40:171–8.
Rindler RS, Chokshi FH, Malcolm JG, et al. Spinal diffusion tensor imaging in evaluation of preoperative and postoperative severity of cervical spondylotic myelopathy: systematic review of literature. World Neurosurg. 2017;99:150–8.
Koskinen E, Brander A, Hakulinen U, et al. Assessing the state of chronic spinal cord injury using diffusion tensor imaging. J Neurotrauma. 2013;30:1587–95.
Li X, Cui JL, Mak KC, Luk KD, Hu Y. Potential use of diffusion tensor imaging in level diagnosis of multilevel cervical spondylotic myelopathy. Spine. 2014;39:E615–22.
Cui JL, Li X, Chan TY, Mak KC, Luk KD, Hu Y. Quantitative assessment of column-specific degeneration in cervical spondylotic myelopathy based on diffusion tensor tractography. Eur Spine J. 2015;24:41–7.
Maki S, Koda M, Ota M, et al. Reduced field-of-view diffusion tensor imaging of the spinal cord shows motor dysfunction of the lower extremities in patients with cervical compression myelopathy. Spine. 2018;43(2):89–96.
Martin AR, De Leener B, Cohen-Adad J, et al. 163 microstructural MRI quantifies tract-specific injury and correlates with global disability and focal neurological deficits in degenerative cervical myelopathy. Neurosurgery. 2016;63(Suppl 1):165.
Martin AR, Aleksanderek I, Cohen-Adad J, et al. Translating state-of-the-art spinal cord MRI techniques to clinical use: a systematic review of clinical studies utilizing DTI, MT, MWF, MRS, and fMRI. NeuroImage Clin. 2016;10:192–238.
Lema A, Bishop C, Malik O, et al. A comparison of magnetization transfer methods to assess brain and cervical cord microstructure in multiple sclerosis. J Neuroimaging. 2017;27:221–6.
Liu H, MacMillian EL, Jutzeler CR, et al. Assessing structure and function of myelin in cervical spondylotic myelopathy: evidence of demyelination. Neurology. 2017;89:602–10.
Holly LT, Freitas B, McArthur DL, Salamon N. Proton magnetic resonance spectroscopy to evaluate spinal cord axonal injury in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;10:194–200.
Taha Ali TF, Badawy AE. Feasibility of 1H-MR spectroscopy in evaluation of cervical spondylotic myelopathy. Egypt J Radiol Nucl Med. 2013;44:93–9.
Salamon N, Ellingson BM, Nagarajan R, Gebara N, Thomas A, Holly LT. Proton magnetic resonance spectroscopy of human cervical spondylosis at 3T. Spinal Cord. 2013;51:558–63.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nguyen, H.S., Kurpad, S.N. (2019). Radiographic Modalities. In: Kaiser, M., Haid, R., Shaffrey, C., Fehlings, M. (eds) Degenerative Cervical Myelopathy and Radiculopathy . Springer, Cham. https://doi.org/10.1007/978-3-319-97952-6_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-97952-6_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-97951-9
Online ISBN: 978-3-319-97952-6
eBook Packages: MedicineMedicine (R0)