Abstract
Purpose
Few studies have reported the characteristics of Modic changes (MCs) in the cervical spine in contrast to the lumbar spine. The purpose of this study was to identify the prevalence of MCs in the cervical spine and to elucidate the relationship of MCs with spinal canal stenosis and angular motion.
Methods
437 consecutive, symptomatic patients with neck pain with or without neurogenic symptoms were included in this study. MRI in multiple positions was performed with dynamic motion of the cervical spine in upright, weight-bearing neutral, flexion and extension positions. Type of MC, intervertebral disc degeneration grade, spinal cord compression grade and sagittal angular motion between flexion and extension for each segment from C2–3 to C6–7 were evaluated.
Results
MCs were observed in 84 out of 437 patients (19.2 %) and in 109 out of 2,185 motion segments (5.0 %) with type 2 changes predominating. Disc degeneration grades and spinal cord compression grades of segments with MCs were significantly higher than those without MCs. Sagittal angular motion of segments with MCs were significantly lower than those without MCs. Multiple logistic regression analysis revealed that significantly elevated odds ratios for MCs were observed in segments with severe disc degeneration, severe spinal canal compression and less angular motion.
Conclusion
The cervical segments with MCs were significantly more likely to have disc degeneration and spinal canal stenosis. In addition, the segments with MCs had significantly less angular motion, which suggests MCs may correlate with loss of mobility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Signal intensity changes of vertebral bodies adjacent to intervertebral discs are often observed in magnetic resonance imaging (MRI) in patients with spinal degenerative diseases. Modic et al. [1, 2] identified and classified them into three types: type 1 (Fig. 1), hypointense signal on T1-weighted sequences and hyperintense signal on T2-weighted sequences; type 2 (Fig. 2), hyperintense signal on T1 sequences and hyper- or isointense signal on T2 sequences; type 3, hypointense signal on T1 and T2 sequences. Histological and radiological studies have demonstrated that type 1 reflects inflammatory changes in the vertebral endplates; type 2 reflects fatty marrow; and type 3 represents sclerotic changes of the endplates [1, 2].
Most of the literature on Modic changes (MCs) focus on the lumbar spine, and only a few studies have reported on the cervical spine [3–5]. In addition, degree of spinal canal stenosis and kinematics of the cervical spine have not been well-studied in patients with MCs. We hypothesize that cervical segments with MCs may have some relationship with spinal canal stenosis and have less motion due to the degenerative changes. The objective of this study was to evaluate how MCs relate to spinal canal stenosis and segmental cervical motion using multiple positional MRI.
Materials and methods
Patients
437 consecutive, symptomatic patients (215 men and 222 women) with an average age 49.8 ± 10.0 years (range 20–79) were examined from March 2011 to November 2011. The inclusion criteria were defined as patients who had neck pain with or without neurologic symptoms (i.e., radiculopathy or myelopathy). The exclusion criteria were: trauma, rheumatoid arthritis, spinal tumors and history of cervical spine surgery. The Institutional Review Board at our institute approved this study and informed consent was obtained from all participants.
MRI positioning
All patients underwent cervical MRI scanning (a 0.6 Tesla MRI scanner: Upright Multi-Position; Fonar Corp., New York, NY), which was performed with dynamic motion of cervical spine in upright, weight-bearing neutral, flexion and extension positions. Each patient was first seated on a bench between the two poles of the MRI magnet in the neutral position with his or her head facing straight ahead without angling his or her chin up or down. Next, each patient was positioned with his or her chin angled toward their chests (flexion position). Finally, each patient was positioned with his or her chin angled towards the ceiling (extension position). For each of these positions, a flexible cervical coil was placed around the participant’s neck and a padded bar and headrest supported the patient’s head and neck.
MRI analysis
All radiologic data obtained from the MRIs were recorded on a computer for subsequent measurements, and all calculations were automatically performed on the MRI by MRI analyzer software (Truemetric Corp., Bellflower, CA), as described previously [6–11]. For each image, 77 points were marked for digitization by three spine surgeons, each with over 5 years clinical experience (C3–T1: 6 points on each vertebral body, 2 on each pedicle, and 2 on the spinal canal diameter at each intervertebral disc level; C2: 1 point on the tip of the odontoid process and 6 on the vertebral body; C1: 4 points on the anterior, superior, and inferior surfaces of the anterior tubercle and the lower end of the spinous process; and occiput (Oc): 2 points on the anterior and posterior baselines).
Intervertebral angular motion was measured at five levels from C2–3 to C6–7 and was defined as the difference of intervertebral angles between two vertebrae from flexion to extension.
Assessment of Modic change
MCs were evaluated for 2,185 segments from C2–3 to C6–7 and classified into: none, type 1, 2 and 3 according to their signal patterns on T1 and T2 sagittal MR images. Type 1, hypointense signal on T1-weighted sequences and hyperintense signal on T2-weighted sequences; type 2, hyperintense signal on T1 sequences and hyper- or isointense signal on T2 sequences; type 3, hypointense signal on T1 and T2 sequences [1, 2]. The intra- and inter-observer reliability of the ratings for MCs were assessed using the κ value, and was assessed using 50 cases (250 intervertebral levels).
Assessment of degenerative change
We evaluated intervertebral disc degeneration from C2–3 to C6–7 using the Grading System for Cervical Intervertebral Disc Degeneration [7, 12] (Table 1). The disc degeneration grades were compared between MC-positive (+) group and MC-negative (–) group for each level. The reliability of this grading system was reported previously [12].
Evaluation of spinal cord compression
The extent of cervical cord compression at each level was assessed by examining the neutral T2-weighted sagittal images. Cervical cord compression was defined as obliteration of the subarachnoid space in the presence of disc herniation, osteophyte formation, or hypertrophy of the ligamentum flavum. Cervical cord compression at each segment was evaluated using a 5-point grading scale (range 0–4, Table 2) modified from Muhle et al. [13, 14]. The reliability of this grading system was also reported previously [14].
Statistical analysis
Mann–Whitney U test was used for comparisons of disc degeneration, spinal cord compression and angular motion between the groups with and without MCs in each segment. After the variables were categorized, Chi-square test was used for the comparisons. Logistic regression analyses were used to detect the association between the existence of MC and related factors. Disc degeneration grade was categorized into mild (grade 1–3) and severe (grade 4–5). Similarly, spinal cord compression grade was categorized into mild (grade 0–1) and severe (grade 2–4). Age and angular motion were divided into two groups, establishing the cutoff points at median. The variables in the multivariate model were those with a p value of <0.05 in Chi-square test and basic characters. Statistical analyses were performed using SPSS (version 20; SPSS, Chicago, IL) computer software and values were expressed as mean ± standard deviation (SD). A p value of <0.05 was considered statistically significant. According to Landis and Koch [15], the reliability of diagnosis of MCs was analyzed using κ statistics and was assessed as follows: κ 0–0.2 showed slight agreement, 0.21–0.4 fair agreement, 0.41–0.6 moderate agreement, 0.61–0.8 substantial agreement, and 0.81–1 excellent agreement.
Results
Prevalence of MCs
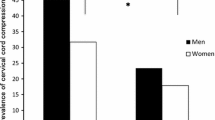
MCs were observed in 84 out of 437 patients (19.2 %). 109 out of 2,185 cervical segments (5.0 %) had MCs: type 1 were seen in 27 segments, type 2 in 72 and type 3 in 10. MCs were most frequent at C5–6 followed by C6–7 and C4–5 (Fig. 3).
Link to disc degeneration, spinal cord compression, and angular motion in motion segments
The relationship for each motion segment between disc degeneration grade and MCs, between spinal compression grade and MCs, and between angular motion and MCs are shown in Table 3. Disc degeneration grade of segments with MCs on average was highest at C5–6. Analysis of disc degeneration revealed significant differences between the MC (+) group and the MC (–) group in each segment, indicating that the segments with MCs had more severe disc degeneration. Spinal cord compression grade of segments with MCs on average was highest at C5–6. A significant difference in spinal cord compression was observed at C4–5, C5–6 and C6–7 between units with MCs and those without MCs, indicating that segments with MCs had more spinal canal stenosis. Average angular motion of segments with MCs was smallest at C6–7; however, the largest decrease in motion between segments with and without MCs was seen at C5–6. A significant decrease in angular motion was found at C4–5 and C5–6 in units with MCs.
Association between MCs and related factors
Factors potentially related to the changes were evaluated using Chi-square test and logistic regression analysis (Table 4). Factors of C2–3 were eliminated because no MC was observed at C2–3. The logistic regression model was adjusted for age, sex, level, disc degeneration, spinal canal compression, and angular motion. After adjustment for potential confounding factors, significantly elevated odds (ORs) ratios were observed in segments with severe disc degeneration, severe spinal canal compression and angular motion of ≤8.6° (OR 3.90; 95 % confidence interval (CI) 2.42–6.30, OR 1.80; 95 % CI 1.18–2.74 and OR 1.84; 95 % CI 1.21–2.82, respectively). Although the crude OR for age >53 years was significantly increased (OR 1.89; 95 % CI 1.27–2.81), it decreased after adjustment (OR 1.39; 95 % CI 0.93–2.11).
Reliability of diagnosis
The inter-observer agreement of MC was substantial with a κ value of 0.78. The intraobserver agreement of MC was also substantial with a κ value of 0.74.
Discussion
A few previous studies have reported on the prevalence of MCs in cervical spine. Peterson et al. [5] reviewed 118 patients (mean age 48 years) with neck pain and reported that MCs were seen in 16 %, with Modic type 1 being the most common. Mann et al. [3] evaluated 426 patients over the age of 50 (mean age 62 years) and reported MCs were observed in 40.4 % of patients, with Modic type 2 being the most common, in contrast to Peterson’s results. Matsumoto et al. [4] assessed 497 asymptomatic healthy volunteers (mean age 39 years) and followed 223 subjects (mean age 51 years) at mean follow-up of 11.6 years. They reported the prevalence of MCs as 4.5 and 13.9 %, respectively, with type 2 developing most frequently over a 10-year period. In our study the prevalence of MCs in the cervical spine was 19.2 % and type 2 was more frequent than type 1. The inconsistent results between studies might be due to the differences in age and clinical symptoms of the study population.
The analysis of spinal cord compression grade showed a significant difference between the MC (+) group and the MC (–) group at C4–5, C5–6, and C6–7. Also, the MC (+) group had significantly more severe disc degeneration at those levels. Moreover, in our multivariate analysis, these two factors showed evidence of an association with MCs. According to these results, patients with MCs were more likely to have spinal canal stenosis and disc degeneration at the same segmental level as the MC compared to patients without MCs. To the best of our knowledge, no previous study has reported the relationship between MCs and spinal canal stenosis in the cervical spine. These findings are consistent with the recent study by Mann et al. [3] who found a link between MCs and disc herniation, and Wang et al. [16] who demonstrated in 313 cadaveric spines that greater vertebral end plate lesion size was associated with more severe adjacent disc degeneration.
To our knowledge, no study has assessed the relationship between motion and MCs. Our multivariate analysis showed a significant correlation between angular motion and MC. The relationship between disc degeneration and motion was studied by Miyazaki et al. [7] who reported a kinematic analysis of the relationship between the grade of disc degeneration and motion of the segmental unit of the cervical spine and demonstrated that in severely degenerated segments (grade 5 discs), angular motion of C4–5 and C5–6 was significantly decreased. Their study was consistent with our results that cervical segments with MCs at C4–5 and C5–6 had less motion than those without MCs, as we showed segments with MCs have significantly severe disc degeneration. The segments with MCs likely tend to ankylose and lose mobility with severe degeneration.
Our study has limitations in methods. The population of our study included only symptomatic patients, whereas some previous papers only included healthy volunteers. Also, the mean age of our population was different from previous papers. Therefore, we cannot simply compare our prevalence rate with those which were published previously. In addition, the reliability results for detecting MCs in this study were good and similar to the previous study [3]; however, it may be relatively difficult to identify MCs of cervical spine because the vertebral bodies of cervical spine are smaller and less degenerative than those of lumbar spine.
Clinically, the current study would be useful because MRI findings of MCs in the cervical spine should make physicians consider the probability of spinal canal stenosis at the same level. Moreover, severe instability might be less likely in segments with MCs because MCs would be observed during the development of ankylosis.
Conclusion
The prevalence of MCs was 19.2 % of our patients, with type 2 predominating. The cervical segments with MCs were significantly more likely to have disc degeneration and spinal canal stenosis at the same level, indicating that MCs may be one of the important signs of spinal stenosis due to disc degeneration. In addition, the segments with MCs had significantly less angular motion, indicating that MCs may correlate with loss of mobility.
References
Modic MT, Masaryk TJ, Ross JS, Carter JR (1988) Imaging of degenerative disk disease. Radiology 168(1):177–186
Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR (1988) Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology 166(1 Pt 1):193–199
Mann E, Peterson CK, Hodler J (2011) Degenerative marrow (Modic) changes on cervical spine magnetic resonance imaging scans: prevalence, inter- and intra-examiner reliability and link to disc herniation. Spine 36(14):1081–1085
Matsumoto M, Okada E, Ichihara D, Chiba K, Toyama Y, Fujiwara H, Momoshima S, Nishiwaki Y, Takahata T (2012) Modic changes in the cervical spine: prospective 10-year follow-up study in asymptomatic subjects. J Bone Joint Surg 94(5):678–683
Peterson CK, Humphreys BK, Pringle TC (2007) Prevalence of Modic degenerative marrow changes in the cervical spine. J Manipulative Physiol Ther 30(1):5–10
Daffner SD, Xin J, Taghavi CE, Hymanson HJ, Mudiyam C, Hongyu W, Wang JC (2009) Cervical segmental motion at levels adjacent to disc herniation as determined with kinetic magnetic resonance imaging. Spine 34(22):2389–2394
Miyazaki M, Hong SW, Yoon SH, Zou J, Tow B, Alanay A, Abitbol JJ, Wang JC (2008) Kinematic analysis of the relationship between the grade of disc degeneration and motion unit of the cervical spine. Spine 33(2):187–193
Miyazaki M, Hymanson HJ, Morishita Y, He W, Zhang H, Wu G, Kong MH, Tsumura H, Wang JC (2008) Kinematic analysis of the relationship between sagittal alignment and disc degeneration in the cervical spine. Spine 33(23):E870–E876
Morishita Y, Falakassa J, Naito M, Hymanson HJ, Taghavi C, Wang JC (2009) The kinematic relationships of the upper cervical spine. Spine 34(24):2642–2645
Morishita Y, Hida S, Miyazaki M, Hong SW, Zou J, Wei F, Naito M, Wang JC (2008) The effects of the degenerative changes in the functional spinal unit on the kinematics of the cervical spine. Spine 33(6):E178–E182
Morishita Y, Naito M, Hymanson H, Miyazaki M, Wu G, Wang JC (2009) The relationship between the cervical spinal canal diameter and the pathological changes in the cervical spine. Eur Spine J 18(6):877–883
Miyazaki M, Hong SW, Yoon SH, Morishita Y, Wang JC (2008) Reliability of a magnetic resonance imaging-based grading system for cervical intervertebral disc degeneration. J Spinal Disord Tech 21(4):288–292
Muhle C, Metzner J, Weinert D, Falliner A, Brinkmann G, Mehdorn MH, Heller M, Resnick D (1998) Classification system based on kinematic MR imaging in cervical spondylitic myelopathy. AJNR 19(9):1763–1771
Suzuki A, Daubs MD, Inoue H, Hayashi T, Aghdasi B, Montgomery SR, Ruangchainikom M, Hu X, Lee CJ, Wang CJ, Wang BJ, Nakamura H (2013) Prevalence and motion characteristics of degenerative cervical spondylolisthesis in the symptomatic adult. Spine 38(17):E1115–E1120
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Wang Y, Videman T, Battie MC (2013) Morphometrics and lesions of vertebral end plates are associated with lumbar disc degeneration: evidence from cadaveric spines. J Bone Joint Surg Am 95(5):e26
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study protocol was approved by the Institutional Review Board of University of California Los Angeles. (Approval number: 10-000968).
Rights and permissions
About this article
Cite this article
Hayashi, T., Daubs, M.D., Suzuki, A. et al. Effect of Modic changes on spinal canal stenosis and segmental motion in cervical spine. Eur Spine J 23, 1737–1742 (2014). https://doi.org/10.1007/s00586-014-3406-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-014-3406-8