Abstract
The high economic value and unique technological properties of the platinum -group metals (PGMs), plus their growing scarcity in the Earth’s crust, justify the crucial importance of developing recycling practices for PGMs end-of-life materials. Examples of top devices relying on the use of PGMs are automotive and industrial catalysts, and electrical and electronics equipment. This article critically describes the most recent research on the use of solvent extraction to recover one PGM, palladium , from spent catalysts . Some groups focus on the development of schemes involving commercial extractants, while others prefer to design specific molecules to efficiently and selectively recover palladium from these particular complex leaching solutions. Examples of commercial extractants proposed for the former schemes are Alamine® 308, TBP and LIX® 84I; while on the other hand, sulfur -containing diamides, thioamides, thiocarbamates, and dithioethers have recently been developed. Ionic liquids have to be mentioned too.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
The mineral deposits of platinum -group metals (PGMs) are generally scarce in the Earth’s crust, being mainly located in a few geographic areas. South Africa and Russia are almost exclusively, the primary producers of platinum , palladium and rhodium worldwide [1].
As a result of their widespread applications in several top technological devices, for example in electrical and electronics equipment, and automotive and industrial catalysts, PGMs are considered critical raw materials [2]. Information in the last European Union (EU) reports on the subject, released in 2014 and 2017, shows that figures for the “substitution index” (a measure of the difficulty in replacing the material) and “end-of-life recycling input rate” (a measure of the ratio of recycling from old scrap to EU demand) for PGMs have worsened in the last three years [2, 3]. An article discussing some ways of improving the supply security of critical metals based on current developments and research in the EU has recently appeared in the literature [4]. Accordingly, the development of PGMs recycling practices from end-of-life materials (so-called urban mining ) is more than ever indispensable, both from the environmental and economic points of view.
Solvent extraction (SX) is the conventional unit operation often applied to separate, purify and concentrate PGMs from leaching solutions when the hydrometallurgical approach for recycling is chosen [5]. Consequently, the development of integrated environmentally-friendly and cost-effective hydrometallurgical processes for PGMs recycling typically count on this separation technique.
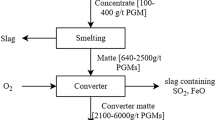
Platinum , palladium and rhodium are the PGMs with a broader worldwide consumption [6]; thus, there is a higher concern for their recycling from urban mining sources than for the other PGMs. Chloride solutions are conventionally used to solubilize the PGMs, as they show better solubility performance than in other matrices (nitric or sulfuric acids) [5].
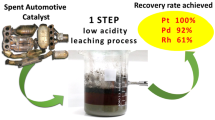
It is very difficult to extract Rh(III), and it is also challenging to separate Pt(IV) from Pd(II) because both metals are often co-extracted [5]. Nevertheless, the co-existence of Pt and Pd in catalysts recovered by urban mining is currently becoming rarer. The most recent automotive catalysts mainly use Pd [6], and its mixtures with Rh are now less common; moreover, industrial catalysts usually do not contain mixtures of Pt and Pd. The most demanding task in the recovery of Pd from catalysts is its separation from other metals present in large excess, such as Al and/or Ce. In fact, there are always excess concentrations of contaminants even for optimized leaching conditions, making selective recovery of Pd(II) challenging.
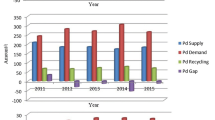
Palladium exhibits the highest supply and demand figures of the three PGMs [6]. This short review aims to highlight some of the most recent research, namely of the last 10 years, on the SX of Pd(II) applied to leaching solutions produced from spent catalysts , either industrial or automotive. Work focusing on the treatment of real catalysts leachates is highlighted, but obviously other articles describing relevant investigation on the use of model leaching solutions will also be referred to. A more extensive review on this specific topic has recently been published in the literature [7].
Solvent Extraction of Palladium
The main academic groups involved in this research area either focus on novel ways to use commercial extractants adapted to these specific leachates or invest in the thoughtful design of new molecules. This latter option is the most widely adopted. Following the development of ionic liquids (ILs) and their increasing utilization in the chemical industry, the investigation of ILs for use as solvents to recover PGMs has recently been increasing.
Commercial Extractants
There are not many recent examples involving commercial compounds as extractants for Pd(II) from real spent catalyst leaching solutions. Two cases where commercial compounds were used for Pd(II) recovery are detailed in Table 1.
LIX® 84I was tested as extractant to efficiently and selectively recover Pd(II) from an automobile catalyst aqueous leach liquor [8]. Probably the leaching solution available was produced from a metallic catalyst, since Al should have been present if catalysts with ceramic monoliths were involved. Authors proposed an integrated flowsheet of the process, arguing that successful Pd(II) recovery may be accomplished using LIX® 84I in an aliphatic diluent, the loaded organic solution being efficiently stripped by an acidic thiourea aqueous phase. Pt(IV) can then be recovered from the Pd(II) free raffinate by Alamine® 336.
Cyanex® 923 was applied for Pd(II) recovery from leaching solutions of Pd coated alumina and coated ceramic honeycomb catalysts [9]. The authors used sulfuric acid instead of HCl because they were not able to achieve a successful separation of Pd(II) from Al(III) in HCl media . They obtained good Pd(II) recovery using 5 mol/L perchloric acid for stripping. The main drawback of the developed schemes are the rather low PGMs concentrations attained in the leachates, as these metals are not efficiently dissolved in media other than chloride .
There are some more articles dealing with commercial extractants in the literature but reporting on the development of process schemes for Pd(II) recovery from model solutions. The model solutions are an attempt by the authors to mimic the real leachates, but the presence of contaminants in higher concentrations than those simulated, or even others that were not expected, may well occur. The following are two examples of such studies. The selective Pd(II) recovery by tributylphosphate (TBP) from a mixture containing Pt, Cr, Mn, Fe, and Ni in 3 mol/L HCl [10] deserves mention, as well as the co-extraction of Pd(II) and Pt(IV) by Alamine® 308 from a complex solution additionally containing Fe(III), Rh(III) and Ce(III) in 6 mol/L HCl [11]. Both PGMs were subsequently separated by selective stripping [11].
Two commercial products known as low-toxicity fungicides, namely propiconazole and penconazole, were also tested for Pd(II) recovery from a simulated leach solution of an aluminopalladium catalyst APK-2 used in the manufacture of nitric acid. In spite of the high Al(III) concentrations in the leachates, the authors showed that it did not affect the efficient and selective Pd(II) uptake. Pd(II) was stripped using a 4 mol/L ammonia solution. From a practical point of view there is still much more work to be carried out with these systems, namely the evaluation of the solvent’s robustness and stability when reutilized, as well as the replacement of chloroform, since chlorinated diluents cannot be used in industrial SX practice [12].
Commercial extractants possess the advantage of being readily available in the market. If a successful SX scheme to recover Pd(II) from spent catalyst leachates is developed (with an individual extractant or under the form of synergistic mixtures, e.g. [13, 14]), then this would be the cheapest way for the recycling industrialists to invest in such a process.
Extensive investigation of Pd(II) extraction using commercial ionic liquids (ILs) has already been carried out, but the developed process schemes have not as yet been applied to real spent catalysts leach solutions. The research focusing on the use of Cyphos® 101 [15,16,17], Cyphos® 102 [17, 18], Cyphos® 104 [15], Cyphos® 105 [17], and hexadecylpyridinium chloride [19] for PGMs recovery and separation deserve mention. When diluted in toluene, all ILs showed a high level of Pd(II) extraction (>96%) from 0.1 mol/L HCl [15, 19], and relatively similar results were achieved for Cyphos® 101 in xylene [16]. The work carried out by Papaiconomou and coworkers [17, 18] is still preliminary, but the authors objectively wish to apply the most promising SX schemes involving ILs to the recycling of PGMs from end-of-life devices such as automobile catalysts.
Synthesized Extractants
Several research groups have been developing SX schemes for Pd(II) recovery from leach solutions of spent catalysts , particularly automotive catalysts. Some of the most relevant SX systems applied for Pd(II) recovery from real leaching solutions are depicted in Table 2.
Holdt and co-workers [20] investigated the complexation of Pd(II) by several unsaturated dithioethers, finding that 1,2-bis(2-methoxyethylthio) benzene—compound 1, Table 2—satisfactorily allows selective Pd(II) recovery over Pt(IV) and Rh(III), as well as over several metals co-existing in a real 2 mol/L chloride ion solution of an automobile catalyst. Taking into account the complexity of the feed solution, the robustness of the extractant in successive extraction -stripping cycles is remarkable, as also is the selectivity of compound 1 for Pd(II). An acidified thiourea aqueous phase was employed to efficiently strip Pd(II) [21]. The main drawbacks are the use of o-dichlorobenzene as diluent and the unfavorable kinetics involved (24 h for extraction , 3 h for stripping) [21].
Several thiocarbamate derivatives have recently been developed by Yamada and coworkers for Pd(II) recovery from leach solutions of automotive catalysts [22, 23]. The structures of the most promising extractants found in these studies are compounds 2 and 3, depicted in Table 2. Both thiocarbamates were thoroughly investigated, showing excellent selectivity values for Pd(II) recovery . However, compounds 2 and 3 are not soluble in commercial diluents, and more concentrated HCl solutions decreased the kinetics of the compound 2 system, since contact times ranging from 10 to 24 h were found necessary to reach equilibrium [22]. For compound 3, its dissolution in o-nitrobenzene guaranteed Pd(II) extraction above 95% for 30 min contacts and for a wider range of HCl concentrations [23]. In addition to these disadvantages, the comparison of the compositions of the leach solutions of automotive catalysts utilized by Holdt et al. [21] and Yamada et al. [22, 23] shows that the latter are far more dilute (in metal ions), also suggesting that the analysis for some other metal ions present may possibly be missing. Moreover, reutilization experiments involving the complex real feed should be performed to fully evaluate the practical potential of SX systems using compounds 2 and 3 for Pd(II) recovery . Acidified thiourea solutions were again employed to strip Pd(II).
Thiodiglycolamide derivatives have been firstly investigated by Narita and collaborators for Pd(II) recovery [28]. The promising results generally obtained motivated other researchers to pursue the development of these sorts of compounds by changing the alkyl groups linked to the nitrogen atoms. Hence, after a thorough study devoted to evaluate the potential of compound 4—Table 2—for Pd(II) recovery [29], this compound was tested to selectively remove Pd(II) from spent petrochemical catalyst leach solutions [24]. Extractant 4 exhibits excellent Pd(II) loading capacity and robustness in sequential extraction -stripping cycles. Nevertheless, due to the high Al(III) concentration in the feed, a steady accumulation of Al(III) in the solvent was noticed [24]. Investigation to find an effective scrubbing agent for Al(III) is currently under way.
The same leaching solution has been used to test a tertiary thioamide derivative that has previously exhibited a high potential to effectively and selectively recover Pd(II) [30]. The overall data collected for extractant 5—Table 2—are generally similar to those achieved for compound 4. In fact, the gradual Al(III) accumulation in the organic phases does not affect Pd(II) recovery for the first few extraction -stripping cycles, but likely reduces the Pd(II) stripping efficiency using thiourea when the solvents are highly loaded with metal ions [24].
Heterocyclic dithioether derivatives were developed by Kondo and coworkers for Pd(II) recovery [25]. The best compound—6, Table 2—was subsequently tested with a real spent catalyst leach solution. Pd(II) was efficiently extracted (>95%), but Pt(IV) was also co-extracted (≈6–8%). Additionally, Al(III) and Ce(III) extractions were lower than 1%. Results from only one extraction -strip cycle do not provide enough information to properly evaluate the adequacy of the system for Pd(II) recovery . A 5% ammonia solution efficiently stripped Pd(II). Additional studies are necessary to clarify the practical potential of this sort of compound, and the chloroform diluent should also be replaced [25].
New dithioether derivatives have recently been investigated by Yamada and collaborators, and one of the best examples is displayed in Table 2 as compound 7 [26]. This comprehensive report contains a methodical research of the SX systems from a more fundamental point of view, and it also demonstrates that compound 7 in an aliphatic diluent is very effective for Pd(II) recovery . Remarkable selectivity values for Pd(II) removal from the real automobile leach solution were found. Very good Pd(II) loading profiles and recyclability data were obtained for compound 7 when it was used in five successive extraction -stripping cycles with a model feed solution, but confirmation that similar results would be achieved treating a real feed solution is still needed. Acidified thiourea solutions were again used as the stripping media [26].
Some dialkyl-amino modified thiophenols have recently been investigated for Pd(II) recovery [27]. A follow-up study to earlier work on these SX systems identified compound 8 in Table 2, as one of the best derivatives. High Pd(II) and Pt(IV) extractions were found (%E > 99%), with rather low extraction values for La(III) and Zr(IV). The excellent Pd(II) and Pt(IV) recyclability data for 8 seem to have been obtained with a real feed solution, with acidified thiourea as stripping agent, but the analysis of the concentration of other metals in the organic and stripping phases should have also been carried out. Moreover, the dilute metal ion automotive leach solutions produced by this group are not fully representative of the typical compositions for these leaches liquors.
The reported data on the use of functionalized thiacalix [4/6] arenes at lower and upper rims as SX compounds for PGMs cannot be ignored. A review published in 2016 summarizes and discusses the most relevant information on the subject [31], and some of the thiacalixarene derivatives revealed a good extractive and selective behavior for Pd(II) recovery . Taking into account the overall results obtained, the collected data achieved for Pd(II) recovery may nevertheless not justify the necessary investment to carry out the elaborate synthesis of these thiacalixarene derivatives.
There are some other types of extractants such as sulfur -functionalized amide derivatives [32] that have shown promising behavior for Pd(II) recovery , although this work was done with synthetic model solutions. Readers can find more information in a recent review [7].
Conclusions
It is well known that the traditional compounds industrially used for Pd(II) extraction from PGMs primary resources, dialkyl sulfides, are readily prone to oxidation and show unfavorable kinetics. Hydroxyoxime derivatives are also used with the same aim. Accordingly, the adjustment of this sort of compound to recycle anthropogenic waste materials appears to be a logical consequence. Given the widespread availability of fundamental and applied data for the extractants already in the market, and information about their solubility in industrial diluents, a strong commitment by researchers to develop SX schemes to recover Pd(II) from spent catalysts could be expected. The promising results already achieved, briefly mentioned in this review , with commercial ILs, may contribute to the preferential choice of these materials for more in-depth research in the near future.
There is currently much activity in looking at the design of novel molecules to work as efficient and selective extractants for Pd(II) recovery from waste catalysts. The majority of these molecules can be obtained through accessible synthetic pathways. A increased effort to apply the best SX systems to real leach solutions of spent catalysts has recently been noticed, but several features should still be addressed prior to any practical application, even for the most promising schemes, such as the thorough evaluation of the loading metal profiles, reutilization stabilities, and data reproducibility when scaled-up. However, in the absence of further investigation and interest from related investors, the potential of some of these promising SX routes for Pd(II) recovery from secondary resources may end up as mere lab exercises.
References
Ndlovu J (2015) Anglo American Platinum–precious metals supply. Paper presented at exchange of good practices on metal by-products recovery–technology and policy challenges. European Commission, Brussels, Belgium, 12–13 Nov 2015. https://ec.europa.eu/growth/tools-databases/eip-raw-materials/en/content/international-conference-%E2%80%9Cexchange-good-practices-metal-products-recovery-technology-and. Accessed 28 Feb 2018
European Commission (2014) In: Report on critical raw materials for the EU. http://ec.europa.eu/DocsRoom/documents/10010/attachments/1/translations. Accessed 28 Feb 2018
European Commission (2017) In: Report on critical raw materials for the EU. http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52017DC0490&from=EN. Accessed 28 Feb 2018
Løvika AN, Hagelüken C, Wägera P (2018) Improving supply security of critical metals: Current developments and research in the EU. Sustain Mat Technol 15:9–18
Cox M (2004) Solvent extraction in hydrometallurgy. In: Rydberg J, Cox M, Musikas C, Choppin GR (eds) Solvent extraction principles and practice, 2nd edn. Marcel Dekker Inc., New York, pp 455–505
Matthey J (2018) In http://www.platinum.matthey.com/about-pgm/applications. Accessed 28 Feb 2018
Paiva AP (2017) Recycling of palladium from spent catalysts using solvent extraction—some critical points. Metals, 7, article 505
Ramachandra Reddy B, Raju B, Lee JY, Park HK (2010) Process for the separation and recovery of palladium and platinum from spent automobile catalyst leach liquor using LIX 84I and Alamine 336. J Hazard Mat 180(1–3):253–258
Gupta B, Singh I (2013) Extraction and separation of platinum, palladium and rhodium using Cyanex 923 and their recovery from real samples. Hydrometallurgy 134–135:11–18
Lee JY, Raju B, Kumar BN, Kumar JR, Park HK, Ramachandra Reddy B (2010) Solvent extraction separation and recovery of palladium and platinum from chloride leach liquors of spent automobile catalyst. Sep Purif Technol 73(2):213–218
Nguyen TH, Kumar BN, Lee MS (2016) Selective recovery of Fe(III), Pd(II), Pt(IV), Rh(III) and Ce(III) from simulated leach liquors of spent automobile catalyst by solvent extraction and cementation. Korean J Chem Eng 33(9):2684–2690
Anpilogova GR, Khisamutdinov RA, Golubyatnikova LG, Murinov YI (2016) Propiconazole and penconazole as effective extractants for selective recovery and concentration of platinum(IV) and palladium(II) from hydrochloric acid solutions formed in leaching of spent aluminoplatinum and aluminopalladium catalysts. Russ J Appl Chem 89(2):206–211
Nguyen TH, Sonu CH, Lee MS (2015) Separation of platinum(IV) and palladium(II) from concentrated hydrochloric acid solutions by mixtures of amines with neutral extractants. J Ind Eng Chem 32:238–245
Truong HT, Lee MS, Son SH (2017) Extraction of palladium(II) from hydrochloric acid solutions by solvent extraction with mixtures containing either Cyanex 301 or LIX 63. Metals, 7, article 541
Cieszynska A, Wisniewski M (2011) Selective extraction of palladium(II) from hydrochloric acid solutions with phosphonium extractants. Sep Purif Technol 80(2):385–389
Nguyen VT, Lee JC, Chagnes A, Kim MS, Jeong J, Cote G (2016) Highly selective separation of individual platinum group metals (Pd, Pt, Rh) from acidic chloride media using phosphonium-based ionic liquid in aromatic diluent. RSC Adv 6(67):62717–62728
Svecova L, Papaiconomou N, Billard I (2016) Quantitative extraction of Rh(III) using ionic liquids and its simple separation from Pd(II). Dalton Trans 45(38):15162–15169
Papaiconomou N, Svecova L, Bonnaud C, Cathelin L, Billard I, Chainet E (2015) Possibilities and limitations in separating Pt(IV) from Pd(II) combining imidazolium and phosphonium ionic liquids. Dalton Trans 44(46):20131–20138
Tong Y, Wang C, Li J, Yang Y (2014) Extraction mechanism, behavior and stripping of Pd(II) by pyridinium-based ionic liquid from hydrochloric acid medium. Hydrometallurgy 147–148:164–169
Traeger J, Tillmann K, Kelling A, Lubahn S, Cleve E, Mickler W, Heydenreich M, Müller H, Holdt HJ (2012) Complexation of palladium(II) with unsaturated dithioethers–a systematic development of highly selective ligands for solvent extraction. Eur J Inorg Chem 14(14):2341–2352
Traeger J, König J, Städtke A, Holdt HJ (2012) Development of a solvent extraction system with 1, 2-bis (2-methoxyethylthio) benzene for the selective separation of palladium(II) from secondary raw materials. Hydrometallurgy 127–128:30–38
Yamada M, Gandhi MR, Sato D, Kaneta Y, Kimura N (2016) Comparative study on palladium(II) extraction using thioamide-modified acyclic and cyclic extractants. Ind Eng Chem Res 55(33):8914–8921
Gandhi MR, Yamada M, Kondo Y, Shibayama A, Hamada F (2016) Rapid and selective extraction of Pd(II) ions using the SCS type pincer ligand 1,3-bis (dimethylthiocarbamoyloxy) benzene, and its Pd(II) extraction mechanism. RSC Adv. 6(2):1243–1252
Paiva AP, Ortet O, Carvalho GI, Nogueira CA (2017) Recovery of palladium from a spent industrial catalyst through leaching and solvent extraction. Hydrometallurgy 171:394–401
Senthil K, Akiba U, Fujiwara K, Hamada F, Kondo Y (2017) New heterocyclic dithioether ligands for highly selective separation and recovery of Pd(II) from acidic leach liquors of spent automobile catalyst. Ind Eng Chem Res 56(4):1036–1047
Gandhi RM, Yamada M, Haga K, Shibayama A (2017) Synthesis of pincer-type extractants for selective extraction of palladium from PGMs: an improved liquid-liquid extraction approach to current refining processes. Sci Rep, 7, article 8709
Yamada M, Gandhi MR, Kaneta Y, Kimura N, Katagiri H (2018) Thiodiphenol-based n-dialkylamino extractants for selective platinum group metal separation from automotive catalysts. Ind Eng Chem Res 57(5):1361–1369
Narita H, Tanaka M, Morisaku K (2008) Palladium extraction with N, N, N’, N’-tetra-n-octyl-thiodiglycolamide. Min Eng 21(6):483–488
Ortet O, Paiva AP (2015) Liquid–liquid extraction of palladium(II) from chloride media by N, N’-dimethyl-N, N’-dicyclohexylthiodiglycolamide. Sep Purif Technol 156–2:363–368
Ortet O, Paiva AP (2015) Development of tertiary thioamide derivatives to recover palladium(II) from simulated complex chloride solutions. Hydrometallurgy 151:33–41
Yamada M, Gandhi MR, Kunda UMR, Hamada F (2016) Thiacalixarenes: emergent supramolecules in crystal engineering and molecular recognition. J Incl Phenom Macrocycl Chem 85(1–2):1–18
Narita H, Morisaku K, Tamura K, Tanaka M, Shiwaku H, Okamoto Y, Suzuki S, Yaita T (2014) Extraction properties of palladium(II) in HCl solution with sulfide-containing monoamide compounds. Ind Eng Chem Res 53(9):3636–3640
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Paiva, A.P. (2018). Recovery of Palladium from Spent Catalysts—A Critical State-of-the-Art Review. In: Davis, B., et al. Extraction 2018. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-95022-8_172
Download citation
DOI: https://doi.org/10.1007/978-3-319-95022-8_172
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95021-1
Online ISBN: 978-3-319-95022-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)