Abstract
The principle behind CEDM is similar to that of breast magnetic resonance imaging as they both assess tumour neoangiogenesis; therefore, with some exceptions, most of the indications for breast MRI apply to CEDM.
CEDM is increasingly being adopted in breast-imaging centres, largely due to early clinical studies demonstrating this technology’s ability to provide both anatomic and functional information of breast parenchyma similar to MRI but at a lower cost and shorter duration of examination. Because such capability can be an added feature to the newer generation mammogram machines, we expect that the use of CEDM will increase. However, there is limited guidance regarding how to best implement this technology, and there are no standard guidelines for the clinical use of CEDM. In this chapter, we discuss the implementation of CEDM into the clinical practice setting, with a review of the available literature and perspectives from our large tertiary academic hospital.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Contrast-enhanced Digital Mammography (CEDM)
- Breast Magnetic Resonance Imaging
- Full-field Digital Mammography (FFDM)
- Careggi University Hospital
- Average Glandular Dose
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Contrast-enhanced digital mammography (CEDM) was first introduced to clinical use by Lewin et al. in 2003 [1]; since then, the use of CEDM in clinical practice has evolved with increasing equipment installation, clinical experience and clinical application.
The principle behind CEDM is similar to that of breast magnetic resonance imaging [2] as they both assess tumour neoangiogenesis; therefore, with some exceptions, most of the indications for breast MRI apply to CEDM.
CEDM is increasingly being adopted in breast-imaging centres, largely due to early clinical studies demonstrating this technology’s ability to provide both anatomic and functional information of breast parenchyma similar to MRI but at a lower cost and shorter duration of examination. Because such capability can be an added feature to the newer generation mammogram machines, we expect that the use of CEDM will increase. However, there is limited guidance regarding how to best implement this technology, and there are no standard guidelines for the clinical use of CEDM. In this chapter, we discuss the implementation of CEDM into the clinical practice setting, with a review of the available literature and perspectives from our large tertiary academic hospital.
Since the implementation of CEDM in 2016, Careggi University Hospital in Florence, Italy, has performed approximately 800 examinations. CEDM is now being conducted almost daily as an essential part of the diagnostic workup, in which a majority (75%) of our cases have a breast cancer diagnosis, and imaging is performed to evaluate the extent of disease. Twenty-five percent of CEDM cases are performed for additional diagnostic evaluation of inconclusive imaging findings on mammography. The remainder of our indications includes an assessment of the response to neoadjuvant chemotherapy (NAC), patients with a history of breast cancer and screening of high-risk patients for whom MRI is contraindicated.
1 Configuring a Contrast-Enhanced Digital Mammography (CEDM) Unit
The U.S. Food and Drug Administration (FDA) approved contrast-enhanced mammography systems in 2011, with the first system to be approved being the GE Essential SenoBright system (GE Healthcare), known as contrast-enhanced spectral mammography (CESM). Subsequently, in 2013, the Hologic Dimensions I-View (Hologic) system was approved, which is known as contrast-enhanced digital mammography (CEDM). Other vendors are currently working on prototypes. Most current-generation mammography systems are frequently delivered with CEDM capability, reflecting the feasibility of implementing CEDM into breast-imaging practice in terms of equipment costs and space allocation.
CEDM can be performed in the existing mammography suite for practices that own a CEDM-capable mammography system. Implementation requires only the acquisition of a standard contrast power injector, purchase of a software upgrade from the vendor and insertion of a copper filter into the existing mammography unit [3, 4]. Hence, this is the beauty of incorporating CEDM, as it is just a technology application that is uploaded into an existing mammogram unit, precluding the need for additional space, and it fits nicely without disruption into the workflow.
For practices that do not own a CEDM-capable mammography system, the acquisition costs are higher. However, implementing a CEDM-capable mammography unit would definitely be beneficial for any breast-imaging centre because the system can provide the unit with standard 2D and 3D mammography as well as a CEDM upgrade capability and can also perform stereotactic-guided procedures.
1.1 Patient Issues
Breast CEDM patients comprise a subset of patients who have issues, typically because they have a known cancer, have a relatively high risk for cancer or have an issue on their mammogram that requires further testing. As a result, these patients are generally anxious. Performing an MRI examination for these patients can exacerbate this anxiety, as they would have to wait for an opening in the MRI examination schedule; in contrast, CEDM can be performed on the same day in the existing mammography suite. The presence of a CEDM unit at our centres has allowed us to image patients quickly because we can perform CEDM on the same day that a patient is referred to us for a suspicious breast mass; therefore, we avoid any delay involved with the patient receiving an MRI examination.
CEDM examinations can be added to a full breast-imaging schedule at our breast units in Careggi University Hospital and Kuala Lumpur Hospital (KLH) with little disruption to our schedule, similar to the case of a breast ultrasound (US). At our centres, we observed that the total “room time” to perform a CEDM is approximately 20 minutes.
Hobbs et al. [5] studied the differences in patient preference and tolerance between CEDM and MRI. Although patients graded breast compression or positioning and contrast administration less favourably for CEDM, in general, they still expressed a preference for CEDM over breast MRI. Based on the experience at our centres, we observed a greater ease of scheduling, faster imaging and interpretation times, and improved patient compliance and tolerance when we performed CEDM compared with those of MRI.
CEDM is particularly useful for patients with claustrophobia, leading to higher satisfaction among patients and referring clinicians than that seen in MRI. CEDM may also be beneficial for patients with other contraindications to MRI such as body habitus, table weight limits and the presence of pacemakers.
Patient anxiety can be reduced by providing information about the CEDM procedure during the consultation along with a patient fact card explaining what to expect (Fig. 7.1). The radiologist or referring physician should inform and counsel patients to ensure that there are no absolute contraindications for CEDM contrast agents. It is also helpful to explain in advance the necessity of injecting contrast; as such a discussion would exclude any prior history of reaction to contrast. Proper patient screening, adequate prophylactic measures and training of staff to cope with hypersensitivity reactions can prevent certain adverse reactions or their complications. Radiologists should be familiar with all potential adverse renal events, including contrast-induced nephropathy, and should plan strategies with the referring physicians to lower their incidences [6].
Before a patient undergoes a CEDM examination, the patient and referring physician should possess a clear understanding of the potential outcomes. The patient must be aware that CEDM is a complementary test that may generate additional workup with a complementary ultrasonography, with the possibility of a core needle biopsy due to findings on the CEDM.
As CEDM is an adjunctive test that is used only in specific clinical situations in view of the added radiation doses received, patient self-referral for breast CEDM should be discouraged. The physicians should also be made aware that there will be an added radiation dose to the breast during the procedure [7]. Therefore, it may be helpful initially to designate a single radiologist to screen and protocol all referred breast CEDM cases to ensure that the appropriate patients are being scanned. This approach would include requests from clinicians who may not initially understand the importance of careful patient selection. We found it helpful to provide referring physicians with a presentation on this new technique available at our centre in addition to providing them with a memo on the appropriate indications for CEDM referrals.
1.2 Radiologist and Technologist Considerations
As with any new imaging modality, training is an important factor in the implementation process. CEDM was implemented at our centres in Careggi University Hospital and KLH by our existing mammography technologists who were trained to perform CEDM, and there was no need to hire additional technologists. The radiologists, technologists and physicists are required to undergo training in CEDM, which is typically offered through application training provided by the vendors.
Initially, it may be useful to scan patients with known cancers to develop a sense of confidence and build a knowledge base. Additionally, these patients are likely going to the operating room, regardless, and any additional information that the CEDM examination provides will likely only help them.
Nursing support may be beneficial for placement of the intravenous (IV) line and assistance with management of contrast reactions; thus, a dedicated nurse is always present during CEDM procedures at our centres. A radiologist or other licensed physician must always be present in the mammography suite during CEDM imaging to evaluate and treat any contrast-associated reaction.
Another important consideration for technologists is that the timeline of 6 minutes to complete all imaging is sufficient to acquire all four standard mammographic views. Emphasizing this point to technologists is important, because patients may experience increased anxiety when they feel rushed through the imaging process, frequently resulting in motion artefacts on the recombined images. Apprehension among the technologists at our centres was particularly apparent when we began implementing the CEDM unit; however, this apprehension lessened with increasing experience.
Contrast splatter mimicking calcifications is another artefact that we occasionally observe. At Careggi University Hospital and KLH, we ensure that a nurse or medical officer inserts the intravenous line and performs contrast administration away from the mammogram machine, after which patient positioning is performed by the technician. Marc Lobbes of the Maastricht University Medical Centre reported that his centre expressly ensures that the technologist handling contrast administration does not perform patient positioning; additionally, the technologist uses gloves that are discarded prior to image acquisition.
1.3 Radiation Dose Considerations
Among the limitations of CEDM is the added radiation dose involved in the examination. Dromain et al. [8] were the first to report on the CEDM radiation dose, for which they observed an increase of a 20% higher radiation dose in CEDM relative to full-field digital mammography (FFDM).
Subsequently, Jeukens et al. [7] demonstrated that the average glandular dose for CEDM was 2.80 mGy relative to 1.55 mGy for routine FFDM.
More recently, James et al. [9] performed phantom studies to observe the radiation exposure in both dense and non-dense breast tissue. In their study, CEDM was found to increase the average glandular dose at a mean breast thickness of 63 mm by approximately 0.9 mGy and 0.5 mGy relative to 2D FFDM and 3D tomosynthesis, respectively; however, the additional radiation exposure was still below the dose limit of 3 mGy set by the US Mammography Quality Standards Act (MQSA) guidelines. James et al. concluded that the benefits obtained by the additional contrast images offset the additional radiation.
Yakoumakis et al. [10] reported that the low-energy mammography is the main contributor to the total glandular breast dose in CEDM. At our centre in KLH, when patients older than 40 years of age are referred for a suspicious breast mass, we immediately perform CEDM because the low-energy image obtained in the CEDM examination is equivalent to that of a standard mammogram. Therefore, the only added radiation dose received would be the high-energy exposure, which is minimal. However, the additional dose should always be kept in mind when deciding to use this examination.
1.4 Examination Interpretation and Results Communication
For radiologists, in addition to training on the technical aspects, a learning curve is also required for image interpretation. Lalji et al. [11] demonstrated that CEDM requires a minimal learning curve to effectively interpret studies in their study, which included ten readers; among which were radiologists who were experienced in reading CEDM exams, experienced breast radiologists without any prior experience in CEDM and radiology residents. Even in our setting, we have observed CEDM to be relatively straightforward to interpret compared with the interpretation of FFDM alone and MRI. Radiologists will generally observe a decrease in recall rates and also fewer short-term follow-up cases. To breast radiologists, CEDM images look familiar and may be faster to interpret than MRI images, with a mean total image interpretation time of 1–2 minutes [3, 4]. As with mammographic interpretation, communication with the referring physician about the final recommendation is necessary in CEDM studies. Considering the ease of interpretation of CEDM examinations, the radiologist can inform patients of the results of the CEDM procedure on the same day. At our centre, we have a consultation room adjacent to the CEDM examination room where we interpret the images immediately after the procedure and provide patients with their reports before they leave. If a new lesion is found on CEDM, we can immediately perform a complementary US, and an US-guided core biopsy procedure can be performed during the same sitting. This capability markedly reduces patient anxiety and waiting time.
2 Performing Contrast-Enhanced Imaging of the Breast
2.1 Patient Handling
Prior to performing a CEDM examination, patients should be provided with thorough instructions regarding the procedure and informed of potential adverse reactions to iodinated contrast media. An informed consent should be obtained from patients before the administration of contrast media. After a careful assessment of the patient’s medical history, including known allergies, particularly to iodinated contrast media, and a laboratory evaluation of renal function, peripheral intravenous (IV) access is obtained in the antecubital fossa, preferably with a 22-gauge needle. A dose of 1.5 mL/kg of iodinated contrast material (300–370 mg iodine/mL) is administered by a power injector at a rate of 2–3 mL/s. A 20-mL saline bolus is administered following contrast injection to achieve a significant bolus administration and optimal delivery of contrast to tissues, thus improving image quality.
After contrast injection is completed, the connecting tubing is detached from the patient, while the catheter remains in place until the end of the examination. The acquisition of the images begins 2 minutes after contrast injection, and care must be taken to complete the examination within 8 minutes following contrast media injection. During this time, the patient is monitored for any adverse reaction to the iodinated contrast material. A 2 minute delay after injection is essential, as applying compression too early may result in retained contrast media within vessels outside the breast, preventing the contrast from flowing in sufficiently and producing a bolus in major vessels that is visible in early images.
At our centres, the entire CEDM procedure takes approximately 20 minutes, after which the patient changes her clothes and waits for us to review the images before she is called to the consultation room, where the results are explained to her. If any areas of abnormal enhancement are detected based on the CEDM, the patient is immediately subjected to a second-look US to locate the lesion, and US-guided core biopsy is performed.
This process takes approximately 30 minutes post-procedure, which is sufficient time for us to observe any contrast reaction that may occur, and the IV line is kept in place in case any severe contrast reactions are observed.
2.2 Contrast Reactions
Although the use of contrast media is generally considered safe and beneficial in medical imaging, such use occasionally results in adverse events in patients. A 0.6% reaction rate to IV injections of non-ionic contrast media has been reported [12].
All life-threatening contrast reactions typically occur immediately or within 20 minutes after contrast administration; therefore, it is necessary to observe the patient post-contrast administration. General precautions should be taken to ensure that the likelihood of severe adverse reactions is minimal, such as ensuring that patients are adequately hydrated and that emergency equipment is available in the CEDM suite [6].
All staff involved with the CEDM unit should have up-to-date anaphylaxis training and the contact information for the institution’s emergency response team. They should know the location of emergency monitoring equipment and medications. The American College of Radiology recently designed a contrast reaction card that summarizes the important steps for managing an acute reaction to contrast agents, which is available on their website. Each card is the size of a driver’s licence, which has been distributed to all our staff associated with the CEDM unit of our centre in KLH. According to Marc Lobbes, the Maastricht University Medical Centre performs annual training to cope with these reactions, which has worked well for their centre.
2.3 Image Acquisition
Image acquisition includes full-field exposures obtained at high and low energies using standard craniocaudal (CC) and mediolateral oblique (MLO) projections of each breast. The imaging protocol is typically configured in the system, and the order of image acquisition has not been shown to be relevant. Each facility must determine their own CEDM protocol to stimulate a consistent protocol. At KLH, we perform the CC projection on the suspected side first, in an attempt to capture early arterial enhancement and minimize false-negative results from early washout; subsequently, the contralateral breast is imaged in CC view and then followed by the MLO projections of both breasts. If enhancement is seen on the suspected side, we obtain an image after 8 minutes in the delayed phase to assess the enhancement kinetics that may provide additional information regarding the likelihood of malignancy. Although there is still no evidence that the kinetics in CEDM is similar to that of breast MRI, based on our experience at Careggi University Hospital and KLH, the incorporation of a delayed acquisition provides a relatively accurate guide to the lesion morphology.
The low-energy mammograms are performed at the same peak kilovoltage (kVp) as full-field digital mammography (FFDM) at 25–33 kVp and with the same filtration of either rhodium or silver. The high-energy acquisition is performed at a higher value of 45–49 kVp, optimized to the k-edge of iodine and using a copper filter. In 2002, Skarpathiotakis et al. [13] compared aluminium, molybdenum and copper filters for CEDM and concluded that copper is a suitable choice because it is relatively transparent to X-rays at energies where iodine attenuates them most heavily, thereby providing high contrast. They also inferred that due to a k-edge of copper at 9 keV, high attenuation occurs at low energies, thereby reducing the dose to the breast from relatively low-energy X-rays.
Recombined images are produced by the cancellation of background breast tissue and sent to the picture archiving and communication system (PACS) together with the low-energy images.
3 Clinical Applications
A detailed description and background of the clinical indications of CEDM are comprehensively discussed in Chapter 6. We provide here a brief summary of the clinical indications with a note on our clinical experience for each indication.
3.1 Inconclusive Findings in Conventional Imaging
Occasionally, a mammographic finding may be inconclusive even when additional problem-solving views and sonograms have been obtained. This situation arises because the diagnostic accuracy of FFDM is highly dependent on the fibroglandular density of breast parenchyma [14]. Additional imaging is typically required in response to difficult-to-interpret mammograms, such as in cases of a focal asymmetry, evaluation of architectural distortion and differentiation between a scar and a recurrent cancer.
Several studies have been conducted to compare the feasibility of CEDM with that of conventional breast imaging in breast cancer detection; these studies showed that CEDM detects more malignant tumours than does mammograms [2, 8, 11, 15,16,17].
Also notable is the comparison of CEDM to MRI in the diagnosis of cancer, regarding which very limited literature is available. A cornerstone in this context was laid by Fallenberg et al. [18], who demonstrated that bilateral dual-energy CEDM and MRI are superior to mammogram in breast tumour detection, with CEDM performing slightly better than MRI, exhibiting an increase in lesion detection by 17.5% relative to FFDM and 2.6% relative to MRI. In this report, the authors also demonstrated that CEDM has an excellent correlation with respect to the evaluation of the extent of the disease. They also observed that CEDM recognized two cancers undetected by MRI, an invasive lobular carcinoma (ILC) and an invasive ductal carcinoma (IDC), plus ductal carcinoma in situ (DCIS). They inferred that in the absence of neoangiogenesis, contrast media moved into the ducts by diffusion as the possible explanation for the enhancement of DCIS in CEDM. That study suggested that longer time delays between contrast injection and CEDM exposure can result in stronger enhancement and better visibility of DCIS in CEDM compared to MRI because the amount of contrast reaching the tissue by diffusion is time-dependent. The authors concluded that the significant role of delayed acquisition of contrast-enhanced images in CEDM allowed better visibility of certain cancers relative to MRI, particularly when neovascularization is absent and only mild or low enhancement is present due to diffusion of the contrast media into the ducts. At Careggi University Hospital, we have also observed that some lower grade tumours are enhanced more vividly in the second mammographic view than in the first view (Fig. 7.2).
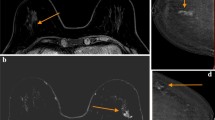
Delayed enhancement. A 59-year-old female, in whom CEDM was done as preoperative staging for a biopsy proven, left infiltrating tubular carcinoma. (a, b) No demonstrable lesion seen on 2D and 3D mediolateral oblique views. (c, d) CEDM recombined image in mediolateral oblique view in early and delayed phase. The examination demonstrates a faint area of non-mass enhancement (NME) in the mid to upper quadrant that is more prominent in the delayed acquisition (circle) corresponding to the region that was previously biopsied. There is another intensely enhancing nodule seen at the axillary tail (arrow), which shows wash-out in the delayed acquisition. A second-look ultrasound was performed where the axillary tail lesion was identified and biopsied.Diagnosis: The pathology of the axillary tail lesion (arrow) was also an Infiltrating Tubular Carcinoma.
However, note that a negative CEDM does not preclude the need to biopsy indeterminate or suspicious calcifications identified on mammography. Whether calcifications are indeterminate or suspicious (thus requiring biopsy) should be determined solely on the mammographic features of the calcifications (Figs. 7.3 and 7.4).
Problem-solving. A 46-year-old woman was recalled from screening for an architectural distortion in the left lower outer quadrant. (a) 2D and 3D mediolateral oblique views. (b) 2D and 3D craniocaudal views. It is observed that the architectural distortions are more clearly seen on the 3D tomosynthesis images (circles) (a 1, b 1) and 3D magnified views of the distortions. (c) CEDM recombined images show no enhancement. (d) Tomosynthesis guided biopsy of the distortion was still performed. Diagnosis: The pathology of the architectural distortion was fibrocystic change
Problem-solving. A 60-year-old female, on follow-up since 12 years post-surgery for left ductal carcinoma in situ (DCIS). (a) 2D craniocaudal and mediolateral oblique views show a focal distortion (circle) seen at the left surgical scar site. (b) 3D craniocaudal and mediolateral oblique views demonstrate a more prominent area of distortion (circle) at the surgical scar. (c) CEDM recombined images show an intensely enhancing lesion in the right breast (box); however, no enhancement is seen at the region of surgical scar (circle) in the left breast. The examination demonstrated an enhancing lesion in the right upper inner quadrant with no enhancement seen at the apparent suspicious lesion of the left breast. Retrospective assessment of the 3D images revealed a subtle architectural distortion in the right breast (magnified view). A second-look ultrasound was performed where this lesion was identified and biopsied.Diagnosis: The pathology of the right beast lesion was an invasive ductal carcinoma
3.2 Preoperative Staging
Breast conservative surgery is the current trend for treatment of small tumours with a favourable lesion-to-breast volume ratio. Therefore, it is essential to provide accurate preoperative radiological quantification of the tumour to prevent early recurrences and to avoid re-excision for positive excision margins on histology. For women in whom the mammogram, ultrasound and physical examination indicate that the tumour is sufficiently small to allow lumpectomy and conservation surgery, further evaluation with additional imaging may demonstrate more extensive tumours than previously suspected. This information can be helpful for the surgeons to accurately plan the amount of tissue to be included in the lumpectomy or in suggesting the need for mastectomy.
At Careggi University Hospital and KLH, the majority of our CEDM examinations are done for preoperative staging. A significant number of cases have undergone a change of management post-CEDM based on the localization of additional lesions. In KLH, all patients undergoing breast conservation surgery are subjected to a CEDM prior to operation, and patients who are referred to us for highly suspicious masses are subjected to a CEDM immediately. The low-energy image is considered the patient’s mammogram. We found that this approach enabled improved biopsy accuracy, particularly in the presence of multifocal lesions (Fig. 7.5).
Preoperative staging. A 45-year-old woman with a biopsy confirmed invasive ductal carcinoma in the left breast. (a, a 1) 2D craniocaudal and mediolateral oblique views with corresponding ultrasound images, demonstrating a dominant mass in the left upper outer quadrant (white arrow) with a small satellite nodule (blue arrow) less than 2 cm away from the index lesion. The patient was scheduled for a breast conservation surgery, and CEDM was performed for preoperative assessment. (b) CEDM-recombined images in craniocaudal and mediolateral oblique views demonstrated a small enhancing nodule (circle) approximately 5 cm from the index lesion. (b 1) A second-look ultrasound was performed, and a subtle suspicious hypoechoic lesion was identified and biopsied.Diagnosis: the pathology was an invasive ductal carcinoma, not otherwise specified
Tennant et al. [19] concluded that CEDM provided immediately available, clinically useful information in patients with suspicious lesions. There was a higher sensitivity, specificity and size accuracy for breast cancer detection and staging demonstrated using CEDM as a primary mammographic investigation in clinically suspicious lesions.
Fallenberg et al. [18] suggested that initially using CEDM alone in symptomatic patients could decrease the radiation dose.
3.3 Assessment of Response to Neoadjuvant Chemotherapy
In women with large primary breast carcinomas, preoperative neoadjuvant chemotherapy (NAC) is increasingly being used to shrink tumour size to facilitate mastectomy or breast conservation. The determination of tumour size after treatment is important to the surgeon to enable complete removal of the tumour without residual cancer in the breast at the time of lumpectomy. MRI [2] is currently the modality of choice for monitoring tumour response and for assessing residual disease after NAC, being more accurate than mammography, ultrasound and clinical examination [20].
A recent study by Iotti et al. [21] compared the diagnostic performance of CEDM with respect to MRI; the authors concluded that CEDM was as reliable as MRI in assessing the response to NAC and can be considered an alternative when MRI is contraindicated or unavailable. In another study, Barra et al. [22] compared CEDM with FFDM in the evaluation of response to NAC and ultimately concluded that a positive CEDM indicates the presence of residual tumour after NAC.
In our clinical experience at Careggi University Hospital, we observed that CEDM could serve as an alternative to breast MRI to monitor the responsiveness to NAC, as depicted in Fig. 7.6.
Response to chemotherapy. A 62-year-old woman with a palpable mass underwent core biopsy yielding invasive ductal carcinoma. CEDM examinations were performed during the patient’s course of preoperative neoadjuvant chemotherapy. (a) Mediolateral oblique and craniocaudal low-energy and (b) recombined images of CEDM done pre-neoadjuvant chemotherapy assessment revealed an irregular heterogeneously enhancing mass (arrow) with associated satellite nodules in the lower outer quadrant. (c) After six cycles of chemotherapy, mediolateral oblique and craniocaudal low-energy and (d) recombined images of a repeat CEDM demonstrated no enhancing areas with complete response of the tumour to chemotherapy
3.4 Screening of High-Risk Patients
When addressing genetic risk, to date, MRI remains the most sensitive examination for the detection of breast cancer in both women at average and increased risk, yielding 15 cancers for every 1000 women at intermediate (15–20%) or high (>20%) risk. Several studies have demonstrated that in approximately 45% of women with intermediate or high genetic risk, breast cancers were detected only by MRI [7, 23, 24]. These results led to specific screening programmes for high-risk women, including annual mammography and MRI, developed and recommended by the American Cancer Society and the European Society of Breast Imaging [25]. However, MRI may not be an option in this group of patients due to its high cost and low availability. Previous studies have suggested CEDM application in the screening of these patients, with the first pilot study performed by Jochelson and colleagues [25], who concluded that this technique could be valuable as a supplemental imaging modality for women at increased risk for breast cancer who do not meet the criteria for MRI or in whom access to MRI is limited.
However, few studies have been published about this topic until now, and the main concern regarding performing CEDM in patients at high risk of developing breast cancer is the radiation exposure involved. There has been no conclusive study for performing CEDM in high-risk patients, and this indication remains to be studied in a larger population.
At our centres, we tend to avoid performing CEDM on patients who are highly sensitive to the effects of radiation. So far, we have performed CEDM on only several patients with the BRCA 1 mutation; such patients had dense breasts and were contraindicated for breast MRI (Fig. 7.7).
High-risk screening. A 40-year-old woman with a strong family history of breast cancer and personal history of BRCA2 gene mutation. She has severe claustrophobia and refused to undergo a breast MRI examination. (a) 2D low-energy and 3D tomosynthesis in MLO projections show relatively dense breast parenchymal pattern with a subtle area of increased density (circle) in the lower quadrant of the left breast. (b) CEDM-recombined image in the early and late phase in MLO projection. The examination shows an irregular enhancing mass demonstrating a rapid wash-out. The lesion was identified on a second look ultrasound and an US-guided biopsy was performed. Diagnosis: the pathology was an infiltrating lobular carcinoma
3.5 Unknown Primary Cancer
Occult primary breast cancer presenting as isolated ipsilateral axillary metastases without evidence of tumour in the breast on physical examination or mammography accounts for approximately 0.3–0.8% of breast cancers [26].
MRI has been the only imaging modality that can reliably identify breast cancers that have evaded detection by mammography and physical examination [27, 28]. To date, there is no literature available for CEDM in identifying unknown primary cancer; however, we assume that the accuracy of breast cancer detection in this group of patients would be similar to MRI. We have performed CEDM in several cases of occult primary malignancy at Careggi and KLH, and we observed satisfactory results.
4 Future of Contrast-Enhanced Mammography
There is a growing body of evidence supporting CEDM use for various clinical indications, with levels of sensitivity and specificity on par with those of breast MRI. CEDM should therefore be considered for expanded clinical use at other breast-imaging centres in the near future. Future research with larger sample populations for CEDM as an adjunct or alternative to mammography, US, MRI or a combination of these modalities will affect the expanded use of CEDM.
The time required to perform a CEDM examination is shorter than that required for MRI, as is the time required for lesion interpretation with CEDM. These are among the main reasons CEDM is being used more frequently in breast cancer diagnosis. Although CEDM is currently available at a minority of breast-imaging practices, widespread adoption could be rapid, given that many current-generation mammography systems are delivered with CEDM capability.
CEDM provides functional information similar to MRI at a lower cost and greater ease of implementation. Bhavika Patel et al. [4], using data obtained from the Mayo Clinic in Arizona, suggested that CEDM is faster to perform and interpret and has lower equipment acquisition and maintenance costs than does MRI. They also observed that if CEDM was deemed a viable substitute for breast MRI, such capability could lower the overall imaging costs of the healthcare system by more than one billion US dollars annually. Even at our centre in Careggi University Hospital, we observed a 60% reduction in the cost of a CEDM examination compared with that of an MRI.
CEDM is likely to be among the modalities offering the best value compared with other costlier emerging imaging technologies, such as automated whole-breast ultrasound, contrast-enhanced breast ultrasound, abbreviated breast MRI, molecular breast imaging, positron emission mammography (PET) and breast computed tomography (CT).
The limitations of CEDM are discussed comprehensively in Chapter 10. The technique’s disadvantages include patient exposure to iodinated contrast material, as well as the potential low-risk associated with contrast-induced reactions and radiation exposure. Unlike MRI, there is no commercially available system to biopsy regions of suspicious enhancement under CEDM guidance [29]. To our knowledge, however, there is evidence that a commercial CEDM-guided biopsy system will become available in due time.
References
Lewin JM, Isaacs PK, Vance V, Larke FJ. Dual-energy contrast-enhanced digital subtraction mammography: feasibility. Radiology. 2003;229(1):261–8.
Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12(1):1–222.
Covington MF, Pizzitola VJ, Lorans R, Pockaj BA, Northfelt DW, Appleton CM, et al. The future of contrast-enhanced mammography. AJR Am J Roentgenol. 2018;210(2):292–300.
Patel BK, Gray RJ, Pockaj BA. Potential cost savings of contrast-enhanced digital mammography. AJR Am J Roentgenol. 2017;208(6):W231–W7.
Hobbs MM, Taylor DB, Buzynski S, Peake RE. Contrast-enhanced spectral mammography (CESM) and contrast enhanced MRI (CEMRI): patient preferences and tolerance. J Med Imaging Radiat Oncol. 2015;59(3):300–5.
Katrina R, Beckett AKM, Langer JM. Safe use of contrast media: what the radiologist needs to know. Radiographics. 2015;35(6):1738–50.
Jeukens CR, Lalji UC, Meijer E, Bakija B, Theunissen R, Wildberger JE, et al. Radiation exposure of contrast-enhanced spectral mammography compared with full-field digital mammography. Investig Radiol. 2014;49(10):659–65.
Dromain C, Thibault F, Muller S, Rimareix F, Delaloge S, Tardivon A, et al. Dual-energy contrast-enhanced digital mammography: initial clinical results. Eur Radiol. 2011;21(3):565–74.
James JR, Pavlicek W, Hanson JA, Boltz TF, Patel BK. Breast radiation dose with CESM compared with 2D FFDM and 3D Tomosynthesis mammography. AJR Am J Roentgenol. 2017;208(2):362–72.
Yakoumakis E, Tzamicha E, Dimitriadis A, Georgiou E, Tsapaki V, Chalazonitis A. Dual-energy contrast-enhanced digital mammography: patient radiation dose estimation using a Monte Carlo code. Radiat Prot Dosim. 2015;165(1–4):369–72.
Lalji UC, Houben IP, Prevos R, Gommers S, van Goethem M, Vanwetswinkel S, et al. Contrast-enhanced spectral mammography in recalls from the Dutch breast cancer screening program: validation of results in a large multireader, multicase study. Eur Radiol. 2016;26(12):4371–9.
Wang CL, Cohan RH, Ellis JH, Caoili EM, Wang G, Francis IR. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am J Roentgenol. 2008;191(2):409–15.
Skarpathiotakis M, Yaffe MJ, Bloomquist AK, Rico D, Muller S, Rick A, et al. Development of contrast digital mammography. Med Phys. 2002;29(10):2419–26.
Carney PA, Miglioretti DL, Yankaskas BC, Kerlikowske K, Rosenberg R, Rutter CM, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138(3):168–75.
Lobbes MB, Smidt ML, Houwers J, Tjan-Heijnen VC, Wildberger JE. Contrast enhanced mammography: techniques, current results, and potential indications. Clin Radiol. 2013;68(9):935–44.
Mori M, Akashi-Tanaka S, Suzuki S, Daniels MI, Watanabe C, Hirose M, et al. Diagnostic accuracy of contrast-enhanced spectral mammography in comparison to conventional full-field digital mammography in a population of women with dense breasts. Breast Cancer. 2017;24(1):104–10.
Cheung YC, Lin YC, Wan YL, Yeow KM, Huang PC, Lo YF, et al. Diagnostic performance of dual-energy contrast-enhanced subtracted mammography in dense breasts compared to mammography alone: interobserver blind-reading analysis. Eur Radiol. 2014;24(10):2394–403.
Fallenberg EM, Dromain C, Diekmann F, Engelken F, Krohn M, Singh JM, et al. Contrast-enhanced spectral mammography versus MRI: initial results in the detection of breast cancer and assessment of tumour size. Eur Radiol. 2014;24(1):256–64.
Tennant SL, James JJ, Cornford EJ, Chen Y, Burrell HC, Hamilton LJ, et al. Contrast-enhanced spectral mammography improves diagnostic accuracy in the symptomatic setting. Clin Radiol. 2016;71(11):1148–55.
Rosen EL, Blackwell KL, Baker JA, Soo MS, Bentley RC, Yu D, et al. Accuracy of MRI in the detection of residual breast cancer after neoadjuvant chemotherapy. AJR Am J Roentgenol. 2003;181(5):1275–82.
Iotti V, Ravaioli S, Vacondio R, Coriani C, Caffarri S, Sghedoni R, et al. Contrast-enhanced spectral mammography in neoadjuvant chemotherapy monitoring: a comparison with breast magnetic resonance imaging. Breast Cancer Res. 2017;19(1):106.
Barra FR, de Souza FF, Camelo R, Ribeiro ACO, Farage L. Accuracy of contrast-enhanced spectral mammography for estimating residual tumor size after neoadjuvant chemotherapy in patients with breast cancer: a feasibility study. Radiol Bras. 2017;50(4):224–30.
Francescone MA, Jochelson MS, Dershaw DD, Sung JS, Hughes MC, Zheng J, et al. Low energy mammogram obtained in contrast-enhanced digital mammography (CEDM) is comparable to routine full-field digital mammography (FFDM). Eur J Radiol. 2014;83(8):1350–5.
Fallenberg EM, Dromain C, Diekmann F, Renz DM, Amer H, Ingold-Heppner B, et al. Contrast-enhanced spectral mammography: does mammography provide additional clinical benefits or can some radiation exposure be avoided? Breast Cancer Res Treat. 2014;146(2):371–81.
Jochelson MS, Pinker K, Dershaw DD, Hughes M, Gibbons GF, Rahbar K, et al. Comparison of screening CEDM and MRI for women at increased risk for breast cancer: a pilot study. Eur J Radiol. 2017;97:37–43.
Morris EA, Schwartz LH, Dershaw DD, van Zee KJ, Abramson AF, Liberman L. MR imaging of the breast in patients with occult primary breast carcinoma. Radiology. 1997;205(2):437–40.
Olson JA Jr, Morris EA, Van Zee KJ, Linehan DC, Borgen PI. Magnetic resonance imaging facilitates breast conservation for occult breast cancer. Ann Surg Oncol. 2000;7(6):411–5.
Lieberman S, Sella T, Maly B, Sosna J, Uziely B, Sklair-Levy M. Breast magnetic resonance imaging characteristics in women with occult primary breast carcinoma. Isr Med Assoc J. 2008;10(6):448–52.
Ali-Mucheru M, Pockaj B, Patel B, Pizzitola V, Wasif N, Stucky CC, et al. Contrast-enhanced digital mammography in the surgical management of breast cancer. Ann Surg Oncol. 2016;23(Suppl 5):649–55.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Kaur, M., Piccolo, C.L., Arasaratnam, S. (2018). Implementation of Contrast-Enhanced Mammography in Clinical Practice. In: Nori, J., Kaur, M. (eds) Contrast-Enhanced Digital Mammography (CEDM). Springer, Cham. https://doi.org/10.1007/978-3-319-94553-8_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-94553-8_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-94552-1
Online ISBN: 978-3-319-94553-8
eBook Packages: MedicineMedicine (R0)