Abstract
Objectives
To compare mammography (MG), contrast-enhanced spectral mammography (CESM), and magnetic resonance imaging (MRI) in the detection and size estimation of histologically proven breast cancers using postoperative histology as the gold standard.
Methods
After ethical approval, 80 women with newly diagnosed breast cancer underwent MG, CESM, and MRI examinations. CESM was reviewed by an independent experienced radiologist, and the maximum dimension of suspicious lesions was measured. For MG and MRI, routine clinical reports of breast specialists, with judgment based on the BI-RADS lexicon, were used. Results of each imaging technique were correlated to define the index cancer. Fifty-nine cases could be compared to postoperative histology for size estimation.
Results
Breast cancer was visible in 66/80 MG, 80/80 CESM, and 77/79 MRI examinations. Average lesion largest dimension was 27.31 mm (SD 22.18) in MG, 31.62 mm (SD 24.41) in CESM, and 27.72 mm (SD 21.51) in MRI versus 32.51 mm (SD 29.03) in postoperative histology. No significant difference was found between lesion size measurement on MRI and CESM compared with histopathology.
Conclusion
Our initial results show a better sensitivity of CESM and MRI in breast cancer detection than MG and a good correlation with postoperative histology in size assessment.
Key points
• Contrast-enhanced spectral mammography (CESM) is slowly being introduced into clinical practice.
• Access to breast MRI is limited by availability and lack of reimbursement.
• Initial results show a better sensitivity of CESM and MRI than conventional mammography.
• CESM showed a good correlation with postoperative histology in size assessment.
• Contrast-enhanced spectral mammography offers promise, seemingly providing information comparable to MRI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Early detection of breast cancer by X-ray mammography has been shown to reduce mortality; however, this method has a low sensitivity and specificity in young patients and women with radiographically dense breasts due to a reduced contrast between a possible tumour and surrounding breast tissue [1–3]. Treatment options for breast cancer depend on the size of the main tumour and the presence or absence of additional foci. Therefore, apart from detection, accurate size estimation is crucial for selecting the best treatment strategy for each patient [4, 5]. At present, MRI is considered the best imaging investigation for the detection of breast cancer and the assessment of the extent of disease in preoperative planning [6–9].
Especially in women with invasive lobular carcinoma (ILC), preoperative MRI can reduce the re-excision rate [10] and is more accurate in size estimation than mammography alone [11]. However, due to high costs and limited availability, preoperative breast MRI may be performed only in a very limited number of cases. In addition, there may be a high number of false-positive findings in preoperative MRI, which have to be ruled out preoperatively, resulting in a high number of additional biopsies that may delay treatment [12].
The introduction of full-field digital mammography has sparked the development of other techniques that are less expensive than MRI and more widely available. One of these is contrast-enhanced spectral mammography (CESM). CESM improves the sensitivity for breast cancer detection without decreasing specificity because it provides higher contrast and better lesion delineation than mammography alone [13–15]. Preliminary results with unilateral CESM examination suggest that, similar to breast MRI, CEDM should be of particular interest for the assessment of the extent of disease, allowing a better evaluation of lesion size and detecting more multifocal breast cancers than mammography alone or combined with ultrasonography [14].
However, so far there are only a few published data on bilateral CESM examinations with comparison to MRI for the detection of breast cancer, lesion size estimation, and preoperative staging. We hypothesise that this technique provides more accurate lesion detection and size assessment than digital mammography and will not be inferior to MRI. Therefore, the objective of our study was to compare digital mammography (MG), bilateral CESM, and breast MRI with regard to the detection and size estimation of histologically proven breast cancer using postoperative histology findings as the gold standard.
Material and methods
This prospective study was performed in accordance with the Declaration of Helsinki and was approved by the Health Authorities and the local ethics committee. All eligible patients were willing to undergo all study procedures and provided written informed consent prior to enrolment.
Inclusion and exclusion criteria
Women aged 21 years and older with histologically proven invasive breast cancer or ductal carcinoma in situ (DCIS) diagnosed on mammography and/or ultrasound within 30 days before inclusion and for whom an MR examination was clinically indicated and performed or planned within 30 days after the diagnosis were enrolled between December 2010 and January 2012.
Patients were excluded if they: were assumed to be pregnant or breastfeeding; had contraindications to MRI or CESM including a history of an anaphylactoid or anaphylactic reaction to any contrast media or impaired renal function of chronic kidney disease stage 3 and higher (e.g. creatinine clearance <60 ml/min); had received any contrast material within 24 h prior to the contrast-enhanced spectral mammography; had breast implants; had already undergone surgery, hormone treatment or radiation therapy for the index lesion; or had already started neoadjuvant chemotherapy before inclusion.
Inclusion mammograms were four analogue (5 %), eight CR (10 %), and 68 (85 %) full-field digital (FFDM).
MR examination parameters
All contrast-enhanced MR examinations were performed on a 1.5-T MR system (Avanto or SymphonyVision, Siemens, Erlangen, Germany) in prone position with no breast compression using a dedicated four-channel breast coil and established T2w turbo spin echo and dynamic T1w FLASH 3D gradient echo sequences (repetition time 7.5 ms, echo time 4.76 ms, flip angle 25°, field of view 320–360 mm, 512 × 512 matrix, scan percentage 100 %, in-plane resolution 0.7 × 0.7 mm, slice thickness 2.0 mm, no intersection gap). After the unenhanced non-fat-saturated, T1w sequence, five contrast-enhanced image sets with no time gap were acquired with a 20-s delay after starting the contrast injection, resulting in an individual duration for one acquisition of 59–77 s depending on breast size. Contrast agents (Gadovist® or Magnevist®, Bayer-Healthcare, Germany or Dotarem®, Guerbet, France) with a dose of 0.1 mmol/kg body weight (BW) were injected using an automated syringe at a rate of 2 ml/s as a single intravenous bolus followed by a 20 ml saline flush.
Subsequently subtractions of unenhanced and contrast-enhanced sequences were conducted. The imaging protocol followed the recommendations of the EUSOBI and EUSOMA working group [12, 16].
CESM examinations
All CESM examinations were performed using a prototype of a full-field digital mammography system derived from a standard Senographe DS (GE Healthcare, Chalfont St. Giles, UK), modified to allow dual-energy exposures, and dedicated software for image acquisition and processing. An automated single-shot intravenous injection of an iodinated contrast agent [300 mg iodine/ml, 1.5 ml/kg BW (Xenetix® 300, Guerbet, France), minimum 50 ml, maximum 120 ml) with a flow rate of 3 ml/s before breast compression was administered to the seated patient.
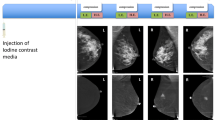
After the injection the patient was disconnected from the injector. The cannula remained within the vein to provide a quick intravenous access in case of any idiosyncratic reaction. Two minutes after the initiation of contrast medium injection, a set of bilateral craniocaudal (CC) and mediolateral oblique (MLO) views was acquired starting with the less suspicious breast. Images of both views and both breasts were completed within 5 min. A diagram of the protocol steps is presented in Fig. 1. Each view consists of two exposures, one with low (26–32 kVp) and one with high energy (45-49kVp). Exposure parameters were adjusted to individual breast size and density using a table of predefined exposure values. After acquisition, low- and high-energy images were recombined to suppress background and highlight uptake of contrast agent.
Image analysis
Images were anonymised and sent to a dedicated mammography workstation with two 5-million-pixel monitors for analysis (AdvantageWindows WS GE Healthcare).
One independent radiologist (E.M.F.) with more than 10 years of experience in reading mammography and breast MR images and trained in reading contrast-enhanced images in earlier studies [15, 17] evaluated the CESM images. The reader was blinded to other imaging findings and clinical information (side of breast cancer, symptoms, medical history). As the CESM examination provides a pair of images for each view and each breast for image interpretation—the low-energy image with exposure parameters and appearance similar to digital mammography and the recombined images displaying contrast enhancement—both were used for diagnosis.
MG and MR examinations were assessed in the routine clinical setting by experienced, board-certified radiologists specialised in breast imaging holding health insurance permission to report diagnostic or screening MG or MRI. On MG examinations a breast density category according to the American College of Radiology (ACR) classification was assigned.
Each detected lesion was specified according to the BI-RADS classification, localisation, and lesion size (maximum diameter) on every imaging investigation and results of CESM were reported in the case report forms of MG and MRI in the patient report. The maximum dimension of the CESM-detected lesions was measured on the recombined images, based on contrast uptake, taking anatomical findings on the low-energy image into consideration; results were reported in the case report form.
On MR examinations measurements were done on the original T1w or subtracted axial images and the results were documented in the patients’ imaging reports.
After the individual evaluation of each imaging investigation the results were correlated to define the index cancer. All findings were compared with postoperative histological results. Only the index cancer of each patient was included in the analysis of sensitivity and size measurements.
Statistical analysis
All calculations were performed using the statistical software SPSS version 20.0. The non-parametrical Wilcoxon test was used to compare lesion sizes measured with CESM, MRI, and MG. P-values < 0.05 were considered statistically significant.
Interclass/Pearson correlation was calculated to correlate the size measurements between the three imaging investigations and postoperative histology. The sensitivities of CESM, MG, and MRI were calculated compared to postoperative histological findings.
Results
Eighty patients with a mean age of 53.6 years ranging from 28 to 79 (SD = 12.5) with histologically proven breast cancer were included in this study. Forty-six women were postmenopausal, 33 premenopausal, and 1 perimenopausal. Mammography and CESM were available for all patients; MRI was available in 78 cases, as 2 patients did not complete the examination because of a panic attack. None of the patients showed any sign of a reaction on the contrast media.
The ACR breast density in the diagnostic MG examinations was classified as follows: fatty in 3 (3.75 %) patients, fibroglandular in 30 (37.5 %) patients, inhomogeneously dense in 27 (33.75 %) patients, and extremely dense in 20 (25 %) patients.
The average dose of the full field digital MG was 1.75 mGy (range 0.43-4.34; SD 0.78); in the CESM investigations the dose levels reached 1.72 mGy (range 0.96-3,56; SD 0.61) on average.
Inclusion histology after minimally invasive biopsies yielded 58 (72.5 %) invasive ductal carcinomas (IDC) [4 combined with ductal carcinoma in situ (DCIS)], 13 (16.25 %) invasive lobular carcinomas (ILC) (1 combined with DCIS), 5 purely DCIS (6.25 %), 1 DCIS (1.25 %) with microinvasion, 1 apocrine carcinoma (1.25 %), and 1 tubular (1.25 %) and 1 medullary carcinoma (1.25 %). All 80 cases could be included assessing the detection rate of the index cancer.
Fourteen index tumours were not visible on mammography, among them10 IDCs (1 combined with DCIS) with a mean size of 17 mm (7–35 mm; SD 9.16) —one in fibroglandular tissue (12 mm) and nine in extremely dense breasts—and four ILCs with a mean size of 35.3 mm (16–50 mm; SD 14.41) —three in inhomogeneously dense and one in extremely dense breasts (size only represents 13 cases, as 1 patient switched to neoadjuvant chemotherapy after imaging) one example is shown in Fig. 2. Two index cancers were missed on MRI, one case of an 8-mm IDC associated with a 12-mm DCIS and one case of a 16-mm ILC (see Fig. 3 and 4). All cancers were detected by the CESM examinations. The sensitivities for the detection of the index tumour were 82.5 % for MG, 100 % for CESM, and 97.4 % for MRI.
Postoperative pathology reports, which served as the gold standard for size assessment, were available for 73 patients. Seven patients were excluded from this subanalysis because of a switch to neoadjuvant chemotherapy after finishing the study imaging procedures.
The 73 index cancers available for size correlation included 48 IDCs (1 associated with DCIS), 13 ILCs, 6 DCISs (1 with microinvasion), 4 invasive medullary carcinomas (1 with LIN2), and 1 mucinous carcinoma and 1 tubular carcinoma.
Finally, size measurements of the index cancer were compared between MG, CESM, MRI and postoperative histology in 59 cases, where the index cancer was depicted with all three imaging investigations and the final histology findings were available.
When the lesion was not detectable in either investigation, sizes could not be compared. These cases included 38 IDCs (1 associated with DCIS), 9 ILCs, 6 DCISs (1 with microinvasion), 4 invasive medullary carcinomas (1 with LIN2), and 1 mucinous carcinoma and 1 tubular carcinoma.
The ACR breast density categories in these 59 cases are summarised in Table 1, the index cancer sizes measured with the three imaging investigations and at histology in Table 2, and the absolute size differences between each imaging technique and histology in Table 3. There was an underestimation of the tumour size using MG and MRI compared to CESM and pathology. There were significant differences in size measurements between CESM and MG (P < 0.001) as well as between CESM and MRI (P < 0.001). The results are summarised in Table 4.
In the comparison of the absolute differences of size measurement for each technique with pathology, the differences between MG and CESM as well as between MG and MRI were also significant (Table 5), whereas no difference was observed between MRI and CESM. The best correlation with pathology was found for CESM; the Pearson coefficients are presented in Table 6. Correlations tended to become poorer with increasing index cancer size (Fig. 2).
Initial mammography (a), low-energy (b), and recombined image (c) of the CESM as well as a MIP of the MRI (d) subtraction images 4 min after contrast administration in a 53-year-old patient with a 3-cm palpable mass in the left breast. In mammography the tumour was not detectable. The low-energy image shows a probably slightly prominent area on the lower part on the MLO view (left); on the recombined images and on the MRI the tumour can be clearly delineated because of a strong contrast uptake
MRI MIP images of the subtractions 1, 2, and 5 min after contrast injection in the patient with a 16-mm ILC in the left breast that was missed by MRI. This was probably due to prolonged enhancement of the lesion resulting in a benign enhancement curve as well as motion artefacts and background enhancement caused misdetection of the tumour
The same patient as in Figs. 3. The CESM images depict the ILC in the upper outer quadrant not detected by MRI
Discussion
In this prospective intraindividual trial we hypothesised that CESM is more accurate in lesion detection and size assessment than digital mammography and that it is not inferior to MRI. Our study has shown that bilateral dual-energy CESM and MRI are superior to MG in breast tumour detection with CESM performing slightly better than MRI. We found the increase in lesion detection using CESM was 17.5 % compared to MG and 2.6 % compared to MRI.
Several earlier studies have shown that contrast-enhanced mammography is able to detect malignant tumours because of contrast enhancement, comparable to tumour enhancement in MRI [13, 17–20].
In line with our study Dromain et al. as well as the Lewin et al. group have shown that contrast-enhanced spectral mammography (CESM) is able to depict tumours in either one or both breasts in the craniocaudal and mediolateral-oblique view after contrast medium injection [14, 15, 19]. Their results suggest that CESM has a similar or even higher sensitivity and better specificity in comparison to mammography alone or in combination with ultrasound for the detection of breast cancer [15].
Our data confirm the findings of Jochelson et al. showing that CESM is feasible with a higher detection rate of the primary breast cancer than mammography and is comparable to MRI in terms of performance. They also showed a higher specificity of CESM compared to MRI. As we only assessed the index lesion in this approach, which was malignant by definition, we cannot comment on specificity with our initial results [21]. However, CESM has not been compared with breast MRI in larger studies so far, and most earlier contrast mammography studies evaluated the temporal subtraction method, which is limited to one view of only one breast [17–20, 22].
Interestingly, in our study, CESM detected two index cancers that were missed by MRI—one ILC (16 mm) and one IDC plus DCIS (8 mm and 12 mm). In the histological workup, the grade 2 ILC was found to be surrounded by fibrous breast tissue and showed no increased vessel density. The grade 2 IDC also had only average neovascularisation and the low-grade DCIS had calcifications 0.03 mm in size. These calcifications were grouped, but more rounded in shape with some sedimentation phenomena, which could not be clearly diagnosed as a suspicious group of calcifications on MG or low-energy images. This gives rise to the question, why they were depicted by CESM but not with MRI?
DCIS and ILC are breast malignancies that tend to be missed by MRI. This may possibly be attributable to a lack of neoangiogenesis in DCIS, resulting in less enhancement or more unspecific enhancement in MRI [23, 24]. The explanation for the enhancement of DCIS in contrast-enhanced imaging, while neoangiogenesis is missing, is that the contrast moves to the ducts by diffusion [25]. As the amount of contrast reaching the tissue by diffusion is time dependent, longer time delays between contrast injection and CESM exposure can result in stronger enhancement and hence better visibility of DCIS compared to MRI. Concerning the time delay between contrast administration and imaging, the CESM image (Xenetix® 300, Guerbet France) is acquired approximately 1–2 min later than the latest dynamic MRI sequence (Magnevist®, Bayer Healthcare, Germany) (time point of the last MRI sequence of both cases: 5 min after enhancement, time point of the last CESM image: case of ILC: 6 min 15 s after enhancement, case of IDC + DCIS: 5 min 46 s). Interestingly, however, the ILC was also visible on the first CESM view acquired after 4 min 57 s. It is known from studies investigating cartilage enhancement, which is due to diffusion as well, that different structures and ionisation of contrast agents influence the enhancement [26]. Therefore, differences in dynamic characteristics and molecular structure between MR and X-ray contrast agents might be another reason for the difference in visibility between CESM and MRI.
In summary, the later acquisition of contrast-enhanced images in CESM seems to contribute to the better visibility of some breast cancers compared with MRI, especially if neovascularisation is absent or only mild and enhancement is due to diffusion. Yet another contributing factor might be misinterpretation of the MR images in the ILC case in which there was a strong parenchymal enhancement but no malignant dynamic curve or prominent enhancing lesion. In addition the investigation also suffered from motion artefacts. In contrast, in CESM, only the malignant tumour was enhanced. Due to the short compression time of about 20 s in the study, the CESM is less sensitive to motion artefacts.
As the low-energy image of CESM is comparable to standard mammography with regard to the visualisation of microcalcifications, this image information together with the indication of contrast uptake might also contribute to the detection of malignant breast lesions compared with MRI. However, in our DCIS case, the additional enhancement of an area of a slightly asymmetric density with more benign calcifications drove the diagnosis to be more suspicious.
Correct size estimation is mandatory for efficient breast cancer treatment and preoperative planning as the treatment depends on the extent of disease, and free margins have to be achieved [27]. Contrast-enhanced MRI has been shown to be an effective technique for preoperative lesion detection and size estimation but tends to overestimate lesion size especially in ILC [10, 11, 28]. Nevertheless, MRI is still an expensive and not widely available imaging technique.
As CESM seems to be a promising tool for increasing the sensitivity of MG, with a performance comparable to that of MRI, it might be expected to also improve size estimation and staging. In terms of lesion size measurement, the best correlation with histology (gold standard) was found for CESM, followed by MRI and MG. MG and MRI underestimated the breast cancer extent compared with CESM. Our results are in agreement with several earlier studies comparing the performance of different investigations in lesion detection and size estimation. Wasif et al., for example, also found MRI to be more accurate than mammography in assessing the size of primary breast cancer. In their study, they showed the best correlation of MRI and pathology with an overestimation in MRI of more than 1 cm in 10 % and an underestimation in 18 % of cases [29]. In contrast, Mann et al. reported that MRI overestimated the size of ILC compared with pathology [28, 30]. Our results also confirm the findings of Fasching et al. that there is an underestimation of lesion size in mammography [5].
Data on the direct comparison of MRI and CESM are still limited. Jochelson et al. reported a good overall estimation of sample size using CESM with an overestimation in two cases but make no statement on statistical significance because of the small study population [21].
We noted an increasing discrepancy between the pathological lesion size and the sizes measured with all three investigations for larger breast cancers. This observation is possibly due to the fact that correct assessment of the extent of a lesion in pathology becomes increasingly complex and difficult when a pathologist has to a assess a lesion that is present in multiple slices of a specimen [31].
The study had several limitations. First, pre- and postmeno-pausal women were included and were examined in different phases of the menstrual cycle, which may have biased the results. So far, no data are available on how much the menstrual cycle influences the contrast uptake in CESM and what the false-positive rate is compared to MRI. Further studies are needed. Second, this study only had one single reader in each technique and data from the clinical workup (MRI, MG) were not collected as a blinded read, but this reflects the routine diagnostic world and workflow. Third, the conventional mammographic results originated from the initial reports from different institutions, so a team agreement was not possible. Nevertheless, there is strong quality control in this country regarding correct image quality and reporting according to the BI-RADS lexicon with annual performance tests so that a comparable quality could be expected. Finally, the number of patients analysed is still small, and the results have to be confirmed further in a study with an independent blinded reading of a larger number of cases.
In conclusion, contrast-enhanced spectral mammography showed a better lesion detection and size estimation than mammography in comparison to postoperative histology as the gold standard. Our initial results showed no significant difference between contrast-enhanced spectral mammography and magnetic resonance imaging in breast cancer detection and size measurement with both investigations correlating well with postoperative histology. These results suggest that contrast-enhanced spectral mammography is a useful imaging investigation that may provide accurate preoperative staging and treatment planning in breast cancer patients and is probably not inferior to magnetic resonance imaging.
References
Kolb TM, Lichy J, Newhouse JH (2002) Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology 225:165–175
Pisano ED, Gatsonis C, Hendrick E et al (2005) Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med 353:1773–1783
Tabar L, Vitak B, Chen TH et al (2011) Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology 260:658–663
Allen SA, Cunliffe WJ, Gray J et al (2001) Pre-operative estimation of primary breast cancer size: a comparison of clinical assessment, mammography and ultrasound. Breast 10:299–305
Fasching PA, Heusinger K, Loehberg CR et al (2006) Influence of mammographic density on the diagnostic accuracy of tumor size assessment and association with breast cancer tumor characteristics. Eur J Radiol 60:398–404
Fischer U, Kopka L, Grabbe E (1999) Breast carcinoma: effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology 213:881–888
Liberman L, Morris EA, Kim CM et al (2003) MR imaging findings in the contralateral breast of women with recently diagnosed breast cancer. AJR Am J Roentgenol 180:333–341
Hollingsworth AB, Stough RG, O'Dell CA, Brekke CE (2008) Breast magnetic resonance imaging for preoperative locoregional staging. Am J Surg 196:389–397
Schell AM, Rosenkranz K, Lewis PJ (2009) Role of breast MRI in the preoperative evaluation of patients with newly diagnosed breast cancer. AJR Am J Roentgenol 192:1438–1444
Mann RM, Loo CE, Wobbes T et al (2010) The impact of preoperative breast MRI on the re-excision rate in invasive lobular carcinoma of the breast. Breast Cancer Res Treat 119:415–422
Mann RM, Veltman J, Barentsz JO, Wobbes T, Blickman JG, Boetes C (2008) The value of MRI compared to mammography in the assessment of tumour extent in invasive lobular carcinoma of the breast. Eur J Surg Oncol 34:135–142
Mann RM, Kuhl CK, Kinkel K, Boetes C (2008) Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol 18:1307–1318
Dromain C, Balleyguier C, Muller S et al (2006) Evaluation of tumor angiogenesis of breast carcinoma using contrast-enhanced digital mammography. AJR Am J Roentgenol 187:W528–537
Dromain C, Thibault F, Muller S et al (2011) Dual-energy contrast-enhanced digital mammography: initial clinical results. Eur Radiol 21:565–574
Dromain C, Thibault F, Diekmann F et al (2012) Dual-energy contrast-enhanced digital mammography: initial clinical results of a multireader, multicase study. Breast Cancer Res 14:R94
Sardanelli F, Boetes C, Borisch B et al (2010) Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer 46:1296–1316
Diekmann F, Freyer M, Diekmann S et al (2011) Evaluation of contrast-enhanced digital mammography. Eur J Radiol 78:112–121
Jong RA, Yaffe MJ, Skarpathiotakis M et al (2003) Contrast-enhanced digital mammography: initial clinical experience. Radiology 228:842–850
Lewin JM, Isaacs PK, Vance V, Larke FJ (2003) Dual-energy contrast-enhanced digital subtraction mammography: feasibility. Radiology 229:261–268
Diekmann F, Diekmann S, Jeunehomme F, Muller S, Hamm B, Bick U (2005) Digital mammography using iodine-based contrast media: initial clinical experience with dynamic contrast medium enhancement. Invest Radiol 40:397–404
Jochelson MS, Dershaw DD, Sung JS et al (2013) Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 266:743–751
Dromain C, Balleyguier C, Adler G, Garbay JR, Delaloge S (2009) Contrast-enhanced digital mammography. Eur J Radiol 69:34–42
Lee AH, Dublin EA, Bobrow LG, Poulsom R (1998) Invasive lobular and invasive ductal carcinoma of the breast show distinct patterns of vascular endothelial growth factor expression and angiogenesis. J Pathol 185:394–401
Teifke A, Hlawatsch A, Beier T et al (2002) Undetected malignancies of the breast: dynamic contrast-enhanced MR imaging at 1.0 T. Radiology 224:881–888
Jansen SA, Paunesku T, Fan X et al (2009) Ductal carcinoma in situ: X-ray fluorescence microscopy and dynamic contrast-enhanced MR imaging reveals gadolinium uptake within neoplastic mammary ducts in a murine model. Radiology 253:399–406
Wiener E, Woertler K, Weirich G, Rummeny EJ, Settles M (2007) Contrast enhanced cartilage imaging: comparison of ionic and non-ionic contrast agents. Eur J Radiol 63:110–119
Singletary SE (2002) Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg 184:383–393
McGhan LJ, Wasif N, Gray RJ et al (2010) Use of preoperative magnetic resonance imaging for invasive lobular cancer: good, better, but maybe not the best? Ann Surg Oncol 17(Suppl 3):255–262
Wasif N, Garreau J, Terando A, Kirsch D, Mund DF, Giuliano AE (2009) MRI versus ultrasonography and mammography for preoperative assessment of breast cancer. Am Surg 75:970–975
Mann RM, Hoogeveen YL, Blickman JG, Boetes C (2008) MRI compared to conventional diagnostic work-up in the detection and evaluation of invasive lobular carcinoma of the breast: a review of existing literature. Breast Cancer Res Treat 107:1–14
Behjatnia B, Sim J, Bassett LW, Moatamed NA, Apple SK (2010) Does size matter? Comparison study between MRI, gross, and microscopic tumor sizes in breast cancer in lumpectomy specimens. Int J Clin Exp Pathol 3:303–309
Acknowledgments
The study was supported by a research grant from GE Healthcare. The investigators had exclusive control of all data, manuscript drafting, and submission of this study.
We are grateful to Nikola Bangemann, Christiane Richter-Ehrenstein, MD, and Angela Reles, MD, for their contribution in the patient recruitment and inclusion.
We are thankful to Prof. Marc Dewey for discussions about this paper and to Bettina Herwig for editorial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fallenberg, E.M., Dromain, C., Diekmann, F. et al. Contrast-enhanced spectral mammography versus MRI: Initial results in the detection of breast cancer and assessment of tumour size. Eur Radiol 24, 256–264 (2014). https://doi.org/10.1007/s00330-013-3007-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-013-3007-7