Abstract
The failures of discs on large steam turbines that occurred in the 1960s presented the electric power generation industry with a formidable problem. These fractures were intergranular and often appeared to initiate in the keyway of the disc as a result of steam condensation and subsequent corrosion. The steels that were used to make these discs were usually of the Cr-Mo type and had the required room temperature and elevated temperature strength. However, in some cases they were also found to be brittle at or even above room temperature. This brittleness was a result of temper embrittlement which in turn was caused by impurity segregation to grain boundaries. Early results also suggested that this impurity segregation might enhance the stress corrosion process as well. Consequently, great advances were made in steelmaking to provide steels of extremely high purity. While these steels did become available, several factors limited their use. These included their cost, the fact that the overall purity of conventional steels improved through appropriate scrap selection and other processing improvements, better control of steam chemistry, designs that eliminated the keyways, and improved plant practice. This chapter reviews the history of this problem, the development of the clean steels, and the ultimate combination of factors that were brought together to solve this problem. In considering such a complex problem, it is useful to think in terms of technology interaction spheres, that is, interactions between technologies brought about by many individuals, that help solve such a problem.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Clean steels
- Fracture
- Steam turbines
- Steel embrittlement
- Steelmaking
- Stress corrosion
- Technology interaction spheres
Introduction
The processing of metals has been performed for millennia. It began with the first uses of native copper and proceeded to smelting of copper ores and intentional alloying of the copper with elements such as tin and arsenic. The fundamentals of metal processing, from mining to use, were in place by approximately 4000 BC. By 1000 BC iron and steel had replaced copper for many applications. These processing technologies spread east and west from their original points of development in the Near East and southeastern Europe. Independent and similar metallurgical discoveries were also made in Mesoamerica (see Kaufman, Chap. 1, this volume; Killick and Fenn 2012).
As summarized in Table 6.1, the metallurgy of copper, iron, lead, silver, and gold were more or less established by 1000 BC (Killick and Fenn 2012). Other metals in great use today, such as aluminum and titanium, were not developed until the nineteenth century because winning them from their ores required either advanced chemical methods or electricity or both. More generally, the availability of electrical power allowed the development of large-scale processing equipment such as forges, rolling mills, and swagers that led, in turn, to processing much larger quantities of metal. In addition to these mechanical processing steps, the twentieth century saw rapid advances in new processes based on techniques such as powder metallurgy , spray deposition , and thin-film coatings .
Each of these processing topics and each type of metal have led to the production of many books, conferences, and patents. Since the motivation for these publications was often to report advancements toward the solution of an ongoing and major technical problem or need, they primarily, and often necessarily, considered only a particular technical aspect of the problem. As a result, they sometimes did not portray the way in which various technologies interacted to solve a major problem facing an industry and how the solution was implemented. Furthermore, they often did not consider the importance of such human factors as maintenance and training in solving such problems or preventing them in the first place.
The relationship between a problem and an industrial advance that occurs through the interaction of different technologies and different groups of people who solve the problem demands more attention for several reasons. Identification of a well-defined need or technology-stopping problem focuses questions and can free up the funds to move the technology forward. Economics almost always plays a role in reaching a solution. The need can also prompt broad discussion and communication among workers in different technologies, each of which have their own cultures. These conversations, which might not ordinarily occur, lead to what I term a technology interaction sphere; that is a sphere of activity in which ideas are generated that integrate multiple technologies in order to address the problem.Footnote 1 These ideas would not evolve if the technologies stayed isolated. It is important to note that these interaction spheres are formed not just between technologists, but across the entire production and implementation chain. In particular, the interaction between managers who oversee the project and make final critical decisions and the workers carrying out the technology manufacturing can be extremely important, an idea developed in its general sense by Bijker (1995).
In this chapter I will take one problem and outline its history. I will do so in terms of how it arose and then how different technologies contributed to the solution. In doing so, I hope to provide at least a rudimentary account of how the problem was addressed and the efforts that were required on many different fronts to achieve a solution. The problem I discuss is the disc fractures in the steam turbines used to provide electricity for the grid (Bodnar and Cappellini 1988; Curran 1986). We will enter the problem with a discussion of a particular fracture event and move on to the resulting effort to manufacture steel with low impurity content, as part of the effort to solve the problem. These impurities, such as phosphorus, tin, and sulfur, can cause the steel to be very brittle, and at least for a time, it was thought that the presence of these elements might greatly enhance stress corrosion cracking, which was seen as one of the primary mechanisms that led to the failures (Gray 1972). This effort to produce steels with low impurity concentrations attracted significant funding and research activity throughout the world in the 1970s and 1980s. Professional organizations and industry consortia played a major role in organizing these efforts. For those of us involved in this particular work, and thus part of the technology culture that formed around the investigation of embrittlement and clean steels,Footnote 2 it was often almost taken for granted that the production and use of these clean steels (i.e., steels with concentrations of these impurity elements below the level of approximately 25 parts per million in concentration) was a solution, perhaps the only solution, to this problem. But the solution to the turbine disc fracture problem, in the end, involved a number of different advances of which steel purity was only a part of the answer. Turbine redesigns to remove the locations of high stress and eliminate crevices that could exacerbate the corrosion were extremely important, as were manufacturing practices, control of the steam cycle, and control of start-up and operational procedures.
One final point should be mentioned before proceeding. As with any technological development or design advance, it is an ongoing process without a clear start and end date. For this chapter, I have begun with a specific failure in 1969 and, except for a brief look back at the history of these failures, proceed to the situation as it stood in approximately 2000, a time when significant technology development had been completed and the advancements had been recorded in the open literature. While these dates are somewhat arbitrary, they do allow us to examine the problem during a particularly active time when many people were engaged with it.
The Problem
We begin this section with a description of the failure of the low-pressure steam turbine at the Hinkley Point power station in Great Britain (Gray 1972; Kalderon 1972). This failure was not the first of this type that had occurred; indeed, the possibility that steels used for rotors and discs could fracture in a brittle manner had been an ongoing concern through the 1950s and 1960s (Emmert 1956; Mochel et al. 1956; Rankin and Seguin 1956; Schabtach et al. 1956; Schaefer 1956; Thum 1956). However, the careful and complete documentation of this particular failure published in the open literature (Gray 1972; Kalderon 1972) makes it an appropriate starting point for our discussion.
On September 19, 1969, a low-pressure steam turbine underwent a catastrophic failure at the Hinkley Point nuclear power station. The turbine had been taken off the grid for standard testing. During this test, the rotor, which had a normal rotational speed of 3000 revolutions per minute (rpm), was to be taken up to a rotational speed of approximately 3600 rpm and then brought back down to the normal operational speed. This testing was referred to as an overspeed test. The explosion occurred as the rotational speed reached 3200 rpm. Although there were operators present, no fatalities or serious injuries were reported. However, the potential for much more serious consequences was obvious and demanded that the problem be addressed in an expeditious manner.
When the explosion occurred, operators reported “a loud bang, flames from the area of the l.p. [low pressure] turbine cylinders with blown lagging and cleading from the l.p. crossover pipes, followed within a few seconds by an explosion and sheets of flames from the generator” (Kalderon 1972). The rotor shaft fractured completely in five separate locations and the attached discs were blown across the machine hall. Some pieces were ejected through the roof. Figure 6.1 shows a picture of the damage, and from this picture, if one notes the individuals in it, one can begin to appreciate the physical dimensions of the turbines and the high stakes of failure.
A picture of the damage caused by the explosion at the Hinkley Point power plant. (From Kalderon (1972). Figure used with permission of SAGE Publications, Ltd.)
As a result of the explosion, a thorough forensic examination was performed. Pieces of the fractured discs were fitted back together (one disc required piecing together of 47 fragments) to try to determine the origin of the fracture. A mechanics analysis was performed on specific parts of the turbine. The temperature, pressure, and composition of the steam in the low-pressure section of the turbine were analyzed, and the steel used to make the turbine was characterized. We now consider the possible role that each of these played in the failure.
Turbine Design and the Mechanics of the Failure
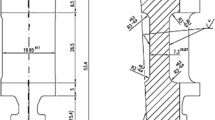
Large steam turbines, of the size shown in Fig. 6.2, were developed to meet the demands for ever-increasing amounts of electrical power in the USA and Europe during the 1950s and were constructed and operated in the following way. A central shaft runs down the center of the turbine. There may be one or several of these in a series in a given turbine design. The discs are placed on this central shaft. Attached to these discs are the blades. High-pressure, high-temperature steam enters the turbine and interacts with the blades, causing the rotor to turn. The specific design of the turbine and the way in which the steam expands in the turbine, transferring its kinetic energy into rotational energy, vary with its size and power rating and the source of the steam. Attached to the end of the rotor is a generator that in its most basic form is a coil of copper wire spinning in a magnetic field. This rotation creates an electric current in the wire which flows to a transformer to provide electricity to the grid.
Steam turbines have been used to generate electricity since the early 1900s, but their size began to increase significantly after about 1940. As the demand for electricity increased, the utility systems had to grow, and this growth, in turn, demanded larger and larger steam turbines. Between 1940 and 1980, the power generation produced by power plants increased from approximately 100 MW to 1000 MW (Curran 1986). This increase in both the demand and the size of the turbine was accompanied by the use of higher-temperature and higher-pressure steam. Between 1950 and 2000, the peak temperatures rose from approximately 400 to above 600 °C, and the highest steam pressure from about 580 pounds per square inch (psi) (4 MPa) to above 4000 psi (27.5 MPa) (Masuyama 2001; Zhou and Turnbull 2003). With each of these increases in pressure and temperature, the efficiency increased by approximately a percent, a significant gain in this technology (see Masuyama 2001 for details of these increases). The steel that was used to construct these turbines had to withstand increased stresses from the larger size of the turbine and be able to maintain that strength at higher temperatures.
Steam turbines are often divided into sections that utilize different steam pressures and temperatures . The turbine that failed at Hinkley Point had both high-pressure and low-pressure steam inlets. The high-pressure steam reached a maximum temperature of 662 °F (350 °C) and a pressure of 615 psi (4.2 MPa), and the low-pressure steam had a maximum temperature of 639 °F (336 °C) and a pressure of 155 psi (1.1 MPa) (Kalderon 1972). The part of the turbine where the failure occurred was in the low-pressure section, where the conditions of the steam were such that it could condense to form water in certain sections of the turbine.
One of the critical manufacturing steps in assembling the turbine is the placement of the discs onto the central rotor. In the early use of steam turbines (before approximately 1940) single forgings, referred to as monoblock forgings, were often used to make the central shaft and discs (Curran 1986). However, following a demand for larger turbines, the discs and shaft were made separately, and the discs were assembled onto the central rotor shaft. The assembly design used for the Hinkley Point turbine, as well as for many other power plants, was referred to as shrunk-on discs. The placement of the disc was achieved by heating the disc to expand the bore, sliding the disc onto the shaft, and allowing it to cool and contract to a snug fit. On the disc and the bore, there were mating slots, called keyways, into which a metal pin or key was placed to help hold the disc in place. Lyle and Burghard (1982) show schematics of different keyway designs.
As the forensic analysis of the Hinkley Point failure proceeded and ruptured discs were pieced back together, it became clear that the fracture had initiated at the keyway crown, as shown in Fig. 6.3. (A second failure also occurred in a turbine that was being tested to replace the original, damaged turbine, and this failure, too, originated in the keyway.) A mechanics analysis of the keyway showed that the tangential stresses at the crown of the keyway in the disc, resulting from the rotation of the turbine, would act to apply a force to separate the material in a line emanating from the crown into the disc. Because of the design of the keyway, the actual stress at a crack in the crown of the keyway would be approximately three times the applied stress that would be present if no crack were present at that point. Kalderon (1972), in his report on the investigation, notes “A small crack at the apex of the keyway could be catastrophic while in the absence of such a crack the presence of a keyway would be of no consequence even in a material of low fracture toughness. Conversely, the presence of a keyway in circumstances where cracking is also encountered could be catastrophic, while in [the keyway’s] absence much deeper cracking of disc bores could be tolerated.” As will be discussed below, changing the rotor design to eliminate the keyways was one of the most important steps in solving this problem.
A fractured disc that has been pieced back together after the Hinkley Point failure. Note the keyway in the upper portion of the inner annulus at approximately 11:30 denoted by the red arrow. (From Kalderon (1972). Figure used with permission of SAGE Publications, Ltd.)
The Steam Cycle and Stress Corrosion
Steam turbines operate by steam at elevated temperatures and pressures interacting with the rotor blades to cause the rotation of the turbine. The steam decreases in temperature and pressure as it expands in the turbine, and it can reach the point where water condenses on the cooler surfaces of the turbine. This point is referred to as the saturation point for the steam. The exact temperature and pressure will depend on the design and operation of the turbine, but condensation would probably occur most easily in occluded regions such as the keyway interface. The importance of understanding this condensation resulted from examination of the fracture surfaces of the discs and the location in the turbine of the specific discs that cracked. Cracking occurred on discs in regions where the low-pressure steam and the metal discs were at a temperature where condensation could occur. The investigation also showed that the part of the fracture surface nearest the keyway was discolored, which indicated that corrosion had occurred. This corrosion must have resulted from the presence of an aqueous solution (Gray 1972, Kalderon 1972). In some areas there were deposits left by the corrosive environment which also led to a concern about possible contaminants in the steam.
Figure 6.4 shows the estimated steam temperature and the disc temperature for the low-pressure turbine where the fracture occurred (Gray 1972). The steam at this point in the cycle was very near its saturation temperature. Contact with any surface below this temperature could cause condensation. The graphic shows that the metal temperature was bordering on this condensation temperature, and may have in some cases gone below it, which would then allow the steam to condense on this cooler surface. It is also important to note that at disc number 4, the metal temperature increased significantly as a result of heating from the high-temperature gland steam and no cracks were ever observed in this disc. Along with mechanical stress, corrosion resulting from the steam condensation appeared to be a central factor in causing the disc fractures.
The temperature of the metal, T m (drawn lines), and the range of steam saturation temperature T s (the gray triangles) at different discs in the low-pressure turbine. Note that at disc 4, the temperature of metal increases significantly. (From Hodge and Mogford (1979). Figure used with permission of SAGE Publications, Ltd.)
The Steel
The third part of the analysis was an investigation of the steels used for the discs. For the low-pressure turbine discs, the steel had a composition of 3 wt% (from now on %) chromium, 0.5% molybdenum, 0.3–0.4% carbon, 0.2–0.3% silicon, and approximately 0.7–0.8% manganese.Footnote 3 This composition would be typical for a steel required to have a moderately high strength and high toughness, the latter a parameter that would describe its resistance to cracking. It was also a composition that was used for discs in power plants throughout the world (Bodnar and Cappellini 1988; Curran 1986). The steels employed in this particular application had been made by two different casting processes. One of these was acid open-hearth casting, and the other was basic electric arc casting. A primary difference between the steels produced by these two manufacturing processes was the impurity content. The acid open-hearth steels contained 0.025–0.034% sulfur and 0.025–0.032% phosphorus. In contrast, the basic electric arc heats contained 0.003–0.015% sulfur and 0.012–0.024% phosphorus (Kalderon 1972). The higher impurity content of the acid open-hearth method resulted from the fact that the slag in this melting process had a lower tendency to float and thus could be entrained in the solidified steel. It was also difficult to perform the refining processes that would be needed to remove elements such as sulfur and phosphorus with this type of process. Better refining could take place in the basic electric arc furnace, and during the 1940s and 1950s, there was a general switch to electric furnaces over the open hearths because of the significantly decreasing costs of electricity (Curran 1986).
It is also important to pay special attention to the heat treatments that had been applied to these forged discs. They had been held at a temperature of 900–930 °C for several hours after forming and then oil quenched to 300 °C. After that heat treatment, they were tempered at 600–650 °C and then slowly cooled at an initial rate where the temperature decreased at 60° per hour, and then at a still slower rate at temperatures below 500 °C (Kalderon 1972). The cooling rate was kept low to avoid the buildup of residual stresses (Bodnar and Cappellini 1988; Curran 1986).
Before continuing to analyze the role of the steel in this failure, two features common to many steels, including those used for discs in steam turbines, need to be described. The first is the ductile-to-brittle transition, and the second is the problem of temper embrittlement.
One of the properties of steels such as those used in these applications is that they undergo a transition from very brittle, low-energy fracture to very ductile, high-energy fracture as the temperature of their environment increases. At extremely low temperatures, a piece of steel that is notched can fracture in a brittle mode more like what one would expect for a ceramic. The temperature at which this type of failure will occur is usually well below even extremely cold outdoor temperatures. As the temperature is raised, the energy absorbed on fracture increases significantly, usually over a range of approximately 50 centigrade degrees. Over this temperature range, the fracture mode changes from a completely brittle to a completely ductile failure mode; a mixture of brittle and ductile fracture is observed within this range. Since this transition in fracture mode from brittle to ductile usually occurs at very low temperatures, an engineer can expect that at room temperature most steels will fail in a ductile manner with high absorption of energy.
The ductile-to-brittle transition temperature of a steel can be increased to well above room temperature if the steel contains sufficient concentrations of impurity elements such as phosphorus and sulfur and undergoes a heat treatment that allows these elements to segregate to the grain boundaries. It was known for many years that this increase in the ductile-to-brittle transition temperature, known as temper embrittlement, occurred if a steel was slowly cooled from a tempering treatment between 600 and 700 °C, but that this embrittlement could be avoided if the steel was rapidly quenched from the temper. For embrittled steels the transition temperature from brittle to ductile fracture can occur well above room temperature, with some transition temperatures measured above 200 °C. The fracture path in the embrittled steel becomes intergranular, as opposed to the transgranular brittle fracture normally observed (Briant and Banerji 1978).
The ductile-to-brittle transition temperatures for the steel samples taken from the fractured discs at Hinkley Point were measured as part of the investigation. The results showed that the transition temperatures for the acid open-hearth materials ranged between 104 and 200 °C and those for the basic electric arc steels were between 14 and 81 °C (Kalderon 1972). The brittle fracture mode in these tests was intergranular, as were the fractures that occurred in the actual disc failures (Fig. 6.5). Three important points should be noted. The first is that the transition temperatures were at or above room temperature in steels manufactured by both processes, and in some steels, they were above the operating temperature of the turbine, estimated to be between 57 and 90 °C in the turbine stages where the fractures occurred (Kalderon 1972). Thus, a brittle material was being used in service. The second point to note is that the higher transition temperatures in the acid open-hearth steels correlated with the higher impurity content in these steels. Third, the slow cool from 600 °C would allow the impurities present in the steel to segregate to the grain boundaries and cause temper embrittlement.
The intergranular cracking that occurred in the Hinkley Point failure. (From Kalderon (1972). Figure used with permission of SAGE Publications, Ltd.)
Other Reports of Failures and Consequences
Before proceeding with the discussion of changes in steelmaking and further analysis of the steam environment and the design of the turbine, it is important to realize that there were a number of other publications both before and around the time of the Hinkley Point event that catalogued and described similar failures (Emmert 1956; Mochel et al. 1956; Rankin and Seguin 1956; Schabtach et al. 1956; Schaefer 1956; Thum 1956). Summaries have also been given in later years of the cracking observed in discs and other parts of the steam turbine (Hodge and Mogford 1979; Lyle and Burghard 1982).
In the February, 1956, issue of Metal Progress, E. E. Thum (1956) summarized a daylong meeting of the American Society of Mechanical Engineers. His opening line states, “A half-century of almost perfect performance of large electrical machinery has been broken by four failures of massive rotor forgings since early 1953.” His article goes on to describe these failures and how they had been interpreted. A brief summary of each of them is as follows:
-
In January 1953, a 125,000-kW steam turbine with a speed of 1800 rpm failed at the Tanners Creek Station of the Indiana and Michigan Electric Co. It began vibrating, and half of the rim of one of the turbine wheels (discs) was broken away. This 1.02 Cr-1.13 Mo-0.27 V-0.36 C steel was tested and found to have only 1–2% elongation at 1000 °F (538 °C), whereas 2–10% elongation was expected. An analysis by GE, the company that built the turbine, suggested that steel quality could have played a role but also suggested that residual stresses from the heat treatment, added to the expected centrifugal and thermal stresses due to a sudden change in steam temperature, contributed to the failure (Rankin and Seguin 1956).
-
On March 4, 1954, a completely finished generator intended for Arizona Public Service Co. burst into a large number of fragments while being tested at the manufacturers. This 2.5 Ni-0.55 Mo-0.32 C-1 Mn-0.06 V steel had a room temperature elongation between 5 and 7% and a reduction in area of 4–6%; these values contrast with the specified minima of 13 and 22%, respectively. It was suggested that the failure resulted from preexisting cracks caused by transformation stresses and trapped hydrogen (Schabtach et al. 1956).
-
On September 17, 1954, a turbine burst at the Cromby Station of Philadelphia Electric Company after a weekend shutdown. This rotor was usually run at 3600 rpm but had been tested to 3780 rpm when the failure occurred. This particular rotor, which had a composition similar to the one that had fractured at the Arizona Public Service Co., had undergone a repair during its manufacture that required threaded holes be drilled into the rotor and threaded studs inserted. The crack started at the bottom of one of these holes where the stress intensity was 7.6 times greater than the normal or designed loading. It was also noted that the material below the hole contained hard brittle material resulting from alloy segregation (Schabtach et al. 1956).
-
On December 19, 1954, a failure occurred at the Ridgeland Station in Chicago at a plant operated by Commonwealth Edison. The low-pressure turbine exploded during a routine overspeed test. When analyzed, the material, which was a Ni-Cr-Mo-V steel, showed insufficient ductility and the presence of numerous small cracks that were apparently already present in the material. The investigation concluded that these preexisting cracks could not be arrested once they started to propagate. It is also interesting that engineers from Allis-Chalmers suggested that materials should be qualified with Charpy testing in addition to smooth bar tensile testing. When measured on this steel, the transition temperature was found to be above the operating temperature (Emmert 1956), as was also the case for the Arizona and Cromby rotors (Thum 1956).
While the full analysis of these failures is interesting, the commentary that Thum (1956) provides after the failure descriptions is also important. He notes, “These recent failures, together with some other incidents where cracked forgings were discovered during periodic inspection, are responsible for the very cautious way in which the electrical industry has approached the problem of bigger and faster generating equipment. Three American manufacturers of such equipment – General Electric, Westinghouse, and Allis-Chalmers, joined whole-heartedly by five steel companies – are intently studying the problem, using not only scale models but also some full-sized forgings made by various steelmaking methods including one rotor from abroad.” After praising the cooperation of the various industries, he concludes, “It would have been easy, in these four instances, for the electric utilities and manufacturing industries to have voted lack of confidence in the steelmakers, and vice versa – thus getting exactly nowhere. It is to the credit of all that this situation has been avoided.”
While Thum’s commentary mentions design and testing, it gives most attention to possible concerns with steelmaking, the best processes for this industry to consider, and the problem of making uniform, high-quality steel for such large ingots. The issue of better steelmaking was also discussed in various ASTM symposia . In 1967, ASTM published a series of papers in a volume titled Temper Embrittlement of Steel (Newhouse 1967). The introduction notes that the larger scale of new steam turbines required more massive sections, produced higher stresses in the steel, and in some cases increased operating temperatures. It further stated that “temper embrittlement is assuming increasing importance as an obstacle inhibiting progress in the design of heavy components.” A second volume issued in 1971 (Newhouse 1971) presented a number of additional studies and pointed to the importance of Auger electron spectroscopy, a then new technique (described below) for detecting the segregants that caused this problem. The metallurgical community was clearly focused on the problem of producing a clean steel to solve the problem of disc fractures in steam turbines.
This emphasis on steel quality arose quite naturally from the realization that the heat treatments that were applied to disc steels that contained what were then typical levels of impurities would cause temper embrittlement. For example, the slow cool from the tempering treatment reported above for the steel used at Hinkley Point would cause segregation of impurities to grain boundaries. Since the slow cool was needed to avoid buildup of residual stresses, it seemed that the most appropriate fix would be to develop steels with extremely low impurity contents, in which the harmful elements would be present in such low concentrations that their segregation would be negligible. Also, there was concern that the hundreds or thousands of hours at the operating temperatures in the range of 400 °C could cause additional segregation of these impurities, and thus, embrittlement might develop during service. Furthermore, some preliminary research by the Central Electric Research Labs in the UK suggested that the condensate in the keyway could have been caustic and that steels that were temper embrittled were more prone to undergo stress corrosion cracking in such an environment (Adams et al. 1975; Atkinson et al. 1979; Gray 1972).
Altogether there seemed to be strong evidence to suggest that prevention of disc cracking lay primarily in preventing impurity segregation which, because of the required heat treatments, meant that the impurities could not be present in the steel in the first place. We therefore begin our discussion of the follow-on research with a discussion of steelmaking practices that allowed the production of clean steels and the laboratory work that was performed to understand in more detail the segregation and consequent embrittlement.
Research on Disc Steels
We divide this section into two parts. The first part concerns advances in steelmaking, and the second the improved understanding of the causes of temper embrittlement.
Steelmaking
At the time that disc cracking was occurring in low-pressure turbines, the steel that had been used for the discs had been made by several different methods. As described above, steels made by the acid open-hearth method tended to have a higher impurity content. However, with the decreasing cost of electricity, electric arc furnaces came into greater use. This practice allowed for better control of the impurity content in the steel because the steelmaker could make use of new refining processes (Bodnar and Cappellini 1988; Curran 1986).
The impetus to make cleaner steels for a number of applications was greatly enhanced by the development and scale-up of two important new processes: vacuum treatment of the melts and ladle metallurgy. By using vacuum pouring of the ingot, levels of hydrogen, oxygen, and nitrogen could be reduced, and with the advent of carbon deoxidation, the levels of oxygen could be reduced further through the evolution of CO (Bodnar and Cappellini 1988; Cramb 1999; Curran 1986; Szekely et al. 1988). Ladle metallurgy, in which the metal is poured into a separate vessel for further treatment, allowed for the use of multiple slags that would react with specific impurities and remove them as well as the use of various stirring and injection methods that would speed up the kinetics of the reactions between the slag and the impurities (Szekely et al. 1988; Fruhan 1985). In addition, as these processes were developed, great progress was made on modeling the solidification process, so an engineer could determine regions of the ingot where compositional inhomogeneities would most likely occur (Kawaguchi et al. 1986; Szekely et al. 1988).
Viswanathan (1997) has provided an excellent summary of the improved methods of steelmaking, and with the general application of these processes, along with careful selection of scrap input material, there was a general decrease in the average concentration of impurities over time and a resultant improvement in the reported ductile-to-brittle transition temperatures . Bodnar and Cappellini (1988) reported that the average phosphorus and sulfur concentration for rotor steels decreased from between 150 and 200 ppm in 1955 to below 50 ppm in 1990, and Viswanathan (1996) reported that over roughly the same time period, the typical ductile-to-brittle transition temperatures decreased from approximately 150 °F (66 °C) to below 0 °F (−18 °C). When all of these processes are optimized, compositions such as those shown in Table 6.2 can be obtained in very large ingots. (This table will be discussed in more detail later in the chapter.)
In addition to control of slag chemistry and vacuum treatment, a number of other practical changes were made in the steel production for turbines. These included better scrap selection which meant that lower impurities were present in the initial melt before refining, removal of up to 25% of the top of the ingot and 12% of the bottom (locations where impurities might concentrate because of solidification patterns), and the use of multiple synthetic slags (Bodnar and Cappellini 1988).
Temper Embrittlement
While research proceeded on how to make steels with low impurity contents, work was begun at a number of turbine manufacturers, steelmakers, and universities to understand all aspects of temper embrittlement . By simply comparing the ductile-to-brittle transition temperatures between tempered steels that were either rapidly quenched after tempering or slowly cooled from the tempering treatment, one could demonstrate that high-purity steels were not embrittled when slowly cooled after tempering, whereas those that contained impurities were embrittled by this slow cool. Neither high-purity nor commercial-purity steels were embrittled when rapidly quenched from the tempering treatment. While one could rightly say that the essential information that was needed had been obtained very early in this research, that the high-purity steels were not susceptible to temper embrittlement, another development allowed one to push this type of examination much further. In 1968, Harris (1968a, b) of the General Electric Company published two papers in which he demonstrated that by detecting low-energy electrons, known as Auger electrons, that are emitted from a material that is bombarded with an electron beam, one could determine the composition of the topmost atomic layers of a solid. That is because these Auger electrons, which occur by a secondary emission process, have very low energies; if they are emitted from atoms even a few layers below the surface, they do not have enough energy to escape. If one could fracture the sample and then examine the grain boundary fracture surface of the embrittled steel, one could demonstrate that the embrittlement was caused by the concentration of these impurity elements on the grain boundaries.
The development of Auger electron spectroscopy , the term by which Harris’ technique became known, required another development that also occurred during the 1960s – the development of high-vacuum technologies. If a surface is exposed to air, it rapidly becomes coated in carbon and oxygen, and a technique such as Auger electron spectroscopy will only reveal those elements, since it only probes the very top atomic layers of the surface. Thus, a clean surface must be prepared in an ultrahigh vacuum and examined without exposure to air. Given that these temper embrittled steels were extremely easy to fracture along the grain boundaries, they could easily be broken in a vacuum chamber and their uncontaminated fracture surfaces analyzed.
These types of experiments were pioneered by Professor C.J. McMahon, Jr., at the University of Pennsylvania, and many years of research in his laboratory clearly determined the basic principles of impurity segregation to grain boundaries and the resultant embrittlement along with other important metallurgical variables such as hardness, grain size, and overall composition (McMahon 1976; Mulford et al. 1976a, b; Ohtani et al. 1976a, b; Ohtani and McMahon 1975).
Of all of the elements that have been found to cause embrittlement, two stand out and require special discussion. These are sulfur and phosphorus. Sulfur has been found to be an extremely potent grain boundary embrittler in iron, but in most practical steels, this element has not played an important role. The reason is that the sulfur is almost always precipitated as a manganese sulfide in the steel matrix and is simply not available to segregate to the grain boundaries. However, removal of sulfur is still extremely important because otherwise there will be such a high density of these sulfides that they will lead to internal cracking and tearing during the processing of the steel and result in lower fracture energies. In addition, these sulfides are preferred sites for corrosive pitting, a process which appeared often to be the initiation of the corrosion crack that gave rise to fracture.
Phosphorus is a different matter. It readily segregates to grain boundaries in steels, and because of its small atomic size, it diffuses more rapidly and at lower temperatures than many of the other embrittling elements such as tin and antimony. For example, even after 100 h at 400 °C, phosphorus segregation will occur and cause significant embrittlement. Furthermore, while most of the other embrittling elements show little tendency to segregate at the high temperatures that would be encountered as the steel solidifies from the melt, phosphorus readily segregates to grain boundaries at temperatures as high as 1200 °C. Finally, there are no elements that can be added to the steel that would precipitate phosphorus in the way that manganese precipitates sulfur. While some elements such as titanium and niobium will form stable phosphides, they have a greater tendency to form carbides in the steels. The only element that appears to counteract phosphorus embrittlement in some way is molybdenum; its positive effect may come from an inherent strengthening of the boundaries rather than inhibiting phosphorus segregation.
Bringing Research and Practice Together
With the result of these studies of segregation and the clear demonstration that impurity elements increase the ductile-to-brittle transition temperature when they have segregated to the grain boundaries, the next question was whether or not steel companies could make castings of the required size that had extremely low impurity concentrations and whether or not these castings would have sufficient strength. The answer to this question came through a series of projects, many sponsored by the Electric Power Research Institute (EPRI) and performed primarily at Japanese and European steel companies. Initially small laboratory heats were made that would have the desired composition. These were then scaled up to larger heats of material.
Viswanathan (1996) summarized a number of these studies. The overall conclusion is that high-purity steels can be made as large castings and that these steels can have the required strength. Table 6.2 shows the chemistries of a series of ingots, two of which weighed over 100 tons. It is clear that phosphorus and sulfur had been kept to a very low level even in these very large castings. The high-temperature strength of these materials was also satisfactory. As an example, a 34-ton heat was prepared by Vereinigte Edelstahlwerke AG, Austria, and tested in a variety of labs as a part of the EPRI-sponsored study (Jaffee et al. 1986). After the first heat was tested, the nickel content was increased to 3.5–3.75% to increase hardenability, and the subsequent heats met all desired mechanical properties. As a result, a 3.5Ni-Cr-Mo-V rotor with extremely low impurity concentrations was installed at the Chubu Electric Company’s Kawagoe Plant in Japan (Viswanathan 1996). The high purity of this steel allowed the low-pressure turbine to operate 30 centigrade degrees higher than the normal operating temperature because there was no concern for embrittlement occurring during operation. This increased temperature improved the efficiency by approximately 0.1% (Viswanathan 1997).
However, an important question was whether or not the turbine manufacturers and utilities would use this high-purity steel. EPRI carried out a survey to examine this question. The survey results reported by Nutting (1996) suggested that among the major producers of steels for electric power generation, only 5% of their capacity was devoted to steels for this application. Of this capacity, only 3.5–5% were produced to an “EPRI superclean specification.” One reason for this lack of orders appeared to be cost. The steelmakers polled in the EPRI survey indicated that a premium of 15–20% would be charged in relation to conventional grades for ingot weights up to 100–150 tons, and a higher premium would be charged for ingot weights in the range of 300–600 tons. While it was clear that most of these steelmakers could make superclean steel if requested, it was also clear, as noted above, that the impurity concentration of conventional steels was decreasing to the point that it might not be necessary to demand the extreme purities proposed by EPRI, especially if input scrap was carefully chosen. Nutting (1996) concludes:
In spite of the clear advantages of using superclean 3.5Ni-Cr-Mo-V steel for LP rotors, only a small proportion of the recently installed rotors are in the new material. It is difficult to understand why. It could be that wider usage will develop from their application to discs and it may be that as more LP rotors are used in nuclear plant[s] where stress corrosion cracking is more prevalent, the advantages of superclean steels will become more apparent. Whatever should develop, there would appear to be no great difficulty in obtaining suitable forgings, the initial cost premium is not high in relation to the finished cost and there appears to be no difficulty in fabrication. Is it the fault of the plant makers for not stressing the advantages? Or should the plant users take a more active role in specifying their needs?
Research on Design, Steam, and Repair
The fact that the turbine manufacturers did not readily choose to use the extremely high-purity steels might at first seem surprising, since it would appear that these steels would provide a major part of the protection from cracking that they needed. However, the story was more complex. While the use of high-purity steel would clearly eliminate temper embrittlement, it was not clear that the use of these steels would eliminate the stress corrosion cracking that had initiated the cracks in the first place. Conventional steels were becoming increasingly cleaner, and it was also becoming apparent that plant practice played an important role in controlling these fractures .
To discuss these other factors, we begin with the immediate steps taken by the Central Electricity Generating Board (CEGB) in the UK immediately after the Hinkley Point failure. The CEGB operated the Hinkley Point power plant, and the CEGB director of operations was charged with coordinating the examination and rehabilitation of existing turbines. These steps are described in detail by Hodge and Mogford (1979) and are summarized here.
Groups were formed to examine the design, manufacturing resources, metallurgical factors, and chemical factors that could lead to cracking. The analysis of the failures at Hinkley Point as well as examination of other rotors of similar design pointed to the keyways as the most susceptible site for cracking. This observation seemed logical based on the increased mechanical stress at the keyway and the fact that the keyway could provide a natural crevice to enhance stress corrosion cracking. Thus, engineers at CEGB could conclude that the most susceptible turbines would be low-pressure turbines with keyed and shrunk-on discs. Those would have the keyway present and have discs that operate in a portion of the steam cycle where condensation might occur. To plan for the rehabilitation program, the turbines were divided into three groups based on the likelihood that they could contain cracks. The three groups were those of the type where cracks had been observed when the rotor was dismantled, those of the type where conditions strongly inferred that cracking should be present, and those of a type where there was a strong inference that cracking should be present but there were also possible alleviating conditions such as lower stresses. As Hodge and Mogford conclude, “it was possible, therefore, even before many factors were understood, to infer that early stage discs on LP rotors of non-reheat turbines ran the greatest risk of cracking.” They also very importantly noted that all of this work had to occur while electricity continued to be generated at a time when there was “little surplus capacity.”
The most immediate recommendation, so that electricity could continue to be generated as the turbines were repaired, was to reduce the amount of overspeed testing (recall that the failure at Hinkley Point had occurred during an overspeed test) and also to have the trip setting for overspeed set as low as possible without giving false indications. This practice would limit the high stress excursions experienced by the discs. It was also recommended to run the turbines at the most constant conditions possible and that during a cold start, the rotors should be pre-warmed before being brought to the operating speed to be sure that the steel was above its ductile-to-brittle transition temperature and to be sure the steam did not condense in the keyway.
The next step was to discontinue the use of any discs that were found to be cracked and to replace them with pressure-reducing plates fitted to the rotor shafts . While this approach decreased the power output of the plant by 7–10 MW, it provided for safe operations. The longer-term solution was to bore the keyway out of the cracked discs and to replace the shaft with one of a larger dimeter that could accept these repaired discs. A cylindrical button-type arrangement was used to keep the discs in place and aligned on the rotor shaft. These cylindrical buttons were placed on less highly stressed areas of the discs. In addition to repairing the rotors with new oversized shafts that could accept the bored out and keyway-free discs, new rotors without keyways were ordered for complete replacement.
While this large-scale repair effort was being performed to provide an immediate solution to this problem, research also continued to understand the fundamental causes of the fractures . This research involved both careful cataloging of observations made in the dismantled turbines along with laboratory experiments to characterize the material behavior in similar conditions of stress and environment. The results of this work led to interesting and not completely expected conclusions that undoubtedly had an impact on the decisions made by turbine manufacturers and users.
The conclusions, primarily summarized by Hodge and Mogford (1979), were the following:
-
1.
Disc cracking was related to rotor design and not steel composition. Cracking always occurred in regions of the turbine where condensation could occur.
-
2.
Ni-Cr-Mo-V and Cr-Mo steels were of similar susceptibility to cracking based on observed cracks in dismantled rotors. Kalderon and Gray, in a discussion of Gray (1972), note that in a wet environment a 3Ni-Cr-Mo steel which had superior fracture toughness to a 3Cr-Mo steel cracked significantly, whereas the 3Cr-Mo steel with a composition similar to those that failed at Hinkley Point did not crack in a dry environment. This example and many others clearly showed that material composition and toughness were not the single root cause of the failures.
-
3.
For one particular design of rotors, there was no difference in susceptibility between acid open-hearth and basic electric 3Cr-Mo discs, again based on observed cracks in dismantled rotors. Thus one could not say that the acid open-hearth steels, which were less pure and had a higher ductile-to-brittle transition temperature, were always more susceptible to cracking in practice. In the same type of rotor, 3Ni steels were not observed to crack.
-
4.
Although many turbine deposits were chemically analyzed, no compositional correlations were found with disc cracking. This result was borne out in the extensive review by Zhou and Turnbull (2003).
-
5.
While crack initiation in the lab was found to be more rapid for steels that were temper embrittled, the crack growth rate was not obviously affected by this embrittlement. However, crack initiation was not eliminated in the high-purity materials tested in the laboratory (Turnbull and Zhou 2003).
-
6.
The dimensions of the crevice played an important role in enhancing crack growth, with larger gaps less susceptible to cracking. Lab specimens exposed to steam for 10,000 h that had a crevice with a gap of 0.05 mm or less had cracks initiated in them. Specimens that had crevices with gaps of 0.25 or 1 mm did not crack (Lyle et al. 1985).
A significant amount of this additional laboratory research was directed at determining whether or not segregated impurities that caused temper embrittlement would also raise the susceptibility to stress corrosion . As mentioned above, this work showed that while high-purity steels were much more resistant to temper embrittlement, one could not make a strong case that they were more resistant to stress corrosion cracking (Lyle et al. 1985; McMinn et al. 1985; Rosario et al. 1998), although they did appear to be more resistant to crack initiation by pitting (Holdworth et al. 1996; Turnbull and Zhou 2003). Overall, the laboratory variables that did appear to enhance stress corrosion cracking were an increase in the yield strength, aeration of water in the test environment (effectively increasing the oxygen concentration), and the presence of a crevice such as would occur in a keyway (Hodge and Mogford 1979).
These results also corresponded well with examination of cracked discs taken out of service. Cracking occurred when a liquid phase could exist, that is at a point when the steam could condense, and the occurrence of cracking could not be correlated with other impurities found in and around the crack. Stress also was found to be a critical factor. Lyle et al. (1985) reported that 31 of 44 rotors with 112-cm last-stage blades developed disc cracks, while 3 of 15 rotors with 102-cm last-stage blades developed cracks. The latter had stress levels at the bore that were 70–80% of those in the large rotor. Lyle and Burghard (1982) also found in their survey of US nuclear plants that the design of the keyway was important. Hemispherical keyways which had a very tight tolerance were prone to initiate cracking. Rectangular keyways which had a larger gap between the raised portion on the shaft and the mating surface on the disc did not crack.
But equally important was the effect of plant practice. Evidence collected on US plants indicates that crack depth increased with the number of plant start-ups and with the occurrence of condenser leakages leading to air in the system (Lyle and Burghard 1982). Hodge and Mogford (1979) also noted that plant operation could be important, since cracks were observed in some discs and not in others that were of the same design. Also, it is interesting to note that in their recommendations, Lyle et al. (1985) emphasized plant operations as critical. Thus, we have a situation where the material, regardless of temper embrittlement and impurity segregation, can be susceptible to cracking in condensed steam, independent of whether the steam is pure or contaminated in some way. The exact crack growth rates can vary, but cracking does appear to occur (McMinn et al. 1985; Turnbull and Zhou 2003).
All of these results point to the importance of lowering stresses and removing tight crevices. With high stress and the presence of crevices, any of these steels, independent of purity level, could crack. Thus, it is not surprising that the elimination of the keyways which removed tight crevices and also removed an area of stress intensification apparently solved this problem for the CEGB (Lyle and Burghard 1982), and that other methods that eliminated the keyways, such as the use of monoblock forgings in which the discs and rotor were forged from a common piece of material, were adopted by other companies (Ikeda et al. 1996; see discussion by Mitchell (1972) to Kalderon and Gray 1972). It is then not surprising that companies were not as interested in the extremely high-purity steels and opted to put less restrictive values on impurity content in the alloy through scrap selection and other methods rather than pay a premium for the clean steels. The importance of temper embrittlement would be to affect the crack length that would lead to unstable rapid fracture. If the stress was lowered and the possibility of crevice corrosion removed through the elimination of keyways and impurities controlled to a reasonable level through scrap selection, the risk of cracking should be greatly reduced. Hodge and Mogford (1979) conclude:
The materials engineer must recognize that the steel in current world-wide use (3½NiCrMoV) is susceptible to stress corrosion in pure steam and if cracks form they may grow at a significant rate. It would be possible to tolerate such a situation if the critical crack size for fast fracture were much larger than the maximum amount of crack growth. The completely safe situation demands a guarantee that cracks could never initiate but in reality it may be necessary to accept the possibility of cracking at a known rate and assume the steel is sufficiently tough or the stress sufficiently low to tolerate the crack.
Technology Interaction Spheres
We now wish to consider this problem in terms of the bigger picture of technology cultures and interaction spheres. Our idea is that around each of the technologies that contributed to the solution of this problem, there are distinct cultures based on training, ideas, and experience but that the larger integrated solution occurs when these cultures interact. These interactions give rise to a new set of ideas that would not have occurred without bringing the technologies together.
Most of what is written on this topic has been about major technology breakthroughs. One can site Bijker’s study (1995) of the bicycle, bakelite, and incandescent bulb. Each of these had a variety of inventors, and each is built on a number of scientific breakthroughs. And each could be identified with a specific material or object that defined the interaction point between technology and society. But the discussion of this problem is different. Since the primary purpose of large steam turbines was to generate electricity, one could argue that society was essentially blind to these underlying developmental advances, since electricity was generally available and the only real impact to society might come through the cost of electricity. The availability of electricity had an enormous positive impact, but when the work reported on here was being performed, electricity was available to almost everyone. Yet, these great advances were being made in various technologies related to steam turbines, and for many individuals working on these advances provided fulfilling careers and salaries for their livelihood. The difference here is that in considering the interaction spheres that gave rise to these advances, one must focus not just on an object (the steam turbine) but on the practice and organization, or development, of technology to solve a problem so that the industry can continue to grow and satisfy the needs of the society that consumes, and in this case relies on, the product. The general concern of these types of industry-limiting problems has been discussed by Hughes (1983) for the power generation industry. He termed these types of problems as reverse salients, a term he borrowed from military history.
In thinking about the problem, it is useful to draw on studies of innovation diffusion and in particular on the studies of innovation in organizations, since these advances in turbine technology were occurring in the context of very large organizations. Schroeder et al. (2000) have outlined a number of observations that they note are common points in terms of innovations made by organizations. While several of these are not directly applicable to our discussion, there are some that are clearly relevant. We briefly discuss these here.
The first observation listed by Schroeder is that the innovation is stimulated by a shock, either internal or external to the organization . Clearly the failure of these large steam turbines provided this shock. While we have described one particular failure here, namely, the one at Hinkley Point, the entire power generation industry was concerned with these failures (Lyle and Burghard 1982). From the fiscal standpoint, the possibility of severe electrical outages and the cost of the turbine were two main reasons for concern. In 1982 it was estimated that replacement of an entire turbine would cost $2–6 million and that the cost to buy replacement electricity for a 1000 MW nuclear plant would run $0.5–1 million per day (Lyle and Burghard 1982). Furthermore, the potential for worker injury in explosions caused by their failure could expose the companies to liability.
Another of Schroder’s observation was that as innovation develops, the old and the new exist concurrently, and over time they are linked together. Unlike a recall of a particular product, large steam turbines could not be completely shut down and replaced but had to be slowly replaced over time. New plants could be configured with new technologies, but the older plants still had to be used. As shown in the report by Hodge and Mogford (1979), it was possible to stage these repairs, beginning with the most suspect rotors, and gradually replace all that were of concern. Over time, engineers recognized what actually needed to be changed and what could remain unchanged.
A third observation is that an initial idea tends to proliferate into several ideas during the innovation process. One can draw a parallel here to the various types of investigations that took place in response to these failures. These included mechanical analysis, steam cycle analysis, corrosion tests, development of clean steel, and new designs of rotors that eliminated the keyway. Around each of these, there is a technology culture of interacting people, but as these groups begin to interact with each other, to provide new ideas to each other, the final solutions were reached that were accepted by multiple organizations.
It is clear that for these interactions to occur there have to be mechanisms to facilitate them. Some of these would come strictly through market forces, the lines of purchasing and the requirements placed by the buyer of the technology or object on the seller. But those types of interactions do not necessarily lead to the idea exchange and debate that are so crucial to solutions of problems of this magnitude. In this particular case, it is clear that the ASME, ASTM, and EPRI helped organize cooperative programs where these ideas could be shared and discussed. The importance of these organizations in using their convening power, and in some cases their funding power, to encourage this integration must be emphasized. An industry approved forum in which ideas can be exchanged is critical for advancement. Viswanathan (1997), who led many of these efforts for EPRI, notes, “It was generally agreed [from the EPRI survey described above] that the need for clean/superclean steels must be placed in the context of design and operational requirements. It is not our objective to make steels as clean as they can get but as clean as we need them” (italics added). Knowing how clean a steel is needed must come through interactions of people who bring all possible contributing solutions to the table, along with operational experience, and then decide how to integrate them in practice.
Let us pursue these ideas further. As one pathway to the solution of the problem discussed here, there was an effort to manufacture high-purity steels. The technology was developed to meet this goal. The development required interaction between steel producers and their suppliers, and the turbine manufacturers who might incorporate this material into their design. But after testing and proof-of-concept trials had been completed, a decision had to be made as to whether or not this new product would be used. In this case, the result of investigations of the mechanics of the turbine design and the stress corrosion of steels in high-purity steam indicated that steels of extremely high purity, which had been the aim of these technology developments, were not required. By placing reasonable, not stringent, requirements on the impurity levels in the steel, one could avoid a brittle material at the turbine operating temperatures, and by elimination of the keyways, high stresses that would lead to fracture would be reduced along with the sites where crevice corrosion began.
It is important to emphasize the point that as a result of this synthesis of ideas, which results from what I term a technology interaction sphere, decisions are made based on the information that is part of this sphere. These decisions determine the direction that a particular technology will take. This outcome points to the fact that those making the decision must have full knowledge of all of the technologies that are coming together. Furthermore, this knowledge must include contributions from all levels in the various contributing organizations. The inputs from the workers tasked with making the product or its parts are as critical as the sophisticated engineering evaluation or the detailed financial considerations. All are important. Establishing an interaction sphere where all of these factors come together is a critical challenge for the decision-makers.
This situation leads to another question and that is one of retention of the knowledge that is developed, particularly the valuable knowledge that has been generated but may not be incorporated into the solution. As part of the practices developed to solve this problem, significant research was performed that elucidated many aspects of the properties of clean steel. The utilities and the turbine manufacturers chose, on the whole, not to use the steels of the very highest purity. Rather they took advantage of the general improvements made in steelmaking that led to production of conventional steels of adequate purity. The question, then, is whether or not all of this knowledge will be retained in some form. There is a vast amount of knowledge developed around making extremely high-purity steels, steel embrittlement, and impurity segregation that appears to be no longer under active discussion. It is important that it be maintained and archived in a readily accessible way.
Finally, it should be noted that when we look back on a problem such as the turbine failures discussed here, it is all too easy to make it sound like everything happened in sequence and that it occurred in a very thoughtful progression. That is undoubtedly not the case. In any problem there are ups and downs, economic and technical competition, pet solutions of different groups, proprietary solutions and applications, and dead ends. However, there is value to looking at the summary of it all and understanding the great importance of shared ideas and the importance of different technologies coming together in interaction spheres to create new solutions and consensus. When Caldwell (1964) first introduced the idea of interaction spheres, his comment was that it was the way civilizations move forward. While turbine cracking is not as grand as a civilization, it is important to note that these interactions are the way technologies move forward.
Conclusions
This paper has traced a central part of the history of catastrophic failures in large steam turbines and the resulting industry solutions to this problem. Particular focus is given to the role that the material of construction, low-alloy steel, played in this problem and the efforts of the steelmaking industry to develop materials that would prevent the problem. Enormous improvements were made in the making of these steels so that they were immune to temper embrittlement, and clear demonstrations were given that these special steels could be made on a scale that was needed for these large turbines. However, the brittle steels were not the entire cause of the problem. Condensed steam, crevices, and mechanical design were also crucial components. The final solution involved dealing with all aspects of the problem. As it turned out, improvements to conventional steels which resulted from this overall effort were sufficient for this application. It is proposed that we can think of these integrated solutions as resulting from technology interaction spheres. The ideas from certain groups come together and lead to new ideas that would not have been possible without the stimulation of thinking from other groups. It is these combining technologies that lead to the big next steps in engineering and design.
Notes
- 1.
I borrow the term “interaction sphere ” from the anthropology/archaeology literature. It was first coined by Caldwell (1964) to describe practices that were adopted by many different cultures, even though these cultures retained other practices that were specific to themselves. Thus, interactions between cultures led to wide and common adoption of some practices but not others.
- 2.
There have been many terms for steels with low impurities. These include clean steels, ultra-clean steels, high-purity steels, ultrahigh-purity steels, etc. Some of these have had specific distinctions at a given time. For clarity, I will just use the term clean steel to denote any steel in which there has been a concerted effort to lower the impurity level. Specific compositions will be given at various points in the paper to indicate how low a concentration of these impurities was obtained.
- 3.
All compositions in this paper are in weight percent.
References
Adams AM, Atkinson JD, Ford FP, Worthington PJ (1975) The effect of potential on stress corrosion cracking crack growth in mild steel and low alloy turbine-disc steels in boiling 35 w/v sodium hydroxide, CERL Note No. RD/LN 36/75. Central Electric Research Laboratories, Leatherhead
Atkinson JD, Adams AM, Khan MAM, Worthington PJ (1979) The effect of de-temper embrittlement on stress corrosion crack propagation in 3% Cr-Mo turbine disc steel in 35 w/v sodium hydroxide, CERL Note No. RD/LN 169/79. Central Electric Research Laboratories, Leatherhead
Bijker WE (1995) Of bicycles, bakelites, and bulbs. MIT Press, Cambridge
Bodnar RL, Cappellini RF (1988) Effects of residual elements in heavy forgings: past, present, and future. In: Bramfit BL, Benn RC, Brinkman CR, Vander Vorrt GF (eds) MiCon 86: optimization and processing, properties, and service performance through microstructural control, ASTM-STP 979. American Society for Testing and Materials, Philadelphia, pp 47–82
Briant CL, Banerji SK (1978) Intergranular failure in steel: the role of grain boundary impurities. Int Met Rev 4:164–199
Caldwell JR (1964) Interaction spheres in pre-history. In: Caldwell JR, Hall RL (eds) Hopewellian studies. Illinois State Museum, Springfield, pp 133–143
Cramb A (1999) High purity, low residual clean steels. In: Briant CL (ed) Impurities in engineering materials: impact, reliability, and control. Marcel Dekker, New York, pp 49–90
Curran RM (1986) The development of improved forgings for modern steam turbines. In: Nisbett EG, Melilli AS (eds) Steel forgings, ASTM-STP 903. American Society for Testing and Materials, Philadelphia, pp 9–32
Emmert HD (1956) Investigation of large steam-turbine spindle failure. Trans ASME 78:1547–1565
Fruhan RJ (1985) Ladle refining furnaces for the steel industry, Prepared for the Center of Metals Production
Gray JL (1972) Investigation into the consequences of the failure of a turbine-generator at Hinkley Point ‘A’ power station. Proc Inst Mech Eng 186:379–390
Harris LA (1968a) Analysis of materials by electron-excited Auger electrons. J Appl Phys 39:1419–1427
Harris LA (1968b) Some observations of surface segregation by Auger electron emission. J Appl Phys 39:1428–1431
Hodge JM, Mogford IL (1979) UK experience of stress corrosion cracking in steam turbine discs. Proc Inst Mech Eng 193:93–108
Holdworth SR, Nougaret M, Vittemant B (1996) Stress corrosion in 3.5 NiCrMoV welded joints. In: Nutting J, Viswanathan R (eds) Clean steel: superclean steel. The Institute of Materials, The University Press, Cambridge, pp 133–155
Hughes TP (1983) Networks of power, electrification in western society 1880–1930. The Johns Hopkins University Press, Baltimore
Ikeda Y, Yoshida H, Tanaka Y, Fukuda T (1996) Production and properties of superclean monoblock LP turbine rotor forgings. In: Nutting J, Viswanathan R (eds) Clean steel: superclean steel. The Institute of Materials, The University Press, Cambridge, pp 71–87
Jaffee RI, Machner P, Meyer W, Steiner JE (1986) Production and properties of a superclean 3.5NiCrMoV LP rotor forging. Ironmak Steelmak 13:322–236
Kalderon D (1972) Steam turbine failure at Hinkley Point ‘A’. Proc Inst Mech Eng 186:341–377
Kawaguchi S, Kanno N, Iwadate T, Ohhashi T (1986) Integrity of full-integral, low pressure nuclear turbine forgings. In: Nisbett EG, Melilli AS (eds) Steel forgings, ASTM-STP 903. American Society for Testing and Materials, Philadelphia, pp 203–214
Killick D, Fenn T (2012) Archaeology: the study of pre-industrial mining and metallurgy. Ann Rev Anthropol 41:559–575
Lyle FF Jr, Burghard HC Jr (1982) Cracking of low pressure turbine rotor discs in US nuclear power plants. Mater Perform 21(11):35–44
Lyle FF Jr, McMinn A, Leverant GR (1985) Low-pressure steam turbine disc cracking – an update. Proc Inst Mech Eng 199:59–67
Masuyama F (2001) History of power plants and progress in heat resistant steels. ISIJ Int 41:612–625
McMahon CJ Jr (1976) Intergranular fracture in steels. Mater Sci Eng 25:233–239
McMinn A, Lyle FF Jr, Leverant GR (1985) Stress corrosion crack growth in NiCrMoV turbine disc steels. Corrosion 41:493–503
Mitchell JM (1972) Discussion of article by Kalderon and Gray. Proc Inst Mech Eng 186:D124
Mochel NL, Peterson RE, Conrad JD, Gunther DW (1956) Large rotor forgings for turbines and generators. Trans ASME 78:1585–1601
Mulford RA, McMahon CJ Jr, Pope DP, Feng HC (1976a) Temper embrittlement of Ni-Cr steel by phosphorus. Metall Trans A 7A:1183–1195
Mulford RA, McMahon CJ Jr, Pope DP, Feng HC (1976b) Temper embrittlement of Ni-Cr steel by antimony: III. Effects of Ni and Cr. Metall Trans A 7A:1269–1274
Newhouse D (1967) Temper embrittlement in steel, ASTM-STP 407. American Society for Testing and Materials, Philadelphia
Newhouse D (1971) Temper embrittlement of alloy steels, ASTM-STP 499. American Society for Testing and Materials, Philadelphia
Nutting J (1996) The EPRI survey on superclean steels. In: Nutting J, Viswanathan R (eds) Clean steel: superclean steel. The Institute of Materials, The University Press, Cambridge, pp 33–52
Ohtani H, McMahon CJ Jr (1975) Modes of fracture in temper embrittled steel. Acta Metall 23:377–386
Ohtani H, Feng HC, McMahon CJ Jr, Mulford RA (1976a) Temper embrittlement of Ni-Cr steel by antimony I: embrittlement at low carbon concentrations. Metall Trans A 7A:87–101
Ohtani H, Feng HC, McMahon CJ Jr (1976b) Temper embrittlement of Ni-Cr steel by antimony II: effects of addition of titanium. Metall Trans A 7A:1123–1131
Rankin AW, Seguin BR (1956) Report of the investigation of the turbine wheel fracture at Tanners Creek. Trans ASME 78:1527–1546
Rosario DA, Viswanathan R, Wells CH, Licina GJ (1998) Stress corrosion cracking of steam turbine rotors. Corrosion 54:531–545
Schabtach C, Fogleman EL, Rankin AW, Winne DH (1956) Report of the investigation of two generator rotor fractures. Trans ASME 78:1567–1584
Schaefer AO (1956) Work of the task group on brittle failure of steel forgings. Trans ASME 78:1623–1626
Schroeder RG, Van de Ven AH, Scudder GD, Polley D (2000) The development of innovation ideas. In: Van de Ven AH, Angle HL, Poole MS (eds) Research on the management of innovation. Oxford University Press, New York, pp 107–134
Szekely J, Carlsson G, Helle L (1988) Ladle metallurgy. Springer-Verlag, New York
Thum EE (1956) Recent accidents with large forgings. Met Progr 69(2):49–57
Turnbull A, Zhou S (2003) Steam turbines part 2 – stress corrosion of turbine disc steels. Corros Eng Sci Technol 38:177–191
Viswanathan R (1996) Application of clean steel/superclean steel technology in the electric power industry – overview of the EPRI research and products. In: Nutting J, Viswanathan R (eds) Clean steel: superclean steel. The Institute of Materials, The University Press, Cambridge, pp 1–32
Viswanathan R (1997) Clean/superclean steel rotors for electric utility applications. In: Nisbett EG, Melilli AS (eds) Steel forgings: second volume, ASTM STP 1259. American Society for Testing and Materials, Philadelphia, pp 280–303
Zhou S, Turnbull A (2003) Steam turbine part 1 – operating conditions and impurities in condensates, liquid films and deposits. Corros Eng Sci Technol 38:97–111
Acknowledgments
The author would like to thank Drs. Alan Turnbull and Shengqi Zhou of the National Physical Laboratory, Teddington, Middlesex, UK, for helpful discussions. He would also like to thank SAGE Publications and the Proceedings of the Institution of Mechanical Engineers for use of Figs. 6.1, 6.3, 6.4, and 6.5 and RWEnpower for use of Fig. 6.2. He would like to thank Professor Brett Kaufman for comments on the manuscript. Finally, he would also especially like to thank Professor C.J. McMahon, Jr., of the University of Pennsylvania who first introduced the author to this problem over four decades ago.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Briant, C.L. (2018). The Development of Clean Steels for Steam Turbine Applications: Their Demand and Use. In: Kaufman, B., Briant, C. (eds) Metallurgical Design and Industry. Springer, Cham. https://doi.org/10.1007/978-3-319-93755-7_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-93755-7_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-93754-0
Online ISBN: 978-3-319-93755-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)