Abstract
Parkinsonian syndromes are a heterogeneous entity of movement disorders, with various described subtypes. This systematic review aimed to examine the available literature on smartphone applications for assessment of Parkinson’s disease motor and nonmotor symptoms and signs. Papers published from 2013 to 2017, listed in two electronic databases—IEEE Xplore and PubMed—were searched, to identify the works related with smartphone use for PD patients’ diagnosis and monitoring. Full-text articles were analyzed to evaluate the quality of the reported methods and results, considering the validity, reliability, and sensitivity of the techniques used in the measurements as well as the Grading of Recommendations Assessment, Development and Evaluation guideline. The data from 26 full-text articles suggest that many and relevant data can be collected automatically and accurately via mobile phone. Inertial measurement units as well as capacitive, force/pressure, acoustic sensors were used for the development of smartphone-based tools to improve assessment and monitor symptoms and signs of Parkinson’s disease. Smartphone-based information on upper limbs tremor, gait, posture, balance, activities, and speech may improve quality of healthcare services for Parkinson’s disease patients and their quality of life.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
I am going to take a nap now. It was more one night of struggle to continue to stay alive, when physical infirmities and torment of soul are worsening. I remember the panic of awakening without capacity to breathe and the effort to breathe again, the sleeplessness produced by fear of dipping in agony of having to traipse while the body functions failures take me through a distant place…where my deep love that I keep in my soul might be lost forever. I got up with the joy of sun rays that wash my sorrow face. I must get up from bed but fear of falling, stop me to think on other things, and to move. Sadness and frustration, I feel when I see my hands tremor…Who, why is producing these movements…It is not me… are not mine….
I am going to spend my day in front of television. Sad, in powerlessness…From time to time I wish a lot to speak with someone… I want to talk about…but now they not understand me, or they haven’t patience to listen what I said. Oh, so many neighbours that so many time when I was healthy came to me to talk on…so many things…So many time have passed without any visit of my neighbours or friends.
I should stand up from my chair… My heart began to beat strongly….if I fall…how many time I would wait in agony of minutes that seams hours or days until someone would help me…The shame, the infirmity, the pain in my soul…The dead is better than life…I stand up after a while and with small and rapid steps I go to kitchen…I need more space…The dead is better than life…I feel the fear…I hope not falling …The dead is better than life…More one day….
What I am going to do today…as yesterday, and the day before yesterday I will spend my time in front of television…sometime falling asleep, sometime bored…More one day will pass. Perhaps, the dead is better than life…So deeply I wish to live for my dears…. More one day…one day…day…
Although it is not a fragment from a written or spoken diary of a person with Parkinson’s disease (PD), as the used words suggest a high degree of PD severity that is characterized by the low ability to write or speak, it can describe the quality of life of a person having this long-term illness. Based on observations and report from a caregiver, the story suggests a health state with a negative score of health-related quality of life (HRQL) measured by Health Utilities Index (HUI) instrument. HUI is a standardized system to measure health status. The two HUI systems HUI2 and HUI3 can describe almost 1,000,000 unique health states [1]. The HUI2 classification system includes seven attributes—Sensation, Mobility, Emotion, Cognition, Self-Care, Pain, and Fertility—each from three to five levels. The HUI3 classification system comprises eight attributes—Vision, Hearing, Speech, Ambulation, Dexterity, Emotion, Cognition, and Pain—each with five or six levels of ability/disability. Negative scores of HRQL represent health state considered by a person as worse than dead.
The first scientific document on Parkinson’s disease—An Essay on the Shaking Palsy [2], reprinted in [3], with more details on the history in [4]—was published by Dr. James Parkinson 200 years ago. Well-known individuals having the disease, such as Pope John Paul II, actor Michael J. Fox, and boxer Muhammad Ali, contributed to wider public awareness and scientific research on PD. Knowledge on Parkinson’s disease seems to be present in India since ancient times. Ayurveda, an ancient system of medicine dating around 5000–3000 B.C., characterized the kampavata disease by symptoms that currently are considered as the main symptoms of Parkinson’s disease as tremors, stiffness, depression, and a depletion of movement [5]. For this disease with strong clinical resemblance with PD, the ancient Indians prescribed diverse therapeutic plants. Moreover, a lower prevalence of PD was registered in India, mainly in some population (i.e., Bangalore district in South Karnataka) [6]. In our knowledge, no data on the factors that produced or are associated with this lower prevalence were published.
Heterogeneity of Parkinsonian syndrome and similarity of various clinical signs and symptoms with those from other diseases increase the error in PD diagnosis. Parkinsonian syndrome is a heterogeneous entity of movement disorders, which can be subdivided into idiopathic Parkinson’s disease, rare genetic forms of Parkinson’s disease, as well as symptomatic and atypical Parkinsonian syndromes (APS) [7]. Multiple system atrophy (MSA), progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and dementia with Lewy bodies are included into APS. Furthermore, many other disorders (i.e., essential tremor, drug-induced Parkinsonism) may show clinical signs of Parkinsonism.
Many PD patients, both from developed or undeveloped countries, mainly those with low accessibility to healthcare services (i.e., those who live in remote rural areas or in countries with unaffordable healthcare services), perceive their quality of life much lower than the age-matched control group (see [8]). A decline in physical function measured with Short-Form Health Status Survey (SF36) in PD patients relative to a cohort of 51,530 male health professionals and 121,701 female registered nurses from the USA began approximately 7.5 years prior to diagnosis in women and 3 years prior to diagnosis in men and continued to decline thereafter with a rate of 2.35 and 1.43 points per year in women and men, respectively (p < 0.001 for both) [9]. The decline in individuals without PD was on average 0.42 and 0.23 points per year in women and men, respectively [9]. All the eight dimensions scored measured with the 39-item Parkinson’s Disease Questionnaire (PDQ-39) were shown to be significantly lower in PD participants; the highest score was found in “bodily discomfort” and the lowest in “social support” [10]. Moreover, many people diagnosed with PD do not see a neurologist [11]. Even in most developed countries, the doctor appointments are once or twice per year (e.g., in Sweden 1.7 times/year with regional variation between 1.1 and 2.1) [12] and accessibility to healthcare services is the worst in rural regions [11].
The advances in hardware miniaturization combined with increased capacity for large data processing and storing, and in implementation of algorithm for higher measurements accuracy and signal pattern recognition, have made the wearable devices important tools for disease diagnosis and long-term health monitoring. Different benefits are envisioned or are already proven: portability of medical devices; customizability and deployment scalability; healthcare cost saving; better communication between patients and healthcare professionals; objective measurements of subjects functioning, disability, and health; increased patient access to health information; medication reminders; to help track progress in physical exercise regime; patient assessment and monitoring for an extended duration in clinic or remotely; opportunities for patients in chronic condition to engage in their healthcare; and capacity to improve the quality of healthcare services and to reduce medical errors while reducing clinician workloads.
Modern smartphones integrate a growing number of sensors, powerful portable media tools, connectivity to the Internet, and cloud computing resources. Major mobile operating systems, such as Android, iOS, and Windows support customizable interfaces and signal processing. In the past years, various smartphone or tablet applications for diagnosis and monitoring of PD signs and symptoms were developed. In this chapter, we present our analysis on smartphone applications for Parkinson’s disease symptoms and signs assessment and monitoring. These sensing technologies may have great potential to improve accessibility to healthcare services, quality of diagnosis, and treatment of patients with Parkinson’s disease as well as to increase their everyday quality of life.
In the Sect. 13.2 of the chapter, a brief literature review on non-electronic methods used for the assessment of Parkinson’s disease motor and nonmotor symptoms and signs and their precision is presented, followed by the Sect. 13.3 by presentation of the methods and results on analysis of literature related to smartphone applications for PD diagnosis and monitoring.
2 Non-electronic Instruments Used for PD Diagnosis
The gold standard diagnosis of PD is based on histopathological analysis, after patient death, and requires cell loss in substantia nigra and the presence of Lewy bodies, which stain for alpha-synuclein and ubiquitin [7]. While these criteria are useful only post mortem, several attempts have been made for real-time effective diagnosis and monitoring. Several biomarkers (i.e., genetic markers, biochemical markers, neuroimaging markers, and clinical biomarkers) were described in the last decades for diagnosis, tracking disease progression, identification of specific therapeutic targets, or determination of the efficacy of agents designed to influence disease progression [13,14,15]. However, the initial diagnosis varies greatly in PD. Clinical diagnosis performed mainly by nonexperts might have an accuracy of 73.8% (95% CI 67.8–79.6%) [16]. The accuracy of PD diagnosis made by a general neurologist was found to be 76% [17], and clinical diagnosis performed by movement disorders experts rose from 79.6% (95% CI 46–95.1%) of initial assessment to 83.9% (95% CI 69.7–92.6%) and up to 90% [18] of refined diagnosis after follow-up [16]. When patients present atypical Parkinsonism, the accuracy of diagnosis is low (41–88% in progressive supranuclear palsy; 50–66% in multiple system atrophy) [19, 20]. Furthermore, in a recent prospective study with 110 subjects [21], in which the accuracy of various technologies for differentiating diagnosis of PD and APS was tested, the clinimetrics of Unified Parkinson’s Disease Rating Scale (UPDRS) have showed more benefits and efficacy for PD diagnosis. In the study, the accuracy of magnetic resonance imaging (MRI), 123I-iodobenzamide single photon-emission computed tomography (IBZM-SPECT) analysis of the cerebrospinal fluid (CSF), and electromyography (EMG) of the anal sphincter to diagnose PD were investigated. This study also analyzed data from: a structured interview, including information on medical history; used medication; presenting complaints and progression of the disease; most affected body site; balance and fear of falling as well as from UPDRS III and IV; Hoehn and Yahr (H&Y) score; International Cooperative Ataxia Rating Scale (ICARS) for cerebellar symptoms; and Mini Mental State Examination (MMSE). Participants underwent a structured interview; detailed and standardized neurological examination; and within 6 weeks after the initial visit brain MRI, IBZM-SPECT, lumbar puncture, and anal sphincter EMG. After 3 years, the examination was made again following the same procedures, for all patients. In 92 out of the 110 patients who completed the 3-year follow-up, the initial diagnosis at baseline was correct. APS diagnosis at baseline was incorrect in 33% of patients. Clinimetric procedures yielded 138 clinical parameters that were potentially able to differentiate between PD and APS. Higher age, rapid disease progression, autonomic dysfunction, impaired tandem gait, abnormal fluency, higher ICARS total score, higher UPDRS axial score, and higher disease stage were the parameters that have shown fair to good accuracy to differentiate between PD and APS. The study has shown that none of the ancillary investigations contribute for better accuracy to differentiate PD and APS but tandem gait and axial UPDRS score yielded a very good accuracy (AUC = 0.92), a sensitivity of 73% and specificity of 92% [21].
The UPDRS scale, published in 1987 [22], and U.K. Parkinson’s Disease Society Brain Bank Clinical Diagnostic Criteria (UKPSBB) [17] are nowadays the most used clinical criteria for PD diagnosis. The diagnosis of PD based on the UKPSBB criteria demands the presence of bradykinesia (slowness of initiation of voluntary movement with progressive reduction in speed and amplitude of repetitive actions) and at least one of the following: muscle rigidity, rest tremor, or postural instability not caused by primary visual, vestibular, cerebellar, or proprioceptive dysfunction. UPDRS have six parts: part I. mentation, behavior, and mood (four items); part II. activities of daily living (13 items); part III. motor examination (13 items); part IV. complication of therapy in which dyskinesia, clinical fluctuation, and other fluctuation are studied; part V. modified Hoehn and Yahr staging; and part VI. Schwab and England activities of daily living scale. A shorter version of UPDRS for the assessment of motor impairment and disabilities in PD—the Short Parkinson’s Evaluation Scale (SPES) [23]—was created. Later, the Scale for Outcomes in Parkinson’s disease (SCOPA) study brought some modification to SPES, improving clinimetric aspect of the SPES scale, which resulted in a new scale—SPES/SCOPA [24, 25]. However, the UPDRS scale has some weaknesses: (i) it is time consuming—mean completion time is 30 min; (ii) the evaluation using this scale is prone to clinicians’ subjectivism; (iii) several ambiguities in the written text are present in UPDRS scale, inadequate instructions for raters, some metric flaws, and the absence of screening questions on several important nonmotor aspects of PD [26]. Founded on the critique that was formulated by the Task Force for Rating Scales in Parkinson’s disease, a new scale was published in 2008 powered by the Movement Disorder Society (MDS) [27, 28]. The MDS–UPDRS has four parts: part I. nonmotor aspects of experiences of daily living (13 items); part II. motor aspects of experiences of daily living (13 items); part III. motor examination (21 items); and part IV. motor complication. In comparison with UPDRS, the new scale includes more nonmotor aspects of PD, and patients reported symptoms and signals.
Despite PD nonmotor symptoms being described for long time ago (e.g., sleep disturbances, gastrointestinal dysfunction, bladder dysfunction, and even fatigue were described by Dr. Parkinson) and extensive demonstration of the importance of nonmotor aspects of experience of daily living, the PD continues to be viewed by most clinicians as a motor disorder, and for simplicity, the UKPSBB criteria are the most used. The nonmotor symptoms received in the last decades an increasing interest by their importance recognition for diagnosis purposes but also because they are the major source of deterioration in quality of life [29]. Various nonmotor aspects were associated with Parkinson’s disease: (i) sensory dysfunction—hyposmia, decreased visual contrast and color discrimination, and decreased visual motion perception, abnormal sensations, such as paresthesias; (ii) dysautonomia—orthostatic hypotension (OH), constipation, urinary dysfunction, sexual dysfunction, excessive sweating, seborrhea, and sialorrhea; (iii) sleep disorders—insomnia, rapid eye movements (REM) behavior disorder (RBD), restless legs syndrome, periodic limbs movements in sleep, and excessive daytime sleepiness; (iv) pain; (v) fatigue; and (vi) neuropsychiatric features—apathy, anxiety, panic attacks, mood disorders, hallucinations, illusions, delusions, cognitive deterioration, and ranging from mild impairment to dementia [29]. Aging may be associated with all PD nonmotor symptoms. However, the nonmotor symptoms are more frequent, more severe, and in a larger number of different aspects, in PD individuals [30, 31]. Some of these nonmotor features may be present before any motor signs are noticeable. In a study with 115 PD patients, it was shown that most frequently self-perceived symptoms in the early and very early prediagnosis phase (>2 years) were hyposmia (23.1%), musculoskeletal pain (21.9%), and depression/anxiety (14.1%). In the late prediagnosis phase (<2 years), mild motor signs, especially asymmetric bradykinesia and rest tremor, increasingly dominated the self-perception [32]. By measurement of the slope of a marker in patients who have already been diagnosed with PD, then back-extrapolating to estimate the time at which the measure crosses normal control values was estimated as a duration of premotor (prodromal) stage, averaging 3–15 years [33]. Prodromal disease refers to the stage wherein early symptoms or signs of PD neurodegeneration are present, but classic clinical diagnosis based on fully evolved motor Parkinsonism is not yet possible [34]. Extrapolation based upon progression of the UPDRS in the early stage of PD suggests an interval of ~5 years before diagnosis [35]. Recently, MDS proposed criteria and probability methodology for the diagnosis of prodromal PD [34]. Nonmotor as well as motor clinical symptoms, clinical signs, and ancillary diagnostic tests were included. Assessment of nonmotor signs as orthostatic hypotension and respiratory dysfunction (not recommended in MDS-UPDRS) were included in recently published MDS clinical diagnostic criteria for PD [36]. However, the nonmotor aspects of PD continue to be more a research issue and less the support for better management of PD patients’ treatment. Therefore, a simple objective and quantitative measure of motor and nonmotor symptoms and signs that may be used for the diagnosis of PD in the early stage may improve the quality of healthcare services and PD treatment outcomes. Moreover, subjectivity impact on the measurements with instruments that mainly use structured observation carried out by clinicians may be related to lower effectiveness of PD diagnosis, monitoring, and treatment. Patient tracking now available via mobile devices that align to the measurements that have shown the greatest ability to predict and diagnose PD and that allow assessment and monitoring of PD symptoms and signs may contribute for better therapeutic approach and increased number of years with better quality of life for the PD patients.
3 Smartphone Use for PD Diagnosis and Monitoring
3.1 Methods
A systematic review on papers related to smartphone applications for Parkinson’s disease symptoms and signs assessment and monitoring was carried out. According to Zenith’s Mobile Advertising Forecasts 2017, in 2018, 66% of individuals in 52 countries will own a smartphone, up from 63% in 2017 and 58% in 2016. Development of many software and hardware technologies for using with smartphone applications turns the smartphone as an affordable, user-friendly tool that may be used in healthcare services.
Papers published from 2013 to 2018 listed in two electronic databases—Medline via PubMed and IEEE/IET Electronic Library, IEEE Xplore—were searched by title and abstract to identify the works related to smartphone use for diagnosis and monitoring patients with Parkinson’s disease. Search was made using the following terms: “smartphone AND Parkinson’s disease,” considering 5 years (2013–2017). To be eligible for inclusion, papers were required to be available in English; include patient(s) with Parkinson’s disease; include assessment of Parkinson’s disease symptoms, signs, and treatment outcomes by using a smartphone. Articles were excluded if they were a systematic review or meta-analysis; described smartphone-based technology for treatment purposes; the articles were published in languages other than English; and were not available in full text. Each record identified through database searching was screened based on their title and abstract and a decision was made based on the criteria above on the suitability of inclusion of the papers in our analysis. The analyzed papers were categorized by technology and the symptoms or treatment outcome that was evaluated. The analysis of full-text articles has taken into account the validity, reliability, and sensitivity to change the technique used in the measurements as well as the GRADE—Grading of Recommendations Assessment, Development and Evaluation guideline (www.gradeworkinggroup.org). The GRADE working group presented its initial proposal for patient management in 2004 [37]. GRADE’s four categories of quality of evidence on diagnostic test and methods of monitoring—very low level of evidence (VLE), low level of evidence (LLE), moderate level of evidence (MLE), and high level of evidence (HLE)—represent a gradient of confidence in estimates of the effect of an assessment method on patient-important outcomes. The quality of reported methods and results was assessed by taking into account: (i) the spectrum of patients representative of who will receive the test in clinical practice; (ii) description of the selection criteria; (iii) what method and how it is used as a reference standard to assess the diagnostic accuracy of the new test; (iv) independence of a new test in relation to reference standard; (v) execution of the new test and reference standard in sufficient detail to permit its replication; (vi) influence of knowledge of reference standard results on the results of the new test; (vii) interpretation of test results based on the same clinical data available as would be available when the test is used in practice; (viii) uninterpretable/intermediate test result; (ix) withdrawals from the study [38, 39]. NVivo10 software was used to manage the data. Decision on the quality of evidence was based on: (i) number of subjects included in study and description of control condition; (ii) clinimetric properties including validity, reliability, responsiveness, and performance; (iii) influence of confounding variables and risk of bias/study limitations; (iv) consistency of results, precision of measurements, and data reporting. For reliability, we required statistically significant correlations of measurements realized using wearable technology, with those instruments considered as reference standard or commonly accepted in clinical studies. Data on Intraclass Correlation Coefficient (ICC) were considered for reliability analysis. For validity, statistically significant correlations with clinical ratings (convergent validity) were required. For responsiveness (sensitivity to change) and performance measurements, the data on overall accuracy, sensitivity, and specificity were considered. Area under the receiver operating characteristic (ROC) curve (AUC) < 0.70 was considered as showing poor accuracy, 0.71–0.80 as fair accuracy, 0.81–0.90 as good accuracy, and > 0.91 as very good accuracy. To evaluate the overall quality of each paper, using above described criteria, a Likert scale of five points was used. A score of four points was assigned for the best quality, zero if the criteria were not met, and one and two if the criteria were unclear. Using these methods, we aimed to summarize the findings related to smartphone-based tools for assessment and monitoring of Parkinson’s diseases symptoms and signs.
3.2 Results
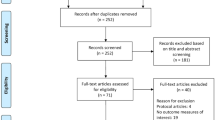
The literature search yielded 68 records, of which 60.3% were obtained from PubMed database. More studies related to smartphone use in Parkinson’s disease context were later identified by “snowballing” method in PubMed and ScienceDirect databases. However, we present in this chapter only data identified directly through searching IEEE Xplore and PubMed by the “smartphone AND Parkinson’s disease” query. A total of 26 articles (38.2%) were included in the full-text format for further evaluation (Fig. 13.1, Table 13.1). Only four papers (15.3%) were categorized by using criteria of our analysis as having moderate level of evidence (see Table 13.1) related to the use of smartphone-based tools for assessment or monitoring PD symptoms and signs. The classification of the data in the papers taking into account GRADE guideline is not intended to recommend any specific commercial product or technology. These categories only stress what technologies need more research to test their effectiveness.
Many clinicians and researchers have used smartphone technology for monitoring various aspects of PD (i.e., bradykinesia, tremor, posture, balance, and speech). Nowadays, many sensors are included in smartphones, and many applications were implemented based on the progress in sensor development, communication features, and data storing capacity of smartphone. Motor system functionalities as gait, posture, tremor, activities, speech, as well as nonmotor functions as sleep, light headedness on standing, fatigue, anxious mood, and depression can be analyzed by using smartphone applications or different wearable devices linked to smartphone.
The larger data from PD patients, using a smartphone, were acquired with the mPower applications for iPhone. Within weeks after its release, over 15,000 participants enrolled in mPower [66]. The participants were asked to respond to a subset of questions from the MDS-UPDRS and to perform short activities such as speeded tapping for 20 s on the screen of the phone or phonating a vowel for 10 s into the microphone, multiple times a day. For those participants with PD and taking medication, the timing of each recording with relation to their last dose of medication was also evaluated. A quantitative measure of movements was obtained by using algorithm that extracts different information from a signal. For example, in addition to reporting bradykinesia by measuring the total number of taps on the screen performed in 20 s, the accuracy of each tap related to targets on the screen was also analyzed to obtain information on tremor. Majority of respondents to survey on mPower app have said that their data can be used for future research, and these data were made available for other research (https://www.synapse.org/mPower). The prototype of mPower was developed, for the Android platform, at Johns Hopkins University in Baltimore, Maryland, by Andong Zhan. Hopkins-PD app is also being assessed in several trials, including one called SmartphonePD, which has been running since 2014 [67]. The app includes also contribution of John Hopkins team in speech processing. A wealth of information related to characteristics of speech in PD patients was published in the last years. The research groups with members from Czech Technical University, Brno University of Technology, and Masaryk University, Czech Republic, stand out by the comprehensive and high-quality methods used for speech research in PD patients [68,69,70,71,72,73,74]. Neurological Disorder Analysis Tool (NDAT) was developed at the Brno University of Technology, Czech Republic.
The position of microphone for speech recording varied in the range of 5–20 cm in different studies with a median at 15 cm. The calibration of the microphone recording is established using a sound-level meter placed at 15 cm from the participant’s mouth while the participant produced for 1–2 s a prolonged “ah” at 70 dBA SPL as indicated on the sound-level meter [75]. In PD speech analysis, the records include vowel phonation (i.e., short vowels pronounced with normal intensity, sustained vowels pronounced with normal intensity, sustained vowels pronounced with maximum intensity, sustained vowels pronounced with minimum intensity but not whispered); counting number from one to 20; word pronunciation; phrase sentence pronunciation; reading a text; reading a text with neutral emotion; stress-modified reading task (interrogative, imperative, and indicative sentence); diadokinetic evaluation through the rapid repetition of the syllables /pa/−/ta/−/ka/; affirmative, interrogative and exclamatory phrases; and conversation.
Many works published in the past years have presented algorithms for automatic identification and classification of speech impairments in PD patients (e.g., [68, 71, 72, 76]). The mean habitual conversational speech intensity level was found being reduced by 5 dB SPL in PD group confirming hypophonia observed in PD patients [75]. The intensity level of conversational speech was 66.86 ± 3.48 db SPL in PD patients versus 71.8 ± 2.5 db SPL in the control group [75]. Speech intensity increases as PD patients increased their walking speed [77]. Using sustained and silent vowel classification within each vowel set allowed classification of PD patients with AUC = 84.2%, sensitivity 85.7%, and specificity 81.6% [69]. By using all vowel realization, and extracting 12 features (where seven of them were obtained by empirical mode decomposition of the signals), the accuracy increased to 94.0%, the sensitivity to 96.4%, and the specificity to 89.8% [43]. Good accuracy (AUC = 88.7%), sensitivity (91.7%), and specificity (83.7%) were observed in the case of classification based on parametrization of sustained vowel [e], although many works have shown good accuracy of differentiation of PD patients by sustained vowel [a] [68]. The highest pith level was significantly lower in the PD group than in the control group, both in males (258.1 Hz vs. 353.0 Hz) and female (361.7 Hz vs. 473.9 Hz), whereas the lowest pitch level was significantly higher in the PD group (126.4 Hz) than that in the control group (110.4 Hz), only in males. Voice pith range was significantly narrower in the PD group. The lowest pitch level in the PD group was as low as that in the female control group, probably because of the tendency of mucosal edema generally found in elderly female subjects [78]. An automatic speech recognition system that assesses the intelligibility deficits of the patients by automatic classification of utterances of patients in comparison with healthy controls was implemented by [79]. The proposed system had an accuracy of up to 92% to detect Parkinson’s disease from speech.
The architecture and tests of a complex smartphone app for PD monitoring were also recently published [56, 64]. The so-called mHealth platform integrates motor and nonmotor assessment including cognitive, speech, sleep monitoring, and treatment adherence monitoring. The clinicians’ platform allows personalized prescription for medication based on periodic reports with major events. The recommendations for modification in medication, diet, physiotherapy, and activity are sent to the patient through the mHealth platform. The platform also has an app for caregivers where they receive information on PD patient symptoms as well as on patient’s adherence to the management plan. Monitoring in these studies was realized with four devices—two insoles (Moticon, Germany), a smartphone (BQ Aquaris E4.5) in the pocket, and one wristband (Microsoft Band, the USA). Based on insole sensors, information on center of foot pressure trajectory (COP), staggering, balance impairments, gait variance, foot loading, freezing of gait, and fall may be obtained. Continuous heart rate patterns (i.e., heart beat signals were acquired with optical blood flow sensor), motion (acquired by three-axes accelerometer and gyroscope), skin temperature, activity (i.e., gait and calories burned), and periods of restful and light sleep were captured with Microsoft wristband. The smartphone captures motion data (finger tapping and alternate finger tapping) and temporarily stores the raw data in the smartphone memory and sends them to the cloud for prolonged storage. This app was developed in the framework of a European Union project (https://ec.europa.eu/programmes/horizon2020/en/news/pdmanager-mhealth-platform-parkinsons-disease). Another project—PD_manager project—aimed to develop a platform that integrates motor and nonmotor assessment including cognitive, speech, sleep monitoring, and treatment adherence monitoring. It is also aimed at delivering different services for the patients, caregivers, and professionals based on this holistic approach. Once the data are processed and symptoms are assessed, a knowledge management platform will be developed to provide a Decision Support System (DSS) that suggests modifications in the medication plan [64]. The research team communicates their plan on testing PD_manager during 2017, involving 200 PD patients, from clinical centers in Ioannina, Surrey, Venice, and Rome. Patients with motor fluctuations and significant disability (Hoehn and Yahr stage 3 or greater) and with at least 3 h OFF time during the day (based on MDS-UPDRS) are considered eligible for this study. All patients will be daily evaluated according to UPDRS and will keep their 3-day diaries [64].
Many wearable technologies for vital signs and activities that are nowadays commercially available (e.g., Withings, France; Polar Electro Oy, Finland; XSens Technologies, Netherland; and FitBit, the USA) and a lot of technical and technological solutions that were presented in the last decade for wearable body sensor network can be combined within a smartphone app. Moreover, design, implementation, and adoption of smart clothes for health monitoring and healthcare are gaining weight in research, healthcare systems, and businesses. A comprehensive review of smart clothes with capability of body vital functions and activity monitoring, with potential use in neuro-motor rehabilitation, might be found in [80]. Figure 13.2 represents various sensing technologies that can be combined in a smartphone platform and their potential position on the body.
Smartphone applications can monitor PD patients’ (i) gait (i.e., by using accelerometer and/or force sensors embedded in insole or shoes and also inertial measurement unit from inside smartphone or attached to legs or waist); (ii) posture (i.e., by using inertial measurement unit or accelerometer attached to ear or lumbar region, and force sensors in shoes); (iii) vital signs (i.e., heart beat and respiration by using smartphone embedded sensors or by using wearable devices attached to the chest or arms); (iv) speech (i.e., by using a microphone from smartphone or attached microphone); (v) daily activities (i.e., by using inertial measurement unit from smartphone or wearable devices that include accelerometer and/or gyroscope, linked to smartphone). Also, smartphone apps (i) may improve communication between patients and health professionals and also between different health professionals and informal caregivers for better PD patient healthcare; (ii) may empower and engage patients in their treatment; (iii) may improve medication and therapy adherence; (iv) may provide access to educational resources related to PD and healthy life style; and (v) may contribute for reduction on PD patients’ social isolation (i.e., facilitating access to online social networks and information on nongovernmental actions related to PD patients or elderly people). These technologies may contribute to increase diagnosis accuracy and to assess fluctuating events (e.g., ON and OFF state of PD), to capture and send alerts on some events (e.g., falls, freezing of gate) and to better define therapeutic strategies. Simple examination using a smartphone-based tool, during tandem walking [21] or hand grip [81] can serve as an effective clinical assessment tool to determine changes in posture, gait, and muscle activity. Moreover, much information on different aspects of the disease can be obtained using smartphone apps. This information may contribute to differentiate PD subtypes. Nowadays, different PD subtypes were described, all having weaknesses highlighted by different clinicians. Recently, analysis of 769 PD patients with mean disease duration of 1.3 years had identified three subtypes that were characterized by (i) psychological well-being features; (ii) nontremor motor features, such as posture and rigidity; and (iii) cognitive features [82]. Their subsequent five-cluster model identified groups characterized by (i) mild motor and nonmotor disease (25.4%); (ii) poor posture and cognition (23.3%); (iii) severe tremor (20.8%); (iv) poor psychological well-being, RBD, and sleep (18.9%); and (v) severe motor and nonmotor disease with poor psychological well-being (11.7%). These subtypes are clearly more adequate than those described in the previous study based on systematic review of the 242 case files of PD patients registered at Queen Square Brain Bank for Neurological Disorders, in which also three subtypes of PD were identified: (i) earlier disease onset (25%), (ii) tremor dominant (31%), and (iii) nontremor dominant (36%) and rapid disease progression without dementia (8%) subgroups [83]. Other classification of PD patients was proposed based on the largest retrospective review of the DATATOP trial [84] considering empiric investigator-determined UPDRS characteristics. The 800 subjects with early PD were classified as exhibiting (i) postural instability and gait difficulty-predominant disease (PIGD; 55.1%); (ii) tremor-predominant disease (29.1%), or an (iii) indeterminate subtype (15.8%). This classification system has now been updated for the MDS-UPDRS motor scale [85]. The formula used to categorize PD patients as having the PIGD subtype involves calculating the ratio of tremor-related items on the MDS-UPDRS to PIGD-related items [85]. Vikas Kotagal [86] questioned these classifications, considering that few patients fit well within these discrete categories, and many patients can exhibit elements that may be characterized as transitory from one subtype to another. He suggests a classification of PD subtype taking into account a model of postural instability and gait difficulty—predominant features that emphasize the overlooked pathological influence of aging and medical comorbidities on the development of axial motor burden and postural instability and gait difficulty predominant features. Also, he proposes thinking the PD postural instability and gait difficulties not as a discrete subtype but rather as multidimensional continuum influenced by several overlapping age-related pathologies. We add to this view the suggestion for a model that takes into consideration the influence of aging and medical comorbidities not as a linear progression from early to severe stage of the disease but as a nonlinear model in which the improvements produced by medication, cues exposure therapy, and environmental factors are represented. These data will better draw upon the new source of data from digital mobile sensors.
The large inter- and intra-subject clinical variability in clinical symptoms of PD patients require development of methods for tailored technology considering PD subtype and patient current and anticipated needs. Affordability of the wearable technology, the increase in data availability related to PD patients (i.e., inclusion and analysis of many data in open databases with acquired signals from patients as Physionet database—www.physionet.org; REMPARK—www.rempark.eu; UC Irvine Machine Learning Repository—Voice Recordings and Daphnet Freezing of Gait Data Set; mPower—www.synapse.org) may contribute for better PD diagnosis and monitoring.
4 Conclusions
There are important advances on smartphone-based tools for objective, relevant, accurate information on motor and nonmotor aspects of PD. Research is still needed to overcome various limitations of nonelectronic and smartphone-based tools for assessments and monitoring of PD symptoms and signs and to build smartphone applications that may improve the quality of life of PD patients. As no high-level evidence was identified related to the use of smartphone-based applications for PD diagnosis or monitoring, more research is needed for the validation of the new technologies, methods, and their performances across various PD subtypes and degrees of disease severity, in clinical laboratory settings, in home, or community settings.
Moreover, in the near future, the development of the valid algorithms and techniques that may allow accurate detection and differentiation of PD symptoms against a background of various activities in home or community settings is required. Building high level of evidence on the effectiveness of these smartphone applications will be essential for adoption in large scale of these technologies. The information from this chapter is therefore important for developers and researchers interested in new technologies for PD assessment. It is also important for clinicians who may define new strategies for improving PD diagnosis accuracy and diagnosis of PD in the early stage, for improving the clinical care reasoning and therapeutic decisions, and for more personalized therapeutic approaches. But for all—the developers, researchers, and clinicians alike—it is essential that a pool of high-level evidence is built up through many studies that people obviously are willing to conduct.
References
Horsman J, Furlong W, Feeny D, Torrance G (2003) The health utilities index (HUI®): concepts, measurement properties and applications. Health Qual Life Outcomes 1(54):1–13
Parkinson J (1817) An essay on the shaking palsy. Sherwood, Neely, and Jones, London
Parkinson J (2002) An essay on the shaking palsy. J Neuropsichiatry Clin Neurosci 14:223–236
Goetz GC (2011) The history of Parkinson’s disease: early clinical description and neurological therapies. Cold Spring Harb Perspect Med 1(1):a008862
India. Parkinson’s disease. Available online: https://graecomuse.wordpress.com/2012/02/09/a-shaky-beginning-parkinsons-disease-in-ancient-history/
Surathi P, Jhunjhunwala K, Yadav R, Pal PK (2016) Research in Parkinson’s disease in India: a review. Ann Indian Acad Neurol 19(1):9–20
Lingor P, Liman J, Kallenberg K, Sahlmann CO, Bahr M (2011) In Diagnosis and Treatment of Parkinson’s disease, Abdul Qayyum Rana (Ed), InTech, http://cdn.intechopen.com/pdfs/20327.pdf
Quinttenbaum BH, Grahn B (2004) Quality of life and pain in Parkinson’s disease: a controlled cross-sectional study. Parkinsonism Relat Disord 10(3), 129–136
Palacios N, Gao X, Schwarzschild M, Ascherio A (2012) Declining quality of life in Parkinson disease before and after diagnosis. J Parkinsons dis 2(2):153–160
Marko-Kucsera M, Kullmann L, Palik E (2017) Measuring quality of life in individuals with Parkinson’s disease attending a self-help club: cross-sectional study in Hungary. Int J Rehabil Res 41:81–83
Willis AW, Schootman M, Evanoff BA, Perlmutter JS, Racette BA (2011) Neurologist care in Parkinson disease. A utilization, outcomes, and survival study. Neurology 77(9):851–857
Lokk J (2011) Lack of information and access to advanced treatment for Parkinson’s disease patients. J Multidiscip Healthc 4:433–439
Horak FB, Mancini M (2013) Objective biomarkers of balance and gait for Parkinson’s disease using body-worn sensors. Mov Disord 28(11):1544–1551
Wang J, Hoekstra JG, Zuo C, Cook TJ, Zhang J (2013) Biomarkers of Parkinson’s disease: current status and future. Drug Discov. Today 18(3–4):155–162
Delenclos M, Jones DR, McLean PJ, Uitti RJ (2016) Biomarkers in Parkinson’s disease: advances and strategies. Parkinsonism Relat Disord 22:S106–S110
Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G (2016) Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology 86(6):566–576
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinic-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55(3):181–184
Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ (2002) The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain J. Neurol. 125(PT(4)):861–870
Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallet M, Jankovic J, Quinn NP, Tolosa E, Zee DS (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47(1):1–9
Litvan I, Goetz CG, Jankovic J, Wenning GK, Booth V, Bartko JJ, McKee A, Jellinger K, Lai EC, Brandel JP, Venny M, Chaudhuri KR, Pearce RK, Agid Y (1997) What is accuracy of the clinical diagnosis of multiple system atrophy? A clinicopathological study. Arch Neurol 54(8):937–944
Aerts MB, Esselink RAJ, Abdo WF, Meijer FJA, Drost G, Norgren N, Janssen MJR, Borm GF, Verbeek MM (2015) Ancillary investigations to diagnose parkinsonism: a prospective clinical study. J Neurol 262:346–356
Fahn S, Elton RL (1987) Members of the UPDRS development committee. Unified Parkinson’s disease rating scale. Macmillan Healthcare Information, Florham Park
Rabey JM, Bass H, Bonuccelli U, Brooks D, Klotz P, Korczyn AD, Kraus P, Martinez-Martin P, Morrish P, van Sauten W, van Hilten B (1997) Evaluation of the short Parkinson’s evaluation scale: a new friendly scale for the evaluation of Parkinson’s disease in clinical drug trials. Clin Neuropharmacol 20:322e37
Marinus J, Visser M, Stiggelbout AM, Rabey JM, Martinez-Martin P, Bonuccelli U, Kraus PH, van Hilten JJ (2004) A short scale for the assessment of motor impairments and disabilities in Parkinson’s disease: the SPES/SCOPA. J Neurol Neurosurg Psychiatry 75:388e95
Martinez-Martin P, Benito-Leon J, Burguera JA, Castro A, Linazasoro G, Martinez-Castrillo JC, Valldeoriola F, Vazquez A, Vivancos F, del Val J, van Blercom N, Frades B (2005) The SCOPA-motor scale for assessment of Parkinson’s disease is a consistent and valid measure. J Clin Epidemiol 58:674e9
Movement disorder Society task force on rating scales for Parkinson’s disease (2003). The unified Parkinson’s disease rating scale (UPDRS): status and recommendations. Mov Disord 18(7):738–50
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N (2008) Movement Disorder Society UPDRS revision task force. Movement Disorder Society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23(15):2129–2170
MDS-UPDRS. Available online: http://www.movementdisorders.org/MDS-Files1/PDFs/MDS-UPDRS-Rating-Scales/NewUPDRS7308final.pdf
Massano J, Bhatia KP (2012) Clinical approach to Parkinson’s disease: features, diagnosis, and principles of management. Cold Spring Harb Perspect Med 2(2):a008870
Krishnan S, Sarma G, Sarma S, Kishore A (2011) Do non-motor symptoms in Parkinson’s disease differ from normal aging? Mov Disord 26:2110–2113
Khoo TK, Yarnall AJ, Duncan GW, Coleman S, O’Brien JT, Brooks DJ, Barker RA, Burn DJ (2013) The spectrum of non-motor symptoms in Parkinson’s disease. Neurology 80:276–281
Walter U, Kleinschmidt S, Rimmele F, Wunderlich C, Gemede I, Benecke R, Busse K (2013) Potential impact of self-perceived prodromal symptoms on the early diagnosis of Parkinson’s disease. J Neurol 260(12):3077–3085
Postuma RB, Lamg AE, Gagnon JF, Pelletier A, Montplaisir JY (2012) How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behavior disorder. Brain 135:1860–1870
Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, Gasser T, Goetz CG, Halliday G, Joseph L, Lang AE, Liepelt-Scarfone I, Litvan I, Marek K, Obeso J, Oertel W, Olanow W, Poewe W, Stern M, Deuschl G (2015) MDS research criteria for prodromal Parkinson’s disease. Mov Disord 30(12):1600–1609
Fearnley JM, Lees AJ (1991) Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114(PT 5):2283–2301
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30(12):1591–1599
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O’Connell D, Oxman AD, Phillips B, Schunemann HJ, Edejer T, Varonen H, Vist GE, Williams JW, Zaza S (2004) GRADE working group. Grading quality of evidence and strength of recommendations. BMJ 328:1490
Schunemann AH, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW, Kunz R, Craig J, Montori UM, Bossuyt P, Guyatt GH (2008) Rating quality of evidence and strength of recommendations. GRADE: grading quality of evidence and strength recommendations for diagnostic tests and strategies. BMJ 336:1106–1110
Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM, Kleijnen J (2003) The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 3(25):1–13
Milosevic M, Jovanov E, Milenkovic A (2013) Quantifying timed-up-and-go test: a smartphone implementation. In: Proc. IEEE international conference on body sensor networks, BSN 2013, Cambridge, MA, USA, 6–9 May 2013
Graça R, Sarmento e Castro R, Cevada J (2014) ParkDetect: early diagnosing Parkinson’s disease. In: Proc. IEEE international symposium on medical measurements and applications (MeMeA), 36th IEEE EMBC, Chicago, Illinois, USA, 26–30 August 2014
Kostikis N, Hristu-Varsakelis D, Arnaoutogloy M, Kotsavasiloglou C (2014) Smartphonebased evaluation of parkinsonian hand tremor: quantitative measurements vs clinical assessment scores. In: Proc. 10th IEEE IASME, Senigallia Ancona, Italy, 10–12 September 2014
Pepa L. Ciabattoni L. Verdini F, Capecci M, Ceravolo MG (2014) Smartphone based fuzzy logic freezing of gait detection in Parkinson’s disease. In: Proc. IEEE/ASME 10th international conference on mechatronic and embedded systems and applications (MESA), 2014, pp 1–6
Printy BP, Renken LM, Herrmann JP, Lee I, Johnson B, Knight E, Varga G, Whitmer, D (2014) Smartphone application for classification of motor impairment severity in Parkinson’s disease. In: Proc. 36th IEEE EMBC, Chicago, Illinois, USA, 26–30 August 2014
Arora S, Venkataraman V, Zhan A, Donohuc S, Biglan KM, Dorsey ER, Little MA (2015) Detecting and monitoring the symptoms of Parkinson’s disease using smartphones: a pilot study. Parkinsonism Relat Disord 21:650–653
Ayena JC, Chapwouo LD, Otis MJD, Menelas BAJ (2015) An efficient home-based risk of falling assessment test based on smartphone and instrumented insole. In: Proc. IEEE MeMeA, Torino, Italy, 7–9 May 2015
Bazgir O, Frounchi J, Habibi SAH, Palma L, Pierleoni P (2015) A neural network system for diagnosis and assessment of tremor in parkinson disease patients. In: Proc. 22nd Iranian Conference on Biomed Engineering, Tehran, Iran, 25–27 November 2015
Ferreira JJ, Godinho C, Santos AT, Domingos J, Abreu D, Lobo R, Gonçalves N, Barra M, Larsen F, Fagerbakke O, Akeren I, Wangen H, Serrano JA, Weber P, Thoms A, Meckler S, Sollinger S, van Uem J, Hobert MA, Maier KS, Matthew H, Isaacs T, Duffen J, Graessner M, Maetzler W (2015) Quantitative home-based assessment of Parkinson’s symptoms: the SENSE-PARK feasibility and usability study. BMC Neurol 15(89):1–7
Ellis RJ, Ng YS, Zhu S, Tan DM, Anderson B, Schlaug G, Wang Y (2015) A validated smartphone based assessment of gait and gait variability in Parkinson’s disease. PLoSONE 10(10):e0141694
Kim H, Lee HJ, Lee W, Kwon S, Kim SK, Jeon HS, Park H, Shin CW, Yi WJ, Jeon BS, Park KS (2015) Unconstrained detection of freezing of gait in Parkinson’s disease patients using smartphone. In: Proc. 37th IEEE EMBC, Milan, Italy, 25–29 August 2015
Kostikis N, Hristu-Varsakelis D, Arnaoutoglou M, Kotsavasiloglou C et al (2015) IEEE Journal of Biomedical and Health Informatics 19(6):1835–1842
Lan K-C, Shih W-Y (2015) Early detection of neurological disease using a smartphone: a case study. In: Proc. 9th international conference on sensing technology ICST, Auckland, New Zealand, 8–10 December 2015
Pepa L, Capecci M, Verdini F, Ceravolo MG, Spalazzi L (2015) An architecture to manage motor disorders in Parkinson’s disease. In: Proc. IEEE World Forum on Internet of Things, Milan, Italy, 14–16 December 2015
Pepa L, Verdini F, Capecci M, Pepa L, Verdini F, Ceravolo MG (2016) A smartphone based architecture to detect and quantify freezing of gait in Parkinson’s disease. Gait & Posture, 50:28–33
Assis S, Costa P, Jose Rosas M, Vaz R, Silva Cunha JP (2016) An adaptive model approach for quantitative wrist rigidity evaluation during deep brain stimulation surgery. In: Proc. 38th IEEE EMBC, Disney’s Contemporary Resort, Orlando, FL, USA, 16–20 August 2016
Cancela J, Mascato SV, Gatsios D, Rigas G, Marcante A, Gentile G, Biundo R, Giglio M, Chondrogiorgi M, Vilzmann R, Konitsiotis S, Antonini A; Arredondo MT, Fotiadis DI (2016) IEEE on behalf of the PD_manager consortium. Monitoring of motor and non-motor symptoms of Parkinson’s disease through a mHealth platform. In: Proc. 38th IEEE EMBC, Disney’s Contemporary Resort, Orlando, FL, USA, 16–20 August 2016
Contreras R, Huerta M, Sagbay G, LLumiguano C, Bravo M, Bermeo A, Clotet R, Soto A (2016) Tremors quantification in Parkinson patients using smartwatches. In: Proc. IEEE Ecuador technical chapters meeting (ETCM), Guayaquil, Ecuador, 12–14 October 2016
Lee CY, Kang SJ, Hong S-K, Ma H-I, Lee U, Kim YJ (2016) A validation study of a smartphone-based finger tapping application for quantitative assessment of bradykinesia in Parkinson’s disease. PLoSONE 11(7):e0158852
Arroyo-Gallego T, Ledesma-Carbayo MJ, Sanchez-Ferro A, Butterworth I, Mendoza CS, Matarazzo M, Montero P, Lopez-Blanco R, Purtas-Martin V, Trincado R, Giancardo L (2017) Detection of motor impairment in Parkinson’s disease via mobile touchscreen typing. IEEE Trans Biomed Eng 64(9):1994–2002
Barrantes S, Sanchez Egea AJ, Gonzalez Rojas HA, Martı MJ, Compta Y, Valldeoriola F, Mezquita ES, Tolosa E, Valls-Solle J (2017) Differential diagnosis between Parkinson’s disease and essential tremor using the smartphone’s accelerometer. PLoS ONE 12(8):e0183843
Cheng W-Y, Scotland A, Lipsmeier F, Kilchenmann T, Jin L, Schjodt-Eriksen J, Wolf D, Zhang-Schaerer Y-P, Garcia IF, Siebourg-Polster J, Soto J, Verselis L, Martin-Facklam M, Boess F, Koller M, Grundman M, Monsch A, Postuma R, Ghosh A, Kremer T, Taylor K, Czech C, Gossens C, Lindemann M (2017) Human activity recognition from sensor-based largescale continuous monitoring of Parkinson’s disease patients. In: Proc. IEEE/ACMinternational conference on connected health: applications, systems and engineering technologies, CHASE, Philadelphia, Pennsylvania, USA, 17–19 July 2017
Lee W, Evans A, Williams DR (2017) Subjective perception of sleep benefit in Parkinson’s disease valid or irrelevant? Parkinsonism Relat Disord 42:90–94
Stamate C, Magoulas GD, Kueppers S, Nomikou E, Daskalopoulos I, Luchini MU, Moussouri T, Roussos G (2017) Deep learning Parkinson’s from smartphone data. In: Proc. IEEE international conference on pervasive computing and communications PerCom, Kona, Hawaii, USA, 13–17 March 2017
Tsiouris KM, Gatsios D, Rigas G, Miljkovic D, Seljac BK, Bohanec M, Arredondo MT, Antonini A, Konitsiotis S, Koutsouris DD, Fotiadis D (2017) PD_Manager: an mHealth platform for Parkinson’s disease patient management. Healthcare Technology Letters 4(3):102–108
Zhang YN (2017) Can a smartphone diagnose Parkinson disease? A deep neural network method and telediagnosis system implementation. Parkinson’s disease 2017:6209703, 1–11
Trister AD, Dorsey ER, Friend SH (2016) Smartphones as new tools in the management and understanding of Parkinson’s disease. NPJ Parkinson’s disease 2:16006
Gravitz L (2016) Monitoring gets personal. Nature 538:S8–S10
Mekyska J, Galaz Z, Mzourek Z, Smekal Z, Rektorova I, Eliasova I, Kostalova M, Mrackova M, Berankova D, Faundez-Zanuy M, L’Opez-de-Ipina K, Alonso-Hernandez JB (2015) Assessing progress of Parkinson’s disease using acoustic analysis of phonation. In: Proceedings of international work conference on bio-inspired intelligence IWOBI, Donostia-San Sebastian, Spain, 9–12 June 2015
Smekal Z, Mekyska J, Galaz Z, Mzourek Z Rektorova I, Faundez-Zanuy M (2015) Analysis of phonation in patients with Parkinson’s disease using empirical mode decomposition. In: Proceedings of ISSCS, Iasi, Romania, 9–10 July 2015
Orozco-Arroyave JC, Vasquez-Correa JC, Honig F, Arias-Londono JD, Vargas-Bonilla JF, Skodda S, Rusz J, Noth E (2016) Towards an automatic monitoring of the neurological state of Parkinson’s patients from speech. In: Proc. IEEE international conference on acoustic, speech and signal processing ICASSP, Shanghai, China, 20–25 March 2016
Arias-Vergara T, Vasquez-Correa JC, Orozco-Arroyave JR, Vargas-Bonilla JF, Haderlein T, Nöth E (2006) Gender–dependent GMM–UBM for tracking Parkinson’s disease progression from speech. In: Proc. speech communication, 12th ITG Conference on Speech Communication, Paderborn, Germany, 5–7 October 2016
Arias-Vergara T, Vasquez-Correa JC, Orozco-Arroyave JR, Vargas-Bonilla JF, Haderlein T, Nöth E (2006) Gender–dependent GMM–UBM for tracking Parkinson’s disease progression from speech. In: Proc. speech communication, 12th ITG Conference on Speech Communication, Paderborn, Germany, October 5–7, 2016
Galaz Z, Mekyskaa J, Mzoureka Z, Smekala Z, Rektorovab I, Eliasovab I, Kostalovac M, Mrackovab M, Berankovac D (2015) Prosodic analysis of neutral, stress-modified and rhymed speech in patients with Parkinson’s disease. Comput Methods Prog Biomed 127:301–317
Galaz Z, Mzourek Z, Mekyska J, Smekal Z, Kiska T, Rektorova I, Orozco-Arroyave J, Daoudi K (2016) Degree of Parkinson’s disease severity estimation based on speech signal processing. In: Proc. 39th International Conference on Telecommunications and Signal Processing, Vienna, Austria, 27–29 June 2016
Dykstra A, Adams SG, Jog M (2015) Examining the relationship between speech intensity and self-rated communicative effectiveness in individuals with Parkinson’s disease and hypophonia. J Commun Disord 56:103–112
Zhang HH, Yang L, Liu Y, Wang P, Yin J, Li Y, Qiu M, Zhu X, Yan F (2016) Classification of Parkinson’s disease utilizing multi-edit nearest-neighbor and ensemble learning algorithms with speech sample. Bio Med Eng OnLine 15(1):1–22
McCaig CM, Adams SC, Dykstra AD, Jog M (2016) Effect of concurrent walking and interlocutor distance on conversational speech intensity and rate in Parkinson’s disease. Gait Posture 43:132–136
Ikui Y, Nakamura H, Sano D, Hyakusoku H, Kishida H, Kudo Y, Joki H, Koyano S, Yamauchi A, Takano S, Tayama N, Hirose H, Oridate N, Tanaka F (2015) An aerodynamic study of phonations in patients with Parkinson disease (PD). J Voice 29(3):273–280
Vasquez-Correa JC, Orozco-Arroyave JR, Noth E (2016) Word accuracy and dynamic time warping to assess intelligibility deficits in patients with Parkinson’s disease. In: Proceedings of 21st Symposium on Signal Processing, Images and Artificial Vision, Bucaramanga, Colombia, August 31–September 2, 2016
Postolache G, Carvalho H, Catarino A, Postolache OA (2016) Smart clothes for rehabilitation context technical and technological issues. In: Postolache OA, Mukhopadhyay SC, Jayasundera KP, Swain AK (eds) Sensors for everyday life: healthcare settings, vol 22. Springer international publishing AG, Berlin, pp 185–219
Jones GR, Roland KP, Neubauer NA, Jakobi JM (2017) Handgrip strength related to long-term electromyography: application for assessing functional decline in Parkinson disease. Arch Phys Med Rehabil 98(2):347–352
Lawton M, Baig F, Rolinski M, Ruffman C, Nithi K, May MT, Ben-Shlomo Y, Hu MTM (2015) Parkinson’s disease subtype in the Oxford Parkinson disease Centre (OPDC) discovery cohort. J Parkinson’s disease 5:269–279
Selikhova M, Williams DR, Kempster PA, Holton JL, Revesz T, Lees AJ (2009) A clinico-pathological study of subtype in Parkinson’s disease. Brain 132:2947–2957
Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, Huber S, Koller W, Olanow C, Shoulson I (1990) Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson study group. Neurology 40:1529–1534
Stebbins GT, Goetz CG, Burn DJ, Jancovic J, Khoo TK, Tilley BC (2013) How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’disease rating scale. Mov Disord 28:668–670
Kotagal V (2016) Is PG a legitimate motor subtype in Parkinson disease? Ann Clin Transl Neurol 3(6):473–477
Acknowledgment
This work was supported by Fundação para a Ciência e a Tecnologia, project PTDC/DTT-DES/6776/2014, and Instituto de Telecomunicações, Portugal.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Postolache, G., Postolache, O. (2019). Smartphone Sensing Technologies for Tailored Parkinson’s Disease Diagnosis and Monitoring. In: Paiva, S. (eds) Mobile Solutions and Their Usefulness in Everyday Life. EAI/Springer Innovations in Communication and Computing. Springer, Cham. https://doi.org/10.1007/978-3-319-93491-4_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-93491-4_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-93490-7
Online ISBN: 978-3-319-93491-4
eBook Packages: EngineeringEngineering (R0)