Abstract
Earth primary productivity reflects the balance between two important biological processes: photosynthesis and respiration (Atkin et al. 2015; Niinemets 2016). Photosynthesis (A) refers to the assimilation of the atmospheric CO2 and its conversion into sugars, the first basic organic compounds entering the metabolism. This process of CO2 fixation uses the sun radiation as the energy source, and water as the electron donor, which in turn releases oxygen in the atmosphere. Dark respiration (R D) or mitochondrial respiration (Atkin and Tjoelker 2003) employs the products of photosynthesis through the glycolysis (cytosol), the tricarboxylic acid cycle (TCA, matrix of mitochondria) and the electron transport rate chain (ETC, inner membrane mitochondria) to produce ATP and carbon skeletons needed for growth, cell maintenance, and other essential cellular processes. During the process of respiration, O2 is consumed, and CO2 is released to the atmosphere within the same order of magnitude than photosynthesis (Jansson et al. 2010), which highlights the importance of considering this process in the leaves, whole-plant and global models of carbon, water, and oxygen fluxes (Valentini et al. 2000; Canadell et al. 2007; Atkin et al. 2015). The velocity and extent of both processes can be assessed at the leaf level using infrared-based gas exchange analysers.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Infrared Gas Analyzer (IRGA)
- Electron Transport Rate (ETR)
- Apparent Photosynthesis
- Leaf Interior
- Mesophyll Conductance
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
1.1 Primary Carbon Metabolism and Gas Exchange in Leaves
Earth primary productivity reflects the balance between two important biological processes: photosynthesis and respiration (Atkin et al. 2015; Niinemets 2016). Photosynthesis (A) refers to the assimilation of the atmospheric CO2 and its conversion into sugars, the first basic organic compounds entering the metabolism. This process of CO2 fixation uses the sun radiation as the energy source, and water as the electron donor, which in turn releases oxygen in the atmosphere. Dark respiration (R D) or mitochondrial respiration (Atkin and Tjoelker 2003) employs the products of photosynthesis through the glycolysis (cytosol), the tricarboxylic acid cycle (TCA, matrix of mitochondria) and the electron transport rate chain (ETC, inner membrane mitochondria) to produce ATP and carbon skeletons needed for growth, cell maintenance, and other essential cellular processes. During the process of respiration, O2 is consumed, and CO2 is released to the atmosphere within the same order of magnitude than photosynthesis (Jansson et al. 2010), which highlights the importance of considering this process in the leaves, whole-plant and global models of carbon, water, and oxygen fluxes (Valentini et al. 2000; Canadell et al. 2007; Atkin et al. 2015). The velocity and extent of both processes can be assessed at the leaf level using infrared-based gas exchange analysers.
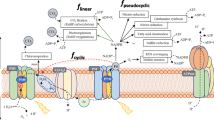
In vascular plants, photosynthesis is a complex interaction between biophysical processes and chemical reactions. Leaves are specialized photosynthetic tissues where the CO2 from the atmosphere can be trapped into the leaf through the stomata to the substomatal cavity, subsequently crossing the mesophyll tissues that comprise several different cell structures (cell wall, plasmalemma, cytosol, chloroplast membrane, stroma), to finally reach the carboxylation sites of the RubisCO (Flexas et al. 2016). RubisCO (ribulose-1,5-bisphosphate carboxylase/oxygenase, EC 4.1.1.39) is the primary enzyme with a central role in photosynthesis, responsible for the CO2 uptake by photosynthetic organisms. It is important to consider that this enzyme presents two different catalytic activities: carboxylation and oxygenation (i.e., fixation of both CO2 and O2). This result in direct competition between O2 and CO2 for the final reaction with RuBP (ribulose-1,5-bisphosphate) and the beginning of the Calvin-Benson cycle (Farquhar et al. 1980). While the carboxylase (fixation of CO2) will end-up in sugar production, the oxygenase activity of Rubisco starts the photorespiration cycle that ends up in the net release of CO2 (instead of CO2 fixation). That is why photorespiration is considered as a counter-efficient process for the leaf regarding carbon balance: the oxygenase decreases RubisCO availability for the carboxylase process, it consumes electrons captured from light radiation, and it releases previously fixed carbon to the atmosphere (Galmés et al. 2005, and references therein).
In consequence, the leaf in vivo net CO2 assimilation (A N), that is measured using an infrared gas analyser (IRGA), is not a true photosynthesis rate, but the net balance between the rates of a carbon flux entering the leaf (the gross photosynthesis) and leaving the leaf simultaneously (the photorespiration and the mitochondrial respiration in the light). The combination of these three processes determines the leaf carbon balance (Valentini et al. 1995; Flexas et al. 2002, 2016) that drives primary productivity for any terrestrial ecosystem. This fact highlights the importance of the gas-exchange techniques when considering carbon fluxes in the context of climate change, agriculture, forestry and the understanding of natural ecosystems.
2 Theoretical Approach
2.1 Measuring Leaf Gas-Exchange: Basic Concepts and Measurements
The key point of these measurements is based on the tight relationship between CO2 assimilation and water losses by transpiration (E) in the leaves through the stomata. By taking profit of this relationship, both specifically responsive infrared wavelengths for CO2 and water vapour were used to develop sensors as the basis of the infrared gas-exchange analyzers (IRGAs). Simultaneous monitoring of the changes in CO2 and water vapour across a leaf, provides a precise and integrated in vivo measurement of the net photosynthesis and the transpiration in illuminated samples, and also of the mitochondrial respiration rate and the residual transpiration in darkened samples (Field et al. 1989, 2000; Flexas et al. 2012b; Evans and Santiago 2014; Montero et al. 2016).
From the 1980s, gas-exchange analysers have become a common tool for plant ecophysiologists, and especially when the first models of “portable” equipment were developed, opening the possibility to measure plants in field conditions (Field et al. 1989, 2000; Long et al. 1996). There are two main approaches available, open and closed path gas-exchange systems. In closed systems, there is no net flow entrance of air in the chamber, and flux estimations are based on the variation of the gas concentration over time inside the closed circuit, which includes a cuvette with a leaf inside. Instead, the open pathways systems have a net flow of air entering and exiting the system, and the estimations are based on differences of concentration of two split fractions of air, one fraction having flown freely from the entrance to an IRGA, and the other one having passed through the leaf cuvette chamber into a second IRGA (Gallé and Flexas 2010). In this chapter, we will use as an example an open system (Fig. 4.1), the LI-COR 6400XT (LI-COR Inc., NE) portable gas-analyser coupled with chlorophyll fluorescence system.
In the LI-6400 Open Flow-Through Systems (bottom), the gas stream is split up into sample and reference flow, which continuous differential measurements without alternating; moreover, IRGAs are located in the head, so that gas measurements take place in the same space in which leaf is located, thus avoiding delay between response and measurement. For comparison of advantages with respect to previous models: (top) One absolute IRGA, switch between in and out, discontinuous measurements; (middle) two absolute IRGAs, continuous measurements, long tubing; (bottom) two absolute IRGAs, continuous measurements, IRGA in the head, shorter tubing
Basically, the open system of the LI-6400 works as follows (Fig. 4.1): a pump forces the air flow to pass through a circuit, where air is split in two: a fraction goes straight to an IRGA, and the other fraction goes through a second IRGA after passing through the measuring chamber or cuvette with a leaf inside. [CO2] and [H2O] are measured in both IRGAs, the reference one reflecting the concentrations entering the chamber (C e and W e), and the sample one determining the concentrations after interaction with the leaf and exiting out of the leaf chamber (C o and W o).
The differences in [CO2] and [H2O] between these two measurements are used to determine the leaf net assimilation and transpiration rates (Fig. 4.2).
Scheme of the gas-exchange measurement chamber with the main calculations. Photosynthesis (A) and transpiration (E) are calculated as differences in CO2 and H2O concentration, based on the readings of the IRGAs in the Reference and Sample circuits (mass balance equations), with F the flow and L a as leaf area inside the measurement chamber. The mass-balances fluxes are hypothesised to reflect the pure physiological fluxes faithfully. From this basics equations, it can be further calculated the stomatal conductance (g s) employing Tleaf (leaf temperature) and CO2 concentration at the sub-stomatal cavity (Ci)
CO2 and water vapour concentrations can be regulated in the equipment. If the user needs a determined concentration of any of the two gases, these can be decreased or even fully removed by passing air through different chemicals. For example, CO2 is absorbed by soda lime (Ca(OH)2 and NaOH granulates), and water vapour by drierite (CaSO4) or silica gel (caution: before manipulating these chemicals check their safety datasheets carefully!). CO2 concentration can be automatically regulated using the sensor readings using a mixer that controls the disposable compressed CO2 gas cylinders to provide the required CO2 air concentration into the already CO2-free air (after previous full depletion using soda lime). In the LI-6400 it is not possible to increase the concentration of water vapour automatically using an analogous system; otherwise, with the new equipment LI-COR 6800 (LI-COR inc., NE), and as well the Walz GSF-3000 (Walz, Effeltrich), water vapour can also be automatically controlled regulating the gas concentration employing desiccant and humidifier chemicals integrated into the air pipe system with electromechanical valves.
CO2 and water vapour leaf fluxes are measured by the difference between the reference and sample circuits (Figs. 4.1 and 4.2) as early proposed by Gaastra (1959) and then modified like in von Caemmerer and Farquhar (1981):
where C e and C o are the CO2 mole fraction at the chamber entrance and output, respectively; ue is the incoming flow air (mol air s−1), L a is the leaf area surface (m2), and E is the transpiration rate (mol H2O m−2 s−1). IRGAs can be used as well to measure leaf dark respiration when the leaf is under darkness conditions: photosynthesis and photorespiration are both suppressed by the absence of light through the chloroplast electron transport chain. When measuring leaf respiration with an IRGA, the “photosynthesis” measured by the device will appear as negative (the system applies the same equation under light or dark conditions). In consequence, that flux must be interpreted as a positive CO2 flux corresponding to the leaf respiration, driven by the mitochondria in darkness.
Stomatal conductance to water vapour (g s) can be calculated from E, by using the leaf temperature – wich is measured by a thermocouple placed inside the cuvette (caution: before each use, it should be tested that the thermocouple is working, well calibrated, and in close contact with the leaf to be measured!) – and accounting for the boundary layer effect. This calculation assumes that within the substomatal cavity the relative humidity is around 100%. This allows to calculate the W i ([H2O] in the sub-stomatal cavity), that in turn allows the estimation of the conductance of the water pathway using the first Fick’s law of diffusion: g sw = E/(W i – W a ) (with W a the [H2O] in the atmosphere – chamber in this case) (Fig. 4.2). Physiologists commonly use g s more than E, since E is sensible to W a (a variable atmospheric condition) and this is not a biological process. In turn, g s is a full biological process mostly reflecting the degree of stomatal aperture (Osmond et al. 1979). Keep in mind that g s can be affected by external factors, but the leaf itself actively controls it. Stomatal conductance can be expressed in terms of H2O (g sw) or CO2 (g sc), with g sw = 1.6 g sc. The 1.6 factor denotes the difference in diffusivity in the air of the two molecules. This allows to calculate the CO2 concentration at the substomatal cavities (C i), applying again the first Fick’s law of diffusion with C i = C a – A N/g sc where C a is the atmospheric [CO2] (inside the chamber in this case) (Fig. 4.2) (Gaastra 1959; von Caemmerer and Farquhar 1981; Gallé and Flexas 2010).
From these measurements, another interesting parameter can be calculated, the water use efficiency (WUE), which represents the balance between carbon gains and the associated costs in water. So, employing instantaneous gas exchange measurements, it is easy to directly estimate it using the ratio between A N and either E (so-called instantaneous WUE) or g s (intrinsic WUE). This parameter drives plant productivity and the interaction of the plant with a changing environment, becoming highly important to improve irrigation and crop breeding strategies to face with the climatic change challenge threat to agriculture in the semi-arid regions (Gago et al. 2014).
These are the basic measurements that can be performed with a gas-exchange system. It is essential to know how the system works, the theory behind and its practical limitations to guarantee the precision and quality of your data. Moreover, these measurements take more relevance when considering that they are at the basis of many other procedures that are used to characterize the leaf physiology completely. We can also recommend excellent practical protocols for gas-exchange and fluorescence measurements already published as Evans and Santiago (2014) “Gas exchange using a LI-COR 6400”, or the Licor LI-6400XT Manual itself.
2.2 Combining Chlorophyll Fluorescence and Gas-Exchange: Opportunities for Deep Photosynthesis Characterization
As mentioned previously, photosynthesis is driven thanks to the energy that comes from the sun. Leaves first capture the photon radiation by the chlorophyll molecules; then, this energy can be transferred through three different main processes: (1) used in photochemistry, where the energy captured is employed in the photosynthetic process; (2) dissipated by an exothermic reaction (heat dissipation); and (3) re-emitted in a longer wavelength (i.e., less energetic radiation than that received), the so-called chlorophyll fluorescence. These processes work in competition, so any decrease in one of them directly imply increases in some of the other two. This theory is employed to estimate the yield of chlorophyll fluorescence, capturing information about photochemistry and heat dissipation.
Currently, theoretical frameworks basically rely on the so-called “Kautsky effect”, early observed when a leaf transferred from dark to light has its fluorescence that rapidly increases (within 1 s or so) and then slowly decreases to steady state. This pattern can be explained as follows: In the dark, heat dissipation processes depending on enzyme activity (e.g., those related to the xanthophyll cycle) are disabled (e.g., Murchie and Niyogi 2011; Demmig-Adams et al. 2012). But chlorophyll fluorescence and the early steps of photochemistry (i.e., light capture by antenna chlorophylls, charge separation in the reaction center, and most of the electron transport in the thylakoid) are active because being physical and not enzymatic processes. Photochemistry can absorb a reduced amount of the incoming energy, and therefore all the remaining leads to a rapid large increase of chlorophyll fluorescence from a basal level (F o) up to its maximum capacity (F m). Then, as the light is kept on, the RubisCO and other enzymes become activated, as well as the xanthophyll cycle-related heat dissipation. Since these two processes compete with chlorophyll fluorescence for the use of the energy absorbed by chlorophylls, their progressive light-induced activation leads to a subsequent slow decrease of chlorophyll fluorescence that will relax until reaching some steady-state value (F s). Such effect reflects the competitive balance between the three processes that depend on the light intensity as well as on the physiological status of the leaf. If a short but intense pulse of light is applied now, photochemistry will become rapidly saturated, and chlorophyll fluorescence will rise again within 1 s or so, but still a lower value than Fm (referred as F m ′). This is because, contrary to darkness, heat dissipation under light condition is competing with chlorophyll fluorescence for the use of the light energy.
Several parameters were defined to determine the PSII photochemistry status, and probably some of the most useful are the following: the maximum efficiency of the PSII (F v /F m = (F m – F o)/F m)) (dark conditions); the quantum efficiency of the PSII photochemistry (light conditions) (ΦPSII = (F m ′ – F s)/F m ′); the fraction of open PSII reaction centers, the photochemical quenching (qP = (F m ′ – F s)/(F m ′ – F o); and the thermal dissipation of energy excess (non photochemical quenching NPQ = (F m – F m ′/F m ′) (see Demmig-Adams et al. 1996 and Maxwell and Johnson 2000 for further information). In a dark-adapted non-stressed leaf F v /F m should range around 0.8, this is the maximum potential fraction of the energy that can be converted in photochemistry. Any decrease of this value would come from either an increase in thermal dissipation (non-photochemical quenching) or photodamage to PSII, indicating different types of photoinhibition process (Genty et al. 1990; Osmond and Förster 2006).
It was reported that the ΦPSII in a light-adapted leaf is a proxy for PSII photochemistry, i.e., for the quantum efficiency of electron transport at the level of PSII. Thus, ΦPSII can be used to estimate the photosynthetic linear electron transport rate (ETR, Genty et al. 1990; Laisk and Oja 2018), with ETR = ΦPSII * PARi * α * β, where PARi is the incident photosynthetically active light radiation (μmol photons m−2 s−1), β the fraction of absorbed light distributed between PSII and PSI, and α the leaf absorbance. Note that ΦPSII and PARi can be directly measured by any gas exchange system coupled to a Fluorometer (like a LI-COR 6400 equipped with an LCF). It also gives a direct estimation of the ETR. Using the by-default parameterization (found in the literature), with β = 0.5 and α = 0.87, the α * β product value will be 0.435, but it is highly recommended to perform a direct estimation of this term. First, because ETR estimates are highly sensitive to the α * β value (and thus all the subsequent variables calculated from ETR, like g m −the mesophyll conductance to CO2,), and, secondly, because they can largely vary among species and conditions, especially α (see Pons et al. 2009 and Martins et al. 2013 for a detailed method description). Note that leaf absorbance can be measured independently employing a spectroradiometer and an integration sphere; nevertheless, there is no robust easy-to-use method for independent estimation of β. The best way to estimate the value of α * β is measuring the relationship between ΦPSII and ΦCO2 through light or CO2 response curves under non-photorespiratory conditions (Valentini et al. 1995; Martins et al. 2013). This procedure will be described below (Sect. 3.2.4).
2.3 Modeling Gas-Exchange: Going Deeper in the Leaf Photosynthetic Characterization
Combining gas-exchange with fluorescence technologies allows going deeper in the leaf physiology understanding. Some of the most important in vivo information that can be retrieved or estimated are: (1) the mesophyll conductance – g m (that directly restricts the CO2 available for the RubisCO at the chloroplast stroma site); (2) the rate of photorespiration (that is an important sink of energy and carbon loss for the leaf); (3) relative photosynthetic limitations occurring for a given leaf (stomatal, mesophyll and biochemical limitation; see Grassi and Magnani 2005); and (4) photosynthetic capacity parameters originally established in the model of Farquhar et al. (1980), i.e., the maximum velocity of carboxylation by RubisCO (V cmax), the maximum capacity for electron transport rate and driving the Calvin cycle (J max) and the triose-phospate use (TPU).
-
1.
Mesophyll conductance estimation is based on combined gas-exchange and chlorophyll fluorescence data: this method was established by Harley et al. (1992) and is based on the basic photosynthetic stoichiometry, i.e., that, in the absence of photorespiration, 4 electrons should be processed in the thylakoid electron transport chain to reduce two molecules of NADPH, which are required to fix one CO2 molecule in a carboxylation event. The idea is to find an estimate of C c ([CO2] at the carboxylation site in the chloroplast stroma), and then apply the Fick’s law of diffusion again with g m = A N/(C i – C c). The complete equation to estimate g m is so:
where R day is the mitochondrial respiration in light and Γ * is the CO2 compensation point in the absence of R day, and it accounts for the fact that measurements are performed under photorespiratory conditions. Keep in mind that even if a model is robust, its correct parameterization (i.e., attributing a value to each parameter in the equation) is crucial to obtain reliable results. So, several methods allow estimating R day, two of them being the Yin et al. (2011) method (requires a light response curve coupled with a Fluorometer), or the more simple Niinemets et al. (2005, 2009) approach, using an empirically-based agreement that R day equals to half R dark. Γ * can also be estimated via several methods: or by gas exchange, that needs two A/Ci curves each performed at 21% and 2% [O2] (see Yin et al. 2009), or by in-vitro estimations of the RubisCO kinetics, from which Γ * is derived (see Galmés et al. 2017 for an extensive comparison of the methods, and Hermida-Carrera et al. 2016 for RubisCO kinetics database in crops). Note that other methods were developed along the years for those two parameters (like the “Kok” method for R day, ot the “Laisk” method for both R day and Γ *), but these are now considered non-reliable. Recent literature is now comparing the different methodologies to establish the robustness of each one (see Walker et al. 2016; Galmés et al. 2017; Walker et al. 2017).
-
2.
Estimation the photorespiration: this was established by Epron et al. (1995), based again on the basic stoichiometry of electrons required for a carboxylation or an oxygenation event: Rp = 1/12[ETR – 4(AN + Rday)].
-
3.
Estimating the relative limitations to photosynthesis: this approach was first proposed by Grassi and Magnani (2005), based on an earlier model by Jones (1985), in which it was not considered the limitation by mesophyll conductance. This kind of analysis can be useful to compare different species (e.g., Carriquí et al. 2015), or compare the photosynthetic performance and limitation under different stressed environments (e.g., Gallé et al. 2009). The aim is to decompose the different factors that limit the photosynthesis at a given moment and to establish a hierarchy of those different limitations. Two of them concern the diffusive limitation: the stomatal (l s) and the mesophyll (l m) limitation. They come from the fact that the A N flow is considered a continuous flow restricted by two resistances (1/conductance) in series, since we assume that A N = g s (C a – C i) = g m (C i – Cc). The third limitation comes from the carboxylation itself (l b). So, we can establish the following equations based on Grassi and Magnani (2005):
where g tot is the total conductance to CO2 between the leaf surface and the carboxylation sites (1/g tot = 1/g s + 1/g m). Note that this model has been improved by Buckley and Díaz-Espejo (2015), but the complexity of the latter is so that, in many cases, parameterizing this model would not be feasible, for which the Grassi and Magnani approach is still useful.
-
4.
Retrieve the biochemical photosynthetic parameters of a leaf: this approach was used by Farquhar and colleagues at the time to establish their extendedly used model (Farquhar et al. 1980). Their idea consisted in seeing the measured photosynthetic rate as if it was a ‘reaction velocity’ in response to ‘reaction substrate availability’ (approached by the C i estimated during IRGA measurements). In this way, by performing gas exchange meaurements along a CO2 gradient (i.e., the A-C i curves), it was possible to apply well known and simple enzyme-reaction equations to estimate the maximum carboxylation by the RubisCO (V cmax, from the portion of the curve where the substrate CO2 is most limiting, under the rule of the Michaelis-Menten law for the case of inhibitory competition by substrate O2), the maximum capacity for electron transport (J max, from the CO2 non-limiting region of the curve, reflecting a limitation by RuBP regeneration and, thus, the activity of photochemistry and the Calvin cycle), and the rate of triose-phosphate utilization (TPU, from the saturated part of the curve at very high [CO2]). All these parameters can be extracted from the analysis of a complete A-C i curve performed at ambient O2 and under saturating light. However, as C i does not reflect the actual CO2 concentration at the chloroplast stroma (C c) it is better to apply the model after considering g m, i.e., to A-C c curves (Flexas et al. 2012a). It can be done using gas exchange and chlorophyll fluorescence estimates of g m and C c as outlined in previous sections, or directly based on pure gas exchange measurements. For the latter, Ethier and Livingstone (2004) modified the equations use for the fitting of the measured data against the theoretical model (to retrieve V cmax, J max, and TPU). They transformed the Farquhar’s model original equation into several non-rectangular hyperbolae, improving the quality of the estimated parameters. They also included g m into their model (originally considered as infinite in the 1980s Farquhar’s model), allowing a g m estimation without the employment of the chlorophyll fluorescence method (see Ethier and Livingstone 2004). The most interesting point of this method is that it provides a second independent approach to estimate g m that can be useful to reinforce its estimation through the other methodologies mentioned. Several tools have been developed to facilitate this purpose, like the Excel sheet of Sharkey et al. (2007; Sharkey 2016).
Other useful physiological parameters can be extracted from gas exchange and fluorescence measurements. Other Excel tools exist, like that provided by Bellasio et al. (2016). These authors propose a systematic analysis of light and CO2 response curves (under ambient and low O2 concentration) and, in a step-by-step approach, the tool provides: R day, initial PSII (photosystem II) photochemical yield, initial quantum yield for CO2 fixation (ΦCO2), fraction of incident light harvested by PSII (α * β product), initial quantum yield for electron transport, electron transport rate (ETR), photorespiration, stomatal limitation, RubisCO (ribulose 1·5- bisphosphate carboxylase/oxygenase) rate of carboxylation and oxygenation, RubisCO specificity factor, g m, light and CO2 compensation point, and RubisCO apparent Michaelis–Menten constant and V cmax (RubisCO CO2-saturated carboxylation rate).
3 Practical Approach: Hands-on Protocol
3.1 Preparing Your Gas-Exchange Analyser for Precise Measurements
At the beginning of the preparation of your equipment, the most important action is the calibration of the sensors to ensure that your device can reproduce reliable results. For this purpose, regular checks of the different sensors of the gas exchange system are crucial; but all sensors do not require the same checking frequency.
At the beginning of this “hands-on protocol”, we split the “checks” of your equipment depending on the frequency that it is recommended to apply them. First, we propose “daily checks” the ones that need absolutely to be done every day before start any measurement, and second “long-term” checks to ensure quality maintenance of the equipment. For this purpose, this “hands-on protocol” employs as an example the LI-COR 6400XT equipped with the fluorescence chamber. All the IRGAs are based on the same concepts, so users of others equipment can also find useful the recommendations that we described below. This protocol is intended to be complementary to the manufacturer’s manual (LI-COR 2012), so we strongly recommend a careful reading of the manual to guarantee the proper utilisation of the equipment. Figure 4.3 will help you to understand what you are checking, where, and why.
3.1.1 Daily Checks Before Measuring
These checks consist of evaluating the most important sensors of the gas exchange system to give the best chance to perform good and reliable measurements.
First of All: “Check Around”
-
Ensure to plug every cable and tube in its right position. For example, the Reference and Sample tubes on the console side have the same connector, check that the black taped tube (Sample) is on its good (Sample) position.
-
Check that the exhaust tube (right-angled semi-transparent 10 cm tube, bottom-side of the chamber) is in place.
-
Check that the gaskets of the chamber are in good state and overlap each other perfectly when you close the leaf measurement chamber.
-
When you are sure that everything is at its place, go to next step.
-
1.
Start the machine, and then Scrub the desiccant and soda lime tubes. Meanwhile, the system is opening and you are doing the other checks, the pump system will empty the airflow circuit of CO2 and water. You may save some time at the moment to check the Zero of the IRGA. You can also place CO2 cartridge to fill the CO 2 mixer if needed.
-
2.
Check the “Match Valve” test visually during the opening sequence of the system (the downside of the head). You can also directly activate it in the measurements menu “Match” button to check its good functioning. This is what happens:

-
3.
Check the Zero of the flow meter. For that, go to “Calib Menu” and select the “Flow meter zero...” wait for the countdown, then the values in mV should be within 1 mV from zero. Adjust it in consequence, but keep in mind this parameter is not likely to change day by day.
-
4.
Check the “ Max ” of the flow. Go to “New Measurements”, and fix the flow to 1000 μmol s−1, then the reading value should be >700 μmol s−1 (menu b). On the contrary, a resistance on the flow is happening through the air circuit: check the air mufflers (white filters) there is two inside the desiccant and two in the soda lime tube. They are very likely to provoke this problem. If ok, fix back the flow to 500 μmol s−1 (for 6 cm2 chamber) or 300 μmol s−1 (for 2 cm2 chamber) as standard measurement flow rates.
-
5.
Check the “ Zero ” of the thermocouple. To do that, disconnect the thermocouple from the LI-6400 head (purple 2-pin connector, see the manual for more information), then the Tleaf (leaf temperature value) value must be close by ± 0.1 °C from the Tblock (block temperature value) value (menu h). If not, this can be adjusted with the small screw of the downside of the head, close to the Reference and Sample tubes connection (see manual for further information). Adjustment of Tleaf zero must be made in stable temperature condition and after a warm-up of the system (ideally ~30 min).
-
6.
Check the sensitivity of the thermocouple. Gently touch the thermocouple with the tip of your finger and check the proper variation in Tleaf (menu h, it must increase). Check later also that each Tblock, Tair, and Tleaf gives reasonable values. For your next measurements set the Tblock or Tleaf as desired.
-
7.
Set the light “ON”, usually with 90% red and 10% blue. Check that LEDs are active, and pay attention to the purplish color that ensures that blue LEDs are active. Check that the reading value of PAR in (menu g) agree with your settings (chamber must be closed).
-
8.
Check the leaf fan (or mixing fan). Change its value (Function Key 1, f1) from 5 to 0 to stop it, and then set it up again at 5. If you listen carefully (place the chamber close to your ear because the noise change is not easy to distinguish), you will hear a change of the sound coming from inside the leaf chamber. If not, check the fuses inside the console, some debris that can obstruct the fan. Unfortunately, if the leaf fan is broken, you need to replace it following the manufacturer’s manual strictly.
-
9.
Check the Zero of the IRGA. This procedure consists of passing CO2 and H2O free air thought the IRGA; then check if the sensor reading is close to zero. Look for values within 5 μmol mol−1 for CO2 and 0.5 mmol mol−1 for H2O (following the LI-COR manual, v 6.2, p 4–5). Focus on the Reference value only, since Sample will take the Reference value after a Match. Zeroing the IRGA must be done with care, essentially with totally free CO2 and H2O air and a perfectly sealed circuit. In most of the cases, non-zero values come from non-fresh chemicals or leaks through the air circuit. The common “guilties” are bad tube connections, the bad seal of the leaf chamber (2 × 3 o-rings at the chamber/head connection), or leaks from the desiccant and soda lime tubes themselves or their connection with the console. An IRGA is unlikely to drift from several CO2 μmol mol−1 between days, as the temperature does not change drastically. Zeroing the IRGA is encouraged to be performed only in laboratory conditions with calibrated gas tanks (pure N 2 , see below).
-
10.
Set the desired CO 2 concentration. You need to close well the chamber, adjust the tight screw and wait for mixer stabilization. Then do a “Match” to get the Sample reading be “calibrated” based the Reference. Now, you can check possible leaks of the chamber gaskets by a blow-test around the chamber. If there is no increase >1 μmol CO2 mol−1, then, after all, you are ready to measure a leaf.
3.1.2 “Long-Term” Maintenance
“Long-term” maintenance procedures are not likely to be performed every day. They are most likely to be done… let’s say, once per month, for example, but this mostly depends on the frequency and intensity employing of your equipment. In general, they can be performed when some problems are detected and can help to solve it. A typical example of these type of maintenance could be the CO2 Mixer calibration: if the CO2 Mixer needs too much time to reach the targeted [CO2] (or the same for Light intensity and the light source), this can be a signal to do the “Mixer Calibration” procedure. However, since “prevention is always better than cure”, it is better to check these procedures periodically.
3.1.2.1 Internal Calibrations (Calibrations that Do Not Require an External Item for the Procedure)
-
1.
Mixer calibration
This routine checks the control signal (mV) of the CO2 mixer and the [CO2] delivered by the mixer itself into the circuit. This procedure is not strictly a calibration itself, in the sense that does not adjust the reading value of a sensor, but helps the CO2 Mixer to reach more rapidly the desired [CO2]. Go to “Calib Menu” and follow instructions detailed in the manual equipment. When the mixer needs too much time to reach the targeted [CO2], this routine can solve this problem. Also, keep in mind to frequently change the filter present inside the mixer (do it without CO2 cartridge inside), because the accumulated oil can also provoke problems of CO2 regulation.
-
2.
Light Source Calibration
The LEDs source and the LCF (Leaf Chamber Fluorometer) can be calibrated in the same way it is for the Mixer. The light source will associate the different voltage feeding the LEDs with the corresponding reading value of PARin. This association will help to reach faster the desired light intensity. There is also a “Zero PARin” procedure that checks the offset reading that can remain in darkness. Check it monthly can avoid this problem.
-
3.
Fluorometer calibration
The Leaf Chamber Fluorometer (LCF) also needs some specific calibration/checks. We will find the same procedure as for classical LEDs light source: a check of the Zero, and a calibration curve mV versus measured light. However, other procedures are specific to the LCF. One of the most important is the “Square Flash Calibration”, that is impaired with the “MultiPhase Flash” method (MPF). It is highly recommended to set the flash method on “MPF” (Function Key 8, f2, type = “Multiphase”), since it will ensure a better estimate of the F m ′ parameter even when saturation values are not easy to reach with your leaf (Loriaux et al. 2013). You will find this in the “Calib menu”, then “LCF source”. Keep in mind that other procedures can help to determine the “Optimum Flash Intensity” and the “Optimum Measuring Intensity”. The first one is less important since using the MPF method avoid the previous commented problems of PSII saturation. The second one helps to determine the ideal intensity for F o determination (in darkness, without inducing photosynthesis).
3.1.2.2 External Calibrations (Calibrations that Required an External Item for the Procedure)
-
1.
Calibrating the IRGA: Zero and Span
Zeroing the IRGA: As said in the LI-COR manual (all versions), “You will do more harm than good, however, if you dutifully re-zero every day using chemicals…”. Another important thing to bear in mind is that “If conditions (temperature, mostly) haven’t changed a great deal since the last time you zeroed the IRGAs, it won’t need adjusting”. So, we recommend to do the check of the Zero on a daily basis, but zeroing the IRGA only in laboratory conditions, with pure N2 tanks. The best way is to connect the N2 tank to a “Y” tube-connector that feed both Reference and Sample circuits to the LI-6400 head (avoiding the console), with a flow of about 0.5 to 1 L min−1. Go to “Calib menu”, then to IRGA, then IRGA Zero. Waiting for stabilization time is crucial at this moment, and more specifically for zeroing the water. The phenomenon of adsorption/desorption of water in many components (plastics, overall), induce a longer stabilization of the water zero. This procedure must also be done with a fully warmed-up machine. This means that a proper “Zeroing” procedure needs at least 30 min for warming and another 15–20 min for fully stable gases concentration.
Setting the Span: The span corresponds to the sensitivity of the sensor (here the IRGA) to the concentration measured. This corresponds to the slope of the relationship between measured concentration and real concentration. So, you will need a tank with a certified CO2 concentration (for CO2 span), or a dew-point generator that fixes a known concentration of water (for H2O span). In principle, you need to use a known concentration that is within or slightly above the concentrations you are likely to measure. For example, the LI-COR factory uses tanks of 1500 μmol CO2 mol−1. The procedure consists on the same set-up as for “Zeroing” the IRGA: use a “Y” tube-connector, set a flow of about 0.5 to 1 L min−1, and plug it directly to the LI-COR 6400XT head, then wait for stabilization. Then use the adjust button to match the reading concentration to the value of the tank (or the dew-point, in case of water span). The water span can be done with the LI-810 dew-point generator, that it was specially designed for this purpose.
-
2.
Light calibration
As times passes, the PAR in sensor or both LCF and LEDs chamber can drift, leading to an over or under- estimation of the real PAR reaching the leaf. Light sources can be sent back to LI-COR factory for calibration, but checks can be done manually by the user. This possible drift can take larger importance when the PAR is used for further calculations, for example for ETR calculation that is subsequently employed for several equations as photorespiration estimation or mesophyll conductance (Valentini et al. 1995; Flexas et al. 2012a). To do that, is it possible to fix a PAR out sensor on the bottom part of the chamber (here it is really important than the sensor are centered and at the same exact position and distance to the light than leaves are placed in the chamber). Obviously, this sensor must be absolutely well calibrated, ideally a new fresh sensor from LI-COR factory or with less than 1-year-old calibration. Then, establish a calibration curve by changing the PAR in value in let’s say 4–5 steps, and record the reading value given by the PAR out sensor. Then it is possible to calculate the slope and the intercept of this relationship, then use those values to set-up your next light intensity set-up.
3.2 Making a Measurement
Now, you are almost ready to perform a precise measurement of your leaves. The equipment is prepared but there are some important considerations that you need to care when you are measuring the leaf gas-exchange and fluorescence in plants.
3.2.1 Plants Need Time to Adapt to Your Measurements Conditions
The ideal case is to measure the plant without affecting its behaviour and physiological status, as leaves and plants are continuously interacting with the environment that means that leaves will need time to adapt to the new conditions in the measurement chamber. Thus, reach leaf steady-state becomes really important for the reproducibility of your data and as well for the comparison with another data from other researchers worldwide.
Also, take in mind that even the photosynthesis chamber is providing stable, uniform and regulated conditions between all your measurements, changing the condition of the rest of the plant will affect the behaviour of the portion of leaf that is inside the chamber. So, it could be an important source of variability in your data measure the photosynthesis inside the chamber meanwhile the rest of the plant is under dark conditions, or the contrary measure the respiration in the targeted leaf tissue inside the chamber meanwhile the rest of the plant is under light conditions.
3.2.2 Selecting Your Target Leaves
Selecting the same type of leaf can avoid a lot of variability between your biological replicates. Usually, the intra-plant variability (among all leaves within the same plant) is much larger than the inter-plant variability (among the same type of leaf among several plants grown in the same conditions). Conventionally, in the literature, the researcher classically uses the “youngest fully expanded leaf”. This selection ensures to have a fully functional leaf that is not affected by ontology (leaf age). This leaf, in the vast majority of cases, must be a direct sun/light exposed leaf (not inside the canopy, not overlapped by other leaves). Of course, the leaf must be healthy and vigorous, not presenting any sign of herbivory, degradation, chlorosis, nitrogen deficiency, or any factor that can affect the physiology of the leaf.
3.2.3 The Leaf Inside the Chamber
3.2.3.1 Ensure Tight Closure Between Gaskets and the Leaf
When you have chosen the “good” leaf, then, how to clamp-it in the LI-6400 chamber? The aim is to tight the leaf enough to reduce leaks (CO2 entry/exit between the chamber and the atmosphere) as much as possible, but not too much to do not damage the leaf. To do so, it is good to use the screw of the head handle to adjust how tight the leaf is: “enough, but not too much”. To ensure that no or few leaks are present, after clamping the leaf and waited for ~30s to ensure a stabilization of the gas Sample circuit, you can gently blow around the chamber gaskets. Check for any variation within 1 μmol mol−1 of the CO2S ([CO2] in the sample = leaf chamber).
3.2.3.2 Reading Correct Leaf Temperature Values
The reading value of Tleaf should be checked after placing the leaf inside the chamber. This value must be coherent with the TBlk and Tair, obviously depends on the light intensity selected (the higher radiation will tend to be higher the leaf temperature). Leaf temperature is a function of transpiration and stomatal conductance, so stressed plants with reduced stomatal conductance would tend to show higher Tleaf values. Keep in mind that measuring at field conditions with the open-top chamber (no light source, direct sun high radiations) and high air temperature will increase leaf temperature importantly over the selected TBlk. If some suspicious values occur or leaf temperature is unstable, check if the leaf perfectly contacts the thermocouple. The ultimate check consists of checking the reading of the Tleaf with a standardised thermocouple (external confirmation).
3.2.3.3 Area Correction
All the gas-exchange calculations are taking into account the area of the leaf. Photosynthesis chambers have a defined area, so if your leaf coverage the entire measurement area then calculations can be done automatically. Several species have leaves that do not allow you to cover the entire measurements area. Thus area correction is needed. Fortunately, the LI-COR output excel file provide all the formulae: so you just have to correct the area values and the rest of calculations change automatically. Area correction is typically done taking a picture of the exact piece of leaf inside your chamber, and then area calculation can be done with an image analysis software, for example, ImageJ (Carriquí et al. 2015; Tosens et al. 2016).
3.2.3.4 Light Saturation Measurements
In most of the cases, you want to measure your plant at saturating light to avoid changes in photosynthesis from intensities below saturating conditions. On the other hand, in some cases like shade species, too high saturating light radiation can induce photoinhibition. For this, it is recommended to determine the correct light intensity at which your plant saturates for light. To do so, perform a light response curve and take the minimum light intensity when the photosynthesis is saturated.
3.2.3.5 Air Vapor Pressure Deficit, Humidity and Stomata Interaction
Once the leaf is stabilized in the chamber and Tleaf reading is correct, others checks must be done concerning the water vapour inside the chamber. This parameter can be assessed when you read the value of H 2 OS (concentration of water vapor in the Sample) or HR_S (relative humidity in the Sample). Preferably, check the relative humidity and ensure that is comprised between 40% and 70%. Dry air will increase the Vapour Pressure Deficit (VPD a) around the leaf provoking a stomatal closure (stomatal conductance, Cond) that can, in turn, induce a possible decrease of the photosynthetic rate (Photo) (Pérez-Martín et al. 2009). In the other hand, relative humidity higher than 80% in the LI-COR 6400XT can affect the stability of the CO2 readings importantly, as well reduce the precision of estimation of the water-exchange.
It is also important to check as well that the difference in water vapor concentration between the surrounding atmosphere and that inside the chamber (H 2 OS). A too large gradient between chamber and atmosphere can provoke leaks of water vapor, which will affect the estimation of stomatal conductance and leaf transpiration rate (Trmmol). To check this, before clamping the leaf, do a check of the H 2 OS when the chamber is open, and let the surrounding air enter the chamber. This will give an idea of the water concentration and the relative humidity of the surrounding atmosphere during the measurements.
3.2.3.6 The Importance of Reaching the Leaf Steady-State
The leaf steady-state means the stabilization of all photosynthetic parameters before starting the measurements. Steady-state is a crucial point for good measurements. Once the leaf is inside the chamber, usually at saturating light, the photosynthesis is very likely to stabilize pretty rapidly. This comes from the fact that carbon fixation depends directly on the light available to feed the electrons transport rate. Those reactions are very fast, so any change in light is almost instantaneously reported on the photosynthetic rate.
The other factor limiting photosynthesis is the availability of CO2 at the RubisCO site. This second factor is directly affected by the degree of aperture of the stomata, i.e., the stomatal conductance. This parameter, in turn, changes very slowly over time. The time needed to change from closed stomata to fully open can take 1 hr for some species. The steady-state is reached when both photosynthetic rate (Photo) and stomatal conductance (Cond) are fully stable. At that moment, the leaf is in steady-state, the measurements can begin. So once the leaf is clamped, wait minimum 15 min and then check the stability of Photo and Cond, over a time scale of 10 min in the LI-6400 graphs menu. If both are stable over a 10 min time lap, there is a good probability that the steady-state is reached. For very fine measurements or specific species, a steady-state of 1 h can be required. Typically, plants at field conditions reach the steady-state faster than plants from growing-chambers.
3.2.4 Ensuring the Precision of Your Measurement
The estimations of photosynthesis and transpiration rate are based on the difference of concentrations between the Reference and the Sample. Knowing that each IRGA has its own error of measurement (maximum deviation of ±5 μmol mol−1 from 0 to 1500 μmol mol−1, and ± 10 μmol mol−1 from 1500 to 3100 μmol mol−1), when the difference of concentration between Reference and Sample is very low, the precision of measure decreases. When the delta (of CO2, for example) gets close to 0.5 μmol CO2 mol−1, then it is comprised within the measurement error. No reliable data can be obtained this way. One solution that can help to avoid or at least reduce this problem is to decrease the air flow through the chamber. This action decreases the air turn-over of the chamber and lets the leaf affecting more the [CO2] and [H2O] in the chamber. This provokes an increased delta, so we obtain a better precision but employing more time to make the measurements. Decreasing the air flow through the chamber is especially useful when measuring leaf respiration, plants with extreme low photosynthetic rates, or plants under a treatment (water stress, low light, low nitrogen, etc.). Since normal operating flows are 500 μmol s−1 (for 6 cm2 chamber) or 300 μmol s−1 (for 2 cm2 chamber), consider that the flow can be decreased up to 200–150 μmol s−1 depending on the chamber. Lower values would affect response time. In certain cases, e.g., for species with low exchange rate such as mosses, lower flow values could be attained. Another possible solution is to increase the area of measurement by choosing larger leaves.
Why do not work all the time at very low flows? Low flows also increase the influence of aside (chamber/surrounding atmosphere) CO2 and H2O exchanges (like leaks, typically). The second limit is the risk of condensation inside the chamber. At very low flow, the water coming from leaf transpiration can accumulate too much inside the chamber (rising air humidity). This accumulation increases the probability to reach the dew-point of the chamber (100% relative humidity), thus provoking water condensation inside the chamber. Condensation is a dramatic problem for the gas exchange user. Condensation will “trap” water inside the chamber inducing a wrong estimation of the transpiration rate. Moreover, once the water has condensed inside the air circuit of the LI-6400 (chamber, or in another part), it is very hard to fully re-evaporate this liquid water to come back to the proper conditions of measurement. If you have problems with condensation in your circuit, you must dry it. This can be done by connecting a dry air source to the system (console inlet or head) with a vent to avoid over or under-pressure. This can take several hours, overnight is recommended.
3.3 Further Considerations and Useful Tips
3.3.1 Leak Corrections
As said above, CO2 (and H2O) exchanges between inside the photosynthesis chamber and the surrounding atmosphere can be present. This is particularly the case during A/C i curves since the chamber (Sample) [CO2] changes dramatically (from 0 to 2000 μmol CO2 mol−1). Leakage can produce an artifactual photosynthesis rate that does not come from the leaf. For example, at low CO2 the CO2 is going from the atmosphere to inside the chamber, decreasing the estimate photosynthesis; at high CO2, the CO2 is entering from the inside of the chamber to atmosphere, increasing the estimate photosynthesis. The importance of the leaks flow will directly depend on the morphology of the measured leaf (thickness, regularity of the shape, size of leaf’s vein).
In order to compensate this effect, “leaks curves” must be performed. The basic idea relies on employing the very same leaf to reproduce the leakage of its surface with the gaskets of the chamber, to check for the physical leakage. However, obviously, you do not want any biological gas-exchange from the leaf disturbing the physical leakage that you want to analyse. So, you can stop the biological gas-exchange using different manners: submerge the leaf in boiling water for 2–3 min, place in an oven at 110 °C for 1–2 min, or employ an oven for 2–3 min at 110 °C. Any case, you must ensure that the tissue is dead (thus no gas-exchange) with a Log with fluorescence measurement, and check the value of the ETR. If it is negative, then your leaf will not interact with the chamber atmosphere. Obviously, the method employed must preserve as much as possible the structure of the leaves to simulate the interaction between leaf surface and chamber gaskets that drives the leakage. Now place the leaf in the chamber and perform a classical A/C i curve (ideally the same used to measure the functional leaf). The A/C i curve performed with the dead leaf will produce a response curve of apparent photosynthesis (Photo) to the [CO2] changes in the chamber (CO 2 S). This relationship is positive, relatively linear, with min and max values of apparent photosynthesis from −1 to 4 μmol m−2 s−1. A trick: in theory, the CO 2 S at which Photo = 0 should correspond to the [CO2] of the surrounding atmosphere at the moment of the measurement. The next step is to calculate the equation of the obtained relationship Photo = a*CO 2 S + b where a and b are the slopes and the intercept of a linear function. This allows calculating the apparent photosynthesis (leaks) that occurred during the A/C i curve performed with the functional leaf, using its own CO 2 S. The apparent photosynthesis – or leak, will be rested to the measured photosynthesis to obtain the leaf photosynthesis corrected for leaks. Keep in mind that any variable calculated from the photosynthesis rate, like C i must be corrected as well. Fortunately, the excel data files (.xls) generated by the LI-COR 6400XT recalculate all those variables in consequence.
3.3.2 Correction of the ETR: ΦPSII and ΦCO2 Under Non Photorespiratory Conditions
Some parameters, like ETR, need some specific parameterization procedure to be correctly estimated. As it was reported previously:
where PAR i is the incident photosynthetically active radiation, ΦPSII is the quantum yield of the PSII, α the leaf absorption (by default 0.87) and β the electrons portioning between PSI and PSII (by default 0.5). There are some methods which estimate them separately, but here we will see the main method used to estimate α * β product.
The aim is to generate a linear relationship between ΦPSII and ΦCO2 under non-photorespiratory conditions (low, ~2% O2 atmosphere). The source of variation can be light of CO2 (knowing that the CO2 method will need leaks correction). See Martins et al. (2013) for an extended description and test of the method. To do so, A N /PAR or A N /C i curves should be performed by feeding the LI-COR 6400XT with air without O2 (typically N2 air-compressed tank). This can be achieved by plugging the inlet of the LI-6400 console to a tank of pure N2, with the caution a place vent (using a “Y”) between them to do not damage the pump because of overpressure. To check that low O2 air truly feeds the leaf, the steady-state photosynthesis at low O2 should be around 20–30% higher than under 21% O2 (because you are inhibiting the photorespiration activity of RubisCO). After checking the increase of photosynthesis (wait ~10 min after plugging to the N2 source), you can start the A/PAR or A/C i curve. Only the very linear part of the relationship will be employed in the calculations. Once the (positive) linear part of the ΦPSII and ΦCO2 relationship is selected, extract the slope to have: α * β = 4/slope.
3.3.3 Physiological “Tricks” to Keep in Mind
Once the machine is well calibrated and the leaf correctly placed in the chamber, there are some tricks that you are better to know to ensure that data provided are reliable.
-
There is a “general rule” about the proportion of photosynthetic rate and the stomatal conductance, for the vast majority of the species. In general, when the photosynthesis is ca. 10 μmol m−2 s−1, the g s is about 0.1 mol H2O m−2 s−1. Species with high photosynthesis (>20 μmol m−2 s−1) will systematically present high g s values (0.2–0.3 mol m−2 s−1). For example, a plant with very high photosynthesis cannot have very low g s. The inverse can be more likely (low photosynthesis, high g s) but this will be true only for specific species (typically from wet/flooded areas, or from shade conditions). Any case, you have plenty of data in the literature analyzing this relationship (for example Flexas et al. 2013; Gago et al. 2014) and, of course, the topic deserves from you a previous search in the literature to know reported photosynthetic data of your species.
-
Combining gas-exchange with fluorescence data is very useful to check the ETR/A N ratio. The theory says that photosynthesis needs at least 4 electrons to fix one molecule of CO2 through the Calvin-Benson cycle; so, knowing that the photorespiration is also present (and also consuming electrons), the ETR/A N ratio range from 8–10 for C3 species (Flexas et al. 2002). For C3 species, low ETR/A N ratio indicates that it could be a problem in the estimation of the ETR. Alternatively, if you are using thick leaves, it could be an impairment between ETR (collected from the upper cell layer of the leaf) and net photosynthesis, that integrates all the layer of the leaf. Wrong estimation of α * β product can be the cause, wrong estimation of the PAR, or non-saturation of the PSII (too low F m′ values). Higher values of this ratio will indicate stress in your plants as typically CO2 assimilation shows a steeper slope reduction under stress than the ETR (Flexas et al. 2002).
References
Atkin OK, Tjoelker MG (2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci 8:343–351
Atkin OK, Bloomfield KJ, Reich PB, Tjoelker MG, Asner GP, Bonal D, Bönisch G, Bradford MG et al (2015) Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. New Phytol 206:614–636
Bellasio C, Beerling DJ, Griffiths H (2016) An excel tool for deriving key photosynthetic parameters from combined gas exchange and chlorophyll fluorescence: theory and practice. Plant Cell Environ 39:1180–1197
Buckley TN, Díaz-Espejo A (2015) Partitioning changes in photosynthetic rate into contributions from different variables. Plant Cell Environ 38:1200–1211
Canadell JG, Le Quere C, Raupach MR, Field CB, Buitenhuis ET, Ciais P, Conway TJ, Gillett NP, Houghton RA, Marland G (2007) Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proc Natl Acad Sci U S A 104:18866–18870
Carriquí M, Cabrera HM, Conesa MÀ, Coopman RE, Douthe C, Gago J, Gallé A, Galmés J, Ribas-Carbo M, Tomás M, Flexas J (2015) Diffusional limitations explain the lower photosynthetic capacity of ferns as compared with angiosperms in a common garden study. Plant Cell Environ 38:448–460
Demmig-Adams B, Adams WW III, Barker DH, Logan BA, Bowling DR, Verhoeven AS (1996) Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol Plant 98:253–264
Demmig-Adams B, Cohu CM, Muller O, Adams WW III (2012) Modulation of photosynthetic energy conversion efficiency in nature: from seconds to seasons. Photosynth Res 113:75–88
Epron D, Godard D, Cornic G, Genty B (1995) Limitation of net CO2 assimilation rate by internal resistances to CO2 transfer in the leaves of two tree species (Fagus sylvatica L and Castanea sativa mill). Plant Cell Environ 18:43–51
Ethier GJ, Livingston NJ (2004) On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar-von Caemmerer-berry leaf photosynthesis model. Plant Cell Environ 27:137–153
Evans JR, Santiago LS (2014) PrometheusWiki gold leaf protocol: gas exchange using LI-COR 6400. Funct Plant Biol 41:223–226
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Field CB, Ball JT, Berry JA (1989) Photosynthesis: principles and field techniques. In: Pearcy RW, Ehleringer JR, Mooney HA, Rundel PW (eds) Plant physiological ecology: field methods and instrumentation. Chapman & Hall, New York, pp 209–253
Field CB, Ball JT, Berry JA (2000) Photosynthesis: principles and field techniques. In: Pearcy RW, Ehleringer JR, Mooney HA, Rundel PW (eds) Plant physiological ecology. Springer, Dordrecht, pp 209–253
Flexas J, Bota J, Escalona JM, Sampol B, Medrano H (2002) Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Funct Plant Biol 29:461–471
Flexas J, Barbour MM, Brendel O, Cabrera HM, Carriquí M, Díaz-Espejo A, Douthe C, Dreyerc E, Ferrio JP, Gago J, Gallé A, Galmés J, Kodama N, Medrano H, Niinemets Ü, Peguero-Pina JJ, Pou A, Ribas-Carbó M, Tomás M, Tosens T, Warren CR (2012a) Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Sci 193:70–84
Flexas J, Loreto F, Medrano H (eds) (2012b) Terrestrial photosynthesis in a changing environment: a molecular, physiological, and ecological approach. Cambridge University Press, Cambridge ISBN: 9780521899413
Flexas J, Scoffoni C, Gago J, Sack L (2013) Leaf mesophyll conductance and leaf hydraulic conductance: an introduction to their measurement and coordination. J Exp Bot 64:3965–3981
Flexas J, Díaz-Espejo A, Conesa MA, Coopman RE, Douthe C, Gago J, Gallé A, Galmés J, Medrano H, Ribas-Carbó M, Tomàs M, Niinemets Ü (2016) Mesophyll conductance to CO2 and Rubisco as targets for improving intrinsic water use efficiency in C3 plants. Plant Cell Environ 39:965–982
Gaastra P (1959) Photosynthesis of crop plants as influenced by light, carbon dioxide, temperature, and stomatal diffusion resistance. Doctoral dissertation, Wageningen University, Veenman
Gago J, Douthe C, Flórez-Sarasa I, Escalona JM, Galmés J, Fernie AR, Flexas J, Medrano H (2014) Opportunities for improving leaf water use efficiency under climate change conditions. Plant Sci 226:108–119
Gallé A, Flexas J (2010) Gas-exchange and chlorophyll fluorescence measurements in grapevine leaves in the field. In: Delrot S, Medrano H, Or E, Bavaresco L, Grando S (eds) Methodologies and results in grapevine research. Springer, Dordrecht, pp 107–121
Gallé A, Florez-Sarasa I, Tomás M, Pou A, Medrano H, Ribas-Carbó M, Flexas J (2009) The role of mesophyll conductance during water stress and recovery in tobacco (Nicotiana sylvestris): acclimation or limitation? J Exp Bot 60:2379–2390
Galmés J, Flexas J, Keys AJ, Cifre J, Mitchell RA, Madgwick PJ, Haslam RP, Medrano H, Parry MA (2005) RubisCO specificity factor tends to be larger in plant species from drier habitats and in species with persistent leaves. Plant Cell Environ 28:571–579
Galmés J, Molins A, Flexas J, Conesa MÀ (2017) Coordination between leaf CO2 diffusion and Rubisco properties allows maximizing photosynthetic efficiency in Limonium species. Plant Cell Environ 40:2081–2094
Genty B, Harbinson J, Briantais JM, Baker NR (1990) The relationship between non-photochemical quenching of chlorophyll fluorescence and the rate of photosystem 2 photochemistry in leaves. Photosynth Res 25:249–257
Grassi G, Magnani F (2005) Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ 28:834–849
Harley PC, Thomas RB, Reynolds JF, Strain BR (1992) Modelling photosynthesis of cotton grown in elevated CO2. Plant Cell Environ 15:271–282
Hermida-Carrera C, Kapralov MV, Galmés J (2016) Rubisco catalytic properties and temperature response in crops. Plant Physiol 171:2549–2561
Jansson C, Wullschleger SD, Kalluri UC, Tuskan GA (2010) Phytosequestration: carbon biosequestration by plants and the prospects of genetic engineering. BioSci 60:685–696
Jones HG (1985) Partitioning stomatal and non-stomatal limitations to photosynthesis. Plant Cell Environ 8:95–104
Laisk A, Oja V (2018) Kinetics of photosystem II electron transport: a mathematical analysis based on chlorophyll fluorescence induction. Photosynth Res 136:63 https://doi.org/10.1007/s11120-017-0439-y
Long SP, Farage PK, Garcia RL (1996) Measurement of leaf and canopy photosynthetic CO2 exchange in the field. J Exp Bot 47:1629–1642
Loriaux S, Avenson T, Welles J, McDermitt D, Eckles R, Riensche B, Genty B (2013) Closing in on maximum yield of chlorophyll fluorescence using a single multiphase flash of sub-saturating intensity. Plant Cell Environ 36:1755–1770
Martins SC, Galmés J, Molins A, DaMatta FM (2013) Improving the estimation of mesophyll conductance to CO2: on the role of electron transport rate correction and respiration. J Exp Bot 64:3285–3298
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence – a practical guide. J Exp Bot 51:659–668
Montero R, Ribas-Carbó M, Del Saz NF, El Aou-ouad H, Berry JA, Flexas J, Bota J (2016) Improving respiration measurements with gas exchange analyzers. J Plant Physiol 207:73–77
Murchie EH, Niyogi KK (2011) Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol 155:86–92
Niinemets Ü (2016) Within-canopy variations in functional leaf traits: structural, chemical and ecological controls and diversity of responses. In: Hikosaka K, Niinemets Ü, Anten N (eds) Canopy photosynthesis: from basics to applications. Springer, Berlin, pp 101–141
Niinemets Ü, Cescatti A, Rodeghiero M, Tosens T (2005) Leaf internal diffusion conductance limits photosynthesis more strongly in older leaves of Mediterranean evergreen broad-leaved species. Plant Cell Environ 28:1552–1566
Niinemets Ü, Díaz-Espejo A, Flexas J, Galmes J, Warren CR (2009) Importance of mesophyll diffusion conductance in estimation of plant photosynthesis in the field. J Exp Bot 60:2271–2282
Osmond B, Förster B (2006) Photoinhibition: then and now. In: Demmig-Adams B, Adams WW III, Mattoo AK (eds) Photoprotection, Photoinhibition, gene regulation, and environment: advances in photosynthesis and respiration. Kluwer, Dordrecht, pp 11–22
Osmond CB, Ludlow MM, Davis R, Cowan IR, Powles SB, Winter K (1979) Stomatal responses to humidity in Opuntia inermis in relation to control of CO2 and H2O exchange patterns. Oecologia 41:65–76
Pérez-Martín A, Flexas J, Ribas-Carbó M, Bota J, Tomás M, Infante JM, Díaz-Espejo A (2009) Interactive effects of soil water deficit and air vapour pressure deficit on mesophyll conductance to CO2 in Vitis vinifera and Olea europaea. J Exp Bot 60:2391–2405
Pons TL, Flexas J, von Caemmerer S, Evans JR, Genty B, Ribas-Carbó M, Brugnoli E (2009) Estimating mesophyll conductance to CO2: methodology, potential errors, and recommendations. J Exp Bot 60:2217–2234
Sharkey TD (2016) What gas exchange data can tell us about photosynthesis. Plant Cell Environ 39:1161–1163
Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL (2007) Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ 30:1035–1040
Tosens T, Nishida K, Gago J, Coopman RE, Cabrera HM, Carriquí M, Laanisto L, Morales L, Nadal M, Rojas R, Talts E, Tomas M, Hanba Y, Niinemets Ü, Flexas J (2016) The photosynthetic capacity in 35 ferns and fern allies: mesophyll CO2 diffusion as a key trait. New Phytol 209:1576–1590
Valentini R, Epron D, Deangelis P, Matteucci G, Dreyer E (1995) In-situ estimation of net CO2 assimilation, photosynthetic electron flow and photorespiration in Turkey oak (Q. cerris L) leaves – diurnal cycles under different levels of water-supply. Plant Cell Environ 18:631–640
Valentini R, Matteucci G, Dolman AJ, Schulze ED, Rebmann C, Moors EJ, Granier A, Gross P, Jensen NO, Pilegaard K, Lindroth A, Grelle A, Bernhofer C, Grünwald T, Aubinet M, Ceulemans R, Kowalski AS, Vesala T, Rannik Ü, Berbigier P, Loustau D, Guðmundsson J, Thorgeirsson H, Ibrom A, Morgenstern K, Clement R, Moncrieff J, Montagnani L, Minerbi S, Jarvis PG (2000) Respiration as the main determinant of carbon balance in European forests. Nature 404(6780):861–865
von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas-exchange of leaves. Planta 153:376–387
Walker BJ, Skabelund DC, Busch FA, Ort DR (2016) An improved approach for measuring the impact of multiple CO2 conductances on the apparent photorespiratory CO2 compensation point through slope–intercept regression. Plant Cell Environ 39:1198–1203
Walker BJ, Orr DJ, Carmo-Silva E, Parry MA, Bernacchi CJ, Ort DR (2017) Uncertainty in measurements of the photorespiratory CO2 compensation point and its impact on models of leaf photosynthesis. Photosynth Res 132:245–255
Yin X, Struik PC, Romero P, Harbinson J, Evers JB, Van der Putten, Vos J (2009) Using combined measurements of gas exchange and chlorophyll fluorescence to estimate parameters of a biochemical C3 photosynthesis model: a critical appraisal and a new integrated approach applied to leaves in a wheat (Triticum aestivum) canopy. Plant Cell Environ 32:448–464
Yin X, Sun Z, Struik PC, Gu J (2011) Evaluating a new method to estimate the rate of leaf respiration in the light by analysis of combined gas exchange and chlorophyll fluorescence measurements. J Exp Bot 62:3489–3499
For Further Details
LI-COR (2012) Using the LI-6400/LI-6400XT portable photosynthesis system – Version 6. LI-COR Biosciences Inc., Lincoln
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Douthe, C., Gago, J., Ribas-Carbó, M., Núñez, R., Pedrol, N., Flexas, J. (2018). Measuring Photosynthesis and Respiration with Infrared Gas Analysers. In: Sánchez-Moreiras, A., Reigosa, M. (eds) Advances in Plant Ecophysiology Techniques. Springer, Cham. https://doi.org/10.1007/978-3-319-93233-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-93233-0_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-93232-3
Online ISBN: 978-3-319-93233-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)