Abstract
Hypertensive disorders of pregnancy are one of the most common medical problems encountered during the management of obstetric patients. Preeclampsia is a type of hypertensive disorder, which is unique to human pregnancy and is a leading cause of maternal and fetal morbidity and mortality. The current widely accepted pathophysiology of preeclampsia is failure of placenta to embed adequately leading to hypoxia due to poor placental perfusion. As a result coagulation cascade is activated with the formation of microthrombi leading to end-organ damage. Preeclampsia therefore involves either one or more than one organ systems including the central nervous, cardiovascular, gastrointestinal, hematological, renal, and uteroplacental systems. Early diagnosis of the disease, accurate monitoring, and timely institution of treatment can prevent complication. In the absence of contraindication, neuraxial anesthesia and analgesia is recommended. Postpartum management includes continuation of monitoring, antihypertensive management, thromboprophylaxis, and analgesia. In the presence of multisystem organ involvement and its related complications, patients need to be managed postoperatively in the intensive care unit. Anesthesiologists being part of the multidisciplinary team are involved in the management of these patients for provision of analgesia/anesthesia and for subsequent management in the postpartum period.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Hypertensive disorders of pregnancy are significant cause of both maternal and fetal morbidity and mortality [1]. It poses a global health threat in both developed and developing countries [2, 3]. However, the impact of the disease is more in the developing countries due to the late presentation of the disease making medical interventions less effective [4, 5]. Anesthesiologists being part of the multidisciplinary team are involved in the initial management for provision of analgesia/anesthesia and for subsequent management in the postpartum period. Therefore, this chapter will outline the role of anesthesiologist as a member of the multidisciplinary team in the anesthetic management of pregnant patients with hypertensive disorders. Since preeclampsia is a multisystem disease unique to human pregnancy, this chapter will focus mainly on this particular disorder.
2.2 Classification of Hypertensive Disorders of Pregnancy
The National High Blood Pressure Education Program (NHBPEP) Working Group on high blood pressure in pregnancy published a classification scheme in 2000 that achieved international acceptance [6]. They classified hypertensive disorders of pregnancy into (1) gestational hypertension, (2) preeclampsia, (3) chronic hypertension, and (4) chronic hypertension with superimposed preeclampsia:
-
1.
Gestational hypertension: It is the most common hypertensive disorder of pregnancy with less severe outcomes almost similar to that of a normotensive parturient [7, 8]. Women are labeled as having gestational hypertension when they present with hypertension after the 20th week of pregnancy with no previous history of hypertension or signs and symptoms of preeclampsia. Hypertension resolves after 12 weeks postpartum [8].
-
2.
Preeclampsia: The diagnosis of preeclampsia is made when hypertension is diagnosed for the first time after the 20th week of gestation and there is involvement of either one or more than one organ systems. The disease processes involving the organ system show resolution by 3 months in the postpartum period. Depending upon its severity, it is further classified into mild or severe. It can progress to eclampsia when the central nervous system involvement leads to seizures [9].
-
3.
Chronic hypertension: Diagnosed as having hypertension prepregnancy or when hypertension fails to resolve after 12 weeks postpartum.
-
4.
Chronic hypertension with superimposed preeclampsia: When a parturient with a prepregnancy diagnosis of chronic hypertension develops sudden new onset of proteinuria or sudden increase in the levels of blood pressure or when the symptoms of severe preeclampsia develop. The outcomes are worse with increased morbidity as compared to patient with preeclampsia alone [10].
2.3 Pathophysiology
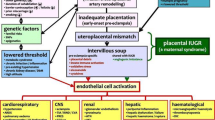
The current widely accepted explanation is failure of placenta to embed adequately leading to hypoxia due to poor placental perfusion [11]. This in turn leads to vasospasm and activation of coagulation cascade with microthrombi formation and end-organ damage [12]. The resultant endothelial damage is responsible for the maternal syndrome of preeclampsia, manifesting as hypertension and proteinuria with or without systemic manifestation [13]. The fetal syndrome of preeclampsia includes fetal growth retardation, oligohydramnios, and abnormal oxygenation [6]. The pathophysiology of eclampsia is due to the presence of placenta and not because of the fetus as it can occur with woman having molar pregnancy.
2.4 Diagnostic Challenges of Preeclampsia
In order to establish the diagnoses of preeclampsia, there has to be a sustained increase in blood pressure on repeated measurement usually done at 4 hourly interval with disease process involving either one or more than one organ systems. The abnormalities of organ systems can either be of central nervous system, cardiovascular system, gastrointestinal system, hematological system, renal system, or uteroplacental systems [14].
Preeclampsia presenting before 20 weeks of gestation occurs in parturient having hydatidiform mole, fetal or placental abnormalities, multiple pregnancy, antiphospholipid syndrome, or severe renal disease [14].
Other causes of hypertension during pregnancy include renal disease, pheochromocytoma, coarctation of aorta, subclavian artery stenosis, aortic dissection, and vasculitis [15]. These conditions are caused by different pathologies, which need to be differentiated from preeclampsia as treatment options are entirely different.
In addition, severe hepatic dysfunctions due to causes other than preeclampsia need to be identified. This includes development of the acute fatty liver in pregnancy, which is not associated with hypertension [16].
One type of severe preeclampsia is HELLP syndrome characterized by hemolysis, elevated liver enzymes, and low platelet count. There is fragmentation of red blood cells and appearance of schistocytes on a blood film, rise in lactate dehydrogenase and total bilirubin levels often combined with declining hematocrit, and evidence of a bleeding diathesis [15].
Conditions like hemolytic uremic syndrome and thrombotic thrombocytopenic purpura, which have features like thrombocytopenia, hemolytic anemia associated with microangiopathies, and abnormalities of the central nervous system and renal system, need to be differentiated from HELLP syndrome, as treatment and/or interventions are entirely different [15].
2.5 Severity of Preeclampsia
This term is used when preeclampsia progresses in severity with rise in blood pressure to a level of systolic blood pressure equal or higher than 160 mmHg and diastolic blood pressure to a level equal or higher than 110 mmHg with severe decline in organ functions of involved body systems. The central nervous system involvement may progress to severe headache, visual disturbances, impaired consciousness, and seizures (eclampsia). Renal function impairment is observed by increase in the excretion of urinary protein to >5 gram (g). Severe preeclampsia also presents itself as HELLP syndrome.
2.6 Anesthetic Management
Early involvement of anesthesiologist is important as woman with mild preeclampsia can progress rapidly to severe category requiring anesthesia management and stabilization before delivery [17]. The importance of experienced multidisciplinary teamwork has been highlighted in literature [18,19,20].
Preanesthetic assessment and preparation should focus on airway examination, reduction of blood pressure, hemodynamic monitoring, fluid balance, and prevention and treatment of seizures. Routine investigations including coagulation profile are required to determine the possible need of any replacement therapy.
2.6.1 Airway
Airway changes in hypertensive parturient are due to soft tissue edema owing to fluid retention, increased cell permeability, and low plasma oncotic pressure [21]. Therefore, anesthesiologists need to anticipate the possibility of difficult airway and be prepared for it.
2.6.2 Control of Rising Blood Pressure
Parturients are labeled as having non-severe hypertension when systolic and diastolic blood pressures rise to a range of 140–159 mmHg and 90–109 mmHg, respectively. Agents considered safe for use in these patients include methyldopa, low-dose diazoxide, nifedipine, and some beta adrenoceptor blockers including labetalol, metoprolol, or pindolol [22]. Atenolol is avoided as it causes fetal growth restriction. Other agents that are contraindicated in parturient include angiotensin-converting enzyme inhibitors and angiotensin type-2 receptor blockers [23, 24]. When systolic pressure rises to more than 160 mmHg and diastolic pressure to more than 110 mmHg, the condition is termed as severe hypertension. Systolic blood pressure rising to more than 180 mmHg is labeled as a hypertensive crisis [18]. This condition is associated with high incidence of intracerebral hemorrhage if immediate medical intervention is not provided [19]. In order to prevent hemorrhagic stroke, various guidelines recommend using oral labetalol as the drug of choice to maintain systolic blood pressure below 150 mmHg and a diastolic blood pressure below 100 mmHg [25]. It is advisable to avoid sudden fall in blood pressure which may lead to adverse maternal and fetal complications. Blood pressure should be lowered at a rate of 10–20 mmHg every 10–20 min to levels of systolic blood pressure between 140 and 150 mmHg and diastolic pressures between 80 and 100 mmHg. Fetal heart rate monitoring should be monitored continuously until the blood pressure is stable [26].
Drugs that are considered to be safe for lowering of blood pressure include labetalol, hydralazine, and nifedipine. However, there is paucity of good randomized controlled trial comparing hydralazine with intravenous labetalol or oral nifedipine [25]. Intravenous hydralazine is usually administered by intermittent bolus of 5 mg or by continuous infusion in the dose of 0.5–10.0 mg/h for more refractory cases. Sodium nitroprusside is known to be associated with adverse maternal side effects of hypotension and paradoxical bradycardia and fetal cyanide toxicity in fetus; therefore it is rarely used in pregnancy [4].
Glyceryl trinitrate is the drug of choice in preeclamptic patients who develop acute pulmonary edema. It is given in an infusion of 5 μg/min increasing every 3–5 min to a maximum dose of 100 μg/min [4].
2.6.3 Hemodynamic Monitoring
Fluctuations in blood pressure are expected in patients with severe preeclampsia as a result of progression of disease and in response to administration of antihypertensive agents. Since patients with preeclampsia are volume contracted and fluid depleted, clinical assessment of intravascular volume can become difficult necessitating the use of invasive monitoring.
Continuous monitoring of blood pressure is indicated in case of poorly controlled maternal blood pressure or a rapid need to lower blood pressure using sodium nitroprusside or glyceryl trinitrate, frequent use of blood gases, or when general anesthesia is required in patients with severe preeclampsia.
Central venous pressure monitoring is advisable in assessment of oliguria and its response to fluid administration [9]. However, there is no indication of central hemodynamic monitoring that is specific to preeclampsia, as it is a disease of peripheral circulation and not of central circulation. Therefore, the indication for invasive central monitoring is the same for any multisystem disorders including severe sepsis, pulmonary edema, and cardiomyopathy [9].
2.6.4 Prevention and Treatment of Seizures
Patients with preeclampsia are at risk of developing seizures. Magnesium sulfate has shown to be the first-line drug treatment for both ongoing seizures (eclampsia) and for prevention of recurrent seizures [27, 28]. When compared to drugs like phenytoin, diazepam, or lytic cocktail (combination of chlorpromazine, promethazine, and pethidine), the use of magnesium sulfate has shown to decrease the incidence of maternal death, seizure recurrence, pneumonia, need for ventilation, or admission to an intensive care unit [27,28,29]. Magnesium sulfate can be used effectively by parenteral route. Collaborative Eclampsia Trial recommends the use of MgSO4 in the dose of 4–5 g intravenously over 5 min, followed by an infusion of 1 g/h for 24 h. An additional 2 g intravenous MgSO4 should be administered in case of recurrent seizures [30].
The use of magnesium sulfate for prevention of seizures is established in severe preeclampsia, but its use in mild disease is controversial. The investigators have found that the number needed to treat to prevent one woman having seizure was approximately 100 in mild disease as compared to 50 in patients with severe disease [31]. However, selective administration of magnesium sulfate to women with severe preeclampsia resulted in increased number of women developing eclampsia who required general anesthesia with adverse neonatal outcomes [32].
Magnesium sulfate is not recommended as an antihypertensive agent, and its clinical use does not reverse or prevent the progression of the disease [24, 26]. Proper guidelines need to be in place in health-care centers for the safe use of magnesium sulfate. This includes monitoring by clinical parameters like measuring urinary output, respiratory rate, oxygen saturation, and patellar reflexes. If any of the clinical parameter is indicated toward magnesium toxicity, it is mandatory to check serum magnesium levels. Magnesium toxicity is often apparent when the serum magnesium levels are above 3.5 mmol/L. Patients having any additional systemic manifestation like renal insufficiency are more likely to develop magnesium toxicity. The drug used for treatment of magnesium sulfate toxicity is 10% calcium gluconate given in the dose of 1 g over 10 min [15].
2.6.5 Fluid Balance
Careful fluid management is an important aspect in the management of patient with preeclampsia. Pulmonary edema is one of the leading causes of morbidity in preeclampsia leading to intensive care admission and death [33]. Evidence suggests that intravenous fluids should not be administered in preeclampsia for the purpose of volume expansion or for the treatment of oliguria when renal functions and serum creatinine concentration are within normal range [34].
2.6.6 Laboratory Investigations Including the Coagulation Status
As there is a marked variation in the normal values during pregnancy at different gestational periods, pregnancy-specific range is accepted for blood tests.
-
(a)
Useful blood tests in preeclampsia include [35]:
-
Full blood count with special emphasis on platelet count.
-
Clotting screen is needed if platelets are <100 × 10−9/L, liver function test (LFT) is abnormal, or unexplained bleeding or bruising is present. A blood film is required if HELLP syndrome is suspected.
-
Serum urea, electrolytes, creatinine, and urate levels.
-
Liver function tests including serum transaminase (AST) and alanine aminotransferase (ALT) are sent to detect liver disorders and liver damage.
-
-
(b)
Glucose needs to be checked in cases of acute fatty liver of pregnancy and diabetes or if patient’s ALT is more than 150 IU/L.
-
(c)
Urine tests in preeclampsia include:
-
Urine dipstick.
-
Urinary protein/creatinine ratio (PCR) from a random sample (<30 mg/mmol excludes significant proteinuria; >30 mg/mmol does not reliably confirm or quantify proteinuria).
-
24-h urine collection for proteinuria (≥300 mg/24 h both confirm and quantify proteinuria).
-
Midstream specimen of urine to exclude infection.
-
Urine output must be accurately monitored and recorded.
-
Further investigations are warranted according to clinical presentation.
2.7 Neuraxial Blockade in Preeclamptic Parturient
Neuraxial blockade is the preferred method for both anesthesia and analgesia in parturient diagnosed with preeclampsia [25, 36, 37]. However, coagulopathy needs to be ruled out before institution of neuraxial block. Common reasons for coagulopathy in preeclampsia include thrombocytopenia and less commonly disseminated intravascular coagulation [15]. Studies using thromboelastography to determine safe levels of platelet count in preeclampsia have recommended that counts less than 100 × 109/L were associated with coagulation abnormality in severe preeclampsia requiring additional investigation of coagulation status [15].
Regarding the lower limit of the platelet count, there is no definitive evidence; however, indirect conclusions from current standard of practice and thromboelastography studies have shown that in the absence of other coagulation abnormalities, the risk of hematoma associated with neuraxial anesthesia with platelet counts >75 × 109/L is very low [15].
2.7.1 Neuraxial Technique and Its Associated Complications in preeclamptic patients
The estimated incidence of major complications after neuraxial techniques in pregnant women in general is approximately 1/20,000–30,000 for spinal anesthesia and 1/25,000 for epidural analgesia [38]. The risk of epidural hematomas increases in preeclampsia as thrombocytopenia is shown to be present in 30–50% of parturient having severe preeclampsia [39]. In addition the presence of engorged epidural venous plexus combined with low platelet count further increases the risk.
Therefore, neuraxial anesthetic techniques may be avoided in pregnancy-induced hypertension if there is concomitant thrombocytopenia and coagulopathy. It is recommended to follow the trend and get a coagulation profile if there is a declining trend.
2.7.2 Neuraxial Labor Analgesia for Labor and Delivery
Currently neuraxial technique for labor analgesia is considered as a gold standard technique for number of reasons. The advantages of neuraxial blocks include improved intervillous blood flow due to sympathetic blockade causing decrease in uteroplacental resistance [40]. In addition it causes reduction in pain-mediated hypertensive responses by causing reduction in serum catecholamine levels. Investigators have shown better neonatal acid-base status and Apgar score at 1 and 5 min with labor epidural analgesia compared to no analgesia and systemic opioids [41]. In addition the presence of a functioning epidural catheter enables the use of the epidural catheter for cesarean delivery.
Epidural labor analgesia is maintained by either continuous infusion, intermittent boluses, or patient-controlled epidural analgesia (PCEA) using a combination of local anesthetics and preservative-free opioids. The PCEA has the advantage over other techniques as parturients are able to self-administer prefixed doses of epidural medication whenever needed and a lock out time interval provides a safety from over dosage of local anesthetics [42]. However, the choice of delivery mostly depends on the availability of equipment.
2.8 Anesthesia for Cesarean Delivery
2.8.1 Technique of Anesthesia
Availability of time, maternal or fetal status, and communication with the obstetrical team have a vital role to play in the decision of the anesthetic technique for cesarean delivery. When there is no maternal and fetal compromise with no time constraint, spinal anesthesia or epidural top-up of a pre-existing epidural catheter is the technique of choice. On the other hand, when there is a maternal and fetal compromise, coagulation abnormality, or a parturient with ongoing seizures, general anesthesia may be considered over regional anesthetic technique.
Neuraxial anesthesia is preferred over general anesthesia as an anesthetic technique for cesarean delivery as the risk of aspiration, possibility of encountering difficult or failed intubations, and sympathetic response to laryngoscopy and intubation associated with general anesthetic can be avoided [15]. A population-based study with a sample size of total 303,862 women who had undergone cesarean delivery showed an increased risk of maternal stroke in preeclamptic women receiving general anesthesia for cesarean delivery [43].
Neuraxial techniques that have been used effectively include single-shot spinal, combined spinal-epidural, and epidural anesthesia. However, no evidence has suggested advantage of one technique over the other. Spinal anesthesia is the most commonly used technique as it has a rapid onset, dense block and can provide an effective postoperative analgesia when long-acting intrathecal opioids are used [44]. Studies have shown two times lower incidence of spinal-induced hypotension and vasopressor requirement in preeclamptic parturient when compared with normal parturient undergoing cesarean delivery [45]. Vasoactive drugs including 3–5 mg bolus of intravenous ephedrine or 50–100 μg bolus of intravenous phenylephrine are used in titrated doses to successfully manage episodes of hypotension [37, 46]. It is best to avoid adrenaline-containing local anesthetic in preeclampsia for the anticipated risk of hypertensive crisis due to absorbed adrenaline [15].
Hypertensive response to intubation during general anesthesia has been identified as a cause of direct maternal mortality [18, 19]. Numbers of drugs have been used to attenuate this response, which includes short-acting narcotics like fentanyl, alfentanil, and remifentanil. In addition MgSO4 lidocaine and esmolol have been used for this purpose. Choice of the drug depends on its availability and physicians’ familiarity with the drug [47]. Special attention needs to be paid to complications like aspiration, hypertensive response, and acute pulmonary edema that are commonly encountered at the time of emergence from anesthesia.
2.8.2 Recommended Monitoring for Cesarean Delivery
Continuous intra-arterial blood pressure monitoring is recommended for cesarean delivery in parturient with severe hypertension [25]. In addition to beat-to-beat arterial pressure recording, it is useful for frequent blood sampling required for the assessment of electrolytes, acid-base balance, and abnormalities of respiratory, hematological, and hepatic systems [15].
Other invasive monitoring like central venous pressure and pulmonary artery pressure are used very occasionally [34]. Transthoracic echocardiography is considered a better option as it has the advantage of providing information related to cardiac structural and functional performance and responses to interventions [48].
2.9 Postpartum Management
Patients with severe preeclampsia should be monitored in the postpartum period by trained staff in an appropriately monitored setting [19]. Important consideration in the postpartum period is for analgesia, thromboprophylaxis, decision to discontinue magnesium sulfate, and identification and management of postpartum complications.
2.9.1 Analgesia
Although analgesic modalities like the use of intravenous opioids, paracetamol, local anesthetic, and neuraxial techniques have been used frequently in the postpartum management, enough literature is not available for preeclampsia [49]. Nonsteroidal anti-inflammatory agents have well-documented adverse effects and are contraindicated in women with preeclampsia [50].
2.9.2 Thromboprophylaxis
It should be considered for all women with preeclampsia, and neuraxial technique should be timed accordingly.
2.9.3 Discontinuation of Intravenous Magnesium Sulfate
There has been recommendation to continue it for 12–24 h [51]. However, it has been suggested to use improvement in clinical parameters rather than time to make a decision to discontinue MgSO4. Clinical improvement includes normalization of blood pressure to less than 150/100 mmHg without the need of antihypertensive medications and urine output of more than 100 mL/h for at least in the last couple of hours and with no headache, pain in the epigastrium, or vision abnormality [52].
2.9.4 Identification and Management of Postpartum Complications
Some of the common postpartum complications observed in the postpartum period among preeclamptic parturient include severe hypertension, acute pulmonary edema, and decrease in urine output and acute renal decompensation.
Postpartum hypertension is a cause of postpartum morbidity and mortality, and medications commonly employed for treatment include hydralazine, methyldopa, furosemide, and nifedipine [53].
Incidence of pulmonary edema in preeclampsia is 2.9% with 70% occurring in the postpartum period [54]. Treatment options are similar to those used in the non-obstetric population.
Oliguria in the postpartum period can be due to a number of causes, and no treatment is required including the use of furosemide or low-dose dopamine if renal and respiratory functions are normal [55].
2.10 Recent Advances
2.10.1 Echocardiography
is emerging as an extremely useful tool to enable more informed decisions in the management of women with preeclampsia especially pertaining to fluid therapy and choice of antihypertensive agents [56]. In addition it can provide a more definitive evaluation of cardiac function in the hypertensive pregnant patient.
2.10.2 Placental Growth Factor
Placenta produces an angiogenic factor called placental growth factor (PlGF) which shows a peak in concentrations between 26 and 30 weeks of gestation. Women with preeclampsia have shown to have reduced levels of PIGF with the lowest levels corresponding to earlier onset and increased severity of the disease. The importance of this biomarker lies in its ability to diagnose preeclampsia before the onset of hypertension and proteinuria, thus allowing earlier treatment and multidisciplinary planning for delivery. As the levels of PlGF naturally decline during the third trimester, therefore the test becomes less reliable after 35 weeks [57]. There is a need for randomized controlled trials to assess whether diagnosing preeclampsia with PlGF rather than current methods can improve maternal and/or fetal outcomes.
Conclusion
Hypertensive disorders of pregnancy especially preeclampsia will continue to remain a significant cause of maternal and fetal morbidity in both developed and developing countries. Anesthesiologists, as part of the multidisciplinary team, play a vital role in the provision of safe anesthesia/analgesia, resuscitation, and critical care management in the postpartum period. Therefore, it is imperative for the anesthesiologists to have a thorough understanding of this disease which is unique to pregnancy. The skill, knowledge, and awareness of potential intra- and postoperative problems and the ability to respond quickly in emergency situation are the key factors for successful management of these patients.
Key Learning Points
-
Hypertensive disorders of pregnancy are significant cause of both maternal and fetal morbidity and mortality.
-
The National High Blood Pressure Education Program (NHBPEP) Working Group on high blood pressure in pregnancy classified hypertensive disorders of pregnancy into (1) gestational hypertension, (2) preeclampsia, (3) chronic hypertension, and (4) chronic hypertension with superimposed preeclampsia.
-
The diagnosis of preeclampsia is made when there is a new onset of hypertension after the 20th week of gestation with one or more organ system involvement and resolution of the disease by 3 months postpartum.
-
The current widely accepted explanation is failure of placenta to embed adequately leading to hypoxia due to poor placental perfusion. This in turn leads to vasospasm and activation of coagulation cascade with microthrombi formation leading to endothelial damage with organ damage responsible for systemic manifestation.
-
Severe preeclampsia is defined as progression in severity with blood pressure showing a substantial increase of systolic >160 mmHg and diastolic >110 mmHg with marked derangements of organ functions.
-
Preanesthetic assessment and preparation should focus on airway examination, reduction of blood pressure, hemodynamic monitoring, prevention and treatment of seizures, fluid balance, and investigations including the coagulation status and need for replacement therapies if needed.
-
Antihypertensive drugs that are considered safe in these patients include labetalol, hydralazine, and nifedipine.
-
Continuous arterial blood pressure monitoring is indicated in cases of poorly controlled maternal blood pressure, frequent use of blood gases especially in the setting of patient with pulmonary edema, rapid need to lower blood pressure using sodium nitroprusside or glyceryl trinitrate, and when using general anesthesia in patients with severe preeclampsia.
-
As preeclampsia is a disease of peripheral circulation and not the central circulation, there is no indication of central hemodynamic monitoring that is specific to preeclampsia.
-
Neuraxial anesthesia is preferred over general anesthesia as an anesthetic technique for cesarean delivery as the risk of aspiration, possibility of encountering difficult or failed intubations, and sympathetic response to laryngoscopy and intubation associated with general anesthetic can be avoided. However coagulopathy needs to be ruled out before institution of neuraxial block.
-
Hypertensive response to intubation has been identified as a cause of direct maternal mortality. Therfore, particular attention needs to be paid to attenuate this response when general anesthesia is used as technique of anesthesia.
-
Important consideration in the postpartum period is for analgesia, thromboprophylaxis, and decision to discontinue magnesium sulfate.
-
In addition there is a need for identification and management of postpartum complications.
-
Some of the common postpartum complications observed in preeclampsia include severe hypertension, acute pulmonary edema, oliguria, and acute renal decompensation.
References
Mammaro A, Carrara S, Cavaliere A, Ermito S, Dinatale A, Pappalardo EM, et al. Hypertensive disorders of pregnancy. J Prenat Med. 2009;3:1.
National Institute for Health and Care Excellence (NICE). Hypertension in pregnancy: the management of hypertensive disorders during pregnancy. In:NICE CG 107. Manchester: National Institute for Health and Clinical Excellence; 2010. Available from http://www.nice.org.uk/guidance/cg107/resources/guidance-hypertension-in-pregnancy-pdf. Accessed 5 Jan 2018.
Bujold E, Morency AM, Roberge S, Lacasse Y, Forest JC, Giguere Y. Acetylsalicylic acid for the prevention or preeclampsia and intra-uterine growth restriction in women with abnormal uterine artery Doppler: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2009;31:818–26.
Regitz-Zagrosek V, Lundqvist CB, Borghi C, Cifkova R, Ferreira R, Foidart JM, et al. European Society of Cardiology Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:3147–97.
Fitzpatrick K, Hinshaw K, Kurinczuk J, Knight M. Risk factors, management, and outcomes of hemolysis, elevated liver enzymes, and low platelets syndrome and elevated liver enzymes, low platelets syndrome. Obstet Gynecol. 2014;123:618–27.
Gifford RW. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:1–22.
Hauth JC, Ewell MG, Levine RJ, Esterlitz JR, Sibai B, Calcium for Preeclampsia Prevention Study Group, et al. Pregnancy outcomes in healthy nulliparas who developed hypertension. Obstet Gynecol. 2000;95(1):24–8.
Barton JR, O’Brien JM, Bergauer NK, Jacques DL, Sibai BM. Mild gestational hypertension remote from term: progression and outcome. Am J Obstet Gynecol. 2001;184(5):979–83.
Polly LS. Hypertension disorders. In: Chestnut DH, Tsen LC, Polly LS, Wong CA, editors. Chesnut’s obstetric anesthesia: principles and practice. 4th ed. Philadelphia: Mosby Elsevier; 2004. p. 975–1008.
Giannubilo SR, Dell’Uomo B, Tranquilli AL. Perinatal outcomes, blood pressure patterns and risk assessment of superimposed preeclampsia in mild chronic hypertensive pregnancy. Eur J Obstet Gynecol Reprod Biol. 2006;126:63–7.
Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM. Preeclampsia: pathophysiology, diagnosis, management. Vasc Health Risk Manag. 2011;7:467–74.
Bell MJ. A historical overview of preeclampsia-eclampsia. J Obstet Gynecol Neonatal Nurs. 2010;39:510–8.
Alladin AA, Harrison M. Preeclampsia: systemic endothelial damage leading to increased activation of the blood coagulation cascade. J Biotech Res. 2012;4:26–43.
Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–61.
Dennis AT. Management of pre-eclampsia: issues for anaesthetists. Anaesthesia. 2012;67:1009–20.
Bacq Y. Liver diseases unique to pregnancy: a 2010 update. Clin Res Hepatol Gastroenterol. 2011;35:182–93.
Healthcare Improvement Scotland. Scottish confidential audit of severe maternal morbidity, 7th annual report. Edinburgh; 2011. http://www.healthcareimprovementscotland.org/programmes/reproductive_maternal_child/programme_resources/scasmm.aspx. Accessed 3 Nov 2017.
Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, et al. Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006–2008. The eighth report of the confidential enquiries into maternal deaths in the United Kingdom. BJOG. 2011;118:1–203.
Lewis G, editor. The confidential enquiry into maternal and child health (CEMACH). Saving mothers’ lives: reviewing maternal deaths to make motherhood safer – 2003–2005. The seventh report on confidential enquiries into maternal deaths in the United Kingdom. London: CEMACH; 2007.
Dyer RA, Piercy JL, Reed AR. The role of the anaesthetist in the management of the pre-eclamptic patient. Curr Opin Anaesthesiol. 2007;20:168–74.
Izci B, Riha RL, Martin SE, Vennelle M, Liston WA, Dundas KC, et al. The upper airway in pregnancy and preeclampsia. Am J Respir Crit Care Med. 2003;167:137–40.
Hennessy A, Thornton CE, Makris A, Ogle RF, Henderson-Smart DJ, Gillin AG, et al. A randomized comparison of hydralazine and mini-bolus diazoxide for hypertensive emergencies in pregnancy: the PIVOT trial. Aust N Z J Obstet Gynaecol. 2007;47:279–85.
Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2007;1:CD002252.
Podymow T, August P. Update on the use of antihypertensive drugs in pregnancy. Hypertension. 2008;51:960–9.
National Collaborating Centre for Women’s and Children’s Health. Hypertension in pregnancy. The management of hypertensive disorders during pregnancy. National Institute for Health and Clinical Excellence Guideline 107. August 2010 revised reprint January 2011 ed. London: RCOG; 2011. http://www.nice.org.uk/nicemedia/live/13098/50475/50475.pdf. Accessed 30 Dec 2011.
Rowe T. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. J Obstet Gynaecol Can. 2008;30:1–48.
Duley L, Gülmezoglu AM, Chou D. Magnesium sulphate versus lytic cocktail for eclampsia. Cochrane Database Syst Rev. 2010;9:CD002960.
Duley L, Henderson-Smart DJ, Walker GJA, Chou D. Magnesium sulphate versus diazepam for eclampsia. Cochrane Database Syst Rev. 2010;12:CD000127.
Duley L, Henderson-Smart DJ, Chou D. Magnesium sulphate versus phenytoin for eclampsia. Cochrane Database Syst Rev. 2010;10:CD000128.
The Collaborative Eclampsia Trial Group. Which anticonvulsant for women with eclampsia? Evidence from the Collaborative Eclampsia Trial. Lancet. 1995;345:1455–63.
Duley L, Gülmezoglu AM, Henderson-Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with preeclampsia. Cochrane Database Syst Rev. 2010;11:CD000025.
Alexander JM, McIntire DD, Leveno KJ, Cunningham FG. Selective magnesium sulfate prophylaxis for the prevention of eclampsia in women with gestational hypertension. Obstet Gynecol. 2006;108:826–32.
Ganzevoort W, Rep A, Bonsel GJ, Fetter WP, van Sonderen L, De Vries JI, et al. A randomised controlled trial comparing two temporising management strategies, one with and one without plasma volume expansion, for severe and early onset pre-eclampsia. BJOG. 2005;112:1358–68.
Duley L, Williams J, Henderson-Smart DJ. Plasma volume expansion for treatment of pre-eclampsia. Cochrane Database Syst Rev. 1999;4:CD001805.
Krishnachetty B, Plaat F. Management of hypertensive disorders of pregnancy. ATOTW Weekly. 2014;304:1–13.
Lucas MJ, Sharma SK, McIntire DD, Wiley J, Sidawi JE, Ramin SM, et al. A randomized trial of labor analgesia in women with pregnancy-induced hypertension. Am J Obstet Gynecol. 2001;185:970–5.
Visalyaputra S, Rodanant O, Somboonviboon W, Tantivitayatan K, Thienthong S, Saengchote W. Spinal versus epidural anesthesia for cesarean delivery in severe preeclampsia: a prospective randomized, multicenter study. Anesth Analg. 2005;101:862–8.
Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990–1999. Anesthesiology. 2004;101:950–9.
Valera MC, Parant O, Vayssiere C, Arnal JF, Payrastre B. Physiologic and pathologic changes of platelets in pregnancy. Platelets. 2010;21:587–95.
Ginosar Y, Nadjari M, Hoffman A, Firman N, Davidson EM, Weiniger CF, et al. Antepartum continuous epidural ropivacaine therapy reduces uterine artery vascular resistance in pre-eclampsia: a randomized, dose-ranging, placebo-controlled study. Br J Anaesth. 2009;102:369–78.
Reynolds F. Labour analgesia and the baby: good news is no news. Int J Obstet Anesth. 2011;20:38–50.
Fettes PD, Moore CS, Whiteside JB, McLeod GA, Wildsmith JA. Intermittent vs. continuous administration of epidural ropivacaine with fentanyl for analgesia during labour. Br J Anaesth. 2006;97:359–64.
Huang CJ, Fan YC, Tsai PS. Differential impacts of modes of anaesthesia on the risk of stroke among preeclamptic women who undergo caesarean delivery: a population-based study. Br J Anaesth. 2010;105:818–26.
Sia AT, Fun WL, Tan TU. The ongoing challenges of regional and general anaesthesia in obstetrics. Best Pract Res Clin Obstet Gynaecol. 2010;24:303–12.
Aya AG, Vialles N, Tanoubi I, Mangin R, Ferrer JM, Robert C, et al. Spinal anesthesia-induced hypotension: a risk comparison between patients with severe preeclampsia and healthy women undergoing preterm cesarean delivery. Anesth Analg. 2005;101:869–75.
Berends N, Teunkens A, Vandermeersch E, Van de Velde M. A randomized trial comparing low-dose combined spinal-epidural anesthesia and conventional epidural anesthesia for cesarean section in severe preeclampsia. Acta Anaesthesiol Belg. 2005;56:155–62.
Dyer RA, Els I, Farbas J, Torr GJ, Schoeman LK, James MF. Prospective, randomized trial comparing general with spinal anesthesia for cesarean delivery in preeclamptic patients with a nonreassuring fetal heart trace. Anesthesiology. 2003;99:561. Discussion 5A-6A.
Dennis AT. Transthoracic echocardiography in obstetric anaesthesia and obstetric critical illness. Int J Obstet Anesth. 2011;20:160–8.
Macintyre PE, Schug SA, Scott DA, Visser EJ, Walker SM, Acute Pain Management: Scientific Evidence Working Group of the Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine. Acute pain management: scientific evidence. 3rd ed. Melbourne: Australian and New Zealand College of Anaesthetists and the Faculty of Pain Medicine; 2010.
Makris A, Thornton C, Hennessy A. Postpartum hypertension and nonsteroidal analgesia. Am J Obstet Gynecol. 2004;190:577–8.
Ehrenberg HM, Mercer BM. Abbreviated postpartum magnesium sulfate therapy for women with mild preeclampsia: a randomized controlled trial. Obstet Gynecol. 2006;108:833–8.
Isler CM, Barrilleaux PS, Rinehart BK, Magann EF, Martin JN Jr. Postpartum seizure prophylaxis: using maternal clinical parameters to guide therapy. Obstet Gynecol. 2003;101:66–9.
Magee L, Sadeghi S, von Dadelszen P. Prevention and treatment of postpartum hypertension. Cochrane Database Syst Rev. 2005;1:CD004351.
Norwitz ER, Hsu CD, Repke JT. Acute complications of preeclampsia. Clin Obstet Gynecol. 2002;45:308–29.
Steyn DW, Steyn P. Low-dose dopamine for women with severe pre-eclampsia. Cochrane Database Syst Rev. 2007;1:CD003515.
Dennis A, Castro J. Transthoracic echocardiography in women with treated severe pre-eclampsia. Anaesthesia. 2014;69:436–44.
Chappell L, Duckworth S, Seed P, Griffin M, Myers J, Mackillop L, et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia. Circulation. 2013;128:2121–31.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Ismail, S. (2018). Anesthetic Management of Pregnant Patients with Hypertensive Disorders. In: Gunaydin, B., Ismail, S. (eds) Obstetric Anesthesia for Co-morbid Conditions. Springer, Cham. https://doi.org/10.1007/978-3-319-93163-0_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-93163-0_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-93162-3
Online ISBN: 978-3-319-93163-0
eBook Packages: MedicineMedicine (R0)