Abstract
Hypertensive disorders of pregnancy, specifically preeclampsia, cause significant morbidity and mortality in both the mother and fetus. Changes in the diagnostic criteria have attempted to improve the ability to identify and initiate treatment. Recently, it has been discovered that a placenta-derived anti-angiogenic environment directly contributes to the pathological cause of preeclampsia. The early identification of these anti-angiogenic markers may enhance diagnosis of the disease in the future. Patients with preeclampsia need to be treated with caution in regard to airway and coagulation; however, neuraxial anesthesia, when appropriate, is preferred. During labor, the increase in placental blood flow secondary to epidural analgesia can improve fetal perfusion. Spinal and epidural anesthesia are safe for cesarean delivery in mothers with preeclampsia, and may be preferred over general anesthesia. Preeclampsia is a complex disorder that impacts day to day decisions of anesthesia care provider.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertensive disorders of pregnancy affect about 20 % of parturients during pregnancy [1]. The most severe form, preeclampsia, causes significant short-term and long-term morbidity, and is one of the leading causes of maternal mortality in the world. Preeclampsia also presents dangers to the fetus, being associated with intrauterine growth restriction, abruption of the placenta, and iatrogenic premature birth with associated risk to the neonate [2].
The anesthesia care provider can be faced with multiple clinical issues when caring for the parturient with preeclampsia—physiological and pathological changes can affect most aspects of anesthetic care. Alterations in the cardiovascular system such as labile hypertension and renal impairment change the way medications need to be administered. The effects of the disease on the hematologic and coagulation systems affect the decision to use neuraxial anesthesia, while the edematous changes to the maternal airway may make intubation challenging. The most worrisome changes are in the central nervous system, where sudden onset of seizures, posterior reversible encephalopathic syndrome (PRES), or intracranial hemorrhage can occur suddenly and be devastating.
The last decade has seen significant developments in preeclampsia, and many of these changes are well documented in the literature. This review will focus on the most recent developments including changes to the categorization of hypertensive disorders aimed at making the identification and prompt treatment of severe preeclampsia easier. Secondly, there has been a dramatic breakthrough in the understanding of the pathophysiologic process that results in the signs and symptoms of preeclampsia. While this breakthrough in the pathogenesis has not yet led to changes in treatment, there is a growing body of literature that may result in a future reduction in morbidity and mortality. Finally, there has been an evolution in the anesthetic care of the hypertensive parturient, with the goal of providing safer care for both mother and fetus.
Definition of Hypertensive Disorders
The American College of Obstetrics and Gynecology updated the categorical definition and diagnostic criteria for hypertensive disorders in 2013 [3••]. This change was an explicit attempt to promote early diagnosis and prompt treatment of preeclampsia, as it was believed that the previous diagnostic criteria were excessively rigid, leading to unnecessary delay in care [3••]. The categories of hypertensive disorders now include only four designations: (1) gestational hypertension; (2) chronic hypertension; (3) chronic hypertension with superimposed preeclampsia; and (4) preeclampsia–eclampsia [3••]. Gestational hypertension is defined as the onset of hypertension after the 20th week of gestation, while patients with chronic hypertension have elevated blood pressure prior to this time. Gestational hypertension and chronic hypertension in pregnancy carry an elevated maternal and fetal risk of morbidity or death compared to normotensive pregnancy, but less than preeclampsia; however, 20–40 % of patients with either disorder will evolve to having proteinuria and the systemic disease known as preeclampsia.
Preeclampsia is a systemic disease affecting multiple organ systems in the mother and fetus, with diverse presentations. Major risk factors include preeclampsia in a previous pregnancy, advanced maternal age, nulliparity, low socioeconomic status, pre-pregnancy obesity or excessive weight gain during pregnancy, Gestational DM, extended birth interval, and vasculitis or renal disease [4]. Preeclampsia is a disease of the placenta, and delivery of the placenta leads to resolution of the signs and symptoms. Especially among women with early-onset preeclampsia, the placenta is incompletely inserted into the maternal endometrium [5, 6]. This pathologic state likely leads to poor fetal perfusion (resulting in intrauterine growth restriction) and also to the expression of maternal systemic factors that cause hypertension and organ injury. Historically, preeclampsia was identified by the coexistence of hypertension, proteinuria, which served as evidence of renal impairment, and edema. Edema was removed as a diagnostic feature, as it is very common in pregnancy. Patients who have preeclampsia prior to 34 weeks of gestation (“early onset” disease) represent only about 10 % of the total incidence, but a greater proportion of maternal and perinatal complications [7].
Preeclampsia is defined as the coexistence of maternal hypertension with either evidence of renal impairment, or any signs or symptoms of systemic disease (see Table 1) [3]. While proteinuria is the most common finding, it is no longer required as part of the formal definition of preeclampsia given the following shortcomings. A single collection of urine to assess for protein via a dipstick or laboratory measure is quick and inexpensive, but may not be adequate to identify renal impairment. The amount of urinary protein excreted by an individual can have great variability throughout the day, leading to both false negatives and false positives [8]. For a more reliable diagnosis, a 24-h collection can be used, but the validity of this test has been questioned [9, 10]. Additionally, a delay in diagnosis while awaiting the results of a 24-h urine collection could delay therapy and lead to morbidity. Recent studies have demonstrated that a single urine sample measuring the protein-to-creatinine ratio may be a rapid and more reliable method of diagnosing renal impairment [11, 12]. A urinary protein-to-creatinine ratio of ≥0.3 may be sufficient evidence of renal involvement that can serve in place of a 24-h collection [13•].
Etiology
The etiology of preeclampsia remains unknown and is likely multifactorial. Genetic factors are clearly involved in the development of preeclampsia [14]. There is a clear familial link to the disease, with greater rates among sisters or daughters of women who were affected [15]. The influence of genetic variables is complex, and involves interactions of the mother, father, fetus, and possibly the environment. Cnattingius et al. [16] studied Swedish births from 1987 to 1997 and estimated that 35 % of variance of preeclampsia was attributable to maternal genetic effects, 20 % to fetal genetics, and 13 % to couple effect (including paternal effects). However, this study found that only 50 % of the risk of developing preeclampsia was due to genetic factors. A recent theory suggested that the genetic impact may be from environmental factors that affect phenotypic or functional expression, without a change in the basic DNA sequence—an effect known as epigenetics. Environmental factors may affect DNA methylation and histone formation, both of which can regulate gene function. While there is no conclusive gene/domain solely responsible for preeclampsia, Chelbi et al. [17] have suggested that abnormal methylation pattern may be a factor in the development of preeclampsia.
An immune source of the disease, which was supported by the observation of a higher rate of preeclampsia in multiparous women having a pregnancy with a new father, was assumed to be the cause of preeclampsia in many patients [18, 19]. However, some authors have suggested that the time between gestations in these pregnancies may be a confounder, and that an immune-based explanation may be inaccurate [20]. It has been suggested that the failure of the placenta to implant into the endometrium may represent an immune etiology in some patients [3••]. While evidence remains debated on both sides of this argument, a purely maternal immune reaction as the cause of preeclampsia does not appear to have the support as in the past [18].
Preeclampsia has been identified to be more prevalent in geographic areas associated with poor nutrition. While malnutrition does not directly cause preeclampsia, women who are predisposed will be more likely to acquire the disease if they are malnourished [2, 21]. For example, dietary supplementation with calcium reduces the incidence of preeclampsia among women with poor calcium intake, but does not change the rates among women with adequate nutritional intake (and may increase the incidence of HELLP syndrome) [22].
Pathophysiology
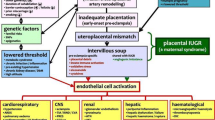
In the last decade, the pathogenesis of preeclampsia has become more clearly defined. Understanding this new theory requires a brief review of the vascular and endothelium system.
Angiogenesis
The creation of new blood vessels from non-vascular stem cells (Vasculogenesis) is controlled by the biomarker, transforming growth factor (TGF) in the developing placenta. This hormone interacts with a receptor involving a multiprotein complex including the protein endoglin, which enhances capillary formation. In addition to de novo blood vessel creation, the growing placenta requires creation of blood vessels from preexisting ones, a process termed, Angiogenesis. The primary angiogenic biomarkers are vascular endothelial growth factor (VEGF) and placental growth factor (PlGF). Primary angiogenesis creates the vascular system tree by branching existing vessels into two new ones. After the primary function of vessel creation, the hormones of angiogenesis continue to be secreted promoting endothelial cell growth, health, and integrity.
VEGF and PlGF are related molecules that share similar structure, and both hormones bind a receptor identified as the VEGF-receptor-1 or the fms-like tyrosine kinase-1 (Flt-1) receptor [23]. This receptor, normally located in vascular endothelial cell surface of placenta, promotes endothelial cell health, and release of nitric oxide and prostacyclin promoting dilation of the surrounding arteriolar smooth muscle [24]. This interaction between circulating molecules of VEGF and PlGF and the endothelial cell-bound receptor, Flt-1, is important in producing adequate placental bed angiogenesis as well as blood flow in the placenta [14]. The Flt-1 receptor is central to the health and function of the organ beds with fenestrated endothelium—specifically, the liver, kidney, and brain [25].
During fetal development at around the 12th–13th week's gestational age, the implanted placenta creates new blood circulatory systems to allow fetal-maternal exchange. This process is driven by locally released hormones and the fetal cytotrophoblasts (CT), which act as pluripotent mesenchymal precursor cells [26]. The CT cells invade the maternal myometrium and replace the intima and media of the maternal spiral arteries and the maternal endothelial and vascular smooth muscle cells. The elimination of the vascular media layer of the spiral arteries leads to low-resistance dilated vessels resulting in a blood supply to the placenta that has minimal response to adrenergic hormones.
Anti-angiogenic Environment
Women with early-onset preeclampsia (<34 weeks gestational age) have been found to have a placenta that is superficially inserted into the maternal myometrium [6, 27]. Patients who have early-onset preeclampsia represent only about 10 % of the total incidence, but a greater proportion of maternal and perinatal complications [7]. Up to 90 % of the placental vessels remain unconverted native maternal arteries, with full responsiveness to adrenergic tone [6, 27]. This failure of the placenta to properly insert can lead to a state of chronic ischemia of the placenta. The response of the vascular system to ischemia is complex, but is intimately involved in the pathogenesis of preeclampsia. In ischemic environments, the endothelial cells (the CT cells in the placenta) reduce production of both VEGF and PlGF, and also alter their receptor expression [28, 29]. The Flt-1 receptor undergoes an alternate splicing becoming a solubilized (s-Flt1) and non-membrane bound receptor [30]. Similarly, the endoglin molecule from the TGF receptor releases into the circulation becoming solubilized (s-ENG) [31]. The circulating s-ENG molecule binds TGF preventing its adherence to the endothelial cell surface receptors, creating an anti-vasculogenic environment [31]. Excess s-Flt1 is absorbed into the maternal circulation, creating an anti-angiogenic state [31, 32]. This anti-angiogenic state was identified in a longitudinal study of patients who developed preeclampsia [33]. The authors demonstrated reduced maternal circulating levels of both VEGF and PlGF (at 11 weeks gestational age), and dramatically elevated maternal serum concentration of sFlt1 (at 5 weeks gestational age) prior to the diagnosis of preeclampsia [28, 29, 33]. The endothelial cell dysfunction resulting from the anti-angiogenic environment leads to maternal vasoconstriction, release of biomarkers of oxidative stress and inflammation, and eventually to capillary bed volume loss and the systemic organ pathology identified as preeclampsia [1, 34, 35••].
Organ Pathology
The maternal anti-angiogenic environment results in systemic physiologic derangements. Parturients with preeclampsia have an elevated systemic vascular resistance caused by systemic vasoconstriction from endothelial cell dysfunction, and from a progressive loss of capillary bed volume due to s-ENG [36]. Endothelial injury also leads to inflammation and creation of oxygen free radicals (oxidative stress) that leads to further end organ injury [35••].
The elevated s-Flt1 and reduced maternal circulating VEGF lead to dysfunction in the fenestrated endothelial cells system in the kidney, which fill with edematous fluid destroying the glomerular structure and function, leading to the classic proteinuria of preeclampsia. Interestingly, the pathognomonic of preeclampsia, mesangial endotheliosis, can be produced in mice infected with retroviral vector that secretes s-Flt1; conversely, administration of VEGF to these mice will return the glomerulus and renal function to normal [33].
The reticular endothelial system found in the liver and spleen also has fenestrated capillaries; loss of endothelial filtration there leads to inflammatory injury, and later parenchymal necrosis. In response to the anti-angiogenic environment, the spleen sequesters platelet leading to relative thrombocytopenia, which is found in about half of patients with severe preeclampsia [37]. HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets) is one of the most severe forms of preeclampsia, with increased morbidity and mortality for both mother and baby; [38] however, liver enzyme abnormalities do not indicate severity of disease.
In the brain, endothelial injury is thought to result in damage to the choroid plexus, leading to interruption of the blood–brain barrier as demonstrated by the presence of stroma-free hemoglobin in the cerebral spinal fluid [39]. Serum magnesium does not pass into the cerebrospinal fluid in patients with an intact blood–brain barrier, but more readily crosses in patient with preeclampsia [40, 41]. In severe cases, endothelial injury can lead to brain edema and altered mental status, a disorder named posterior reversible encephalopathy syndrome (PRES), often found in malignant hypertension [42, 43]. Interestingly, patients receiving anti-VEGF therapy for malignancies develop a disorder strikingly similar to preeclampsia, including hypertension-induced PRES syndrome [44, 45].
The effect of preeclampsia on the maternal heart is controversial. In normal, non-hypertensive pregnancy, elevated preload conditions lead to mild thickening of left ventricle and increased cardiac output throughout gestation [46]. In women with preeclampsia, the hypertrophy of the myocardium and cardiac output are increased compared to normal pregnancy [47, 48]. The hypertrophy is likely a response to elevated loading condition and increased cardiac work, possibly due to myocardial stress from the anti-angiogenic environment [49•, 50]. Sophisticated echocardiographic techniques including myocardial performance index (MPI) or Tei index (which reflects cardiac function independent of blood pressure) have been used to show that patients with preeclampsia have cardiac dysfunction. There is disagreement regarding the degree of cardiac dysfunction in preeclampsia, although the presence of diastolic dysfunction is thought to be common [47, 50–52]. In some studies, women with preeclampsia have had subclinical systolic dysfunction [53, 54]. These changes appear to be worse in patients with early-onset preeclampsia and may persist even after delivery.
In susceptible patients, the vascular dysfunction and organ damage may progress to peripartum cardiomyopathy [49•, 52]. A meta-analysis by Bello et al. [55] confirmed an elevated prevalence of preeclampsia among patients with peripartum cardiomyopathy. Although 80 % of woman with peripartum cardiomyopathy do not have preeclampsia, and most woman with preeclampsia (>90 %) do not develop peripartum cardiomyopathy, these disease entities appear to share a common pathophysiology, perhaps by similar mechanisms of anti-angiogenic process [49•]. One of the more disconcerting findings of the past decade has been the strong association between preeclampsia during pregnancy and an increased risk of heart disease later in life, including metabolic syndrome, hypertension, ischemic heart disease, and stroke [56, 57, 58•• 59]. Whether the higher incidence of vascular disease later in life is due to pathologic injury during pregnancy, or conversely, whether the susceptibility to preeclampsia during the stress of pregnancy foreshadows how a patient will respond to aging remains to be discerned.
Anesthetic Considerations
The anesthetic care for the patient with severe preeclampsia can pose a significant challenge. In addition to often requiring urgent or emergency care, these patients must be considered at risk for increased airway edema, decreased glomerular filtration rate dysfunctional platelets and thrombocytopenia, and in some cases with HELLP syndrome or disseminated intravascular coagulopathy.
While preeclampsia is commonly associated with relative thrombocytopenia, coagulopathy is uncommon unless the platelet count is very low, is decreasing rapidly, or is associated with other etiologies of coagulopathy (e.g., placental abruption, HELLP syndrome). Whereas prothrombin time (PT/INR) and partial thromboplastin time (PTT) measure in vitro portions of coagulation cascade, thromboelastography (TEG) can be used to assess whole blood reflecting interaction among clotting factors, platelets, and fibrinogen. Normotensive parturients have normal-appearing TEG values until the platelet count is reduced below 75,000/ml [60]. Parturients with mild preeclamptic women are hypercoagulable compared to healthy pregnant controls according to TEG; however, severely preeclamptic women with platelet counts below 100,000/ml can be slightly hypocoagulable compared to controls [61]. The exact platelet count at which the risk of complications from a spinal/epidural hematoma increases is not known, but is likely lower than where the TEG values begin to change. Unfortunately, evidence-based recommendations are impossible because the incidence of epidural hematoma is very low (approximately 1:250,000) [62] and little published data are available to guide the clinician. Beilin et al. [63] recorded a series of 30 uncomplicated neuraxial procedures in parturients with platelet counts between 69,000 and 100,000/ml. In a letter to the editor, Frenk et al. [64] reported 170 patients with thrombocytopenia (<100,000/ml) who received uncomplicated neuraxial procedures, including five with counts of between 50,000 and 60,000 ml. These reports, while impressive, do not define the minimum platelet count that increases the risk of injury.
Labor Analgesia
The parturient with preeclampsia can receive great benefit from epidural analgesia during labor. Effective epidural analgesia reduces the maternal serum catecholamine levels, which can improve blood flow to the placenta by up to 80 % [65]. A similar improvement in Doppler indices of uterine artery blood flow has been demonstrated after epidural analgesia among women with preeclampsia, but not with normotensive patients [66]. Patients with severe preeclampsia may have compromised placental blood flow, as measured by Doppler indices. In a study by Ginosar et al. [67], the control patients (standard care) required prompt delivery within 2 days for fetal indications but in patients treated with antepartum epidural analgesia, delivery was able to be delayed for an average of 19 days. While the sample size was small, and the maternal risk to benefit ratio remains to be determined, this may be an important finding.
Cesarean Anesthesia
Should cesarean delivery be necessary, careful consideration of possible comorbid conditions, such as obesity, and laboratory values (including current platelet count and trend), should be applied prior to choosing method of analgesia. Regional techniques for delivery via cesarean section are preferred over general anesthesia, as there appears to be fewer morbid complications [68]. In the patient with severe preeclampsia, spinal anesthesia results in similar hemodynamic stability as either epidural or general anesthesia [69–71]. Traditional concerns over avoiding spinal anesthesia due to excessive hypotension do not appear to have been warranted. Although Visalyaputra et al. [69] found that significant hypotension (systolic <100 mmHg) was more frequent among patients receiving spinal rather than epidural anesthesia (51 vs. 23 %; P = 0.004), these episodes of hypotension lasted less than one minute and were easily treated (with 6 vs. 12 mg ephedrine). In addition, newborn outcomes were similar in both groups. Aya et al. [72] found that patients with severe preeclampsia actually had less hypotension following spinal anesthesia than healthy, normotensive parturients for elective cesarean delivery. Normotensive parturients had more episodes of hypotension following spinal than patients with severe preeclampsia (53.3 vs. 16.6 %; P = 0.006), as defined as systolic <100 mmHg or 30 % below baseline.
Trends for the Future
While there is no current therapy to prevent preeclampsia, early and effective diagnosis is believed to bestow the best maternal and fetal outcome. Multiple authors have proposed using the ratio of s-Flt1 to PlGF, which rises in mid-gestation and seems to correlate with the severity of preeclampsia for more timely diagnosis [73–75]. This is currently not possible due to the lack of consistent methodology and a lack of a Federal Drug Administration-approved machine for measurements. Based on the current hypothesis of the pathology of preeclampsia, a future treatment other than delivery may be conceivable. Early work has already begun on techniques for removing s-Flt1 from the maternal circulation using extracorporeal plasmapheresis [76]. While this does not alter the placental source of the disease, it may improve maternal care and reduce morbidity.
Conclusion
Hypertensive disorders of pregnancy are a diverse spectrum of disease that increase the risk to both the mother and fetus. The most severe form of the spectrum, preeclampsia, is one of the leading causes of maternal morbidity and mortality, and the leading cause of iatrogenic premature birth. The last decade has seen several developments in this spectrum of disease. The diagnostic criteria and categories have recently been altered to improve timely diagnosis. The reliance on evidence of renal injury, and especially on ensuring a 24-h urine collection for protein, has been eliminated in favor of broader criteria. A second important development has occurred in the understanding of the pathophysiology of preeclampsia. While the etiology of the disease remains elusive, the discovery that placental ischemia induces an anti-angiogenic maternal environment has redefined the disease. While therapy and treatment remain remote, using this information for early diagnosis may soon be possible. Finally, the impact of preeclampsia on anesthetic care and decision making cannot be overstated. Several recent investigations have found that neuraxial anesthesia is safe and effective for labor and cesarean delivery in women with preeclampsia.
References
Papers of particular interest, published recently have been highlighted as: • Of importance •• Of major importance
Practice ACoO. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 2002;77(1):67–75.
Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):1066–74.
•• American College of O, Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–31. This report of the American College of Obstetrics and Gynecology updated the society’s position on preeclampsia. Findings include re-categorization of the hypertensive disorders, and changes to the recommendations for management and delivery of patients.
Coghill AE, Hansen S, Littman AJ. Risk factors for eclampsia: a population-based study in Washington State, 1987–2007. Am J Obstet Gynecol. 2011;205(6):553 e1-7.
Brosens I, Dixon HG. The anatomy of the maternal side of the placenta. J Obstet Gynaecol Br Commonwealth. 1966;73(3):357–63.
Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–91.
Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early-versus late-onset disease. Am J Obstet Gynecol. 2013;209(6):544; e1–e12.
Meyer NL, Mercer BM, Friedman SA, Sibai BM. Urinary dipstick protein: a poor predictor of absent or severe proteinuria. Am J Obstet Gynecol. 1994;170(1 Pt 1):137–41.
Brown MA, Lindheimer MD, de Swiet M, Van Assche M, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy. 2001;20(1):IX–XIV.
Cote AM, Firoz T, Mattman A, Lam EM, von Dadelszen P, Magee LA. The 24-hour urine collection: gold standard or historical practice? Am J Obstet Gynecol. 2008;199(6):625.
Waugh JJ, Bell SC, Kilby MD, Blackwell CN, Seed P, Shennan AH, et al. Optimal bedside urinalysis for the detection of proteinuria in hypertensive pregnancy: a study of diagnostic accuracy. BJOG Int J Obstet Gynaecol. 2005;112(4):412–7.
Cote AM, Brown MA, Lam E, von Dadelszen P, Firoz T, Liston RM, et al. Diagnostic accuracy of urinary spot protein:creatinine ratio for proteinuria in hypertensive pregnant women: systematic review. BMJ. 2008;336(7651):1003–6.
• Morris RK, Riley RD, Doug M, Deeks JJ, Kilby MD. Diagnostic accuracy of spot urinary protein and albumin to creatinine ratios for detection of significant proteinuria or adverse pregnancy outcome in patients with suspected pre-eclampsia: systematic review and meta-analysis. BMJ. 2012;345:e4342. This is a meta-analysis and review of the use of protein-to-creatinine ratio for the diagnosis of renal impairment in preeclampsia.
Pijnenborg R, Brosens IA, Romero R. Placental bed vascular disorders: basic science and its translation to obstetrics. New York: Cambridge University Press; 2010.
Chesley LC, Annitto JE, Cosgrove RA. The familial factor in toxemia of pregnancy. Obstet Gynecol. 1968;32(3):303–11.
Cnattingius S, Reilly M, Pawitan Y, Lichtenstein P. Maternal and fetal genetic factors account for most of familial aggregation of preeclampsia: a population-based Swedish cohort study. Am J Med Genet Part A. 2004;130A(4):365–71.
Chelbi ST, Vaiman D. Genetic and epigenetic factors contribute to the onset of preeclampsia. Mol Cell Endocrinol. 2008;282(1–2):120–9.
Dekker G, Robillard PY. Pre-eclampsia: Is the immune maladaptation hypothesis still standing? An epidemiological update. J Reprod Immunol. 2007;76(1–2):8–16.
Trupin LS, Simon LP, Eskenazi B. Change in paternity: a risk factor for preeclampsia in multiparas. Epidemiology. 1996;7(3):240–4.
Klungsoyr K, Morken NH, Irgens L, Vollset SE, Skjaerven R. Secular trends in the epidemiology of pre-eclampsia throughout 40 years in Norway: prevalence, risk factors and perinatal survival. Paediatr Perinat Epidemiol. 2012;26(3):190–8.
Leeners B, Rath W, Kuse S, Irawan C, Neumaier-Wagner P. The significance of under- or overweight during childhood as a risk factor for hypertensive diseases in pregnancy. Early Hum Dev. 2006;82(10):663–8.
Hofmeyr GJ, Lawrie TA, Atallah AN, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. 2010(8):CD001059.
Silasi M, Cohen B, Karumanchi SA, Rana S. Abnormal placentation, angiogenic factors, and the pathogenesis of preeclampsia. Obstet Gynecol Clin North Am. 2010;37(2):239–53.
He H, Venema VJ, Gu X, Venema RC, Marrero MB, Caldwell RB. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J Biol Chem. 1999;274(35):25130–5.
Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol. 1998;140(4):947–59.
Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46(5):1077–85.
Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994;101(8):669–74.
Nagamatsu T, Fujii T, Kusumi M, Zou L, Yamashita T, Osuga Y, et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145(11):4838–45.
Gu Y, Lewis DF, Wang Y. Placental productions and expressions of soluble endoglin, soluble fms-like tyrosine kinase receptor-1, and placental growth factor in normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2008;93(1):260–6.
Banks RE, Forbes MA, Searles J, Pappin D, Canas B, Rahman D, et al. Evidence for the existence of a novel pregnancy-associated soluble variant of the vascular endothelial growth factor receptor, Flt-1. Mol Hum Reprod. 1998;4(4):377–86.
Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. New Engl J Med. 2006;355(10):992–1005.
Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Investig. 2003;111(5):649–58.
Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. New Engl J Med. 2004;350(7):672–83.
Levine RJ, Karumanchi SA. Circulating angiogenic factors in preeclampsia. Clin Obstet Gynecol. 2005;48(2):372–86.
•• Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123(24):2856–69. This is a review of the leading hypothesis of the role of placental-derived anti-angiogenic hormones in the pathogenesis of preeclampsia.
Gammill HS, Lin C, Hubel CA. Endothelial progenitor cells and preeclampsia. Front Biosci. 2007;12:2383–94.
Rakoczi I, Tallian F, Bagdany S, Gati I. Platelet life-span in normal pregnancy and pre-eclampsia as determined by a non-radioisotope technique. Thromb Res. 1979;15(3–4):553–6.
Martin JN Jr, Blake PG, Perry KG Jr, McCaul JF, Hess LW, Martin RW. The natural history of HELLP syndrome: patterns of disease progression and regression. Am J Obstet Gynecol. 1991;164(6 Pt 1):1500–9; discussion 9–13.
Norwitz ER, Tsen LC, Park JS, Fitzpatrick PA, Dorfman DM, Saade GR, et al. Discriminatory proteomic biomarker analysis identifies free hemoglobin in the cerebrospinal fluid of women with severe preeclampsia. Am J Obstet Gynecol. 2005;193(3 Pt 2):957–64.
Altman D, Carroli G, Duley L, Farrell B, Moodley J, Neilson J, et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359(9321):1877–90.
Thurnau GR, Kemp DB, Jarvis A. Cerebrospinal fluid levels of magnesium in patients with preeclampsia after treatment with intravenous magnesium sulfate: a preliminary report. Am J Obstet Gynecol. 1987;157(6):1435–8.
Easton JD. Severe preeclampsia/eclampsia: hypertensive encephalopathy of pregnancy? Cerebrovasc Dis. 1998;8(1):53–8.
Wagner SJ, Acquah LA, Lindell EP, Craici IM, Wingo MT, Rose CH, et al. Posterior reversible encephalopathy syndrome and eclampsia: pressing the case for more aggressive blood pressure control. Mayo Clin Proc. 2011;86(9):851–6.
Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21(1):60–5.
Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. New Engl J Med. 2003;349(5):427–34.
Laird-Meeter K, van de Ley G, Bom TH, Wladimiroff JW, Roelandt J. Cardiocirculatory adjustments during pregnancy—an echocardiographic study. Clin Cardiol. 1979;2(5):328–32.
Dennis AT, Castro J, Carr C, Simmons S, Permezel M, Royse C. Haemodynamics in women with untreated pre-eclampsia. Anaesthesia. 2012;67(10):1105–18.
Bamfo JE, Kametas NA, Chambers JB, Nicolaides KH. Maternal cardiac function in normotensive and pre-eclamptic intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;32(5):682–6.
• Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485(7398):333–8. This paper examines the link between preeclampsia and peripartum cardiomyopathy, and demonstrates that an anti-angiogenic environment is a sufficient source for both.
Simmons LA, Gillin AG, Jeremy RW. Structural and functional changes in left ventricle during normotensive and preeclamptic pregnancy. Am J Physiol Heart Circ Physiol. 2002;283(4):H1627–33.
Rafik Hamad R, Larsson A, Pernow J, Bremme K, Eriksson MJ. Assessment of left ventricular structure and function in preeclampsia by echocardiography and cardiovascular biomarkers. J Hypertension. 2009;27(11):2257–64.
Melchiorre K, Thilaganathan B. Maternal cardiac function in preeclampsia. Curr Opin Obstet Gynecol. 2011;23(6):440–7.
Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;58(4):709–15.
Shahul S, Rhee J, Hacker MR, Gulati G, Mitchell JD, Hess P, et al. Subclinical left ventricular dysfunction in preeclamptic women with preserved left ventricular ejection fraction: a 2D speckle-tracking imaging study. Circ Cardiovasc Imaging. 2012;5(6):734–9.
Bello N, Rendon IS, Arany Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: a systematic review and meta-analysis. J Am Coll Cardiol. 2013;62(18):1715–23.
Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974.
Harskamp RE, Zeeman GG. Preeclampsia: at risk for remote cardiovascular disease. Am J Med Sci. 2007;334(4):291–5.
•• Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre-eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol. 2014;63(18):1815–22. This paper discusses the elevated life-long increased risk of ischemic heart disease, metabolic syndrome, and stroke among women who develop severe preeclampsia.
Mannisto T, Mendola P, Vaarasmaki M, Jarvelin MR, Hartikainen AL, Pouta A, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127(6):681–90.
Wong CA, Liu S, Glassenberg R. Comparison of thrombelastography with common coagulation tests in preeclamptic and healthy parturients. Reg Anesth. 1995;20(6):521–7.
Sharma SK, Philip J, Whitten CW, Padakandla UB, Landers DF. Assessment of changes in coagulation in parturients with preeclampsia using thromboelastography. Anesthesiology. 1999;90(2):385–90.
D’Angelo R, Smiley RM, Riley ET, Segal S. Serious complications related to obstetric anesthesia: the serious complication repository project of the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2014;120(6):1505–12.
Beilin Y, Zahn J, Comerford M. Safe epidural analgesia in thirty parturients with platelet counts between 69,000 and 98,000 mm(-3). Anesth Analg. 1997;85(2):385–8.
Frenk V, Camann W, Shankar KB. Regional anesthesia in parturients with low platelet counts. Can J Anaesth. 2005;52(1):114.
Jouppila P, Jouppila R, Hollmen A, Koivula A. Lumbar epidural analgesia to improve intervillous blood flow during labor in severe preeclampsia. Obstet Gynecol. 1982;59(2):158–61.
Ramos-Santos E, Devoe LD, Wakefield ML, Sherline DM, Metheny WP. The effects of epidural anesthesia on the Doppler velocimetry of umbilical and uterine arteries in normal and hypertensive patients during active term labor. Obstet Gynecol. 1991;77(1):20–6.
Ginosar Y, Nadjari M, Hoffman A, Firman N, Davidson EM, Weiniger CF, et al. Antepartum continuous epidural ropivacaine therapy reduces uterine artery vascular resistance in pre-eclampsia: a randomized, dose-ranging, placebo-controlled study. Br J Anaesth. 2009;102(3):369–78.
Huang CJ, Fan YC, Tsai PS. Differential impacts of modes of anaesthesia on the risk of stroke among preeclamptic women who undergo Caesarean delivery: a population-based study. Br J Anaesth. 2010;105(6):818–26.
Visalyaputra S, Rodanant O, Somboonviboon W, Tantivitayatan K, Thienthong S, Saengchote W. Spinal versus epidural anesthesia for cesarean delivery in severe preeclampsia: a prospective randomized, multicenter study. Anesth Analg. 2005;101(3):862–8; table of contents.
Hodgkinson R, Husain FJ, Hayashi RH. Systemic and pulmonary blood pressure during caesarean section in parturients with gestational hypertension. Can Anaesth Soc J. 1980;27(4):389–94.
Wallace DH, Leveno KJ, Cunningham FG, Giesecke AH, Shearer VE, Sidawi JE. Randomized comparison of general and regional anesthesia for cesarean delivery in pregnancies complicated by severe preeclampsia. Obstet Gynecol. 1995;86(2):193–9.
Aya AG, Mangin R, Vialles N, Ferrer JM, Robert C, Ripart J, et al. Patients with severe preeclampsia experience less hypotension during spinal anesthesia for elective cesarean delivery than healthy parturients: a prospective cohort comparison. Anesth Analg. 2003;97(3):867–72.
Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125(7):911–9.
Sunderji S, Gaziano E, Wothe D, Rogers LC, Sibai B, Karumanchi SA, et al. Automated assays for sVEGF R1 and PlGF as an aid in the diagnosis of preterm preeclampsia: a prospective clinical study. Am J Obstet Gynecol. 2010;202(1):40 e1-7.
Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Maternal Fetal Neonatal Med. 2008;21(1):9–23.
Thadhani R, Kisner T, Hagmann H, Bossung V, Noack S, Schaarschmidt W, et al. Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation. 2011;124(8):940–50.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical collection on Obstetric Anesthesia.
Rights and permissions
About this article
Cite this article
Sanders, C.E., Hess, P.E. Updates in Preeclampsia. Curr Anesthesiol Rep 5, 74–81 (2015). https://doi.org/10.1007/s40140-014-0091-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-014-0091-4