Abstract
In nature, an important example of chemical communication and synchronicity can be found in cell populations where long-range chemical communication takes place over micrometer distance. In vitro laboratory systems can be useful to understand and control such complex biological mechanisms and, in a biomimetic approach, we present in this paper a model based on three basic features, namely (i) the compartmentalization of chemical information (using microfluidics), (ii) a stable emitter of periodic chemical signals inside compartments (Belousov-Zhabotinsky oscillating reaction) and (iii) a suitable spatio-temporal monitoring of the emitted chemical signal. In particular, starting from our recent work on the communication among oscillators via chemical intermediates in networks of lipid-stabilised droplets, we discuss here the role of compartments and of the geometry of the system. We present 3 different experimental configurations, namely liposomes (water-in-water dispersions), double emulsions (water-in-oil-in-water dispersions) and simple emulsions (water-in-oil dispersions) and we show that the global behaviour of networks can be influenced and controlled by several experimental parameters, like the nature of the collecting solvent, the presence of dopants and the network geometry. Numerical models supporting and explaining the experimental findings are also discussed.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
- Belousov-Zhabotinsky reaction
- Microfluidics
- Lipid droplets
- Chemical oscillators network
- Chemical coupling
1 Introduction
Biological systems are the most fascinating expression of self-organisation phenomena taking place in nature. After Prigogine’s work, self-organisation is interpreted as the tendency of far-from-equilibrium systems, also known as Dissipative Structures, to spontaneously organise in more complex assemblies, starting from simple elements. Such kind of organisation bears new features that are in a stationary state, kept far from thermodynamic equilibrium by a constant flux of energy and/or matter [1,2,3]. As an extension, several researchers considered Life and many of its manifestations as dissipative structures, providing, for instance, a possible solution to the evolutionary problem of order out of disorder in the transitional stages between abiotic and prebiotic ages [4]. Beside the high hierarchical structures, like in biological systems, there are simple physical and chemical systems that manifest self-organising properties, such as, for example, the Belousov-Zhabotinsky (BZ) chemical oscillator [5, 6]. Starting from the seventies, chemical oscillators quickly became a simple model for studying complex phenomena typical of the living realm, such as oscillations, bistability, excitability and pattern formation [7, 8].
However, equilibrium dynamics is also fundamental for understanding the beautiful complexity of nature. In this respect, similarly to self-organisation, self-assembly has been defined as the tendency of single components to spontaneously aggregate in complex structures while tending to a minimum (or a maximum) of a thermodynamic potential [9,10,11]. In chemical and biochemical fields, dispersed media like micelles, emulsions and liposomes are genuine examples of self-assembling systems [12]. Unlikely to Dissipative Structures, such systems do not need a continuos flux of energy to survive [9, 10, 13].
In this context, blending the structural properties of self-assembled matrixes together with the evolutive peculiarities of dissipative system, allows to study an important aspect of biological systems, namely the transmission of signals across an amphiphilic boundary layer (membrane) and the synchronisation and communication among coupled chemical systems in networks [14, 15]. The Epstein’s group in Brandeis pioneered the study of coupled chemical oscillators in water-in-oil nano size domain (microemulsions), where the interfacial film was a simple AOT monolayer [16, 17] to find that, the exchange of molecules among water compartments dispersed in a nonpolar solvent and containing the BZ reaction, produced a rich variety of structured patterns at the macroscopic level. The cooperative behaviour of nano-droplets, mediated by diffusion (or cross-diffusion [18, 19]) of chemical messengers, resulted in an unexpected and emergent global behaviour at a higher hierarchical level. By using microfluidics technique, the microemulsion system was then upscaled to an emulsion system with the droplets having a characteristic size of hundreds of micrometers; here it was found that a network of diffusively coupled oscillators could produce global in-phase and out-of-phase oscillations, or more complex dynamical behaviours, depending whether the messenger molecules were activators, inhibitors or a mix of the two, respectively [20,21,22]. Other groups used microfluidics devices to explore similar configurations [23,24,25].
In a more realistic biomimetic approach, our group substituted synthetic surfactants with phospholipids to stabilise droplets in dispersed systems. We could thus study pattern formation in membrane model systems [26,27,28] and, by using microfluidics [29], chemical communication in liposomes [30], double emulsions [31] and emulsions [32]. The oscillating BZ reaction was employed as the signal generator. The overall reaction is driven by the oxidation of an organic substrate, e.g. malonic acid (MA), by bromate in acidic solution in the presence of a catalytic species in the form of an organometal complex, such as ferroin [a phenanthroline-iron(II) complex]. The oscillatory dynamics, however, is governed by the amount of the inhibitory intermediate bromine and the excitatory intermediate bromous acid. These BZ intermediates might diffuse between individual microdrops, thus affecting the overall oscillatory dynamics and synchrony of multiple drop arrays. As such, the intermediates serve as messenger molecules between individual drops. More details about the kinetic mechanism responsible for oscillations will be given in Sect. 3. 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) is known to form stable bilayers in water and to self-assemble spontaneously at the water-oil interface giving resistant, yet internally fluid, membranes [33, 34]. DMPC was thus used in our experiments as the principal component of the membranes.
In this paper we present 3 different experimental configurations, namely liposomes (water-in-water dispersions), multi-core double emulsions (water-in-oil-in-water dispersions) and simple emulsions (water-in-oil dispersions), whose structures are sketched in Fig. 1, and we discuss how the global behaviour of networks can be influenced and controlled by the nature of the collecting solvent, the presence of dopants and the network geometry. Numerical models supporting and explaining the experimental findings will be also discussed.
2 Experimental Approach: Microfluidic Techniques for the Generation of Oscillating Droplets
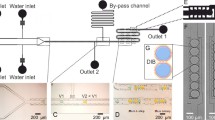
Microfluidic techniques are a reliable and easy method to synthesize droplets (either single and double emulsions, liposomes or polymersomes) with controllable size, monodispersity and composition [29]. In particular, to generate both liposomes and emulsions loaded with the BZ reaction, we employed a home-made coaxial flow microfluidic device adapted from the setup devised by the group of D. Weitz [35]. The geometry of our setup varied depending on the experimental configuration we explored. In particular, we studied three different systems: planar 2-D liposomes and multi-core double emulsions and planar 1-D arrays of single emulsions. Figure 2 resumes the experimental conditions and the experimental observations of the most significative results in liposomes (left panel) and multi-core double emulsions (right panel). Experimental details are in the figure captions and in references [30, 31]. From a physical point of view, the two systems have different solvents separating the oscillating droplets; this fact implies the presence of an osmotic pressure in the case of liposomes that has to be balanced by adding an electrolyte in the collecting solution. Moreover, the different solubility properties of the surrounding solvents might affect the communication among the oscillators. In fact, these features are reflected in the dynamical behaviour of the two systems. In the case of liposomes we observed the development of autocatalytic fronts in single droplets, that could be transmitted from one compartment to the neighbours. In the series of pictures b–e of Fig. 2 an autocatalytic front starts to oxidise the droplet 1, wherein the colour change from dark (reduced form of the catalyst, ferroin) to bright (oxidised form of the catalyst, ferriin). The oxidative front is then propagated via the transduction of a chemical signal through the liposomes membrane to the surrounding droplets, as it is evident from the colour change of droplets 2, 5 and 6. The signal transmission sequence among liposomes 2, 5 and 6 was analysed by means of the space-time (ST) plot reported in Fig. 2f. Thin slices cut from each frame along the white line in Fig. 2e were vertically stacked, so that the horizontal axis represents the actual space spanned by the oxidation pulse (\(\sim \)820 \(\upmu \)m) and the vertical axis represents the time elapsed from the generation of the first pulse in liposome 2 to the end of the last pulse in liposome 6 (\(\sim \)23 s). The reciprocal of the slope of the diagonal borders between dark and bright areas represents the speed at which the chemical pulses travel inside the water compartment of the liposome and was calculated to be in the range 110–150 \(\upmu \)m s\(^{-1}\). From the ST plot we could also quantify the lag time in about 5 s, during which a liposome remains in an oxidised state before transmitting the impulse to its neighbour.
Experimental setup and dynamical behaviour in liposomes (left panel) and multi-core double emulsions (right panel). (a) Microfluidic device for liposomes generation: the inner phase was a BZ aqueous mixture having \(\text {H}_2\text {SO}_4\) (300 mM), \(\text {NaBrO}_3\) (120 mM), MA (30 mM) and ferroin (5 mM), the middle phase contained DMPC solubilised in a mixture of chloroform: cyclohexane (40:60; v/v), the external phase was an aqueous solution of PVA (Polyvinyl alcohol) (7%, wt), an aqueous solution of \(\text {NaBrO}_3\) (0.4 M) was used to recuperate the liposomes at the exit of the microfluidic device; (b)–(e) pulse transmission across the touching liposomes after solvent evaporation. White arrows indicate the direction of pulse propagation; (f) space-time plot of liposomes 2, 5, 6 along the white bar in panel (e); (g) Microfluidic device for double emulsion generation. Conditions are the same as in (a) but in this case the droplets and the surrounding oil phase where collected in a PVA solution only at the end of the encapsulation process, preventing the evaporation of the organic solvents, \(\text {H}_2\text {SO}_4\) (350 mM), \(\text {NaBrO}_3\) (180 mM), MA (150 mM) and ferroin (2.5 mM); (h) Final configuration of the oscillating droplets in double emulsions; (i) Time series of the oscillating dynamics of the two droplets in the red circlet in (h); (j) Temporal behaviour of the phase difference between the two droplets calculated by using the Eq. (1). (Color figure online)
(a) Sketch of the microfluidic device used to generate the droplet arrays; (b) 1D array of BZ containing droplets collected in a PTFE tube for monitoring. (c) Array of six oscillating droplets in the simple emulsion system. Space-Time plots of each droplet were reconstructed from the movie frames (sampling time 1 s). (d) Time-series extracted from the Space-Time plots by converting the pixels in grey scale values; \(\text {H}_2\text {SO}_4\) (300 mM), \(\text {NaBrO}_3\) (120 mM), MA (300 mM) and ferroin (5 mM), The suspending oil phase consisted of a mixture of chloroform/cyclohexane (1:2), DMPC (0.8% w/w), and STS (0.2% w/w).
In liposomes experiments, we demonstrated an actual communication among different single oscillators. The oxidative pulse transmission suggested the autocatalytic species, \(\text {HBrO}_2\), as the main messenger molecule able to cross the DMPC bilayers and this was confirmed by an electrochemical investigation of the membranes during the oscillatory cycles [30, 36]. However, mainly because of the osmotic pressure, the stability of liposomes was not long enough (<10 min) to study the network dynamics during a series of sustained oscillations. Therefore we devised a series of experiments in a double emulsion system as depicted in Fig. 2g and h, where the DMPC stabilised oscillating droplets were dispersed in an organic solvent (the same used for the encapsulation process) and, in turn, in a PVA (Polyvinyl Alcohol) solution. In contrast to liposomes, in double emulsions the DMPC membrane around each droplet is a thick layer (30–50 nm) with disordered internal structure. By avoiding the osmotic pressure problems, we could obtain droplets stable enough to record more than 10 oscillatory cycles (\(\sim \)30 min) and observe an in-phase synchronisation tendency between touching droplets. Figure 2i shows the timeseries extracted from the two droplets in the red circlet of Fig. 2h; it is quite evident that, after few oscillations, the two droplets tend to spike with the same period and phase. This is also confirmed by the evolution of the phase difference between the two oscillators (\(\phi _{12}=\phi _1-\phi _2\)) reported in Fig. 2j and calculated by means of the Eq. (1) [37, 38].
where \(t_k\) is the time of the k-th peak of the oscillatory time series of the oscillator i. The diagonal lines in Fig. 2j mean that the phase difference is changing in time, while horizontal lines indicate that both oscillators have the same period and oscillate with a constant phase difference. This represents a phase lock state, i.e. a coherent behaviour of the two oscillators. The phase difference, in particular, shifts from an initial value of about 0.5 p during the first cycles to 0 at the end, indicating that the two oscillators adjust their oscillation frequency until they reach a synchronistic behaviour. The in-phase oscillations of the communicating droplets reveals an activatory coupling path, that can be brought about by the exchange of the autocatalytic intermediates [39], in agreement with the electrochemical investigations and with the pulse transmission in liposomes. Simulations presented in the Sect. 3 confirmed this hypothesis.
1-D arrays of oscillating droplets were built to explore a linear connection geometry in a controlled and tunable configuration. By taking advantage of the microfluidic setup shown in Fig. 3a, it was possible to obtain a reliable and robust network of oscillators that could be monitored for longer periods with respect to double emulsions. In this case, we dealt with a single emulsion system obtained by keeping the droplets inside of the collection tube (Fig. 3b). Experimental details are in the figure caption and in reference [32]. In order to work at a low lamellarity of the membranes between the touching droplets, thus facilitating the exchange of messenger molecules, we used sodium tetradecyl sulfate (STS), since charged surfactants are known to favour oligo- (or mono-) lamellar structures in liposomes, to dope the DMPC [32, 40]; cholesterol (CHOL) was also used to interact with brominated BZ intermediates and tune the communication between droplets [32, 40].
As an example of the operative setup, Fig. 3c shows a simple emulsion array of six oscillating droplets loaded with BZ and surrounded by the mixture of cyclohexane/chloroform containing DMPC+STS. On the right of the array, the ST plots display the oscillating dynamics of each droplet. The vertical bright lines correspond to a firing of the oscillator (oxidized catalyst), whilst the dark regions represent the recovery period (reduced catalyst) between single oscillations. From the ST plots of four droplets (2–5), the time-series were extracted; the corresponding time-series are reported in Fig. 3d.
The analysis of the timeseries for the DMPC+STS system revealed an anti-phase global dynamics among adjacent droplets (\(\Delta \phi \sim \pi \)) and an in-phase synchronisation among alternating droplets (\(\Delta \phi \sim 0\)), as highlighted in Fig. 4a–b. This behaviour is typical of networks of oscillators coupled via inhibitory signals, that in the BZ case are generally \(\text {Br}_2\) or Br\(^{-}\) intermediates. To confirm the prominent role of brominated species as inhibitory messengers in the simple emulsions system, we modified the membrane composition by inserting CHOL molecules in order to modulate the communication pathway and to seek a difference in the global behaviour of the array. We expected, in fact, that cholesterol-doped DMPC membranes act as a barrier for \(\text {Br}_2\), thus preventing, or at least mitigating, inhibitory coupling. The analysis of the phase difference of the droplets couples reported in Fig. 4c–d, shows a substantially erratic behaviour for all the permutated couples except for a weak coupling of the droplets 3,4.
(a) Phase difference of the alternate droplets 2,4 and 3,5, as a function of time. The inset shows the count distribution for various intervals of phase differences in the DMPC+STS system; (b) Phase differences of adjacent droplet couples vs time, displaying the progressive phase shift to anti-phase oscillations in the DMPC+STS system; (c) Phase difference of the droplets 3,4 as a function of time, the inset shows the count distribution for various intervals of phase differences in the DMPC+CHOL system; (d) Phase differences of the droplet couples 2,3; 2,4; 4,5; and 3,5 vs time, displaying an uncorrelated coupling for these adjacent and alternate droplets in the DMPC+CHOL system.
In this section, we showed that the global behaviour of a network of coupled oscillators can be influenced and controlled by several experimental parameters: (i) the nature of the collecting solvent determines the type of dispersion (liposomes, double and single emulsions) and control the osmotic pressure; (ii) the presence of dopants like STS favour communication among droplets by decreasing the lamellarity of the membranes; (iii) CHOL in the membranes selectively interacts with messenger molecules; (iv) finally, also the network geometry exerts a certain influence on the type of communication pathways among individual oscillators.
In the next section we propose few models that can explain and reproduce some of the observed results.
3 Modeling
The first step for understanding the dynamics of the droplets network is to model the chemical system responsible for the oscillations. A minimal reaction mechanism that reproduces the complex chemistry of the ferroin catalysed BZ reaction is represented by the reaction scheme (R1)–(R14) with relative kinetic constants reported in Table 1 [32, 41,42,43]. According to the classic interpretation of the oscillatory mechanism [44], the reactions (R1)–(R14) can be simplified in three main processes as sketched in the lower panel of Fig. 5. Process A accounts for the reactions (R1)–(R4) and it is dominated by the inhibitor Br\(^{-}\) chemistry, process B accounts for the reactions (R5)–(R8) and represents the autocatalytic reactions that involve the activator \(\text {HBrO}_2\), process C accounts for reactions (R9)–(R14) and it is responsible for the regeneration of the catalyst (\({\text {M}^{(\text {o}x)}}\) \(\rightarrow \) \(\text {M}^{\text {(red)}}\)) and the production of the inhibitor that, in turn, restarts the cycle. A kinetic scheme can then be derived from the three processes and numerically integrated for simulating the dynamics of the systems (legend for the symbols is reported in the Fig. 5 caption).
(a) Sketch of two touching droplets. \((d_i)\) represents the droplet, \(d_d\) is a droplet diameter and \(d_c\) is the diameter of the contact surface between two droplets, which has been approximated to a circle; (b) Model of the BZ oscillator array.
The lower part of the panel shows the most import processes accounting for the chemistry of the BZ reaction: \(X_i\), \(Y_i\) and \(Z_i\) represent the concentration of \(\text {HBrO}_2\), Br\(^{-}\) and \(\text {M}^{\text {o}x}\) in droplet i, respectively; the kinetic constants derived from Table 1 are \(k_1\) (M\(^{-1}\)s\(^{-1}\)) = 0.245, \(k_2\) (M\(^{-1}\)s\(^{-1}\)) = \(1.05 \times 10^6\), \(k_3\) (M\(^{-1}\)s\(^{-1}\)) = 14.7, \(k_4\) (M\(^{-1}\)s\(^{-1}\)) = \(1.05 \times 10^3\), \(k_z\) (M\(^{-1}\)s\(^{-1}\)) = 1, f = 0.5 is a stoichiometric factor which account for the Br\(^{-}\) regeneration. \(k_c^i = k_{ - c}^i\) (s\(^{-1}\)) are the transfer kinetic constants related with the permeability of the i-th species, \(P_m^i\) (cm/s), towards the phospholipid membranes by the relation \(k_c^i= P_m^i A_c/V_d\), where \(V_d\) is the droplet volume and \(A_c\) is the contact surface area between two droplets. The values for \(V_d\) and \(A_c\) were determined from experiments, the value for \(P_m^1\) is 0.07 cm/s [45], the value for \(P_m^2\) was chosen as \(1 \times 10^{-4}\) cm/s [46], so that \(k_c^1\) = 0.15 s\(^{-1}\) and \(k_c^2\) = \(2 \times 10^{-4}\) s\(^{-1}\).
Colours map the state and the transition of the catalyst forms, red for the reduced and blue for the oxidised state. (Color figure online)
(a)–(b) Signal transmission between two liposomes. At t = 400 s a signal has been triggered in droplet 1 (\(\text {[HBrO}_{2}]_\text {ex}\) = \(1.1 \times 10^{-6}\) M). After about 4 s the signal reaches droplet 2 and causes the production of the autocatalytic species and the consequent oxidation of the ferroin; (c) Numerical simulations of the coupled dynamics of two BZ droplets in a double emulsion system. (d) Time evolution of the phase difference calculated from the time series in the panel (c); (e)–(h) Simulated phase dynamics for four droplets in an array of linearly coupled oscillating simple emulsions: (e) Phase difference for the synchronised droplets in the DMPC+STS system; (f) Phase difference for the adjacent droplets oscillating in anti-phase in the DMPC+STS system; (g) phase difference for the weakly coupled droplets (3,4) in the DMPC+CHOL system; (h) phase difference for adjacent and alternate droplets showing uncorrelated phase behaviour over time.
When communication among droplets is considered, the system can be modelled as sketched in Fig. 5a for liposomes and double emulsions and b for the droplets array: each droplet contains the BZ reaction and it is free to exchange the activator, \(\text {HBrO}_2\), and the inhibitor, \(\text {Br}_2\), with neighbours through a simple equilibrium reaction following mass action kinetics. The coupling reactions are reported in the lowest part of Fig. 5 with the corresponding kinetics to be included in the BZ scheme. Details are reported in the figure caption. DMPC was
always used as the major structural unit for the mono- and bilayer membranes, the latter present at all the droplet interfaces, alternatively intercalated with CHOL and STS molecules in the case of arrays.
When cholesterol was intercalated in the DMPC membranes, a fast bromination reaction was added to the scheme, similarly to the bulk systems we investigated in reference [40]
with a kinetic rate constant k = 340 M\(^{-1}\)s\(^{-1}\).
Numerical simulations were performed by integrating the kinetic scheme by means of the CO.PA.SI. software [47]. All data used in simulations reflected the real experimental parameters, and the different initial conditions of the droplets were reproduced by introducing a small delta in the concentration of the reactants (±0.1%). All the simulations details can be found in references [30,31,32, 40], Fig. 6 resumes the most important numerical results that reproduce the experimental findings presented in Sect. 2.
The pulse transmission between two communicating liposomes (Fig. 6a and b) was clearly reproduced on a timescale comparable with the experiments (\(\sim \)4 s). In this case we simply perturbed one droplet in its excitable state and we followed the time evolution of the impulse in a second droplet. Both the autocatalysis and the oxidation of the catalyst take place with a lag time consistent with the experimental data reported in Fig. 2f.
Phase synchronisation of two communicating droplets in a double emulsion system was also reproduced by numerical simulations (Fig. 6c and d), both the oscillation profiles and the evolution of the phase difference are in excellent agreement with the experimental results reported in Fig. 2i and j.
Finally the typical behaviour of the arrays of simple emulsions, either in the presence of STS or CHOL as dopants in the membranes (Fig. 4), was reproduced by the model as showed by the results in Fig. 6e–h.
4 Conclusions
In this paper we discussed how the nature of the compartments, of the solvents and of the network geometry influenced the communication among chemical oscillators in networks of lipid-stabilised droplets. We employed the Belousov-Zhabotinsky reaction as the source/sink of chemical signals transmitted from and to single network elements. The chemical signals directly influenced the time evolution of each droplet, that, in turn, creates a feedback to the network. Microfluidic techniques allowed precise and reliable control over the experimental conditions, thus we could explore several network configurations in different chemical environments. By using this approach, it can be relatively simple to follow the global dynamics of large networks of far-from-equilibrium reactions, that can mimic the complex behaviours typical of the biological systems (self-organisation and self-regulation, oscillations, pattern formation etc.). Moreover, with respect to the previous work on similar systems, the use of lipids as barrier-forming molecules confers to the overall structure a enhanced biomimetic character.
We investigated 3 different experimental configurations as a function of the environment where the droplets were dispersed, namely liposomes (water-in-water dispersions), double emulsions (water-in-oil-in-water dispersions) and simple emulsions (water-in-oil dispersions). The lipid molecule DMPC was always used as the major structural unit alternatively intercalated with STS or CHOL molecules to tune the communication properties.
We showed that the global behaviour of networks can be influenced and controlled by several experimental parameters, like the nature of the collecting solvent, the presence of dopants and the network geometry. The most important molecules responsible for communication were identified in the brominated species, being the inhibitor \(\text {Br}_2\) and the autocatalytic activator \(\text {HBrO}_2\) the ones chosen for numerical simulations. In liposomes and double emulsions the communication was dominated by the activators (pulse transmission and in-phase oscillations), in contrast to the 1-D arrays where the communication between adjacent droplets mainly exhibited an inhibitory character (anti-phase oscillations), governed by the prominent role of \(\text {Br}_2\). In the presence of mono-lamellar membranes, in fact, molecular bromine has a higher permeability with respect to the activator \(\text {HBrO}_2\). This is also confirmed by the experiments with bromine-blocking molecule (i.e. cholesterol) intercalated in the membrane structure; in this case, the global dynamics resulted in a weakly coupled array with an erratic global behaviour. Numerical simulations of coupled oscillators (up to 6 units) confirmed our hypothesis and could reproduce, qualitatively and quantitatively, the experimental observations.
References
Prigogine, I.: Time, structure and fluctuations. In: Nobel Lectures, Chemistry 1971–1980, pp. 263–285. World Scientific Publishing Co., Singapore (1977)
Nicolis, G., Prigogine, I.: Self-organization in Nonequilibrium Systems. Wiley, New York (1977)
Field, R.J., Burger, M.: Oscillations and Traveling Waves in Chemical Systems. Wiley, New York (1985)
Ruiz-Mirazo, K., Briones, C., de la Escosura, A.: Prebiotic systems chemistry: new perspectives for the origins of life. Chem. Rev. 114(1), 285–366 (2014)
Belousov, B.P.: A periodic reaction and its mechanism. In: Sbornik Referatov po Radiatsonno Meditsine, Moscow, Medgiz, pp. 145–147 (1958)
Zhabotinsky, A.M.: Periodic liquid phase reactions. Proc. Acad. Sci. USSR 157, 392–395 (1964)
Winfree, A.T.: The Geometry of Biological Time. Springer, Heidelberg (2001). https://doi.org/10.1007/978-3-662-22492-2
Tiezzi, E.: Steps Towards an Evolutionary Physics. WIT Press, Southempton (2006)
Yamaguchi, T., Suematsu, N., Mahara, H.: Nonlinear dynamics in polymeric systems. In: Pojman, J.A., Tran-Cong-Miyata, Q. (eds.) Nonlinear Dynamics in Polymeric Systems. Volume 869 of ACS Symposium Series, Washington DC, pp. 16–27 (2004)
Yamaguchi, T., Epstein, I.R., Shimomura, M., Kunitake, T.: Introduction: engineering of self-organized nanostructures. Chaos 15(4), 047501-1–047501-3 (2005)
Gompper, G., Domb, C., Green, M.S., Schick, M., Lebowitz, J.L.: Phase Transitions and Critical Phenomena: Self-assembling Amphiphilic Systems. Academic Press, Cambridge (1994)
Cevc, G.: Phospholipids Handbook. CRC Press, Boca Raton (1993)
Fennell-Evans, D., Wennerström, H.: The Colloidal Domain: Where Physics, Chemistry, Biology, and Technology Meet. Wiley, Hoboken (1999)
Lach, S., Yoon, S.M., Grzybowski, B.A.: Tactic, reactive, and functional droplets outside of equilibrium. Chem. Soc. Rev. 45, 4766–4796 (2016)
Ashkenasy, G., Hermans, T.M., Otto, S., Taylor, A.F.: Systems chemistry. Chem. Soc. Rev. 46(9), 2543–2554 (2017)
Vanag, V.K., Epstein, I.R.: Pattern formation in a tunable medium: the Belousov-Zhabotinsky reaction in an aerosol OT microemulsion. Phys. Rev. Lett. 87(22), 228301–4 (2001)
Epstein, I.R., Xu, B.: Reaction-diffusion processes at the nano- and microscales. Nat. Nanotechnol. 11(4), 312–319 (2016)
Rossi, F., Vanag, V.K., Epstein, I.R.: Pentanary cross-diffusion in water-in-oil microemulsions loaded with two components of the Belousov-Zhabotinsky reaction. Chem. Eur. J. 17(7), 2138–2145 (2011)
Budroni, M.A., Lemaigre, L., De Wit, A., Rossi, F.: Cross-diffusion-induced convective patterns in microemulsion systems. Phys. Chem. Chem. Phys. 17(3), 1593–1600 (2015)
Toiya, M., Vanag, V.K., Epstein, I.R.: Diffusively coupled chemical oscillators in a microfluidic assembly. Angew. Chem. Int. Ed. 47(40), 7753–7755 (2008)
Delgado, J., Li, N., Leda, M., González-Ochoa, H.O., Fraden, S., Epstein, I.R.: Coupled oscillations in a 1D emulsion of Belousov-Zhabotinsky droplets. Soft Matter 7(7), 3155 (2011)
Tompkins, N., Li, N., Girabawe, C., Heymann, M., Ermentrout, G.B., Epstein, I.R., Fraden, S.: Testing Turing’s theory of morphogenesis in chemical cells. Proc. Natl. Acad. Sci. 111(12), 4397–4402 (2014)
Thutupalli, S., Herminghaus, S., Seemann, R.: Bilayer membranes in micro-fluidics: from gel emulsions to soft functional devices. Soft Matter 7(4), 1312 (2011)
de Souza, T.P., Perez-Mercader, J.: Entrapment in giant polymersomes of an inorganic oscillatory chemical reaction and resulting chemo-mechanical coupling. Chem. Commun. 50(64), 8970–8973 (2014)
Guzowski, J., Gizynski, K., Gorecki, J., Garstecki, P.: Microfluidic platform for reproducible self-assembly of chemically communicating droplet networks with predesigned number and type of the communicating compartments. Lab Chip 16(4), 764–772 (2016)
Magnani, A., Marchettini, N., Ristori, S., Rossi, C., Rossi, F., Rustici, M., Spalla, O., Tiezzi, E.: Chemical waves and pattern formation in the 1,2-dipalmitoyl-sn-glycero-3-phosphocholine/water lamellar system. J. Am. Chem. Soc. 126(37), 11406–11407 (2004)
Ristori, S., Rossi, F., Biosa, G., Marchettini, N., Rustici, M., Tiezzi, E.: Interplay between the Belousov-Zhabotinsky reaction-diffusion system and biomimetic matrices. Chem. Phys. Lett. 436, 175–178 (2007)
Rossi, F., Ristori, S., Rustici, M., Marchettini, N., Tiezzi, E.: Dynamics of pattern formation in biomimetic systems. J. Theor. Biol. 255(4), 404–412 (2008)
Torbensen, K., Rossi, F., Ristori, S., Abou-Hassan, A.: Chemical communication and dynamics of droplet emulsions in networks of Belousov-Zhabotinsky micro-oscillators produced by microfluidics. Lab Chip 17(7), 1179–1189 (2017)
Tomasi, R., Noel, J.M., Zenati, A., Ristori, S., Rossi, F., Cabuil, V., Kanoufi, F., Abou-Hassan, A.: Chemical communication between liposomes encapsulating a chemical oscillatory reaction. Chem. Sci. 5(5), 1854–1859 (2014)
Rossi, F., Zenati, A., Ristori, S., Noel, J.M., Cabuil, V., Kanoufi, F., Abou-Hassan, A.: Activatory coupling among oscillating droplets produced in microfluidic based devices. Int. J. Unconventional Comput. 11(1), 23–36 (2015)
Torbensen, K., Ristori, S., Rossi, F., Abou-Hassan, A.: Tuning the chemical communication of oscillating microdroplets by means of membrane composition. J. Phys. Chem. C 121(24), 13256–13264 (2017)
Nii, T., Ishii, F.: Properties of various phosphatidylcholines as emulsifiers or dispersing agents in microparticle preparations for drug carriers. Colloids Surf. B: Biointerfaces 39(1), 57–63 (2004)
Di Cola, E., Torbensen, K., Clemente, I., Rossi, F., Ristori, S., Abou-Hassan, A.: Lipid stabilized water- oil interfaces studied by micro focusing small angle X-ray scattering. Langmuir 33(36), 9100–9105 (2017)
Utada, A.S., Lorenceau, E., Link, D.R., Kaplan, P.D., Stone, H.A., Weitz, D.A.: Monodisperse double emulsions generated from a microcapillary device. Science 308(5721), 537–541 (2005)
Stockmann, T.J., Noël, J.M., Ristori, S., Combellas, C., Abou-Hassan, A., Rossi, F., Kanoufi, F.: Scanning electrochemical microscopy of Belousov-Zhabotinsky reaction: how confined oscillations reveal short lived radicals and auto-catalytic species. Anal. Chem. 87(19), 9621–9630 (2015)
Pikovsky, A.S., Rosenblum, M.G., Osipov, G.V., Kurths, J.: Phase synchronization of chaotic oscillators by external driving. Phys. D: Nonlinear Phenom. 104(3–4), 219–238 (1997)
Fukuda, H., Morimura, H., Kai, S.: Global synchronization in two-dimensional lattices of discrete Belousov-Zhabotinsky oscillators. Phys. D: Nonlinear Phenom. 205(1–4), 80–86 (2005)
Vanag, V.K., Epstein, I.R.: Excitatory and inhibitory coupling in a one-dimensional array of Belousov-Zhabotinsky micro-oscillators: theory. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 84(6 Pt 2), 066209 (2011)
Torbensen, K., Rossi, F., Pantani, O.L., Ristori, S., Abou-Hassan, A.: Interaction of the Belousov-Zhabotinsky reaction with phospholipid engineered membranes. J. Phys. Chem. B 119(32), 10224–10230 (2015)
Benini, O., Cervellati, R., Fetto, P.: Experimental and mechanistic study of the bromomalonic acid/bromate oscillating system catalyzed by [Fe(phen)3]2+. Int. J. Chem. Kinet. 30(4), 291–300 (1998)
Rossi, F., Varsalona, R., Liveri, M.L.T.: New features in the dynamics of a ferroin-catalyzed Belousov-Zhabotinsky reaction induced by a zwitterionic surfactant. Chem. Phys. Lett. 463(4–6), 378–382 (2008)
Rossi, F., Lombardo, R., Sciascia, L., Sbriziolo, C., Liveri, M.L.T.: Spatio-temporal perturbation of the dynamics of the ferroin catalyzed Belousov-Zhabotinsky reaction in a batch reactor caused by sodium dodecyl sulfate micelles. J. Phys. Chem. B 112, 7244–7250 (2008)
Noyes, R.M., Field, R., Koros, E.: Oscillations in chemical systems. I. Detailed mechanism in a system showing temporal oscillations. J. Am. Chem. Soc. 94(4), 1394–1395 (1972)
Zhang, J., Unwin, P.R.: Kinetics of bromine transfer across Langmuir monolayers of phosphatidylethanolamines at the water/air interface. Phys. Chem. Chem. Phys. 5(18), 3979–3983 (2003)
Xiang, T.X., Anderson, B.D.: Permeability of acetic acid across gel and liquid-crystalline lipid bilayers conforms to free-surface-area theory. Biophys. J. 72(1), 223–237 (1997)
Kummer, U., Hoops, S., Sahle, S., Gauges, R., Lee, C., Pahle, J., Simus, N., Singhal, M., Xu, L., Mendes, P.: COPASI-a COmplex PAthway SImulator. Bioinformatics 22(24), 3067–3074 (2006)
Acknowledgments
F.R. gratefully acknowledge the University of Salerno for the grants ORSA158121 and ORSA167988. F.R. and A.A-H. acknowledge the support through the COST Action CM1304 (Emergence and Evolution of Complex Chemical Systems).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this paper
Cite this paper
Rossi, F., Torbensen, K., Ristori, S., Abou-Hassan, A. (2018). Signal Transduction and Communication Through Model Membranes in Networks of Coupled Chemical Oscillators. In: Pelillo, M., Poli, I., Roli, A., Serra, R., Slanzi, D., Villani, M. (eds) Artificial Life and Evolutionary Computation. WIVACE 2017. Communications in Computer and Information Science, vol 830. Springer, Cham. https://doi.org/10.1007/978-3-319-78658-2_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-78658-2_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-78657-5
Online ISBN: 978-3-319-78658-2
eBook Packages: Computer ScienceComputer Science (R0)