Abstract
Diphtheria, a highly contagious disease, is now rare in most countries but still endemic in many developing nations. Clinical features on presentation include sore throat, difficulty swallowing, low-grade fever, and an adherent pseudomembrane in the throat. Cardiac and neurologic complications may result from the potent exotoxin produced by Corynebacterium diphtheriae. Early diagnosis and prompt treatment with antitoxin, along with antibiotics, are essential for decreasing the risk of death. The only way to reduce the prevalence of diphtheria in endemic countries and prevent future epidemics is through complete vaccination of all children and booster vaccination of adults. This chapter reviews the epidemiology, clinical features, diagnosis, treatment, and prevention of diphtheria.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
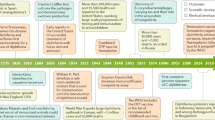
Diphtheria , a contagious disease first recognized by Hippocrates in the fifth century BCE, is caused by the Gram-positive bacillus, Corynebacterium diphtheriae . The word “diphtheria” comes from the Greek word for leather and refers to the membrane that develops in the throat of an infected person. Klebs was the first to identify the organism (1883) and Loeffler the first to cultivate the organism (1884). The bacterium has been called the “Klebs-Loeffler ” bacillus as a consequence. Roux and Yersin first demonstrated that the organism produced a toxin (1888), and von Behring and Kitasato (1890) demonstrated that administering an “anti-serum” or “antitoxin,” the antibody-containing serum produced in animals infected with an attenuated strain, would prevent mortality. The first successful treatment of a child with diphtheria using the antitoxin occurred in Germany in 1891. The antitoxin was produced in horses and soon became commercially available. The mortality rate, which had been 50%, fell dramatically as a consequence. It became quickly evident, however, that the antitoxin had to be administered soon after the onset of symptoms in order to be effective.
Large scale vaccination programs began after Ramon and others demonstrated in the 1920s that diphtheria toxin could be rendered safe for vaccination by exposure to heat and formalin, producing a “toxoid.” Because of widespread vaccination since then, diphtheria is rarely seen in developed countries today although the disease is still endemic in many developing countries (e.g., India, Nepal, Bangladesh, and Myanmar). Modern vaccination schedules include a 3-dose “primary” vaccine series before age 1, a fourth dose (“booster”) during the second year of life, a fifth dose (booster) in early childhood (age 4–7 years), and another booster dose either in adolescence (age 9–15 years) or early adulthood [1, 2]. Booster doses are also recommended every 10 years during adulthood.
Bacteriology
Corynebacterium diphtheriae bacilli are Gram-positive, unencapsulated, non-sporulating, rod-like organisms with a tendency to club at one or both ends (Fig. 19.1). The genus name comes from the Greek “koryne,” or club. Diphtheria may be either toxigenic (toxin-producing) or non-toxigenic. Non-toxigenic strains usually cause a mild form of disease without any major organ damage. Toxigenic strains produce a powerful exotoxin that causes severe disease with both local tissue damage and remote organ dysfunction. Toxin production only occurs by bacteria that have been infected (“lysogenized”) by a virus carrying the tox gene. Production of the toxin by the bacterium occurs following depletion of local extracellular iron stores, as high iron concentrations cause a bacterial suppressor gene to be activated that suppresses toxin production.
Stains of Corynebacterium diphtheriae , after growth on culture media. (a) Gram stain, demonstrating that it is a Gram-positive bacillus with “clubbed” ends. (b) Albert’s stain, taken from an 18-h culture. The clubbed ends are easily seen. From the U.S. Centers for Disease Control and Prevention (CDC) Public Health Image Library, photograph (a) attributed to CDC, Dr. Graham Heid, 1965, photograph (b) attributed to CDC, 1979
Diphtheria has four biotypes , gravis, intermedius, mitis, and belfanti, and any may produce toxin [3]. Historically, biotype gravis has been associated with severe illness and a high case fatality rate while mitis has been associated with milder disease. However, mortality may occur from any biotype. In an outbreak of diphtheria in Krygystan in 1995, the mitis biovar caused two-thirds of culture-positive cases and over 40% of the deaths [4].
Corynebacterium diphtheriae will grow on blood agar plates but it is difficult to detect on throat cultures without use of special media. The microbiology laboratory must be alerted to the possibility of diphtheria when throat cultures are sent, as selective media can be used to detect the organism. The throat culture swabs must be transported to the laboratory quickly and media inoculated promptly to optimize growth. Loeffler’s media will grow the organism rapidly, but the tellurite-containing Tinsdale media is used more often. Tinsdale media inhibits normal oral flora and C. diphtheriae colonies appear black with brown halos (Fig. 19.2). Once there is growth of C. diphtheriae, a test for toxin production, such as the modified Elek test, should be performed.
Rarely, another stain of Corynebacterium, C. ulcerans, may produce diphtheria toxin and produce a respiratory illness identical to diphtheria [5]. Culture methods are similar.
Epidemiology
Humans are the only known reservoir for diphtheria, and cases are spread by contact with respiratory droplets, exudate from skin lesions, or fomites [3]. Diphtheria is highly contagious and patients remain contagious for 48–72 h after starting antibiotic therapy. In untreated or partially treated respiratory cases, patients may be contagious for 2–6 weeks. Humans can be asymptomatic carriers of toxigenic or non-toxigenic diphtheria strains, and these chronic carriers may be contagious for 6 months or more. Immunity in the individual, either natural or from vaccination, does not prevent asymptomatic carriage. Patients with cutaneous diphtheria may contribute to the transmission of diphtheria in a population, as skin lesions are often chronic and C. diphtheriae may be transmitted by contact with the exudate.
Diphtheria may occur throughout the year although in temperate climates, the disease occurs mostly in the colder months. This may be due to the increased risk of transmission in crowded indoor conditions.
Diphtheria occurs globally and remains a health threat, particularly in developing nations. The disease is endemic in many developing countries, primarily due to incomplete childhood vaccinations including booster doses. Children have been the major victims of diphtheria historically [6]. Several recent series also reported that the majority of cases (60–85%) occurred in children ages 0–10 years old [7,8,9]. In contrast, an outbreak during the 1990s in the New Independent States of the former Soviet Union included a large number of adults, with 70% of cases occurring in patients older than 15 [10, 11]. Table 19.1 compares the epidemiology of diphtheria reported by six representative studies from different countries and decades [4, 6,7,8,9, 12].
Many adults remain susceptible to diphtheria, primarily due to waning immunity and failure to get the recommended booster doses [13]. A study from the United Kingdom found that only 31% of adults in 1995 were fully immune (titer >0.1 IU/ml) while 39% lacked any detectable antibodies (titer <0.01 IU/ml) [14]. Over 50% of adults age 50–59 lacked immunity in that study. A study from the United States that evaluated immunity in adults 1988–1994 found that only 30% of 70-year-olds were fully immune [15]. A report from Brazil in 2003 found that only 31% of adults age 18–61 had adequate immunity [16].
A decline in vaccination rates in the countries of the former Soviet Union contributed to the largest epidemic of diphtheria in the past 50 years. This epidemic began in 1990 in Russia (Russian Federation), spread to the Ukraine in 1991, then to 12 of the 13 remaining New Independent States (e.g., Baltic states, central Asian nations) during 1993–1994 [11]. The epidemic peaked in 1995 and ultimately resulted in 157,000 cases and 5000 deaths [17]. More than 40% of cases occurred in adults older than 40. The epidemic was controlled by mass immunizations along with early detection and management of cases and close contacts. As a result, new cases in the affected regions decreased by over 95% between 2000 and 2009, from 1.82 to 0.07 cases per million population [18]. By 2009, only Latvia still had an incidence of over one case per million population, the WHO target incidence, but Latvia’s rate had fallen dramatically from 111 cases per million in 2000 to 2.67 per million in 2009 [18]. The major age groups affected in the epidemic were adolescents and adults, many of whom had been at least partially vaccinated, but deaths occurred primarily in unvaccinated adults and infants. Biovar gravis caused 60–80% of cases overall and 99% of cases in Latvia [18].
In Western Europe and the U.S., rates of diphtheria have been low in the vaccine era. In the U.S., widespread vaccination programs that began in the 1920s led to a dramatic decrease in the incidence of diphtheria, from 200,000 cases in 1921 to 0–2 cases annually since 2000. A study of U.S. cases 1971–1981 found that only 5% of all U.S. counties reported any cases of non-cutaneous diphtheria during that decade, nearly all from western states, and Native Americans (22.6 cases per million) and children age 14 and younger (0.8 cases per million) had the highest rates [19]. The mortality rate in unvaccinated patients was tenfold higher than in fully vaccinated patients (13.4% versus 1.3%). The diphtheria incidence declined over the decade, and there were only two cases in the U.S. in 1980 (0.01 cases per million population) [19]. The incidence has decreased even further since then, and only two cases of diphtheria have occurred in the U.S. 2004–2015 [20]. This reflects the high U.S. DTP3 (three doses of diphtheria, tetanus, pertussis vaccine) vaccination rate [21]. However, vigilance for diphtheria must be maintained even in countries with low rates of diphtheria, as imported cases in visitors and returned travelers may occur. One such case occurred in Massachusetts in 2004 when a 60-year-old woman of unknown vaccination status developed a sore throat (positive on culture for toxigenic C. diphtheriae) following a trip to a diphtheria-endemic Caribbean island [22]. A family member who accompanied her on the trip was found to be a carrier.
Worldwide, approximately 5000–8000 cases of diphtheria have been reported to the WHO annually over the past decade. Diphtheria is still endemic in several tropical countries, with the greatest number of cases reported to the WHO most recently (2015) from India (2365 cases), Madagascar (1627 cases), and Lao People’s Democratic Republic (194 cases) [21]. Considering the populations of those countries, the incidence in 2015 was highest in Madagascar (67 cases per million population), followed by Loa People’s Democratic Republic (29 cases per million) and India (1.8 cases per million). The true incidence of worldwide diphtheria may be higher, as cases in rural areas may be underreported. The main cause of the persistence of diphtheria in developing countries is incomplete childhood vaccination including booster vaccinations. However, significant progress has been made over the past decade. According to a WHO report, in 2005 only 65% of the population in India and 49% in Madagascar had received DPT3 but this rate increased to 87% and 89%, respectively, in 2015 [21]. As a consequence, diphtheria cases in India and elsewhere are gradually declining [23].
A recent concern has been inadequate immunologic response to vaccination in infants and children in some countries. Diphtheria-containing vaccines usually produce protective immunity in over 90% of children who have received three doses. However, a recent study found that only 56% of 1100 children in Lao People’s Democratic Republic had protective diphtheria antibody levels after three doses of vaccine although 90% had detectable levels [24]. The authors of that study hypothesized that various factors, such as freezing of the vaccine during transportation (vaccine should be refrigerated not frozen), inaccurate record-keeping, suboptimal timing of vaccine administration, and childhood malnutrition, may have contributed to the low vaccine response rate.
Pathogenesis
In a susceptible host, C. diphtheriae colonizes the mucosa of respiratory tract and within the first few days, toxigenic strains produce a potent exotoxin that causes local tissue inflammation and necrosis resulting in the formation of dense pseudomembrane (exudate, inflammatory cells, necrotic tissue, and organisms). Toxin is also absorbed into systemic circulation causing dysfunction of various organs (heart, nervous system, kidneys). The exotoxin has two protein fragments (A and B), of which the B fragment binds to cell receptors enabling fragment A to enter the cell cytosol and inhibit protein synthesis.
Non-toxigenic strains usually cause mild to moderate pharyngitis and do not form the typical pseudomembrane. Non-toxigenic strains very rarely become toxigenic (by lysogenic conversion). Occasionally non-toxigenic strains cause invasive infections such as bacteremia, endocarditis, mycotic aneurysms, septic arthritis, and osteomyelitis [25].
Clinical Manifestations
Respiratory Diphtheria
Classic or “Faucial” Diphtheria
This is the most common form of diphtheria, and symptoms typically begin after an incubation period of 2–5 days (range 1–10 days). Patients initially complain of sore throat (approximately 80%), malaise, pain with swallowing, and mild fever (usually less than 101 °F). In a study from Thailand, 100% of patients had fever but this was less than 100.4 °F in nearly 70% of cases [7]. Pharyngeal injection and cervical lymphadenopathy may be present. After 2–3 days, a bluish-white membrane develops covering one or both tonsils. This membrane sometimes extends to the soft palate, pharynx, or even larynx. The membrane gradually becomes grayish or even black (in the presence of bleeding) (Fig. 19.3). The membrane is adherent to the underlying tissues and attempts to remove it resulting in bleeding. This extensive pseudomembrane formation along with tissue edema or associated local bleeding may result in airway obstruction, which may lead to wheezing, a choking sensation, or development of stridor. The clinical features reported by several series are noted in Table 19.1.
In an undiagnosed or very severe case, there may be marked enlargement of cervical lymph nodes and this, along with soft tissue swelling, produces a characteristic “bull neck” appearance (Fig. 19.4). In such cases more toxin is absorbed into the circulation causing severe prostration, tachycardia, hypotension, stupor, coma, or even death despite adequate medical treatment. A bull neck presentation is associated with a high mortality rate (>50%).
The severity of infection on presentation usually correlates with the extent of the pseudomembrane. In 1954, Naiditch and Bower reported clinical features of 1433 cases of diphtheria treated in the 1940s in Los Angeles, California, and the 61% of cases with tonsillopharyngeal involvement alone had a mortality of 1.8%, while cases with extension to other areas (e.g., larynx, tracheobronchial tree) had a mortality of 24%, [6]. Bull neck cases had a mortality of 33%.
A pseudomembrane is a major clinical feature of diphtheria but may be absent in rare cases. A pseudomembrane was reported as absent in 2.6% of cases in the Naiditch study but mortality was still 5% in these patients [6]. In most series, a pseudomembrane is present in over 95% of diphtheria cases. However, in diphtheria outbreaks in which patients with sore throat are routinely screened for diphtheria, a higher percentage of cases lack a pseudomembrane. These patients may represent either diphtheria carriers (sore throat not due to diphtheria) or “pre-membranous” diphtheria. In the 1995 Kyrgystan outbreak, 24% of cases with positive diphtheria cultures presented without a pseudomembrane, but all patients with sore throat were screened for diphtheria during that outbreak so carriers would be included in that number [4].
Nasal Diphtheria
Primary nasal diphtheria is a mild form of diphtheria seen mainly in infants and young children. Patients present with catarrhal symptoms, unilateral nasal discharge (mostly blood stained), and excoriation of nostril. After careful examination, a grayish-white pseudomembrane is usually seen on the nasal septum that bleeds on attempts to dislodge it. Only 1.9% of cases in the 1954 Los Angeles study had primary nasal diphtheria: 22% were younger than 12 months old, 44% were 1–5 years old, and none died [6].
Laryngeal or Tracheobronchial Diphtheria
Isolated primary laryngeal diphtheria is very rare, comprising 1.4% of diphtheria cases in one study [6]. Most cases with laryngeal involvement are due to faucial diphtheria with secondary extension to the larynx. Cases of primary laryngeal diphtheria are diagnosed only by an otolaryngologist when a patient is referred with history of low grade fever, barking cough, and early development of hoarse voice and dyspnea. The main danger of laryngeal diphtheria is the development of sudden and early airway obstruction requiring either urgent endoscopic removal of pseudomembrane or tracheotomy, or both.
Tracheobronchial diphtheria occurs as a downward extension of faucial or laryngeal diphtheria. In the 1954 Los Angeles study, the tracheobronchial tree was involved in 6.7% of cases and had a mortality of 67% [6]. Urgent intubation or tracheostomy, allowing removal of diphtheria membranes, may be life-saving.
“Malignant” Diphtheria (Diphtheria Gravis)
This is an older term but still used occasionally today. Patients with malignant diphtheria present with high fever, severe sore throat, diffuse swelling of the neck (bull neck), tachycardia, hypotension, stridor, or cyanosis. The typical patient is unvaccinated or only partially vaccinated, malnourished, and has a history of presenting late to clinical care, or of significant delay in the correct diagnosis of diphtheria. Early myocardial involvement with advanced conduction abnormalities and severe bleeding from local sites contribute to a high mortality (>50%). A 1943 study by Frobisher reported similar features of malignant diphtheria, emphasizing the sudden onset, high fever, bull neck, and frequent cardiac toxicity seen in these cases [26]. Frobisher noted that tonsils are usually very large but that pseudomembrane formation may be “slight.”
Complications of Respiratory Diphtheria
Airway Complications
Airway complications may arise in respiratory tract diphtheria as a result of local obstruction by the pseudomembrane or by secondary aspiration pneumonia. Acute airway obstruction is a potentially lethal complication and requires urgent laryngoscopic removal of membrane and/or tracheostomy. The causes are either extension or aspiration of pseudomembrane into the larynx, laryngospasm, edema, or bleeding.
Cardiac Toxicity
Evidence of cardiac toxicity occurs in up to two-thirds of patients but many cases are subclinical [27]. A vaccination history does not preclude cardiac toxicity. In a series from India involving 200 diphtheria cases treated from 2009 to 2011, 75% of patients had a vaccination history but evidence of cardiac involvement developed in 68% [8]. In most of these cases, myocarditis was subclinical and detected by electrocardiographic screening. In another study from India involving 180 pediatric patients (ages 0–20 years old), cardiac complications occurred in 30% and were associated with a 52% mortality [9]. Over half of the patients in that study lacked immunity.
Diphtheria myocarditis with clinical manifestations is associated with a high mortality (60–70%). Myocarditis typically develops at the end of the first week of illness, but may develop 2–3 weeks after onset [28]. Risk factors include extensive pseudomembrane formation, presence of a bull neck, and delayed initiation of treatment [29,30,31]. Diphtheria toxin is directly cardiotoxic, causing DNA fragmentation, cytolysis that leads to hyaline degeneration, and necrosis of the myocardium [32]. The majority of patients with cardiac involvement from diphtheria remain asymptomatic and have only electrocardiogram (ECG) changes and/or elevation of aspartate tranaminase (AST). But symptomatic patients develop shortness of breath, disproportionate tachycardia, and in severe cases, features of cardiac failure. The transient nonspecific ECG abnormalities (e.g., repolarization abnormality, abnormal Q wave, QTc prolongation, T wave inversion, ST segment elevation) are frequent but “sickle-like sagging” of the ST segment is more specific. First degree heart block may occur and progress to more severe forms of block, such as hemiblock, bundle branch block, atrioventricular (A-V) dissociation, or complete heart block. In a study from Chile of 167 patients with diphtheria 1976–1986, 27% developed myocarditis including 11% with severe forms of heart block [33]. Several patients died within 48 h of pacemaker insertion due to cardiogenic shock or ventricular fibrillation. In a more recent study from Vietnam, 21% of the 154 children with diphtheria 1995–1996 had cardiac involvement: this developed 2–20 days after onset of diphtheria [34]. Mortality in children with cardiac involvement was 38%, and ischemic changes and complete heart block on initial ECG were risk factors for mortality. The severity of diphtheria myocarditis usually parallels the elevation of AST so AST levels may be helpful in monitoring cases [27]. Survivors of diphtheritic myocarditis eventually recover normal cardiac function. In the Vietnam study, 15 survivors of myocarditis returned for 1 month follow-up and all had normal ECGs [34].
Neurologic Toxicity
Neuropathy in diphtheria is due to toxin-mediated demyelination of nerves and occurs in 5–15% of cases ([4, 6, 9, 12]). The incidence is directly related to the severity of local disease; mild disease rarely produces neuropathy [27, 35], while up to 75% of patients with severe disease may develop neuropathy. The initial features, which usually relate to local effects of the toxin, include palatal paralysis, perioral numbness, and bulbar findings. Patients complain of numbness in the lips, tongue, or gums [36]. Paralysis of the soft palate and posterior pharynx may cause regurgitation of swallowed fluids through the nose [27]. Dysphonia (nasal voice) and dysphagia occur due to involvement of the ninth and tenth cranial nerves (bulbar involvement). Involvement of the third cranial nerve may occur, causing diplopia, ptosis, and anisocoria. The seventh cranial nerve may be affected and lead to facial palsy. Involvement of the third and seventh cranial nerves occurred in 30% and 10% of patients, respectively, in one series of patients with neurologic complications of diphtheria [37].
Neurologic involvement usually develops at the end of the second week of illness but may occur weeks to months later. Logina and Donaghy reported clinical features of 50 patients from Latvia with diphtheria neuropathy [37]. The first neurologic symptoms were bulbar in 98% of patients and began a median of 10 days (range 2–50) after onset of respiratory diphtheria. Limb symptoms also occurred in most (90%) patients, developing at a median of 37 days (range 12–63) after onset of diphtheria. Limb symptoms followed bulbar symptoms in all but one patient, and developed as bulbar symptoms were improving in 30% of cases. Peak severity of neurologic symptoms occurred at a median of 49 days and improvement began at a median of 73 days.
Peripheral nerve involvement usually develops 10 days to 3 months later [27]. Weakness begins in the proximal muscles of the extremities and extends distally. Rarely the presentation may be as acute flaccid paralysis, mimicking poliomyelitis. In many countries (e.g., India) the existing surveillance for acute flaccid paralysis identifies a small portion of diphtheria cases with neurological involvement [35]. Severe motor involvement may occur and patients may develop either quadriparesis or quadriplegia with hypotonia, areflexia, and rarely diaphragmatic paralysis. This presentation may mimic acute demyelinating polyradiculoneuropathy (Guillain-Barré syndrome), although the much higher prevalence of bulbar involvement and the descending pattern indicate diphtheria polyneuropathy [37]. Peripheral sensory involvement is common. In Logina and Donaghy’s study , 38% of patients had mild and 46% moderate sensory involvement [37]. The involvement may be in a stocking-glove pattern.
Various combinations of autonomic disturbances (sympathetic and parasympathetic) may develop and may cause sudden death as a result of fulminant autonomic dysfunctions.
Rarely, neurological involvement may be biphasic, with secondary worsening of bulbar symptoms occurring after initial recovery [38, 39]. Eventually, however, most patients with diphtheria neuropathy recover full neurologic function although recovery may be very slow. Logina and Donaghy reported that 97% of patients still had some neurologic symptoms at one year follow-up, but these symptoms were mild in nearly all cases (mild numbness, paresthesias, or weakness) [37]. Only 6% of patients required a cane or other walking aids.
Renal and Other Complications
Renal injury usually occurs either from hypotension or from a toxin-mediated effect on the renal tubules. The incidence varies from less than 1% to 16% [6, 9]. Serum sickness occurs in a few cases following antitoxin therapy and is often manifested as mild, urticaria-like localized cutaneous lesions that persist for a few hours or days. Bacteremia may occur from toxigenic or non-toxigenic diphtheria. Diphtheria may also produce encephalitis.
Cutaneous Diphtheria
Cutaneous diphtheria is usually caused by non-toxigenic strains but may be caused by toxigenic strains. This type of diphtheria is more common than respiratory diphtheria in many developing countries, but the incidence has decreased following socioeconomic improvement in these countries. Cases may also be seen in developed countries with a very low incidence of diphtheria. From 1995 to 2000, 17 cases of cutaneous diphtheria caused by toxigenic strains were detected in the United Kingdom, and all were imported cases [40].
The disease manifests as a scaling rash or a nonhealing, ulcerated lesion with an elevated margin (Fig. 19.5). Epidemiologically, cutaneous diphtheria is important primarily because of its role in the spread of diphtheria strains. Cutaneous diphtheria can be more contagious than the respiratory type. Skin lesions typically follow an indolent course, persisting for weeks to months, and rarely cause respiratory diphtheria [41, 42]. Diagnosis is usually suspected on the basis of clinical features and epidemiology, particularly if there is a history of travel to a diphtheria-endemic regions, and is confirmed by bacterial culture.
Cutaneous diphtheria is treated with erythromycin or penicillin. Antitoxin is not routinely indicated because toxigenic complications are rare and most cases are caused by nontoxigenic strains. Following treatment, lesions usually heal within 12 weeks but sometimes require more than a year [13, 25, 43].
Non-respiratory, Non-cutaneous Diphtheria
Rarely diphtheria may involve other mucous surfaces such as the conjunctiva (purulent ulcerative conjunctivitis) and vulvo-vagina (ulcerative vulvovaginitis), or may involve the external auditory canal (recurrent otitis externa). The incidence of these manifestations is very rare. The 1433 cases reported in the 1954 Los Angeles study included only three cases involving the eye, five involving the ear, and 1 involving the vagina [6].
Diagnosis
Diphtheria is diagnosed on the basis of the clinical presentation, with confirmation by culture of the organism. Treatment with antitoxin and antibiotics should not be delayed in a suspected case of respiratory diphtheria, because urgent therapy is necessary to avoid fatal complications. Cultures may require 72 h or more to turn positive, and fail to grow in some cases (e.g., prior antibiotic therapy). In developed countries, early diagnosis may be difficult because of atypical presentations and because the disease is very rare. Routine laboratory tests are usually nonspecific and the white blood count is usually only moderately elevated. A checklist may be helpful for physicians who rarely see cases of diphtheria (Table 19.2) [44].
An accurate bacteriological diagnosis is crucial for therapy and epidemiological purposes. A culture should be obtained from the nose and throat, including beneath the membrane if possible. Culture swabs must be transported to the microbiology laboratory quickly for prompt inoculation on appropriate media. The laboratory should be alerted about the possibility of diphtheria and the need for special media. The throat swabs should be inoculated on both tellurite media such as Tinsdale agar (for selective growth of C. diphtheriae) and blood agar (for routine throat culture). In cases of prior antibiotic therapy, the culture may be negative and in such cases, a positive polymerase chain reaction (diphtheria tox gene) or isolation of the organism from close contacts is helpful for diagnosis. Once C. diphtheriae bacilli are isolated, they must be tested for toxin production either by polymerase chain reaction assay or Elek’s immunoprecipitation test [45].
The differential diagnosis of diphtheria includes other causes of acute pharyngitis (see Chap. 17). Table 19.3 lists some of the features of various types of pharyngitis and tonsillitis. The distinctive feature of diphtheria is the pseudomembrane, although this may be missing or patchy in some patients with early diphtheria.
It is worth noting that Corynebacterium ulcerans, a species usually associated with animal infections, may produce diphtheria toxin and cause a disease in humans identical to that caused by C. diphtheriae. Any throat culture that grows C. ulcerans should be tested for toxin production [46].
Treatment of Respiratory Diphtheria
In a suspected case of diphtheria, the patient should be assessed carefully with special attention to airway patency and any cardiovascular instability (hypotension or conduction abnormality). The patient should be referred urgently to a hospital where specialists are available (e.g., infectious disease physicians, otolaryngologists, cardiologists, and anesthesiologists), along with a skilled nursing staff and the facility for barrier nursing care. Most importantly, diphtheria antitoxin must be obtained on an emergency basis and administered as soon as possible.
All suspected diphtheria cases should be isolated immediately in the emergency department and hospital and treated with droplet precautions and contact precautions. Of note, the CDC recommends only droplet precautions for respiratory diphtheria [47], although it notes that fomite transmission is possible. Therefore, it seems prudent to use both droplet and contact precautions until the patient is no longer contagious (two negative cultures obtained 24 h apart).
Patients should be evaluated promptly, including evaluation for severity of symptoms, overall clinical status, vital signs, and development of any stridor. A throat swab should be collected, from the pseudomembrane if possible, by a physician or other trained healthcare professional. The throat swab should be sent without delay to the laboratory for stains (Gram stain and Albert’s stain) plus culture (routine blood agar plus specialized media for C. diphtheriae such as Loeffler’s slant and/or Tinsdale agar). The laboratory should be alerted about the potential case of diphtheria.
Treatment with diphtheria antitoxin and antibiotics should be started immediately in cases that are clinically compatible with diphtheria, without waiting for results of cultures. All patients should be monitored closely for the development of any respiratory or cardiac complication. Severe cases having a toxic appearance require continuous monitoring, and may require resuscitative measures (e.g., tracheostomy or temporary cardiac pacing). In cases with airway compromise, immediate action should be taken (e.g., tracheostomy, intubation, clearing of pseudomembrane endoscopically by an otolaryngologist).
Diphtheria Antitoxin
Diphtheria antitoxin , the mainstay of therapy, is a specific agent used to neutralize unbound toxin, thus preventing further progression of organ damage (Fig. 19.6). Since the antitoxin is prepared from horse serum, every patient must be first tested for hypersensitivity by a skin test. Patients with positive or equivocal skin tests will require a desensitization protocol to receive the antitoxin safely. A skin test protocol is listed in the CDC website [48]. Informed consent is mandatory (parents or legal guardian in cases of minors), unless the patient cannot give consent, no next-of-kin or legal guardian is available, the illness is life-threatening and therapy with antitoxin cannot be delayed [48]. The physician should enquire about an atopic history (asthma, allergic rhinitis, urticaria), or allergy to horse serum: the CDC recommends using a desensitization protocol for these patients as well, regardless of the result of the skin test [48]. A negative skin test does not always preclude the chance of a severe allergic reaction. In addition, patients who have received antihistamines within the previous 24 h or more (depending on the antihistamine) may have falsely negative skin test results. The CDC notes that “possibly other drugs such as tricyclic antidepressants” may interfere with the skin test [48]. The recommended desensitization protocol may vary by manufacturer; the protocol recommended by the CDC involves administering an increasing dose of dilute antitoxin every 15 min over a 3-h period, beginning with 0.1 ml of a 1:1000 dilution [48]. Trained personnel and necessary medications and equipment (e.g., epinephrine, intubation kit) should be readily available to treat any severe allergic reactions such as anaphylaxis.
The recommended dose of antitoxin depends mainly on the time elapsed since the onset of the first symptoms of diphtheria, sites of involvement, and the presence or absence of a bull neck. The recommended doses are:
-
Systemic disease manifestations of three or more days’ duration, or patients with bull neck: 80,000–100,000 units
-
Nasopharyngeal disease: 40,000–60,000 units
-
Pharyngeal or laryngeal disease of two days duration: 20,000–40,000 units
The dose of antitoxin is the same for all the ages. Antitoxin must be warmed to 32–34 °C before administration [48]. The preferred route is intravenous but intramuscular route may be used only in mild to moderate cases. The dose of antitoxin should be mixed with 250–500 ml of normal saline and infused over 2–4 h. Pregnancy is not a contraindication to antitoxin administration. Repeat doses of antitoxin are not recommended because of serious allergic reactions as a result of antibody formation. The possible adverse reactions following antitoxin administration include anaphylactic reactions, febrile reactions, serum sickness (skin rash, arthralgia, angioedema), and injection site pain.
Antibiotics
Antibiotics are necessary to kill the organism, limiting further toxin production that will hasten early recovery and also reduce spreading the disease to others. Procaine penicillin G is the preferred antibiotic and should be administered for a period of 14 days. In cases of penicillin allergy, erythromycin is given for the same duration. The doses recommended by the CDC are: procaine penicillin G daily, intramuscularly (300,000 units every 12 h for those weighing 10 kg or less, and 600,000 units every 12 h for those weighing more than 10 kg) for 14 days, or erythromycin orally or by injection (40 mg/kg/day; maximum, 2 g/day) for 14 days [20]. The disease usually becomes non-contagious 48 h following antibiotic therapy, but for epidemiological purposes, two consecutive negative cultures (24 h apart) are required to demonstrate elimination of the organism.
Isolates should be tested for susceptibility. Reduced susceptibility to penicillin for both toxigenic and non-toxigenic strains has been reported [12, 49].
Monitoring
All diphtheria patients need close monitoring with respect to any abnormalities in pulse (rate, rhythm), changes in blood pressure (hypotension), respiratory rate, development of stridor or cyanosis, any bleeding, or serum sickness-like illness.
Specialists’ consultations are required in certain emergency situations and these must be available around the clock in the referral hospital.
-
ENT specialist: in difficult cases for airway protection, for the removal of a pseudomembrane endoscopically. Tracheostomy may be required.
-
Cardiologist: in cases of advanced cardiac conduction abnormality due to severe myocarditis requiring temporary pacing.
-
Pulmonologist and anesthesiologist: in cases of severe laryngeal obstruction by a pseudomembrane with associated aspiration pneumonia and cyanosis. Intubation may be required.
-
Infectious disease specialist: in cases of atypical presentation of diphtheria or any unusual disease complications.
Follow-Up Vaccination
Respiratory diphtheria does not produce immunity and patients should be vaccinated against diphtheria as appropriate for their age.
Management of Close Contacts and Carriers
All household contacts require a throat swab for culture and should receive chemoprophylaxis regardless of age or immunization status. Confirmation of eradication of carriage state following treatment is necessary for those found to be culture positive [3]. Chemoprophylaxis of household contacts should be with either oral erythromycin (40 mg/kg per day for children and 1 g per day for adults) for 7–10 days, or if compliance is uncertain, with a dose of intramuscular benzathine penicillin (600,000 units for children under age 6 and 1.2 million units for patients age 6 and older) [20]. In addition, all contacts require close supervision and should be treated with antitoxin at the earliest signs of illness. Carriers require treatment with the same antibiotics as contacts, and should also be kept under strict surveillance for any signs of the disease. Both contacts and carriers should be vaccinated as appropriate for their age.
Prognosis
The case fatality rate of diphtheria has been unchanged for several decades, remaining at 5–10% in most series. The mortality rate may be higher (60–90%) in unvaccinated or partially vaccinated patients, patients with clinical evidence of myocardial involvement, age less than 5 years or above 40 years, and those who received the antitoxin very late. Patients with bull neck are also at higher risk of death. The most important predictors for mortality are the time interval of antitoxin administration from the onset of first respiratory symptom and vaccination status of the affected individuals.
Prevention
The only way to control diphtheria effectively is through immunization. The goal should be to immunize all infants before they lose their maternal antibody, and then give booster doses to enhance and maintain immunity. Diphtheria vaccine has been combined with pertussis and tetanus vaccines since the 1940s as DTP. Pertussis vaccines are now designated as “whole cell” (the original vaccine) or “acellular” (introduced in the 1980s) so the combination may be written as DTwP (whole cell pertussis) or DTaP (acellular pertussis), although the WHO uses DTP to designate either. Acellular pertussis-containing vaccines are preferred by most countries and are the only pertussis-containing vaccines recommended in patients age 7 years and older [1].
There are pediatric formulations, DTaP and DT, given to infants and children younger than 7, and “adult” formulations, Tdap and Td, given to patients age 7 and older. The pediatric formulations contain a similar amount of tetanus toxoid as the adult formulations but several times as much diphtheria toxoid. Adults and all children age 7 and older, including those who missed the usual vaccine schedule, should be prescribed the adult formulations. Diphtheria toxoid is highly efficacious and the protective antibody (>0.1 IU of anti-toxin/mL) is produced in more than 95% of cases following complete vaccination. Immunosuppression is not a contraindication to vaccination, although it may reduce the immune response to vaccination. Pregnancy is also not a contraindication.
The World Health Organization (WHO) recommends that all children receive a 3-dose “primary” series of DTP before age 1 (starting as early as 6 weeks old), a 4th DTP dose (“booster”) at age 12–23 months, and Td booster doses at ages 4–7 years and ages 9–15 years [1]. The Centers for Disease Control and Prevention (CDC) recommends five doses of DTaP given at ages 2 months, 4 months, 6 months, 12–15 months, and 4–6 years [2]. Adults age 19 and older should receive booster doses of Td every 10 years. Recommendations for interrupted or delayed vaccinations are given in the WHO and CDC websites [1, 2]. Diphtheria toxoid-containing vaccines should be stored in the refrigerator (at 2–8 °C) but should not be frozen; most manufacturers state that frozen vaccines should be discarded.
Conclusion
Diphtheria is still with us and causes a high mortality, despite the availability of an effective vaccine. The main cause of the persistence of diphtheria is inadequate immunization coverage, including booster shots, in developing countries. In developed countries, where the disease is very rare, diphtheria must be considered in the differential diagnosis of patients presenting with membranous pharyngitis, especially if there is a history of recent travel to an endemic country or recent close contact with such a traveler. Early diagnosis, effective therapy, and close monitoring are all essential to reducing mortality.
References
World Health Organization (WHO). WHO recommendations for routine immunization – summary tables. http://www.who.int/immunization/policy/immunization_tables/en/. Accessed May 2017.
Centers for Disease Control and Prevention (CDC). Recommended vaccination schedule for children and adolescents age 18 years or younger, United States, 2017. http://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html. Accessed May 2017.
Centers for Disease Control and Prevention. The pink book: epidemiology and prevention of vaccine-preventable diseases. http://www.cdc.gov/vaccines/pubs/pinkbook/dip.html. Accessed May 2017.
Kadirova R, Kartoglu HU, Strebel PM. Clinical characteristics and management of 676 hospitalized diphtheria cases, Kyrgyz Republic, 1995. J Infect Dis. 2000;181(Suppl 1):S110–4.
Centers for Disease Control and Prevention. Manual for surveillance for vaccine-preventable diseases; Chapter 1: Diphtheria. http://www.cdc.gov/vaccines/pubs/surv-manual/chpt01-dip.html, accessed May 2017.
Naiditch MJ, Bower AG. Diphtheria: a study of 1,433 cases observed during a ten-year period at the Los Angeles County Hospital. Am J Med. 1954;17:229–45.
Pantukosit P, Arpornsuwan M, Sookananta K. A diphtheria outbreak in Buri Ram, Thailand. Southeast Asian J Trop Med Pub Health. 2008;39:690–6.
Kole AK, Roy R, Kar SS, Chanda D. Outcomes of respiratory diphtheria in a tertiary referral infectious disease hospital. Indian J Med Sci. 2010;64:373–7.
Jain A, Samdani S, Meena V, Sharma MP. Diphtheria: It is still prevalent. Int J Pediatr Otorhinolaryngol. 2016;86:68–71. https://doi.org/10.1016/j.ijporl.2016.04.024.
Vitek CR, Wharton M. Diphtheria in the former Soviet Union: reemergence of a pandemic disease. Emerg Infect Dis. 1998;4:539–50.
Centers for Disease Control and Prevention. Diphtheria epidemic – new independent states of the former Soviet Union 1990–1994. MMWR. 1995;44:177–81.
Santos LS, Sant'anna LO, Ramos JN, et al. Diphtheria outbreak in Maranhão, Brazil: microbiological, clinical and epidemiological aspects. Epidemiol Infect. 2015;143:791–8.
Centers for Disease Control and Prevention. FDA approval of expanded age indication for a tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine. MMWR Morb Mortal Wkly Rep. 2009;58(14):374–5.
Maple PA, Efstratious A, George RC, et al. Diphtheria immunity in UK blood donors. Lancet. 1995;345:963–5.
McQuillan GM, Kruszon-Moran D, Deforest A, et al. Serologic immunity to diphtheria and tetanus in the United States. Ann Intern Med. 2002;136:660–6.
Damasco PV, Pimenta FP, Filardy AA, et al. Prevalence of IgG diphtheria antitoxin in blood donors in Rio de Janeiro. Epidemiol Infect. 2005;133:911–4.
Mattos-Guaraldi AL, Moreira LO, Damasco PV, Hirata Júnior R. Diphtheria remains a threat to health in the developing world—an overview. Mem Inst Oswaldo Cruz. 2003;98:987–93.
Wagner KS, White JM, Lucenko I, Mercer D, Crowcroft NS, Neal S. Diphtheria in post epidemic period, Europe, 2000–2009. Emerg Infect Dis. 2012;18:217–25.
Chen RT, Broome CV, Weinstein RA, Weaver R, Tsai TF. Diphtheria in the United States, 1971–81. Am J Public Health. 1985;75:1393–7.
Centers for Disease Control and Prevention. Diphtheria. http://www.cdc.gov/diphtheria/clinicians.html. Accessed May 2017.
World Health Organization. WHO vaccine-preventable diseases monitoring system, 2016 global summary. http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tsincidencediphtheria.html. Accessed May 2017.
Massachusetts Department of Public Health, Bureau of Communicable Disease Control. http://www.mass.gov/eohhs/docs/dph/disease-reporting/guide/diphtheria.pdf. Accessed May 2017.
Murhekar MV, Bitragunta S. Persistence of diphtheria in India. Indian J Community Med. 2011;36:164–5.
Evdokimov K, Sayasinh K, Nouanthong P, et al. Low and disparate seroprotection after pentavalent childhood vaccination in the Lao People’s Democratic Republic: a cross-sectional study. Clin Microbiol Infect. 2017;23:197–202.
Zasada AA, Baczewska-Rej M, Wardak S. An increase in non-toxigenic Corynebacterium diphtheriae infections in Poland – molecular epidemiology and antimicrobial susceptibility of strains isolated from past outbreaks and those currently circulating in Poland. Int J Infect Disease. 2010;14:907–12.
Frobisher M Jr. The etiology of malignant diphtheria. Am J Public Health Nations Health. 1943;33:1244–56.
Macgregor RR. Corynebacterium diphtheria. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. 8th ed. Philadelphia: Elsevier; 2015. p. 2366–72.
Singh J, Harit AK, Jain DC, Panda RC, Tewari KN, Bhatia R, et al. Diphtheria is declining, but continues to kill many children: analysis of data from a sentinel center in Delhi, 1997. Epidemiol Infect. 1999;123:209–15.
Kole AK, Roy R, Kar SS. Cardiac involvement in diphtheria: study from a tertiary referral infectious disease hospital. Ann Trop Med Pub Health. 2012;5:202–6.
Lumio JT, Groundstroem KW, Melnick OB, Huhtala H, Rakhmanova AG. Electrocardiographic abnormalities in patients with diphtheria: a prospective study. Am J Med. 2004;116:78–83.
Jayashree M, Shruthi N, Singhi S. Predictors of outcome in patients with diphtheria receiving intensive care. Indian Pediatr. 2006;43:155–60.
Hadfield TL, McEvoy P, Polotsky Y, Tzinserling VA, Yakovlev AA. The pathology of diphtheria. J Infect Dis. 2000;181(Suppl 1):S116–20.
Stockins BA, Lanas FT, Saavedra JG, Opazo JA. Prognosis in patients with diphtheric myocarditis and bradyarrhythmias: assessment of results of ventricular pacing. Br Heart J. 1994;72:190–1.
Kneen R, Dung NM, Solomon T, et al. Clinical features and predictors of diphtheritic cardiomyopathy in Vietnamese children. Clin Infect Dis. 2004;39:1591–8.
Manikyamba D, Satvavani A, Deepa P. Diphtheritic polyneuropathy in the wake of resurgence of diphtheria. J Pediatr Neurosci. 2015;10:331–4.
Sanghi V. Neurologic manifestations of diphtheria and pertussis. Handb Clin Neurol. 2014;121:1355–9.
Logina I, Donaghy M. Diphtheritic polyneuropathy: a clinical study and comparison with Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 1999;67:433–8.
Mateen FJ, Bahl S, Khera A, SutterRW FJ. Detection of diphtheritic polyneuropathy by acute flaccid paralysis surveillance. India Emerg Infect. 2013;19:1370–3.
Lumio J, Olander RM, Groundstroem K, Suomalainen P, Honkanen T, Vuopio-Varkila J. Epidemiology of three cases of severe diphtheria in Finnish patients with low antitoxin antibody levels. Eur J Clin Microbiol Infect Dis. 2001;20(10):705.
de Benoist AC, White JM, Efstratiou A, Kelly C, Mann G, Nazareth B, et al. Imported cutaneous diphtheria, United Kingdom. Emerg Infect Dis. 2004;10(3):511–3.
Hofler W. Cutaneous diphtheria. Int J Dermatol. 1991;30:845–7.
Belsey MA, Sinclair M, Roder MR, LeBlanc DR. Corynebacterium diphtheriae skin infections in Alabama and Louisiana. A factor in the epidemiology of diphtheria. N Engl J Med. 1969;280(3):135–41.
Pickering LK Red Book, diphtheria: report of the committee on infectious diseases, 25. Elk Grove Village, IL: American Academy of Pediatrics; 2000: 230–234.
Centers for Disease Control and Prevention. http://www.cdc.gov/diphtheria/downloads/dip-cklist-diag.pdf. Accessed May 2017.
Efstratiou A, Maple PAC. Manual for the laboratory diagnosis of diphtheria. Copenhagen: Expanded Programme on Immunization in the European Region of World Health Organization; 1994. ICP/EPI 038 (C).
Konrad R, Hörmansdorfer S, Sing A. Possible human-to-human transmission of toxigenic Corynebacterium ulcerans. Clin Microbiol Infect. 2015;21(8):768–71.
Siegel JD, Rhinehart E, Jackson M, Chiarello L, Healthcare Inefection Control Practices Advisory Committee. Centers for Disease Control and Prevention. Guidelines for prevention of transmission of infection in healthcare settings. 2007. https://www.cdc.gov/infectioncontrol/pdf/guidelines/isolation-guidelines.pdf. Accessed May 2017.
Tiwari T, Clark T, Centers for Disease Control and Prevention. Use of diphtheria antitoxin (DAT) for suspected diphtheria cases, 3/14/2016. https://www.cdc.gov/diphtheria/downloads/protocol.pdf. Accessed May 2017.
Benamrouche N, Hasnaoui S, Badell E, et al. Microbiological and molecular characterization of Corynebacterium diphtheriae isolated in Algeria between 1992 and 2015. Clin Microbiol Infect. 2016;22:1005e1–7.
Acknowledgement
The authors would like to thank Dr. Sumit Chakrabarty for his critical review of the manuscript and Mr. Asish Sikder for his technical support.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Kole, A.K., Kole, D.C. (2018). Diphtheria. In: Durand, M., Deschler, D. (eds) Infections of the Ears, Nose, Throat, and Sinuses. Springer, Cham. https://doi.org/10.1007/978-3-319-74835-1_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-74835-1_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-74834-4
Online ISBN: 978-3-319-74835-1
eBook Packages: MedicineMedicine (R0)