Abstract
Diphtheria is a paradigmatic example of a toxigenic infectious disease. It was Klebs who first identified Corynebacterium diphtheriae as the causative agent for diphtheria in 1883. It is an acute respiratory infection characterized by pseudo-membrane formation in the throat but can also cause cutaneous infections. Systemic effects are a result of the production of diphtheria toxin, which is an exotoxin that inhibits protein synthesis and leads to cell death. The toxin can commonly cause myocarditis and neuropathy, which are associated with increased mortality. Clinical diagnosis is of utmost importance and timely diagnosis and management are lifesaving. An attempt to confirm the diagnosis by isolating and identifying Corynebacterium diphtheriae by microbiological culture should be made. Enzymatic and toxin detection tests should confirm the isolate. Treatment consists of the administration of diphtheria antitoxin and antimicrobial therapy. Mainly a vaccine-preventable childhood disease, this disease has re-emerged in countries where the recommended vaccination programs are not sustained, and not only children but also adults are becoming prey to the disease. In the South East Asia region, thousands of diphtheria cases are reported annually. Globally, small pockets of outbreaks still occur in developed countries. There has been a change in the epidemiological trend of diphtheria around the world. In order to prevent the spread of such toxigenic strains in communities, clinical and epidemiological investigations are necessary along with strict public health measures. Recent outbreaks have highlighted the importance of vaccination in reducing the incidence in children and its re-emergence in adults.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 History
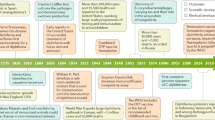
The origin of diphtheriae is from the Greek word for “leather” or “hide,” which describes the coating that appears in the throat that is called the pseudo-membrane. Diphtheria gained its official name from French physician Pierre Bretonneau (1778–1862), who called the disease diphtérite (Bretonneau 1826).
Bretonneau also distinguished diphtheria from scarlet fever. It was Klebs who was the first to identify the organism in 1883 (Klebs 1940), and Loeffler was the first to cultivate the organism in 1884 (Loeffler 1884). The bacterium came to be called the “Klebs-Loeffler” bacillus. Roux and Yersin demonstrated that the organism could produce a toxin in 1888, and von Behring and Kitasato in 1890 demonstrated that after administering an “anti-serum” or “antitoxin,” the antibody-containing serum produced in animals infected with an attenuated strain would prevent mortality (Behring 2013). The first successful treatment of a child with diphtheria using the antitoxin occurred in Germany in 1891. Antitoxin was invented in the late nineteenth century but the toxoid was developed further in 1920 (Smith 1909).
2 Epidemiology
In 1921, the United States recorded 206,000 cases of diphtheria, resulting in 15,520 deaths. But the disease rate dropped quickly due to the widespread use of vaccines, which resulted in two cases in 2004 and 2017. Since 1980, globally, there has been a decline in the number of diphtheria cases. However, between the mid-1990s, there occurred a re-emergence of diphtheria cases in the former Union of the Soviet Socialist Republic. From 1980 to 2015, the number of cases reported to the World Health Organization (WHO) has declined to 2500 from nearly 100,000. In 2016, globally, 7100 cases of diphtheria were reported to the World Health Organization (WHO 2016).
A recent evaluation of the literature suggests several outbreaks from sub-Saharan Africa (e.g., Nigeria and Madagascar) since 2000. In 2014, for example, 22 cases of confirmed diphtheria were reported in the European Union, and about half of these cases were in Latvia. According to the Global Epidemiology of Diphtheria 2000–2017, the final dataset consisted of 15,380 cases of diphtheria (15,068 including age data and 7242 including vaccination status data) from 34 countries. Recently, an outbreak has been experienced in Bangladesh among the Rohingya’s refugee camps in the year 2017 (Rahman and Islam 2019). So, in recent years, Venezuela, Yemen, and Bangladesh are the three countries where diphtheria has been in the headlines. Although cases of diphtheria have been reported from across the globe, it is an endemic primarily in developing regions of Africa, Asia, and South America (Clarke et al. 2019).
2.1 Indian Scenario of Diphtheria
Over the last 25 years, diphtheria has continued to persist in India without much decline, according to the Government of India’s data on vaccine-preventable diseases. During 2001–2015, India accounted for nearly half of all diphtheria cases reported worldwide (Dabbagh et al. 2007). From 2005 to 2014, the Central Bureau of Health Intelligence (CBHI) reported 41,672 cases with 897 deaths with case fatality ratio of about 2.2% whereas Joint Reporting Form on Immunisation (JRF) data since 2000 reported 64% (CBHI 2005). However, on reviewing the literature, >50% of cases were from India. According to the database, 84% cases have been reported from 10 states namely, Andhra Pradesh, Assam, Delhi, Gujarat, Haryana, Karnataka, Nagaland, Maharashtra, Rajasthan, and West Bengal. Collectively, in short, India, Nepal, and Indonesia reported 96–99% of the cases from the South-East Asia region since 2000 (Singh et al. 1999). According to the authors, there have been few published reports on adult diphtheria outbreaks in India in the past few years, suggesting a shift in the incidence of diphtheria from children to adolescents, as shown in Table 30.1.
Murheka et al. reported that the median age of diphtheria cases in most of the published studies was 5 years. In some Indian states, Muslim children were affected more. Most of the diphtheria cases were unvaccinated. The coverage of the primary diphtheria vaccine in the country is around 80%, whereas the coverage of diphtheria boosters, although not available, is expected to be low (Murhekar and Bitragunta 2011).
The most probable reasons for the resurgence in cases are lack of awareness among parents in following up on immunization, and ignoring the booster doses. To prevent this infection, the World Health Organization (WHO) introduced Expanded Program of Immunization (EPI), which recommends three doses of DPT—diphtheria, pertussis, and tetanus—vaccine starting at 6 weeks of age with booster doses of diphtheria vaccine in countries with adequate resources. Besides the routine doses given at 6, 10, and 14 weeks of age, the Universal Immunization Program (UIP) in India offers two booster doses at 18 months and 56–72 months of age (World Health Organization 2016). Historically, children have been the major victims of diphtheria, but recent trends have shown a rise in adult diphtheria also. Several recent series also reported that the majority of cases (60–85%) occurred in children aged 0–10 years. In contrast, an outbreak during the 1990s in the New Independent States of the former Soviet Union included many adults, with 70% of cases occurring in patients older than 15 years of age (Vitek and Wharton 1998).
3 Pathogenesis
The only known reservoir for Corynebacterium diphtheriae is humans, and the disease is transmitted by respiratory droplets, exudate from skin lesions, or fomites. Pathogenesis of diphtheria is based on two determinants: one that is associated with colonization of the host and is encoded by the bacteria, and the other determinant that is associated with virulence and is encoded by corynebacteriophages:
-
The first ability of Corynebacterium diphtheriae is to colonize the nasopharyngeal cavity and/or the skin and the second ability is to produce toxins. Thus, in a susceptible host, it colonizes on the mucosa of the respiratory tract and within the next few days, the toxigenic strains produce a potent exotoxin that causes inflammation and necrosis of the local tissue resulting in the formation of pseudo-membrane that consists of exudate, inflammatory cells, necrotic tissue, and the organism itself. Epidemiologic studies have demonstrated that a given isotype may be supplanted by another isotype and the persistence and the emergence of a new isotype is presumably due to its ability to colonize.
-
Toxin when gets absorbed into systemic circulation causes dysfunction of various organs (heart, nervous system, kidneys). The exotoxin has two protein fragments, A and B, of which the B fragment binds to cell receptors enabling fragment A to enter the cell cytosol and inhibit protein synthesis. Diphtheria toxin specifically cleaves its protease-sensitive loop into two polypeptide fragments, A and B. Fragment A is the N-terminal 21 kDa component of the toxin and contains the catalytic center for the Adenosine Diphosphate (ADP)-ribosylation of elongation factor 2 (EF-2), whereas fragment B carries the transmembrane and the receptor-binding domains of the toxin. Non-toxigenic strains usually cause mild to moderate pharyngitis and do not form the typical pseudo-membrane and by lysogenic conversion, these strains can become toxigenic with corynebacteriophage-ß (Tao et al. 1994).
4 Diphtheria Toxin Production
As is well-known, diphtheria is a vaccine-preventable disease that causes systemic organ damage due to the production of toxins, which leads to the development of both an effective antitoxin-based therapy and a highly successful toxoid. The tox gene, which is the structural gene for the diphtheria toxin, is carried by a family of closely related corynebacteriophages, among which the phage has been the subject of the most research about diphtheria. A DtxR-like iron-activated repressor is responsible for controlling gene expression. Iron dissociates from DtxR in circumstances when it becomes the growth-rate limiting substrate, depressing tox; hence the expression of the gene is dependent on the physiological state of C. diphtheriae. Because of its extreme potency, diphtheria toxin can be fatal in doses as little as 100–150 mg/kg of body weight. A 535 amino acid long polypeptide chain makes up the diphtheria toxin (Parveen et al. 2019). Additionally, X-ray crystallography and biochemical genetic studies reveal that the toxin is made up of three structural/functional domains, namely:
-
1.
A catalytic domain, i.e., N-terminal ADP-ribosyl transferase.
-
2.
A transmembrane domain, which is a region that facilitates the delivery of the catalytic domain across the cell membrane.
-
3.
The receptor-binding domain.
The following mechanism is involved in intoxication. First, the toxin is internalized by receptor-mediated endocytosis as a result of binding to its cell surface receptor, which causes charged receptors to adhere to the coated pits. Here, an Adenosine Triphosphate (ATP)-driven proton pump causes the endocytic vesicle to become acidic, the transmembrane domain to be inserted into the membrane, and the catalytic domain to be more easily delivered into the cytosol. Finally, the ADP-ribosylation of EF-2 causes the irreversible inhibition of protein synthesis (Greenfield et al. 1983).
5 Treatment
Diphtheria is highly contagious but a vaccine-preventable disease. The patient may remain contagious for about 48–72 h even after starting antibiotic therapy and for up to 2–6 weeks in case of untreated or partially treated patients (Truelove et al. 2020).
Patients should be evaluated promptly including evaluation for severity of symptoms, overall clinical status, vital signs, and development of any stridor or other systemic complication. Most importantly, diphtheria antitoxin (DAT) must be obtained on an emergency basis and administered as soon as possible. Treatment with diphtheria antitoxin and antibiotics should be started immediately in cases that are clinically compatible with diphtheria, without waiting for culture results. All patients should be monitored closely for the development of any respiratory or cardiac complications. Severe cases having a toxic appearance require continuous monitoring with special attention to airway patency and any cardiovascular instability. Sometimes it may require resuscitative measures like tracheostomy or rarely temporary cardiac pacing. In cases with airway compromise, immediate action should be taken (e.g., tracheostomy, intubation, clearing of pseudo-membrane endoscopically by an otolaryngologist).
All suspected diphtheria cases should be kept in isolation immediately in the emergency department of the healthcare facility. The patient should be treated following all droplet precautions and contact precautions. Of note, the Centre for Disease Control and Prevention (CDC) recommends only droplet precautions for respiratory diphtheria, although it notes that fomite transmission is also possible. Therefore, it seems prudent to use both droplet and contact precautions until the patient has two negative cultures obtained 24 h apart (Atkinson et al. 2007).
6 Antitoxin
Diphtheria antitoxin (DAT) was first produced in the 1890s and is still being produced using serum from horses that are hyperimmunized with diphtheria toxoid. The evidence for the efficacy of DAT for the treatment of diphtheria is found in studies done several decades ago. Mortality rates exceeded more than 50% in the pre-antitoxin era for clinical diphtheria. Almost as soon as antitoxin was available, a dramatic decline in mortality was noticed after the introduction of DAT. In a controlled trial when patients at a hospital were allocated with antitoxin treatment or no antitoxin treatment on an alternating day schedule, it was noticed that mortality in treated patients was 3.3% as compared to 12.2% among the untreated patients. So, it was concluded that mortality increased progressively from the onset of illness to treatment, with a pointy increase from 4% in those treated with antitoxin to 16.1% in those treated on their third day of illness. With prolonged intervals, mortality also continued to increase reaching 29.9% in those treated on day 7 or more days after the onset of illness. Current thinking is that toxin fixes to susceptible cells early in the disease and the fixed toxin is not neutralized by antitoxin, so it was proved that early treatment with DAT is inversely associated with the duration of clinical illness preceding its administration (Sevigny et al. 2013).
7 Vaccination
Immunity in an individual can be acquired either naturally or by vaccination, but it does not prevent asymptomatic carriage in an individual. Diphtheria, a vaccine-preventable disease, remains a global health threat, more so in developing countries. The reason for it being endemic is incomplete childhood vaccinations and thereafter inadequate boosters. Toxoid-containing diphtheria vaccines stay the oldest vaccines and continue to be in use. The United States was the first to start with active immunization against diphtheria based on a mixture of toxin and antitoxin both in 1914. In 1923, the diphtheria toxoid vaccine was developed by formaldehyde detoxification. In 1926, a more immunogenic alum-precipitated diphtheria toxoid was developed and it was in the 1940s that diphtheria toxoid was combined with tetanus toxoid and pertussis antigens and labeled as DPT/DTP, which was widely used throughout the world. Diphtheria toxoid is usually included with other antigens in combination with vaccines of varying strengths. For the description of diphtheria vaccines, uppercase letters like D, P, or T denote the full strength of diphtheria, pertussis, and tetanus whereas lowercase letters d and p indicate the reduced concentrations of diphtheria. Acellular components of a vaccine are represented by the lowercase “a” (DTaP). A combined vaccine of DTP, which contained whole-cell pertussis (DTwP), was introduced in 1948 whereas in 1990, acellular pertussis equivalent (DTaP) became available (Chitkara et al. 2019).
Co-administration of vaccines containing DTaP or DTwP does not interfere with antibody response, so such vaccines can be combined with Bacille Calmette-Guerin (BCG), conjugate pneumococcal vaccine (PCV), inactivated polio vaccine (IPV), oral polio vaccine (OPV), and measles and rubella vaccines (Skibinski et al. 2011)
The advantage of conjugate vaccines is that it contains diphtheria toxoid or a protein carrier that induces a booster response in previously immunized individuals, so they will be safe when co-administered with other conjugate vaccines. According to the WHO recommendations, the higher potency of the diphtheria vaccine (D) is used for the immunization of children up to 6 years of age. Tetanus-diphtheria (Td), low-dose diphtheria toxoid formulations, and tetanus-diphtheria-acellular pertussis (Tdap) formulations are licensed for use from 5 years of age and 3 years of age, respectively. This reduction in diphtheria toxoid potency is sufficient to provoke an antibody response in older children and adults. DTP vaccines have been combined with other vaccine antigens of Haemophilus influenzae type b (Hib), poliomyelitis, and hepatitis B (HepB) virus (HBV) to make it a pentavalent vaccine and labeled as DPT-HBV-Hib and DTaP-IPV/Hib. Antigens manufactured against all six diseases offer the general benefits of higher valent combination vaccines for children, parents, and to healthcare providers and these are available as hexavalent vaccines. Three IPV-containing hexavalent vaccines are available in India: DTwP-Hib/HepB-IPV (Panacea Biotec), DTaP-IPV-HB-Polyribosylribitol phosphate (PRP)~T (Sanofi Pasteur), and DTaP-HBV-IPV/Hib (GSK). The main difference in their composition is that DTwP-Hib/HepB-IPV contains a wP component, DTaP-IPV-HB-PRP~T contains two aP components, and DTaP-HBV-IPV/Hib contains three aP components. We will therefore refer to them as wP-hexa, 2aP-hexa, and 3aP-hexa, respectively. wP-hexa has been available since 2017 and is only available in India. 2aP-hexa was launched in 2013 and has been available in India since 2016. 3aP-hexa has been available in India since 2018. All hexavalent vaccines are well tolerated, although whole-cell pertussis-containing vaccines may result in more solicited local reactions and fever than those with acellular pertussis components (Madhi et al. 2011).
Dosage is scheduled in India during the first 2 years of life. The Universal Immunization Program (UIP) recommends vaccination against the six diseases covered by the hexavalent vaccines: oral poliovirus (OPV) and HBV vaccines are given at birth, the pentavalent vaccines like DPT-HBV-Hib plus OPV are given at the age of 6, 10, and 14 weeks, while fractional doses, i.e., 1/5 full dose, via the intradermal route of inactivated polio vaccine (IPV) are given at 6 and 14 weeks; and the boosters of OPV and DTP are provided at 16–24 months (Mohanty et al. 2018). Most diphtheria toxoid-containing vaccines are administered as a 0.5 mL dose, by intramuscular injection only (Lalwani et al. 2017).
However, the Indian Academy of Pediatrics (IAP) recommends OPV and HBV vaccines at birth; DTP, HBV, and Hib (or pentavalent vaccine), and intramuscular IPV at age 6–10–14 weeks; and DTP, Hib, and IPV at 16–18 months. The most common solicited local adverse events (AEs) were pain/tenderness and pain while the most common solicited systemic AEs were fever (wP-hexa), irritability (2aP-hexa), and temperature (3aP-hexa). Serious adverse events were rare (<2% in each study) and none were judged to be related to vaccination. All these studies have reported that the hexavalent vaccines were well tolerated (Madhi et al. 2011).
A finding on the epidemiology of diphtheria reported by six representative studies from different countries and decades documented that not only children but also many adults remain susceptible to diphtheria, primarily due to waning immunity and failure to get the recommended booster doses and pertussis in the vaccine. DTaP and DT vaccines are used through 6 years of age. The DTaP is also formulated with inactivated polio–hepatitis B (Pediarix). Adult Td can vaccinate individuals either 7 years or older than that, Tdap vaccines are available for young people aged 10 and 18 years, and Adacel is approved for individuals aged 11–64 years (Kulkarni et al. 2011).
In a study by Grasse et al., it was stated that in multivalent tetanus or diphtheria vaccines, diphtheria is more frequently left unprotected than tetanus, with disastrous results. According to a recent outbreak in Yemen where more than 333 cases were reported, the mortality rate was over 10%, and 39% in suspected cases who were vaccinated against diphtheria, indicating a need to improve the current vaccination strategy and/or the vaccine. Grasse et al. found a correlation between antibody levels against diphtheria in both young and old persons and a number of immunological markers. The study came to the conclusion that the immune responsiveness to the diphtheria component in multivalent vaccinations would be impacted by the in vivo administration of Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF). Recombinant GM-CSF will therefore enhance the humoral and cellular immune responses to diphtheria while leaving the response to tetanus unaffected when administered in vivo. Additionally, GM-CSF raised antibody titers against human immunodeficiency virus (HIV), Chlamydia trachomatis, and H5N1 influenza virus and functions as an adjuvant for diphtheria, but not tetanus toxoid (Grasse et al. 2018).
Revised recommendations for diphtheria vaccination were introduced in August 2017 by the WHO, which stated that in addition to the three-dose primary series in infancy, three diphtheria toxoid-containing booster doses were to be given at 12–23 months of age, 4–7 years of age, and 9–15 years of age. These recommendations, which harmonize with the updated recommendations for tetanus boosters released in February 2017, emphasize the need for a life course vaccination approach and present new opportunities for synergies with other vaccines. According to the Global Epidemiology of Diphtheria, it was noticed that vaccination coverage in a country has increased and the percentage of case patients of more than 15 years has also increased. In India, the national-level health surveys depicted that coverage of three doses of the diphtheria vaccine was 80% in the year 2015–2016 (World Health Organization 2016).
Immunization programs also ensure providing booster doses of diphtheria toxoid-containing vaccine during childhood and adolescence, which will protect adolescence and adulthood. The diphtheria booster should be given in combination with tetanus toxoid at the same schedule at 12–23 months of age, 4–7 years of age, and 9–15 years of age (Michel and Lang 2011). However, it remains a patch whether a booster dose later in life will be necessary to ensure life-long protection or not.
8 Summary
To summarize, Corynebacterium diphtheriae is an aerobic Gram-positive bacillus. It has three biotypes, namely gravis, intermedius, and mitis. All strains may produce toxins and only toxigenic strains can cause severe disease. Toxigenicity occurs when the bacillus is lysogenic by a specific virus bacteriophage carrying the tox gene. The toxin, if absorbed systemically, can affect organs and tissues distant from the site of invasion resulting in myocarditis and neuritis. The global incidence of diphtheria has decreased by >90% during 1980–2016 after the initiation of the WHO Expanded Program on Immunization in 1974. Recently, there is a changing trend regarding the age distribution of cases, from children to adolescents and adults. This reminds us of non-vaccination and failure to receive boosters among specific subpopulations, which even today remain vulnerable to this severe vaccine-preventable disease. Clinicians and public health practitioners should remember that primary prevention is through proper vaccination coverage and completing boosters as well.
References
Atkinson W, Hamborsky J, McIntyre L (2007) Epidemiology and prevention of vaccine-preventable disease, vol 10. Public Health Foundation, Washington, DC, pp 59–70
Behring EV (2013) Ueber das zustandekommen der diphtherie-immunität und der tetanus-immunität bei thieren. Drucke 16:1113–1114
Bretonneau P (1826) Des inflammations spéciales du tissu muqueux, et en particular de la diphthérite, on inflammation pelliculaire. Chez Crevot, excerpt in Major. Classic Descriptions, Paris, pp 159–161
CBHI (2005) Onwards. http://www.cbhidghs.nic.in/index1.asp?linkid=267. Accessed 15 Apr 2017
Chitkara AJ, Parikh R, Mihalyi A et al (2019) Hexavalent vaccines in India: current status. Indian Pediatr 56:939–950
Clarke KE, MacNeil A, Hadler S et al (2019) Global epidemiology of diphtheria, 2000–2017. Emerg Infect Dis 25:1834–1842
Dabbagh A, Eggers R, Cochi S et al (2007) A new global framework for immunization monitoring and surveillance. Bull WHO 85:904–905
Das PP, Patgiri SJ, Saikia L (2016) Recent outbreaks of diphtheria in Dibrugarh district, Assam, India. J Clin Diagn Res 10:1–3
Grasse M, Meryk A, Miggitsch C et al (2018) GM-CSF improves the immune response to the diphtheria-component in a multivalent vaccine. Vaccine 36:4672–4680
Greenfield L, Bjorn MJ, Horn G (1983) Nucleotide sequence of the structural gene for diphtheria toxin carried by corynebacteriophage beta. Proc Natl Acad Sci U S A 80:6853–6857
Klebs E (1940) Ueber Diphtherie. Bull Hist Med 8:509
Kulkarni PS, Raut SK, Dhorje SP (2011) Diphtheria, tetanus, and pertussis immunity in Indian adults and immunogenicity of Td vaccine. Int Sch Res Notices 28:1–4
Lalwani SK, Agarkhedkar S, Sundaram B (2017) Immunogenicity and safety of 3-dose primary vaccination with combined DTPa-HBV-IPV/Hib in Indian infants. Hum Vaccin 13:120–127
Loeffler F (1884) Utersuchung uber die Bedeutung der Mikroorganismen fir die Entstehung der Diptherie beim Menschen, bei der taube und beim Kalbe. Mitth Adkaiserl 2:421–499
Loganathan T, Bin Yusof MP (2018) Adult diphtheria in Malaysia: a case report. Med J Malaysia 73:340–341
Lurie P (2004) Fatal respiratory diphtheria in a U.S. traveler to Haiti Pennsylvania. JAMA 291:937–938
Madhi SA, Mitha I, Cutland C et al (2011) Immunogenicity and safety of an investigational fully liquid hexavalent combination vaccine versus licensed combination vaccines at 6, 10, and 14 weeks of age in healthy South African infants. Pediatr Infect Dis J 30:68–74
Michel JP, Lang PO (2011) Promoting life course vaccination. Rejuvenation Res 14:75–81
Mohanty L, Sharma S, Behera B (2018) A randomized, open label trial to evaluate and compare the immunogenicity and safety of a novel liquid hexavalent DTwP-Hib/Hep B-IPV (EasySix™) to licensed combination vaccines in healthy infants. Vaccine 36:2378–2384
Murhekar M, Bitragunta S (2011) Persistence of diphtheria in India. Indian J Community Med 36:164–166
Parveen S, Bishai WR, Murphy JR (2019) Corynebacterium diphtheriae: diphtheria toxin, the tox Operon, and its regulation by Fe2+ activation of apo-DtxR. Microbiol Spectr 7:7–4
Rahman M, Islam K (2019) Massive diphtheria outbreak among Rohingya refugees: lessons learnt. J Travel Med 26:1–3
Sangal L, Joshi S, Anandan S, Balaji V et al (2017) Resurgence of diphtheria in North Kerala, India, 2016: laboratory supported case-based surveillance outcomes. Front Public Health 5:218
Sevigny LM, Booth BJ, Rowley KJ (2013) Identification of a human monoclonal antibody to replace equine diphtheria antitoxin for treatment of diphtheria intoxication. Infect Immun 81:3992–4000
Singh J, Harit AK, Jain DC et al (1999) Diphtheria is declining but continues to kill many children: analysis of data from a sentinel centre in Delhi, 1997. Epidemiol Infect 123:209–215
Singhal L, Kour I, Gupta V et al (2021) Diphtheria in an adult: a paradigm of waning immunity. J Clin Diagn Res 15:1–3
Skibinski DA, Baudner BC, Singh M et al (2011) Combination vaccines. J Glob Infect 3:63–72
Smith T (1909) Active immunity produced by so called balanced or neutral mixtures of diphtheria toxin and antitoxin. J Exp Med 11:241–256
Swarna S, Sivagurunathan S, Bharathi S (2020) Diphtheria in an adult - a case report. Ind J Case Rep 6:160–162
Tao X, Schiering N, Zeng HY et al (1994) Iron, DtxR, and the regulation of diphtheria toxin expression. Mol Microbiol 14:191–197
Truelove SA, Keegan LT, Moss WJ (2020) Clinical and epidemiological aspects of diphtheria: a systematic review and pooled analysis. Clin Infect Dis 71:89–97
Vitek CR, Wharton M (1998) Diphtheria in the former Soviet Union: reemergence of a pandemic disease. Emerg Infect Dis 4:539–550
WHO (2016) Immunization, vaccines and biologicals. reported cases of selected vaccine preventable diseases (VPDs). http://www.who.int/immunization/monitoring_surveillance/data/en/
World Health Organization (2016) Summary of WHO position papers: recommendations for routine immunization, Geneva. http://www.who.int/immunization/documents/positionpapers/en/
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kour, I., Singhal, L., Gupta, V. (2023). Diphtheria: A Paradigmatic Vaccine-Preventable Toxigenic Disease with Changing Epidemiology. In: Singh, P.P. (eds) Recent Advances in Pharmaceutical Innovation and Research. Springer, Singapore. https://doi.org/10.1007/978-981-99-2302-1_30
Download citation
DOI: https://doi.org/10.1007/978-981-99-2302-1_30
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-2301-4
Online ISBN: 978-981-99-2302-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)