Abstract
Diphtheria, the ‘strangling angel of children’, plagued mankind for thousands of years. With the discovery of its etiological agent, Corynebacterium diphtheriae, it became a paradigm of an infectious disease. According to Koch’s postulates C. diphtheriae was isolated by Klebs and Loeffler from infected patients, grown in pure culture and used to re-infect guinea pigs as test animals. Loeffler also recognized that the bacterium predominantly colonizes the nasopharyngeal cavity and, based on this observation, postulated that the secretion of a toxin might cause the often fatal damage of distant organs, a hypothesis, which was further supported by Roux and Yersin. While toxin production is the most dangerous aspect of diphtheria infection, the diphtheria toxin has also been the basis for effective diphtheria treatment and control. Already in 1890, von Behring suggested antitoxin application as a means of diphtheria treatment; in 1913 he developed a first vaccine against diphtheria toxin and in 1920 first mass vaccinations started. Mass immunization is still the most efficient means to prevent and control diphtheria, while antibiotics are effective to eradicate the bacteria from infected patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
Diphtheria of the upper respiratory tract is characterized by pseudomembrane , which renders breathing difficult. Eventually, coughing can remove parts of the pseudomembrane, easing the situation of the patient temporarily, and after several fits of coughing the pseudomembrane might even be removed and healing might be achieved. More often obstruction of airways results in suffocation, agony and death.
In tropical and subtropical regions respiratory tract diphtheria is outnumbered by cutanous diphtheria, which is characterized by skin lesions located predominantly on feet, lower legs and hands. Typically this infection is persistent for months.
Treatment of diphtheria is unproblematic today since C. diphtheriae can be eliminated easily using antibiotics, and the diphtheria toxin can be neutralized by antitoxin application. Nevertheless, mass immunization is the means of choice for diphtheria control. A highly effective toxoid vaccine is available which made diphtheria an extremely rare disease in industrialized countries. However, local outbreaks and even a full scale epidemic have been observed during the last three decades. Several thousand diphtheria cases are reported to the World Health organization each year, showing that diphtheria is not completely eradicated and that reservoirs still exist. Putative carriers are people with insufficient access to medical care including vaccination programs, for example poor parts of the population in developing countries. Consequently, for citizens of industrialized countries a major risk factor for infection with C. diphtheriae might be travel to an endemic country; however, animals also seem to play an increasing role in transmission of the infection. Isolation of C. diphtheriae strains was reported from domestic cats and horses. Animal reservoirs are even more common for other toxigenic Corynebacterium species, which might also be connected to diphtheria-like illness. Corynebacterium ulcerans has been primarily recognized as a commensal bacterium in domestic and wild animals; however, an increasing frequency and severity of human infections associated with C. ulcerans is observed and C. ulcerans strains producing diphtheria toxin are reported with rising frequency from industrialized countries.
1.2 A brief History of Diphtheria
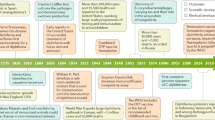
Diphtheria has been well known since Babylonian and Sumerian times and documents describing this disease can be found in the Talmud and in writings by Hippocrates. Its alternative designation ‘strangling angel of children’ refers to the dreadful death by suffocation especially of infants infected by classical diphtheria of the upper respiratory tract. Based on the appraisal of written reports, diphtheria seems to originate from the Middle East and was most likely disseminated from there first to Europe and later to all other continents. Numerous waves of epidemic outbreaks of diphtheria occurred in Europe, often connected to times of war. The cycles include gaps of 100 years and more (English 1985) and outbreaks were reported, e.g. in the sixteenth century in France and Germany, in the seventeenth century in Italy and Spain, and in the eighteenth century in Germany and Sweden. During the eighteenth century, diphtheria also reached the East coast of North America, where in 1799 George Washington became maybe the most prominent victim of this disease. At the end of the eighteenth and the beginning of the nineteenth century, diphtheria became more prevalent and developed into a leading cause of infant mortality. Up to four fifth of children infected with diphtheria died. The reason for this increase in infection numbers and mortality might be the concomitant rise of urban population with beginning industrialization coupled with inferior housing and nutrition conditions of working class people.
The start of industrialization at the end of the nineteenth century brought not only an increase of diphtheria and other diseases connected with poverty, overcrowding and malnutrition, such as tuberculosis, but also the rise of bacteriology. In fact, diphtheria became a paradigm of an infectious disease and provided key evidence for Koch’s postulates on the germ theory (Dolman 1973; Levy 1975; English 1985). In 1883 Klebs delivered the first description of C. diphtheriae isolated from a patient’s swab (Klebs 1883) and based on Klebs’ work, Loeffler was the first to grow C. diphtheriae in pure culture. In consistence with Koch’s postulates, this pure culture was able to evoke diphtheria in guinea pigs used as test animals. Furthermore, Loeffler passed the disease from guinea pig to guinea pig and found that even after more than twenty of these passes the animals still developed diphtheria-like symptoms. In his groundbreaking work, Loeffler described that C. diphtheriae colonizes the membranes of the nasopharyngeal cavity but not deeper parts of the body. Based on this observation, he postulated the secretion of a toxin being responsible for the often fatal damage of internal organs connected with upper respiratory tract diphtheria (Loeffler 1884). His hypothesis was supported by Roux and Yersin (1888), who injected bacteria-free filtrates of C. diphtheriae cultures in animals and observed that these developed organ damages indistinguishable from those of human diphtheria cases.
While toxin production is the most dangerous aspect of diphtheria infection, leading to severe, often fatal damages of heart, nervous system and muscles (see below), the diphtheria toxin has also been the key to successful treatment, vaccination and control, based on scientific advances made in the late nineteenth century. Already in 1890, von Behring and Kitasato suggested antitoxin application as a means of choice to treat diphtheria (von Behring and Kitasato 1890; for overview, see Grundbacher 1992). In fact, von Behring was also the first who used antitoxin from a horse to successfully treat diphtheria and in 1913 he developed the vaccine against diphtheria toxin. The basis of the vaccine and the vaccination programs starting in the 1920s is the diphtheria toxoid , a formaldehyde-inactivated diphtheria toxin, produced and secreted in vast amounts by strain Park-Williams no. 8 (PW8) , originally isolated from a mild case of diphtheria (Park and Williams 1896; Iwaki et al. 2010). Immunization with the inactivated toxin that remains antigenetically intact is extremely effective and changed diphtheria from a former main cause of infant mortality to an extremely rare disease, which will never be seen by most pediatricians or other physicians (English 1985).
Despite this extraordinary success story, diphtheria is not eradicated today. From 2000 to 2008 between three to eleven thousand cases were reported to the World Health Organization per year. Furthermore, especially the epidemic outbreak starting after the breakdown of the former Soviet Union in 1989 showed impressively that diphtheria is still a serious health hazard.
This large scale epidemic started in 1990 with the Russian Federation and Ukraine being centers of the outbreak. 15,211 diphtheria cases were reported for Russia and further 2,987 cases for Ukraine in 1993 (Galazka et al. 1995; Eskola et al. 1998; Popovic et al. 2000), from where the disease spread quickly to neighboring countries. The most characteristic feature of the outbreak was the infection of adolescents and adults, instead of infants being victims of the disease. In addition to Belarus, Russia and Ukraine, this was especially pronounced in the Baltic States Estonia, Latvia and Lithuania, where diphtheria cases among people from 15 years and older ranged from two thirds to four fifths of total cases (Hardy et al. 1996; Dittmann et al. 2000). Consequently, mass immunization of adults, which was started in 1993, was one crucial step to eradicate the disease and stop the epidemic.
The 1990s epidemic showed impressively that socioeconomic instability, large-scale population movements and absence or break-down of health infrastructure favor the spread of diphtheria. It also indicated, together with constant numbers of case reports from various countries, that C. diphtheriae might persist in a population for a long time (for example see Marston et al. 2001). In many cases, carriers of infection are unknown, sometimes these are groups of people with insufficient access to medical care including vaccination programs (John 2008) or drug abusers (Lowe et al. 2011). For inhabitants of industrialized countries, a major risk factor for infection with C. diphtheriae might be travel to a country where diphtheria is endemic (Connell et al. 2005; Bonmarin et al. 2009; Wagner et al. 2010).
1.3 Transmission and Clinical Manifestations of Diphtheria
1.3.1 Classical Diphtheria of the Upper Respiratory Tract
Diphtheria is a classical infectious disease which typically spreads from person to person by respiratory droplets produced by an infected person by coughing or sneezing (Fig. 1.1). Establishment of the disease takes 2–5 days and patients are infectious for two to three weeks. Besides people showing diphtheria symptoms, asymptomatic carriers may also contribute to spreading of the disease. Loeffler showed before that about five percent of the school children in Berlin (Germany) were carriers of C. diphtheriae without showing diphtheria-like symptoms.
Transmission of toxigenic corynebacteria. Transmission of Corynebacterium diphtheriae from person to person occurs by close physical contact. Respiratory diphtheria is mainly disseminated by respiratory droplets; however, smear infections are also possible, especially in case of cutaneous diphtheria. C. ulcerans infections occur via close physical contact with pets and farm animals or animal bites. Man-to-man transmission was not reported. C. pseudotuberculosis is transferred from infected cattle, especially sheep and goats, to humans by close physical contact.
Classical diphtheria is an infection of the upper respiratory tract that causes sore throat and low fever. Symptoms range from mild pharyngitis to suffocation due to airway obstruction by pseudomembrane formation . In severe cases, the air passages might be completely blocked. Typically, infection starts with the colonization of epithelial cells of the upper respiratory tract by C. diphtheriae . The bacteria multiply on the surface of the mucous membrane, but do not advance into deeper parts of the body, as already described by Loeffler (1884). Tissues infected show inflammatory symptoms, later edema and necroses develop due to the detrimental action of the diphtheria toxin. The toxin is also responsible for inflammation of deeper lying capillaries which results in fibrin secretion into the damaged epithelium. The released fibrin protein, destroyed epithelial cells and bacteria together form the so-called pseudomembrane, a grayish or yellowish-white coating. Later during infection, the damage of the capillaries due to inflammatory processes causes bleedings, and due to decaying erythrocytes, the color of the pseudomembrane can turn into a dirty brown and the breath of the patients becomes sweetish-putrid, a characteristic indication of diphtheria. With progressive pseudomembrane formation reaching the larynx, a barking cough develops. Furthermore, the voice of the patients becomes affected and hoarseness develops which can even result in complete loss of the voice. Later, trachea and bronchi may be covered by firmly or loosely attached pseudomembranes. At this stage, breathing renders difficult for the patients and their lips turn blue. With increasing dyspnea the patients become restless, their pulse beats become faster and the look of their faces becomes timid and frightened. Sometimes coughing can remove parts of the pseudomembranes at this stage of the disease, easing the situation of the patient temporarily. After several of these fits of coughing, the pseudomembranes might even be completely removed and healing might be achieved in some cases. More often, suffocation results in death agony and, finally, the patients faint and heart failure results in death.
As mentioned above, the colonization of the human host by C. diphtheriae remains localized to the upper respiratory tract, although satellite infections may occur in the esophagus, stomach or lower airpassages. The severe complications observed in later stages of infection are the result of the diphtheria toxin which is secreted by C. diphtheriae and distributed by the circulatory system to remote parts of the body.
Diphtheria toxin is synthesized depending on the iron concentration in the environment. When iron becomes limiting, as it is the case in the human host, the bacteria start to synthesize the toxin which is then secreted into the extracellular medium as a single polypeptide chain (Pappenheimer 1977; Holmes 2000). In this form the toxin is inactive, can be absorbed into the circulatory system and disseminated to distant parts of the body. When binding to its receptor, the uncleaved precursor of the heparin-binding EGF-like growth factor (Naglich et al. 1992), it can enter the cell by endocytosis. Once inside the endosome, the inactive diphtheria toxin, consisting of an A and B chain, is cleaved, and the A chain is released into the cytoplasm, where it is activated by further cleavage into the active toxin and inactivates its cellular target elongation factor 2 (EF-2) by ADP-ribosylation (Pappenheimer 1977; Lord et al. 1999; Falnes and Sandvig 2000).
Especially myocardium and peripheral motor neurons are affected by diphtheria toxin (Harrison et al. 1972; Murray and Noble 1985; Hadfield et al. 2000; Perles et al. 2000). Up to two thirds of patients show some evidence of myocarditis and 10–25 % of cases develop clinical cardiac dysfunctions. Cardiac symptoms are directly correlated to the extent and severity of local C. diphtheriae infection in the patient’s upper respiratory tract and often may prove fatal weeks later during convalescence (MacGregor 1995; Hadfield et al. 2000; Perles et al. 2000). Histological changes in the heart differ significantly from patient to patient and may include edema, congestion, infiltration by mononuclear cells and presence of fat drops. The myocardium may show areas of granular degradation, hyaline degradation, necrosis, inflammation and loss of cross striation (Kline and Kaplan 1998; Hadfield et al. 2000; Perles et al. 2000). The heart may be dilated, pale and flabby (Hadfield et al. 2000) and as a result of toxin damage to the cardiac conduction, muscle and nervous system electrical disturbances such as bradyarrhthmia, tachyarrythmia, artioventricular and bundle branch blocks are often found (Perles et al. 2000).
The nervous system is—besides the heart—a main target of the toxin. About three-fourths of patients with severe diphtheria infection develop neurologic complications. First symptoms of neuropathy are paralysis of the soft palate and posterior pharyngeal wall, resulting in regurgitation of swallowed fluids through the nose. Additionally, often a paralysis of the muscles of the eyes and dysfunction of facial, pharyngeal or laryngeal nerves are observed. In later stages, the nerves of trunk, neck, arms and hands might be affected leading to paralysis. Histological investigations to characterize the basis of the neuropathies led to the observation of paranodal and segmental demyelination, resulting in degeneration of myelin sheaths and axons (Baba et al. 1984; Hadfield et al. 2000).
1.3.2 Cutaneous Diphtheria
In tropical and subtropical regions, cutaneous diphtheria is more common and prevails over the respiratory tract form (Höfler 1991). It is still endemic in some African and Asian countries, where climate, overcrowding, poverty, poor hygiene, and frequent, slowly healing wounds favor the infection. Cutaneous diphtheria is easily spread by contact with infected skin, respiratory droplets of a patient infected with respiratory tract diphtheria or exposure to dust or clothing contaminated with C. diphtheriae (Höfler 1991) .
Common sites for cutaneous diphtheria are feet, lower legs and hands (for example see Hamour et al. 1995; Connell et al. 2005). Due to the various skin lesions that can be colonized by the bacteria and frequently occurring co-infections by different other bacterial pathogens the clinical manifestation of cutaneous diphtheria can be extremely variable.
The infection typically starts with a painful, fluid-filled pustule which breaks down later and progresses as a punched-out ulcer. The diameter of the ulcer might range between a few millimeters to centimeters. During the first weeks of infection lesions are covered by a smeary grayish-brown pseudomembrane, which might change color to a dirty or dark reddish brown over time. Later, the infection becomes anesthetic and the pseudomembrane falls off, leaving a hemorrhagic base with a surrounding of edematous grayish-white, pink or purple-colored tissue (Höfler 1991; Hadfield et al. 2000). Spontaneous healing takes several weeks to months, cases lasting one year have been observed (Höfler 1991). This long persistence might favor dissemination of the disease and might explain the extremely high infection rates of skin lesions with C. diphtheriae observed previously. Infection rates of up to 60 % were found in some African and Asian countries (Liebow et al. 1946; Livingood et al. 1946; Bezjak and Farsey 1970a, 1970b; Höfler 1991) .
1.3.3 Systemic Infections
C. diphtheriae is not only the etiological agent of classical diphtheria of respiratory tract and skin, but can also cause—although generally rare—systemic infections. Cases of bacteraemia, endocarditis , hepatic and splenic abcesses, meningitis, mycotic aneurysm, osteomyelitis, pneumonia as well as septic arthritis caused by non-toxigenic and toxigenic C. diphtheriae were reported (Isaac-Renton et al. 1981; Puliti et al. 2006; Hirata et al. 2008; Honma et al. 2009; Muttaiyah et al. 2011; and references therein). The best documented systemic infections are related to C. diphtheriae endocarditis. Endocarditis as a result of Corynebacterium infection has been described as aggressive disease often requiring surgical intervention (Mishra et al. 2005). Typically, the left heart of adult males is infected and underlying valvular disease is frequently found. Up to 28 % of patients require valve replacements and more than 40 % die (Belmares et al. 2007).
1.4 Diagnosis, Treatment and Control
The diagnosis of respiratory tract diphtheria is—as in former times—still based on the classical symptoms of this disease: sore throat, formation of a pseudomembrane and the typical sweetish-putrid smell of the patients’ breath . With the identification of C. diphtheriae as its etiological agent by Loeffler (1884) and the development of modern biochemistry and molecular biology, the diagnostic toolbox was constantly improved. Besides different screening and identification tests, the classical Elek test (Efstratiou et al. 1998) for toxicity testing is most commonly used (for a overview of tests and quality evaluation, see Neal and Efstratiou 2009). Furthermore, also a number of other toxigenic Corynebacterium species (see below) can also be reliably identified today (Schuhegger et al. 2008; Sing et al. 2011).
Before the introduction of antitoxin and antibiotics, physicians were restricted to treatments preventing suffocation such as tracheostomy introduced by Bretonneau in 1825 and intubation introduced by Bouchut in 1859 (English 1985). Despite some success, mortality stayed high, since—besides the severe side-effects of the proposed treatments—the detrimental action of the toxin could not be avoided by these treatments. The situation improved dramatically with the introduction of antitoxin, which is able to neutralize the toxin in the circulatory system. Even more effective for controlling diphtheria is the immunization with diphtheria toxoid, formaldehyde-inactivated diphtheria toxin that remains antigenetically intact. With increasing levels of immunity, the annual incidence of diphtheria dropped to 0.1–0.2 per million (Kwantes 1984; Höfler 1991; Murphy 1996; Vitek 2006) (Roush and Murphy 2007). Diphtheria toxoid is widely used as a component of DPT (diphtheria, pertussis, tetanus) vaccine. Immunization typically starts with early childhood; after four doses of the vaccine within the first 2 years immunization against diphtheria is effective up to 97 %. Since antibody titers wane over time, a large percentage of adults in the United States and Europe have antitoxin levels below the protective level (Murphy 1996; von Hunolstein et al. 2000). Therefore, booster immunization of adults is recommended .
The discovery of antibiotics was the next hallmark of effective diphtheria treatment. In contrast to other corynebacteria such as Corynebacterium jeikeium, which causes severe infections in intensive care units, multi-resistance against antibiotics is not the problem in C. diphtheriae and penicillin and erythromycin are first line antibiotics used for its eradication (Begg 1994; Kneen et al. 1998; Pereira et al. 2008; Zasada et al. 2010). In cases of cutaneous diphtheria , additional local application of drugs such as bacitracin or gentian violet is recommended (Höfler 1991). The therapy with antibiotics might become more difficult in future with the emergence of multidrug resistant strains in some countries. While a recent study on antimicrobial resistance found no multidrug resistant strains among isolates circulating in Poland (Zasada et al. 2010), a considerable number of isolates resistant against one or more antibiotics were observed among Brazilian C. diphtheriae strains and reservations about the use of penicillin were risen (Pereira et al. 2008).
To avoid complications due to action of diphtheria toxin, e.g. myocarditis, antitoxin is normally given. However, even if properly treated with antibiotics and antitoxin , five to ten percent of cases can end fatally. Therefore, mass immunization of the entire population is the best means to control diphtheria .
1.5 Diphtheria as a Zoonotic Infection
Besides humans, animals seem to play a role as a reservoir of the disease (Fig. 1.1) . Isolations of C. diphtheriae strains were reported for example from domestic cats (Hall et al. 2010), cows (Corboz et al. 1996) and horses (Henricson et al. 2000; Leggett et al. 2010). The existence of animal reservoirs is even more common, when other toxigenic Corynebacterium species are taken into consideration (Bonmarin et al. 2009). C. diphtheriae is closely related to two further Corynebacterium species, Corynebacterium pseudotuberculosis and Corynebacterium ulcerans (Riegel et al. 1995). The three species can be lysogenized by similar corynebacteriophages and, if these carry a tox gene, all three species produce diphtheria toxin (Groman 1984; Buck et al. 1985; Cianciotto and Groman 1996), making them a potential health threat.
Infections due to C. pseudotuberculosis, which causes caseous lymphadenitis in sheep and goat populations worldwide (Dorella et al. 2006; Baird and Fontaine 2007), are rare in humans and classical diphtheria of the upper respiratory tract or skin connected with C. pseudotuberculosis have not been observed. However, C. pseudotuberculosis may serve as a reservoir of corynebacteriotoxphages . In contrast, during the last decade, the frequency and severity of human infections associated with C. ulcerans appear to be increasing in various countries. Cases of respiratory tract diphtheria caused by toxigenic C. ulcerans strains were reported from various industrialized countries (Wagner et al. 2001; Hatanaka et al. 2003; De Zoysa et al. 2005; Tiwari et al. 2008) and became even more common than C. diphtheriae infections in the United Kingdom (Wagner et al. 2010). Infections with toxigenic C. ulcerans can be fatal in unvaccinated patients and usually occur in adults. Besides upper respiratory tract diphtheria, C. ulcerans can also cause severe skin and pulmonary infections (Dessau et al. 1995; Nureki et al. 2007; Mattos-Guaraldi et al. 2008). Transmission by person to person contact was not reported up to now. In contrast, close contact with domestic animals (Wagner et al. 2010) and consumption of raw, unpasteurized milk (Bostock et al. 1984; Hart 1984) seem to be risk factors. This observation is in accordance with the identification of C. ulcerans strains commensals in various domestic and wild animals (Schuhegger et al. 2008; Dixon 2010; Sykes et al. 2010) , which may serve as a reservoir for zoonotic infections .
1.6 Development and Persistence of C. diphtheriae Populations
As described above, there seems to be a shift from C. diphtheriae to C. ulcerans as etiological agent of diphtheria in at least some Western countries. Interestingly, a shift within C. diphtheriae populations has been observed as well. With the introduction of diphtheria toxoid vaccines, not only the number of diphtheria cases but also the number of isolated toxigenic C. diphtheriae strains decreased, suggesting a protection by the vaccine not only against the fatal action of the toxin but, at least partially, against the bacteria itself. Interestingly, anti-parallel to this development, an increasing number of non-toxigenic strains has been isolated (Zuber et al. 1992; Gilbert 1997; Hadfield et al. 2000; Wagner et al. 2011). These non-toxigenic C. diphtheriae are persisting over years in different, often poor populations (Romney et al. 2006; Lowe et al. 2011; Shashikala et al. 2011), where they are connected especially to skin infections. Unfortunately, also an increasing number of systemic infections by non-toxigenic strains has also been observed.
Following the outbreak by a unique clonal group of C. diphtheriae in Russia in 1990 (Popovic et al. 2000), a rising heterogenicity of circulating strains after the epidemic, emergence of new toxigenic variants, and persistence of invasive non-toxigenic strains were observed (Mokrousov 2009).
All in all, the emergence of new diphtheria-causing corynebacteria such as C. ulcerans and the adaptation of C. diphtheriae populations to medical treatment are supporting the need of continuous surveillance of C. diphtheriae and its relatives and justify, besides the basic scientific interest, experimental efforts to characterize these pathogens.
References
Baba M, Gilliatt RW, Harding AE, Reiners K (1984) Demyelination following diphtheria toxin in the presence of axonal atrophy. J Neurol Sci 64(2):199–211
Baird GJ, Fontaine MC (2007) Corynebacterium pseudotuberculosis and its role in ovine caseous lymphadenitis. J Comp Pathol 137(4):179–210
Begg N (1994) Manual of the management and control of diphtheria in the European region. Vol ICP/EPI 038 (B). World Health Organization, Copenhagen
Belmares J, Detterline S, Pak JB, Parada JP (2007) Corynebacterium endocarditis species-specific risk factors and outcomes. BMC Infect Dis 7:4
Bezjak V, Farsey SJ (1970a) Corynebacterium diphtheriae carriership in Ugandan children. J Trop Pediatr 16(1):12–16
Bezjak V, Farsey SJ (1970b) Corynebacterium diphtheriae in skin lesions in Ugandan children. Bull World Health Organ 43(5):643–650
Bonmarin I, Guiso N, Le Fleche-Mateos A, Patey O, Patrick AD, Levy-Bruhl D (2009) Diphtheria: a zoonotic disease in France? Vaccine 27(31):4196–4200
Bostock AD, Gilbert FR, Lewis D, Smith DC (1984) Corynebacterium ulcerans infection associated with untreated milk. J Infect 9(3):286–288
Buck GA, Cross RE, Wong TP, Loera J, Groman N (1985) DNA relationships among some tox-bearing corynebacteriophages. Infect Immun 49(3):679–684
Cianciotto NP, Groman NB (1996) Extended host range of a beta-related corynebacteriophage. FEMS Microbiol Lett 140(2–3):221–225
Connell TG, Rele M, Daley AJ, Curtis N (2005) Skin ulcers in a returned traveller. Lancet 365(9460):726
Corboz L, Thoma R, Braun U, Zbinden R (1996) Isolation of Corynebacterium diphtheriae subsp. belfanti from a cow with chronic active dermatitis. Schweiz Arch Tierheilkd 138(12):596–599
De Zoysa A, Hawkey PM, Engler K, George R, Mann G, Reilly W, Taylor D, Efstratiou A (2005) Characterization of toxigenic Corynebacterium ulcerans strains isolated from humans and domestic cats in the United Kingdom. J Clin Microbiol 43(9):4377–4381
Dessau RB, Brandt-Christensen M, Jensen OJ, Tonnesen P (1995) Pulmonary nodules due to Corynebacterium ulcerans. Eur Respir J 8(4):651–653
Dittmann S, Wharton M, Vitek C, Ciotti M, Galazka A, Guichard S, Hardy I, Kartoglu U, Koyama S, Kreysler J, Martin B, Mercer D, Ronne T, Roure C, Steinglass R, Strebel P, Sutter R, Trostle M (2000) Successful control of epidemic diphtheria in the states of the Former Union of Soviet Socialist Republics: lessons learned. J Infect Dis 181(Suppl 1):S10–22
Dixon B (2010) Sick as a dog. Lancet Infect Dis 10(2):73
Dolman CE (1973) The Donald T. Fraser Memorial Lecture, 1973. Landmarks and pioneers in the control of diphtheria. Can J Public Health 64(4):317–336
Dorella FA, Pacheco LG, Oliveira SC, Miyoshi A, Azevedo V (2006) Corynebacterium pseudotuberculosis: microbiology, biochemical properties, pathogenesis and molecular studies of virulence. Vet Res 37(2):201–218
Efstratiou A, Engler KH, Dawes CS, Sesardic D (1998) Comparison of phenotypic and genotypic methods for detection of diphtheria toxin among isolates of pathogenic corynebacteria. J Clin Microbiol 36(11):3173–3177
English PC (1985) Diphtheria and theories of infectious disease: centennial appreciation of the critical role of diphtheria in the history of medicine. Pediatrics 76(1):1–9
Eskola J, Lumio J, Vuopio-Varkila J (1998) Resurgent diphtheria-are we safe? Br Med Bull 54(3):635–645
Falnes PO, Sandvig K (2000) Penetration of protein toxins into cells. Curr Opin Cell Biol 12(4):407–413
Galazka AM, Robertson SE, Oblapenko GP (1995) Resurgence of diphtheria. Eur J Epidemiol 11(1):95–105
Gilbert L (1997) Infections with Corynebacterium diphtheriae—changing epidemiology and clinical manifestations. Report of the third international meeting of the European Laboratory Working Group on Diphtheria (ELWGD), Institute Pasteur, Paris 7–8 June 1996. Commun Dis Intell 21(12):161–164
Groman NB (1984) Conversion by corynephages and its role in the natural history of diphtheria. J Hyg (Lond) 93(3):405–417
Grundbacher FJ (1992) Behring’s discovery of diphtheria and tetanus antitoxins. Immunol Today 13(5):188–190
Hadfield TL, McEvoy P, Polotsky Y, Tzinserling VA, Yakovlev AA (2000) The pathology of diphtheria. J Infect Dis 181(Suppl 1):S116–S120
Hall AJ, Cassiday PK, Bernard KA, Bolt F, Steigerwalt AG, Bixler D, Pawloski LC, Whitney AM, Iwaki M, Baldwin A, Dowson CG, Komiya T, Takahashi M, Hinrikson HP, Tondella ML (2010) Novel Corynebacterium diphtheriae in domestic cats. Emerg Infect Dis 16(4):688–691
Hamour AA, Efstratiou A, Neill R, Dunbar EM (1995) Epidemiology and molecular characterisation of toxigenic Corynebacterium diphtheriae var mitis from a case of cutaneous diphtheria in Manchester. J Infect 31(2):153–157
Hardy IR, Dittmann S, Sutter RW (1996) Current situation and control strategies for resurgence of diphtheria in newly independent states of the former Soviet Union. Lancet 347(9017):1739–1744
Harrison BM, McDonald WI, Ochoa J (1972) Remyelination in the central diphtheria toxin lesion. J Neurol Sci 17(3):293–302
Hart RJ (1984) Corynebacterium ulcerans in humans and cattle in North Devon. J Hyg (Lond) 92(2):161–164
Hatanaka A, Tsunoda A, Okamoto M, Ooe K, Nakamura A, Miyakoshi M, Komiya T, Takahashi M (2003) Corynebacterium ulcerans Diphtheria in Japan. Emerg Infect Dis 9(6):752–753
Henricson B, Segarra M, Garvin J, Burns J, Jenkins S, Kim C, Popovic T, Golaz A, Akey B (2000) Toxigenic Corynebacterium diphtheriae associated with an equine wound infection. J Vet Diagn Invest 12(3):253–257
Hirata R, Pereira GA, Filardy AA, Gomes DL, Damasco PV, Rosa AC, Nagao PE, Pimenta FP, Mattos-Guaraldi AL (2008) Potential pathogenic role of aggregative-adhering Corynebacterium diphtheriae of different clonal groups in endocarditis. Braz J Med Biol Res 41:986–991
Höfler W (1991) Cutaneous diphtheria. Int J Dermatol 30(12):845–847
Holmes RK (2000) Biology and molecular epidemiology of diphtheria toxin and the tox gene. J Infect Dis 181(Suppl 1):S156–S167
Honma Y, Yoshii Y, Watanabe Y, Aoki N, Komiya T, Iwaki M, Arai H, Arakawa Y, Takahashi M, Kimura H (2009) A case of afebrile pneumonia caused by non-toxigenic Corynebacterium diphtheriae. Jpn J Infect Dis 62(4):327–329
Isaac-Renton JL, Boyko WJ, Chan R, Crichton E (1981) Corynebacterium diphtheriae septicemia. Am J Clin Pathol 75(4):631–634
Iwaki M, Komiya T, Yamamoto A, Ishiwa A, Nagata N, Arakawa Y, Takahashi M (2010) Genome organization and pathogenicity of Corynebacterium diphtheriae C7(-) and PW8 strains. Infect Immun 78(9):3791–3800
John TJ (2008) Resurgence of diphtheria in India in the 21st century. J Med Res 128:668–670
Klebs E (1883) Über Diphtherie. Verh Cong Inn Med 2:139–154
Kline MW, Kaplan SL (1998) Infections, immunizations, and principles of antimicrobial therapy. In: Garon A, Bricker JT, Fisher DJ, Neish SR (eds) The Science and practice of pediatric cardiology. Williams and Wilkins, Baltimore, pp 2856–2857
Kneen R, Pham NG, Solomon T, Tran TM, Nguyen TT, Tran BL, Wain J, Day NP, Tran TH, Parry CM, White NJ (1998) Penicillin vs. erythromycin in the treatment of diphtheria. Clin Infect Dis 27(4):845–850
Kwantes W (1984) Diphtheria in Europe. J Hyg (Lond) 93(3):433–437
Leggett BA, De Zoysa A, Abbott YE, Leonard N, Markey B, Efstratiou A (2010) Toxigenic Corynebacterium diphtheriae isolated from a wound in a horse. Vet Rec 166(21):656–657
Levy FM (1975) The 50th anniversary of diphtheria and tetanus immunization. Prev Med 4(2):226–237
Liebow AA, Maclean PD, Bumstead JM (1946) Tropical ulcers and cutaneous diphtheria. Arch Intern Med (Chic) 78:255–295
Livingood CS, Perry DJ, Forrester JS (1946) Cutaneous diphtheria; a report of 140 cases. J Invest Dermatol 7(6):341–364
Loeffler F (1884) Untersuchungen über die Bedeutung der Mikroorganismen für die Entstehung der Diphtherie beim Menschen, bei der Taube und beim Kalbe. Mitt Klein Gesundheitsamte Berlin 2:421–499
Lord JM, Smith DC, Roberts LM (1999) Toxin entry: how bacterial proteins get into mammalian cells. Cell Microbiol 1(2):85–91
Lowe CF, Bernard KA, Romney MG (2011) Cutaneous diphtheria in the urban poor population of Vancouver, British Columbia, Canada: a 10-year review. J Clin Microbiol 49(7):2664–2666
MacGregor RR (1995) Corynebacterium diphtheriae. In: Mandell GL, Douglas JE, Dolin R (eds) Principles and practice of infectious diseases. 4th edn. Churchill Livingston, New York, pp 1866–1869
Marston CK, Jamieson F, Cahoon F, Lesiak G, Golaz A, Reeves M, Popovic T (2001) Persistence of a distinct Corynebacterium diphtheriae clonal group within two communities in the United States and Canada where diphtheria is endemic. J Clin Microbiol 39(4):1586–1590
Mattos-Guaraldi AL, Sampaio JL, Santos CS, Pimenta FP, Pereira GA, Pacheco LG, Miyoshi A, Azevedo V, Moreira LO, Gutierrez FL, Costa JL, Costa-Filho R, Damasco PV, Camello TC, Hirata Jr R (2008) First detection of Corynebacterium ulcerans producing a diphtheria-like toxin in a case of human with pulmonary infection in the Rio de Janeiro metropolitan area, Brazil. Mem Inst Oswaldo Cruz 103(4):396–400
Mishra B, Dignan RJ, Hughes CF, Hendel N (2005) Corynebacterium diphtheriae endocarditis—surgery for some but not all!. Asian Cardiovasc Thorac Ann 13(2):119–126
Mokrousov I (2009) Corynebacterium diphtheriae: genome diversity, population structure and genotyping perspectives. Infect Genet Evol 9(1):1–15
Murphy JR (1996) Corynebacterium diphtheriae. In: Baron S (ed) Medical Microbiology. 4th edn. University of Texas Medical Branch at Galveston, Galveston, pp 99–100
Murray K, Noble M (1985) In vitro studies on the comparative sensitivities of cells of the central nervous system to diphtheria toxin. J Neurol Sci 70(3):283–293
Muttaiyah S, Best EJ, Freeman JT, Taylor SL, Morris AJ, Roberts SA (2011) Corynebacterium diphtheriae endocarditis: a case series and review of the treatment approach. Int J Infect Dis 15(9):e584–e588
Naglich JG, Metherall JE, Russell DW, Eidels L (1992) Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell 69(6):1051–1061
Neal SE, Efstratiou A (2009) International external quality assurance for laboratory diagnosis of diphtheria. J Clin Microbiol 47(12):4037–4042
Nureki S, Miyazaki E, Matsuno O, Takenaka R, Ando M, Kumamoto T, Nakano T, Ohkusu K, Ezaki T (2007) Corynebacterium ulcerans infection of the lung mimicking the histology of Churg-Strauss syndrome. Chest 131(4):1237–1239
Pappenheimer AM Jr (1977) Diphtheria toxin. Annu Rev Biochem 46:69–94
Park WH, Williams AW (1896) The production of diphtheria toxin. J Exp Med 1:164–185
Pereira GA, Pimenta FP, Santos FR, Damasco PV, Hirata Junior R, Mattos-Guaraldi AL (2008) Antimicrobial resistance among Brazilian Corynebacterium diphtheriae strains. Mem Inst Oswaldo Cruz 103(5):507–510
Perles Z, Nir A, Cohen E, Bashary A, Engelhard D (2000) Atrioventricular block in a toxic child: do not forget diphtheria. Pediatr Cardiol 21(3):282–283
Popovic T, Mazurova IK, Efstratiou A, Vuopio-Varkila J, Reeves MW, De Zoysa A, Glushkevich T, Grimont P (2000) Molecular epidemiology of diphtheria. J Infect Dis 181(Suppl 1):S168–177
Puliti M, von Hunolstein C, Marangi M, Bistoni F, Tissi L (2006) Experimental model of infection with non-toxigenic strains of Corynebacterium diphtheriae and development of septic arthritis. J Med Microbiol 55(Pt 2):229–235
Riegel P, Ruimy R, de Briel D, Prevost G, Jehl F, Christen R, Monteil H (1995) Taxonomy of Corynebacterium diphtheriae and related taxa, with recognition of Corynebacterium ulcerans sp. nov. nom. rev. FEMS Microbiol Lett 126(3):271–276
Romney MG, Roscoe DL, Bernard K, Lai S, Efstratiou A, Clarke AM (2006) Emergence of an invasive clone of nontoxigenic Corynebacterium diphtheriae in the urban poor population of Vancouver, Canada. J Clin Microbiol 44(5):1625–1629
Roush SW, Murphy TV (2007) Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA 298(18):2155–2163
Roux E, Yersin A (1888) Contribution à l’etude de la diphtérie. Annales de l’Insitut Pasteur 2:629–661
Schuhegger R, Lindermayer M, Kugler R, Heesemann J, Busch U, Sing A (2008) Detection of toxigenic Corynebacterium diphtheriae and Corynebacterium ulcerans strains by a novel real-time PCR. J Clin Microbiol 46(8):2822–2823
Shashikala P, Reddy PV, Prashanth K, Kanungo R, Devi S, Anitha P, Rajarajeshweri N, Cherian TM (2011) Persistence of nontoxigenic Corynebacterium diphtheriae biotype gravis strains in Pondicherry, Southern India. J Clin Microbiol 49(2):763–764
Sing A, Berger A, Schneider-Brachert W, Holzmann T, Reischl U (2011) Rapid detection and molecular differentiation of toxigenic Corynebacterium diphtheriae and Corynebacterium ulcerans strains by LightCycler PCR. J Clin Microbiol 49(7):2485–2489
Sykes JE, Mapes S, Lindsay LL, Samitz E, Byrne BA (2010) Corynebacterium ulcerans bronchopneumonia in a dog. J Vet Intern Med 24(4):973–976
Tiwari TS, Golaz A, Yu DT, Ehresmann KR, Jones TF, Hill HE, Cassiday PK, Pawloski LC, Moran JS, Popovic T, Wharton M (2008) Investigations of 2 cases of diphtheria-like illness due to toxigenic Corynebacterium ulcerans. Clin Infect Dis 46(3):395–401
Vitek CR (2006) Diphtheria. Curr Top Microbiol Immunol 304:71–94
von Behring EA, Kitosato S (1890) Über das Zustandekommen der Diphtherie-Immunität und der Tetanus-Immunität bei Thieren. Dtsch Med Wochenschr 16:113–114
von Hunolstein C, Rota MC, Alfarone G, Ricci ML, Salmaso S (2000) Diphtheria antibody levels in the Italian Population. Eur J Clin Microbiol Infect Dis 19(6):433–437
Wagner J, Ignatius R, Voss S, Höpfner V, Ehlers S, Funke G, Weber U, Hahn H (2001) Infection of the skin caused by Corynebacterium ulcerans and mimicking classical cutaneous diphtheria. Clin Infect Dis 33(9):1598–1600
Wagner KS, White JM, Crowcroft NS, De Martin S, Mann G, Efstratiou A (2010) Diphtheria in the United Kingdom, 1986–2008: the increasing role of Corynebacterium ulcerans. Epidemiol Infect 138(11):1519–1530
Wagner KS, White JM, Neal S, Crowcroft NS, Kupreviciene N, Paberza R, Lucenko I, Joks U, Akbas E, Alexandrou-Athanassoulis H, Detcheva A, Vuopio J, von Hunolstein C, Murphy PG, Andrews N, Efstratiou A (2011) Screening for Corynebacterium diphtheriae and Corynebacterium ulcerans in patients with upper respiratory tract infections 2007–2008: a multicentre European study. Clin Microbiol Infect 17(4):519–525
Zasada AA, Baczewska-Rej M, Wardak S (2010) An increase in non-toxigenic Corynebacterium diphtheriae infections in Poland—molecular epidemiology and antimicrobial susceptibility of strains isolated from past outbreaks and those currently circulating in Poland. Int J Infect Dis 14(10):e907–e912
Zuber PL, Gruner E, Altwegg M, von Graevenitz A (1992) Invasive infection with non-toxigenic Corynebacterium diphtheriae among drug users. Lancet 339(8805):1359
Acknowledgements
The author’s work was supported by the Deutsche Forschungsgemeinschaft (SFB796, B5). Help with the manuscript and preparation of Fig. 1.1 by S. Morbach (Friedrich-Alexander-Universität Erlangen-Nürnberg) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht (outside the USA)
About this chapter
Cite this chapter
Burkovski, A. (2014). Diphtheria and its Etiological Agents. In: Burkovski, A. (eds) Corynebacterium diphtheriae and Related Toxigenic Species. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7624-1_1
Download citation
DOI: https://doi.org/10.1007/978-94-007-7624-1_1
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7623-4
Online ISBN: 978-94-007-7624-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)