Abstract
Silver (Ag) has been known for its antimicrobial properties for centuries but has received renewed interest in recent years primarily due to the rise of antibiotic-resistance and the development of Ag nanoparticles (AgNPs). In orthopedics, multiple applications containing Ag are being developed including Ag dressings, Ag-coated prosthetic implants, and Ag-based bone cements. In this chapter, we review the antimicrobial mechanisms as well as the delivery and metabolic pathways of Ag and the primary uses of Ag and AgNPs in orthopedic applications, focusing on their antimicrobial activity, toxicity, and clinical uses.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Nanotechnology
- Nanoparticle
- Silver
- Antimicrobial
- Infection
- Bacteria
- Toxicity
- Nanotoxicity
- Clinical use
- Antibiotic resistance
- Megaprosthese
- Bone cement
- In vitro
- In vivo
1 Introduction
Silver (Ag) is a soft, metallic transition metal with unique chemical and physical properties that make it an attractive metal for various applications. Ag is an excellent conductor of heat and electricity and has been used in photography and the electrical and electronic industries. In addition, Ag has been used in the production of coins, jewelry, silverware, and decorative items due to its malleability, ductility, bright color, and resistance to oxidation. Ag also has well-known antimicrobial properties which could be important in the prevention and treatment of infection. The antimicrobial properties of Ag have been known for centuries, as Ag has been used to treat water supplies via Ag coins since ancient Egypt and to craft antiseptic surgical instruments since the Middle Ages [1]. Recently, interest in the use of Ag as an antimicrobial agent has attracted renewed interest as antibiotic resistance is increasing [2] and researchers are searching for new options to treat antibiotic-resistant bacteria.
Ag has found increased utility in medical and healthcare applications , with products such as Ag-coated medical devices, wound dressings, and topical creams and solutions. Figure 1 presents some major clinical applications of Ag and some emerging uses of Ag nanoparticles (AgNPs). Certain applications are suited to the field of orthopedics, as the potential to reduce infection rates has great implications in terms of economic costs and quality of life for patients. In this chapter, we review the uses of Ag and AgNPs in orthopedic applications with evaluations that focus on their antimicrobial activity , toxicity, and clinical uses . A summary of these studies is presented in Table 1.
Uses of Ag (right-hand side) and AgNPs (left-hand side) in medicine . Traditionally, silver nitrate is used in a number of clinical contexts, including stemming the flow of blood from nosebleeds, inducing pleurodesis when closing chest tube wounds and cauterization of granulomas. C.S.F. Crede’s introduction of 1% silver nitrate eye drops in 1881 prevented neonatal conjunctivitis and is still used clinically in developing countries. Silver sulfadiazine cream is used in the widespread treatment of burns, although argyria (discoloration of the skin) remains a prevalent side effect. AgNPs are emerging as a next-generation antibacterial agent, augmenting antibiotics and disinfectants for the coating of medical devices. AgNP-based wound dressings are already commercially available (e.g., ActicoatTM) and in current clinical use. AgNPs are used as an antibacterial additive or coating in a range of catheters and in bone cement. AgNPs can also be used in hand gels and paints as a prolonged antibacterial disinfectant. Reprinted from [3] with permission from Elsevier
2 Antimicrobial Mechanisms, Delivery, and Metabolic Pathways of Ag
Ag is inert in its metallic form, but becomes biologically active when ionized, which typically occurs upon exposure to an aqueous media [1]. Ag ions work in three ways as an antimicrobial agent. First, they bind and denature the bacterial cell wall causing cell lysis; secondly, they bind to ribosomes thus inhibiting protein synthesis. Lastly, they bind to DNA preventing bacterial replication (Fig. 2) [3]. The unique and multi-modal mechanisms of action that Ag employs against bacteria make the development of resistance less likely.
Mechanisms of the antibacterial activity of Ag ions. It is widely accepted that the major antibacterial effect of AgNPs is mediated by its partial oxidation and release of Ag ions. Ag ions interact with: the peptidoglycan cell wall and the plasma membrane, causing cell lysis; bacterial (cytoplasmic) DNA, preventing DNA replication; and bacterial proteins, disrupting protein synthesis. Multifaceted antibacterial activity is the key to low bacterial resistance rates observed for Ag and AgNPs. AgNPs also can directly damage and penetrate the cell wall and plasma membrane. Reprinted from [3] with permission from Elsevier
Ag delivery strategies have largely focused on applications that achieve local delivery, such as Ag-coated medical devices and topical applications. Local delivery of Ag is favorable as it allows for a targeted delivery at the site of interest at lower concentrations than systemic delivery to reduce toxicity. The concentration at which Ag is bactericidal but not toxic to host cells is termed the therapeutic window and studies on this topic suggest that such therapeutic windows exist [4]. Systemic delivery of Ag through oral formulations is generally avoided due to the higher concentrations required and the greater potential for toxic side effects, which most notably include argyria or argyrosis [5].
Ag-coatings and topical applications exert their biological action through Ag ions which are released after contact of the application with an aqueous environment; their release depends on the solubility of Ag in the aqueous environment [6, 7]. It is generally favorable for Ag applications to have low release rates to prevent short-term adverse effects from taking place. Released Ag ions are free to interact with microbial pathogens and may be sequestered by binding to serum proteins or anions [6, 7]. There is an equilibrium of Ag ions between the Ag application and aqueous environment; whenever Ag ions are sequestered, additional Ag ions are released from the Ag application to keep a low but steady concentration in the aqueous environment [7].
The metabolic fate of Ag ions in the body is dependent upon the organic and metallic complexes that it may form with other materials. Walker et al. published a review article detailing the metabolic pathways of Ag [7]. Depending on the biomolecules or anions to which Ag ions bind, they may be stored in nearby tissues and/or organs as inert complexes or excreted from the body via natural tissue turnover or via urinary and fecal routes. Ag ions bound to anions, typically chloride ions, remain at the site and may deposit near the surrounding tissues. Ag ions may also bind to extracellular matrix proteins, which is particularly relevant for topical applications on the skin, and may remain in the tissue as inert sulfide compounds or be removed through natural tissue turnover. Ag ions bound to mobile proteins may enter the systemic circulation, whereby they are excreted from the urine, complexed with bile in the liver to be excreted in fecal matter, or uptaken by organs and deposited as inert Ag sulfide and selenide complexes [7].
3 Ag Dressings
Ag dressings are comprised of standard wound dressings [8], such as gauze, alginates, films, foams, hydrocolloids, or hydrogels, with the addition of Ag. Ag may be found as a coating on the surface of the dressing, impregnated within the material of the dressing, or a combination of the two [9]. The Ag found within these dressings may come in a variety of forms that include elemental Ag, inorganic compounds with Ag, or organic complexes with Ag [9].
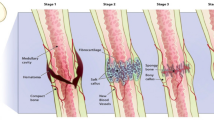
In general, Ag dressings may be classified as Ag-delivery dressings or Ag-containing dressings [10]. Ag-delivery dressings act to deliver Ag to the wound site after contact with the dressings [9, 10]. Ag-containing dressings typically act by absorbing wound exudate containing bacteria [9, 10]. A detailed look at the mechanism of Ag-containing dressings , specifically the action of Aquacel Ag toward biofilm disruption, is described by Parsons et al. and is shown in Fig. 3 [11]. In brief, Ag-containing dressings first absorb wound exudate and disrupt the biofilms; the exposed bacteria become susceptible to the action by ionic Ag within the dressings. The dressings absorb the bacteria and remain there until removal of the dressings.
Functionality of the next-generation antibiofilm carboxymethylcellulose Ag-containing wound dressing (NGAD) [11]. Phase 1: The applied NGAD dressing hydrates and gels in contact with wound fluids, contacting intimately the wound bed and surface biofilm. Phase 2: Biofilm is loosened and dispersed due to the synergistic action of the disodium ethylenediaminetetraacetate and benzethonium chloride in combination with sodium silver carboxymethylcellulose (CMC) fibers. Phase 3: Exposed microorganisms become highly susceptible to killing by the action of ionic Ag. Phase 4: Residual biofilm and cells are immobilized within the gelled dressing. Phase 5: Biofilm biomass is reduced by dressing removal. Reference 11 is an open access article which permits unrestricted use, distribution, and reproduction
There are many commercially-made Ag dressings available, with around 50 different formulations and brands on the market [9]. Some of the more commonly cited formulations found in studies include Aquacel® Ag (ConvaTec), Acticoat™ (Smith & Nephew), Urgotul® SSD (Urgo), PolyMem® Silver (Ferris), and Contreet® (Coloplast). A brief description of these silver dressings is shown in Table 2.
3.1 In Vitro and In Vivo Studies of Ag Dressings
Ag dressings have been shown to be effective in reducing bacterial growth as demonstrated by the in vitro reduction of common wound pathogens, including methicillin-sensitive and -resistant Staphylococcus aureus (S. aureus) [12,13,14], Pseudomonas aeruginosa (P. aeruginosa) [12, 13, 15], Escherichia coli (E. coli) [12], Enterobacter cloacae [12], Enterococcus faecalis [12], and Acinetobacter baumannii (A. baumannii) [12]. Ag dressings have also been found to reduce simple biofilm formation from P. aeruginosa [11, 13] and polymicrobial biofilms consisting of S. aureus and Klebsiella pneumoniae [11]. Studies in the literature have investigated a wide variety of laboratory-made or commercially-available Ag dressings and have generally shown that Ag dressings have efficacious antimicrobial properties . However, Ag dressings may show differing results when comparing the antimicrobial efficacies among different formulations.
Ip et al. [12] examined the antibacterial activity of five commercially available Ag dressings (Aquacel® Ag, Acticoat™, Urgotol® SSD, PolyMem® Silver, and Contreet®) against many common Gram-positive and -negative bacteria using an in vitro culture broth method. The Ag dressings varied in terms of antimicrobial spectrum and rapidity of action. All dressings were effective against Gram-negative bacteria; however, only Acticoat and Contreet were also effective against S. aureus. Overall, Acticoat and Contreet had the most rapid and broad spectrum of antimicrobial activity. Rapid antimicrobial activity is desired to reduce interference from bacteria in wound healing and to reduce likelihood of bacterial resistance.
Lin et al. [15] found that Ag-containing dressings (Aquacel® Ag, Acticoat 7, and KoCarbonAg) prevented external bacteria from entering the wound and had the ability to retain bacteria within the dressing in vitro. In addition, the authors showed that use of Ag-containing dressings led to improved wound healing compared to gauze dressings in vivo, which can be attributed to a reduced bacterial burden at the wound, as prevention of critical colonization of bacteria prevents interference from wound healing.
Certain Ag dressings have also been shown to reduce biofilm formation . Parsons et al. [11] investigated the use of Aquacel Ag+ Extra toward biofilm disruption and compared its efficacy against two other commercially-available Ag dressings (Acticoat 7 and Silvercel Non-Adherent ). Staining procedures were done on the biofilms followed by confocal laser scanning microscopy to capture the image for analysis. The results showed that Aquacel Ag+ Extra was more effective in reducing biofilm growth compared to the other two Ag dressings. In addition, Aquacel Ag+ Extra significantly reduced the growth of viable biofilm cells and significantly reduced biofilm thickness with significantly fewer extracellular polymeric substances. Bourdillon et al. [13] also investigated the use of Ag dressings against biofilm disruption . In their study, three commercially-available Ag dressings (Aquacel Ag, Acticoat 7, and Promogran Prisma) were examined for their efficacy at reducing bacterial burden and biofilm formation. The results showed that all Ag dressings reduced S. aureus and P. aeruginosa vegetative cultures. However, when tested against P. aeruginosa biofilms, only Promogran Prisma significantly reduced the biofilm population and its protease production.
Despite the antimicrobial efficacy of Ag dressings in vitro, there is concern that Ag dressings could result in adverse side effects from too much silver being delivered to tissues. In addressing this concern, many studies have investigated the in vitro cytotoxicity of Ag dressings. Paddle-Ledinek et al. [16] investigated the in vitro cytotoxicity of several Ag dressings. Keratinocytes were exposed to Ag dressing extracts and cell survival was determined by MTT assays. They demonstrated that extracts from the Ag dressings induced significant reductions in keratinocyte viability, with variable effects depending on the dressing formulations. It was found that Acticoat, Aquacel Ag, Avance, and Contreet-H induced the greatest cytotoxicity among all the Ag dressings tested.
Burd et al. [10] investigated the cytotoxicity of five commercially available Ag dressings (Aquacel Ag, Acticoat, Contreet Foam, PolyMem Silver, and Urgotol SSD). In the monolayer cell culture , their results showed that PolyMem Silver and Urgotol SSD were relatively safe for keratinocytes and fibroblasts. However, Aquacel Ag, Acticoat, and Contreet Foam produced significant cytotoxic effects on keratinocytes and fibroblasts with varied effects depending on the media. In the tissue explant culture model, delayed reepithelization was observed in all Ag dressings as measured by the reepithelization index upon histological examination. In the mouse excisional wound model, delayed or inhibited wound reepithelization was observed in all Ag dressings as measured by the ratio of the epithelial gap to wound gap upon histological examination.
One study indicated that the in vitro cytotoxicity of Ag dressings does not necessarily predict how Ag dressings may function in wound healing in vivo. Hiro et al. [17] investigated multiple different Ag dressings and primarily evaluated their in vitro cytotoxicity and in vivo process of wound healing. The in vitro cytotoxicity was determined by evaluating fibroblast function using fibroblast-populated collagen lattices and fibroblast viability using the Trypan Blue assay and MTT assay. The in vivo process of wound healing was evaluated utilizing a rat model. Their results showed that all Ag dressings were cytotoxic to fibroblasts. However, despite the cytotoxicity , their in vivo model demonstrated that all of the Ag dressings, except Contreet Foam and Acticoat, resulted in improved wound healing.
3.2 Clinical Studies
Ag dressings have been shown to be effective in wound care, particularly in the treatment of burn wounds [18, 19], diabetic ulcers [20], and chronic ulcers [21]. In the field of orthopedics, Ag dressings have found applications in the prevention of periprosthetic joint infections (PJI ) and traumatic injuries . PJI is a potentially catastrophic complication that is estimated to occur in about 0.92% of cases following total knee arthroplasty (TKA) and 0.88% of cases following total hip arthroplasty (THA) [22]. PJIs are associated with significant morbidity and mortality, and may lead to costly surgical revisions, long-term disability, and even death [23]. Ag dressings have shown promise in lowering the infection burden following surgery and there have been a number of clinical studies demonstrating the effectiveness of Ag dressings post-TKA and -THA. Of note, the majority of studies in the literature regarding the use of Ag dressings in clinical cases post-arthroplasty have primarily investigated the effects of Aquacel Ag. As a result, there remains a need for more clinical studies investigating the effects of different Ag dressings post-arthroplasty.
A few clinical studies have investigated the incidence of acute PJI using Ag dressings. Grosso et al. [24] conducted a retrospective cohort trial in 1173 patients who underwent TKA or THA and received either an Aquacel Ag dressing or standard xeroform dressing. Their results showed that the Aquacel Ag dressing significantly reduced the incidence of acute PJI, as the incidence of acute PJI was 1.58% in the standard xeroform dressing group and 0.33% in the Aquacel Ag dressing group (p = 0.03). In a similar study, Cai et al. [25] conducted a retrospective case-control study of patients who underwent a TKA or THA and received either the Aquacel Ag dressing or standard gauze dressing. The authors reviewed 903 cases who received the Aquacel Ag dressing and 875 cases with the standard gauze dressing. They found that the Aquacel Ag dressing significantly reduced the incidence of acute PJI, as the incidence of acute PJI was 1.7% in the standard gauze dressing group and 0.44% in the Aquacel Ag dressing group (p = 0.005).
Some clinical studies have also investigated other aspects of Ag dressings including wound complications, blistering of skin, number of dressing changes, and patient satisfaction. Springer et al. [26] conducted a randomized controlled trial in 300 patients who were scheduled to undergo TKA or THA and were randomized to receive Aquacel Ag or a standard surgical dressing. In patients who received Aquacel Ag, there were statistically significantly fewer wound complications (p = 0.015), fewer blisters (p = 0.026), fewer dressing changes (p < 0.0001), and greater overall patient satisfaction (p < 0.0001). Ravenscroft et al. [27] conducted a prospective, randomized controlled trial in 200 patients who were scheduled to undergo TKA , THA , or surgery for a femoral neck fracture, and were randomized to receive either Cutiplast (a commonly used dressing for orthopedic procedures that is comprised of an absorbent perforated dressing with adhesive borders) or Aquacel Ag covered with Tegaderm. It was determined that, in patients who received the Aquacel/Tegaderm dressing , there was statistically significantly fewer wound complications (p < 0.00001), which included dressing failure, skin blistering, or any signs of infection. There was also statistically significantly fewer wound dressing changes (p = 0.03) and statistically lower pain scores at the time of dressing change (p = 0.001) in patients who received the Aquacel/Tegaderm dressing .
Ag dressings have also found applications in traumatic injuries. Traumatic injuries present challenges to surgeons as they must address infection control and appropriate bone and/or soft tissue healing. Ag dressings are a potential solution to this problem and there have been a number of clinical studies investigating the use of Ag dressings in traumatic injuries.
Several clinical studies have shown that Ag dressings may lead to reduced infection and improved wound healing . Jurczak et al. [28] conducted a prospective, randomized clinical trial assessing the use of a Hydrofiber dressing (Aquacel Ag) compared to povidone-iodine gauze for the treatment of open surgical and traumatic wounds. There were 35 patients in the Hydrofiber Ag dressing group and 32 patients in the povidone-iodine gauze group. Results demonstrated that the Hydrofiber Ag dressing group was significantly better than the povidone-iodine gauze treatment group in terms of pain management (p < 0.001), comfort (p < 0.001), and exudate management (p < 0.001). For wound healing, there was a 23% and 9% healing rate in the Hydrofiber Ag and povidone-iodine group , respectively. Keen et al. [29] conducted a retrospective case series of Gustilo/Anderson type II and III open fractures that received a unique treatment protocol including irrigation and debridement, intravenous antibiotics, and a nanocrystalline Ag dressing (Acticoat) placed within the wound with an overlying negative pressure dressing. They found only one sign of clinical infection among the 17 patients who met inclusion criteria.
However, several clinical studies have also shown that Ag dressings did not have positive outcomes in traumatic injuries. Kadar et al. [30] conducted a prospective, randomized clinical trial assessing the use of a Ag dressing (SilvalGuard) compared to a regular dressing (OPSITE) in elderly patients undergoing surgery for hip fractures. A matched group of 55 patients were randomized to receive the Ag dressing or regular dressing and were followed for 1 week to monitor for signs of clinical infection . There were no significant differences in signs of clinical infection between the two groups, as infection was seen in 2 of 31 patients in the Ag dressing group and 2 of 24 patients in the regular dressing group. Fries et al. [31] conducted a prospective, randomized clinical trial assessing the use of nanocrystalline Ag dressings (Acticoat) compared to standard of care dressings (plain gauze) upon traumatic military wounds. There were 76 patients in the trial who were randomized to receive the Ag dressing or standard of care dressing, and the results showed no statistical differences between the groups in terms of wound colonization (p = 0.1384, Fishers) and time to wound healing (p = 0.5009, Mann-Whitney).
4 Ag-Coated Prosthetic Implants
Prosthetic implants are commonly used in the field of orthopedics in procedures such as internal fixation and arthroplasty [32, 33]. However, use of these implants may increase the risk for implant-associated infections , as implanted foreign bodies are more susceptible to infection. It has been reported that the incidence of infected joint prosthesis and fracture-fixation devices is 2% and 5%, respectively [34]. Implant-associated infections have many clinical and economic consequences, and typically require expensive surgical revisions [34].
Implant-associated infections are caused by a triad of factors, which involve the interaction between the microorganism, implant, and host [35]. Formation of biofilms is believed to be one important factor in the pathogenesis of implant-associated infections; biofilms are difficult to eradicate due to their resistance to internal and external environmental factors that allow for survival of the microorganisms [35]. Foreign body implants have key characteristics that promote the formation of biofilms. Foreign body implants lack microcirculation, which is important in delivering therapeutic agents and the host response to infection [35]. In addition, there are many surface characteristics of foreign body implants that can promote biofilm formation, which include the composition of the material, surface charge, surface roughness , and hydrophobicity [36].
One potential solution to reduce the risk of infection involves coating the prosthetic implants with Ag. Thin layers of Ag coating on prosthetic implants can help reduce biofilm formation. In orthopedics, Ag-coated prosthetic implants are primarily being used in external fixation devices and megaprostheses .
4.1 Ag-Coated External Fixation Pins: In Vitro, In Vivo, and Clinical Studies
External fixation is often used in settings of trauma or limb reconstruction to stabilize the bone or joint and involves the placement of percutaneous pins and wires. The use of external fixation pins may put patients at risk for infection because the pins may act as a conduit for the transfer of bacteria on the skin to the underlying soft tissue and bone; the incidence of pin tract infections is high and has been reported to be as high as 63%. In vitro and in vivo studies have shown that Ag-coated external fixation pins could significantly reduce bacterial colonization and infection rates; however, no clinical advantages have been observed in several clinical studies [37, 38].
4.1.1 In Vitro and In Vivo Studies
One property that Ag-coated prosthetic devices may have in reducing infection is through inhibiting the initial stages of colonization by decreasing the adhesion of bacteria to their surfaces. This was shown in an in vitro study by Wassall et al. [39], where it was demonstrated that Ag-coated external fixation pins had significantly less bacterial adhesion compared to control stainless steel pins (p < 0.05). Reduced bacterial adhesion on Ag-coated pins was also shown with E. coli, P. aeruginosa, and S. aureus, but not with Staphylococcus haemolyticus.
One of the initial in vivo studies investigating the prevention of infection using Ag-coated external fixation pins was done by Collinge et al. [40] In their in vivo study, six sheep were inoculated with S. aureus and had Ag-coated and uncoated stainless steel pins inserted in the iliac crest for 19 days. The pin tips were removed and cultured for bacterial growth; Ag-coated pins led to less infection than uncoated pins (confidence interval [CI] > 85%). The infection rate in the Ag-coated pins was 62% (22 of 36 pins) compared to 84% (10 of 12 pins) in the uncoated pins. The pins were also assessed for mechanical anchorage and inflammation. The results supported an association between mechanical anchorage and infection, as infected pins were more likely to be loose. Biofilm growth on the pin tips was examined with scanning electron microscopy (SEM) and decreased biofilm colonization was seen in the Ag-coated pins when compared with the uncoated pins.
Bosetti et al. [41] assessed the cytotoxic and genotoxic properties of Ag-coated external fixation pins in vitro. Their study showed that Ag-coated pins were not cytotoxic nor genotoxic when compared to control stainless steel pins. Genotoxicity studies were done by examining the frequency of sister chromatid exchanges (SCE) and kinetics in human peripheral blood lymphocytes. Results of the SCE exchange showed no significant differences between Ag-coated pins and control stainless steel pins. The lymphocyte kinetics data further suggested that Ag-coated pins did not cause cell-cycle delay. Cytotoxicity studies showed that, after 4 days, human osteoblast cells cultured with Ag-coated pins displayed typical morphology and cell spreading.
4.1.2 Clinical Studies
One of the initial clinical studies performed to assess the effects of Ag-coated external fixation pins was done by Massè et al. [38]. The authors conducted a prospective, randomized study to assess the efficacy of Ag-coated screws in preventing external pin tract infection. Twenty-four male patients with diaphyseal fractures of the tibia or femur underwent external fixation with Ag-coated screws and uncoated stainless steel screws. The rate of positive culture from the screw tips was 30.0% (15 of 50 screws) in the Ag-coated group and 42.9% (24 of 56 screws) in the uncoated group; however, this difference was not statistically significant (p = 0.243). There were no significant differences between the Ag-coated group and uncoated group in terms of inflammation and mechanical anchorage scores. The differences between pre-operative and post-operative blood Ag levels were statistically significant (p < 0.001).
In another prospective, a randomized study conducted by Coester et al. [37], 22 patients underwent external fixation for tibial fractures. They received Ag-coated pins and uncoated stainless steel pins that were randomized by clamp position to allow for a side-by-side comparison. The results showed no significant differences in the performance of the Ag-coated pins and uncoated stainless steel pins in regard to the rate of pin tract infection, bacterial growth of the pins, clinical appearance of the pin sites, and mechanical integrity of the pins upon removal.
4.2 Ag-Coated Megaprostheses: In Vitro, In Vivo, and Clinical Studies
Typically , megaprostheses are used to reconstruct skeletal defects after bone or soft tissue tumor resection, major trauma, or end-stage revision arthroplasty [42]. The most serious complication after the implantation of megaprostheses is periprosthetic infection, with reported incidence rates of infection between 11% and 23%, depending on the site of replacement [43]. Previously attempted preventative measures have had limited success [43]. In this section, we review the in vitro, in vivo, and clinical studies regarding the efficacy of Ag-coated megaprostheses in reducing infection and also examine the cytotoxicity of Ag toward host cells.
4.2.1 In Vitro and In Vivo Studies
Megaprostheses are typically composed of titanium due to their high resistance to corrosion and excellent biocompatibility [42]. However, plain titanium megaprostheses are susceptible to bacterial adhesion. To reduce the development of infection on titanium surfaces , a number of strategies have been investigated and include the use of Ag coatings. Ewald et al. [44] developed Ag-coated titanium alloys via physical vapor deposition to coat titanium surfaces with a titanium alloy containing 0.7–9% Ag and investigated the in vitro antimicrobial and biocompatibility properties . There was a significant decrease in the adhesion of bacteria on the Ag-coated titanium surface when compared to uncoated titanium controls, with up to a 64% decrease against Klebsiella pneumoniae and up to a 52% decrease against Staphylococcus epidermidis . In addition, there was no observed cytotoxicity against epithelial and osteoblast cell lines, as cell activity and protein content were not significantly changed.
Hardes et al. [45] incubated Ag and titanium supplements with osteosarcoma cell lines and evaluated the effect of Ag on cell viability and cell function in vitro. Their results showed that Ag was non-cytotoxic at low concentrations (e.g., 5 mg) and significantly stimulated more alkaline phosphatase production, indicative of osteogenic differentiation, than titanium. However, at higher concentrations (e.g., >10 mg), Ag was found to be cytotoxic with a drastic decrease in viability and alkaline phosphatase production, and thus it was less biocompatible when compared to titanium.
Gosheger et al. [46] reported that Ag-coated megaendoprostheses reduced infection rates and had no significant toxicological side effects when used in an in vivo animal model. The study design involved inserting either Ag-coated prostheses or uncoated titanium-vanadium prostheses into the diaphyseal femur of rabbits. The rabbits were infected with S. aureus and were observed for 90 days. The results showed that the infection rate in the Ag-coated group was significantly lower than the uncoated group (p < 0.05), as the infection rate in the Ag-coated group was 7% compared to 47% in the uncoated group. Regarding the potential toxicity of Ag-coated prostheses , elevated Ag levels of the Ag-coated group were seen in the blood (median 1.883 ppb) and in organs (0.798 to 86.002 ppb) when compared to the uncoated group. However, these levels of Ag were considered to be non-toxic since no toxicological side effects were found and no histological abnormalities were observed in any of the organs studied.
4.2.2 Clinical Studies
Wafa et al. [47] conducted a retrospective case-control study to evaluate the incidence of periprosthetic infection in patients who underwent endoprosthetic replacement for bone tumors and received either Ag-coated prostheses (Alguna-coated) or uncoated prostheses. Their results suggest that Ag-coated prostheses are successful in reducing post-operative infection rates, particularly evident in two-stage revisions. The Ag-prostheses group had no infection in 85% of patients (17/20) whereas the control group had no infection in 57.1% (12/21). The authors conclude that the use of silver coating on prostheses in addition to antibiotic treatment and debridement are more successful in controlling infection and they currently use silver implants in cases of high infection risk.
Hardes et al. [48] carried out a prospective case study with comparison to retrospective controls regarding the incidence of periprosthetic infection using Ag-coated megaprostheses in patients with bone sarcoma. In their study, 51 patients with sarcoma underwent placement of a Ag-coated megaprosthesis in the proximal femur or tibia, and were followed prospectively for 5 years. A control comparison group was formed retrospectively in 74 patients who received an uncoated titanium megaprosthesis placed in the proximal femur or tibia. The incidence of periprosthetic infection was found to be lower in patients who received a Ag-coated megaprosthesis, with an infection rate of 5.9% compared to 17.6% of the control patients who received a titanium prosthesis (p = 0.062).
Scoccianti et al. [49] performed a prospective case study evaluating the efficacy of a Ag-coated megaprostheses (Porag MegaC) to reduce post-operative periprosthetic infection and to monitor toxicity. From 2010 to 2014, Ag-coated megaprostheses were implanted in 33 patients following trauma surgery, arthroplasty, or tumor resection; 21 patients required a prostheses due to previous septic complications. Patients were followed for 12–56 months and results showed a low incidence of post-operative infection. In the 12 patients with no previous septic complications, only one case of post-operative infection occurred at 25 months post-operation. In the 21 patients with previous septic complications, there were two cases of post-operative infection, at 7 and 24 months. Ag was found to be safe in this study group, as no clinical abnormalities or systemic side effects of Ag were observed. Mean levels of Ag in the blood and urine were monitored from 24 h to 36 months post-operation and were 0.41–5.33 μg/L and 0.28–0.86 μg/L, respectively.
Glehr et al. [50] also conducted a prospective case study to examine possible local argyria with the use of Ag-coated megaprostheses . From 2004 to 2011, Ag-coated megaprostheses were implanted in 32 patients following revision arthroplasty or resection of a bone or soft tissue tumor. Patients were monitored for toxicity through clinical signs of systemic or local argyria, liver and renal function markers, and levels of Ag in the blood and seroma. The results showed that, of the 32 patients, 7 developed local argyria after a median of 25.7 months. Of the seven patients with local argyria, no neurological symptoms were evident and electronystagmography revealed no signs of systemic argyria. In 20 patients, the median level of Ag in the blood was 15.9 μg/kg. In 14 patients, the median levels of Ag in the seroma was 545.0 μg/kg. There was no association between the development of local argyria and levels of Ag in the blood or seroma. All liver and renal parameters were within normal limits with no significant differences between patients who had developed local argyria and those who did not.
In addition to being applied to external fixation devices and megaprostheses , Ag coatings have also been studied for internal fixation and reduction of biofilms. Kose et al. [51] investigated the use of Ag doped hydroxyapatite coated titanium nails to prevent infection in a rabbit open fracture femur model. This in vivo study involved inserting uncoated, hydroxyapatite coated, or Ag doped hydroxyapatite coated titanium nails in the femurs of rabbits that were injected with MRSA in the intramedullary canal. After 10 weeks, significantly reduced bacterial growth was observed in swab cultures (p = 0.003) from the intramedullary canal and samples (p = 0.001) cultured from the Ag-coated nails in the Ag-coated group compared to the other groups. Histopathological evaluation of the Ag-coated nails presented no cellular inflammation around the nail, no Ag particles in the surrounding tissue, and no toxicity against osteoblast cells.
Secinti et al. [52] studied the use of Ag-coating to reduce the formation of biofilms on the surface of titanium screws. An in vivo animal model was utilized; rabbits were infected with coagulase-positive S. aureus at the screw insertion site in the iliac crest, and had either a Ag-coated titanium screw or uncoated titanium screw inserted at the site. After 28 days, SEM imaging of the screws showed no biofilm formation for the Ag-coated screws, whereas biofilms were detected in all uncoated screws. There was a significant difference in bacterial growth between Ag-coated screws and uncoated screws (p < 0.001), with no bacterial growth on the Ag-coated screws and up to 2200 colony forming units/mL (CFU/mL) on the uncoated screws.
5 Ag-Based Bone Cements
In orthopedics bone cement has been widely used as a space filler to anchor the implant and bone in joint replacement surgeries. Bone cement typically consists of the chemical compound polymethyl methacrylate (PMMA) , and other formulations may include calcium phosphate and glass polyalkenoate. PMMA is formed upon mixing liquid and powder components in an exothermic polymerization reaction. Various additives include stabilizing agents, contrast agents, and antimicrobial agents [53]. As for antimicrobial additives, ideally, the antimicrobial agent should have a broad antimicrobial spectrum, be heat resistant, persist for a long period of time after implantation, and not affect the mechanical properties of the bone cement [54]. Traditionally, many antibiotics have been used in bone cements including gentamicin, tobramycin, erythromycin, cefuroxime, and vancomycin [53].
Ag has been studied as a substitute for antibiotics. One of the first studies investigating the use of Ag in bone cement was done by Spadaro et al. [54] In this study, low concentrations (0.05–1 wt.%) of different inorganic Ag compounds (AgCl, Ag-AgCl, Ag2O, Ag2SO4, Ag3PO4) were added to PMMA and tested in vitro against S. aureus, E. coli, and P. aeruginosa. The compressive strength of the cement, persistence of antibacterial activity, and biocompatibility were tested as well. Their results showed that the Ag formulations inhibited bacterial growth in vitro with dose-dependent zones of inhibition. Among the formulations studied, Ag2SO4 persisted the longest with a duration of antibacterial activity of at least 49 days. The Ag-based bone cements had similar compressive strength as the control bone cements. The biocompatibility tests in rabbit muscle tissues showed no significant differences between the Ag bone cement and the control bone cements.
Dueland et al. [55] compared the efficacy of Ag bone cement (Ag-PMMA) , gentamicin bone cement (gentamicin-PMMA), and plain bone cement using an in vivo animal model. The Ag-PMMA rods were made with the addition of 1 wt.% Ag2SO4. New Zealand rabbits were inoculated with S. aureus in the proximal tibial medullary canal and had either Ag-PMMA rods, gentamicin-PMMA rods, or control rods without antimicrobial agents implanted in the intramedullary canal. Evaluation of the treatment and control groups was done by quantifying the bacterial growth from the tibias after 6 weeks and mortality rates of the rabbits. The results showed that bacterial growth found in the Ag-PMMA group (35.6 ± 43 × 106 CFU) and gentamicin-PMMA group (8.6 ± 13 × 106 CFU) were significantly reduced when compared to the control group (89.4 ± 60 × 106 CFU). The mortality rate of the Ag-PMMA group, gentamicin-PMMA group, and control group was 22%, 6%, and 61%, respectively. There was a significantly lower mortality rate of the Ag-PMMA group and gentamicin-PMMA group when compared to the control group, but no significant differences were found when comparing the two treatment groups with each other.
AgNPs were also incorporated within bone cements . Alt et al. [56] found that AgNP-loaded bone cements had a dose-dependent inhibition of bacterial growth , with complete inhibition at a concentration of 1% AgNPs against MRSA , S. epidermidis, and methicillin-resistant S. epidermidis. In comparison, plain bone cements did not inhibit bacterial growth and gentamicin-loaded bone cements showed incomplete inhibition. The AgNP-loaded bone cements displayed no in vitro cytotoxicity and no significant differences were found when comparing to controls. Oei et al. [57] reported that AgNP-PMMA had 99.9% bacterial inhibition against S. aureus, A. baumannii, P. aeruginosa, and Proteus mirabilis when compared to plain PMMA controls. Prokopovich et al. [58] developed a PMMA bone cement impregnated with tiopronin-capped AgNPs and found that it had antimicrobial activities against MRSA. In addition, no cytotoxicity was observed against osteoblasts via MTT assays and the mechanical strength was comparable to the control PMMA without nanoparticles. Slane et al. [59] showed that AgNP-PMMA bone cements significantly reduced biofilm formation for S. epidermidis and S. aureus when compared to the bone cement control (p < 0.001).
Despite studies demonstrating the efficacy of AgNP-loaded bone cements against bacteria in vitro , studies completed in vivo suggested a different outcome. In a study done by Moojen et al. [60], the in vivo efficacy of AgNP-loaded bone cement against methicillin sensitive S. aureus infection was determined. In the design of the study, New Zealand rabbits were infected with S. aureus in the medullary canal of the femur, and implanted with AgNP-PMMA , tobramycin-PMMA, or plain-PMMA. After 14 days, the rabbits were analyzed for infection based on infection rates, bacteriology, and histology. All rabbits in the AgNP-PMMA cement and plain cement groups were infected, whereas only 17% in the tobramycin-PMMA group were infected. The bacteriology showed no differences in the growth between the AgNP-PMMA cement group and the plain cement group, whereas no growth was observed in the tobramycin group. The histopathology study revealed that all signs of infection (periosteal thickening, enlargement of Haversian canals, and cortical destruction) were present in all rabbits of the AgNP-PMMA and plain cement groups. In contrast, signs of infection were absent in the tobramycin-PMMA group, except for two rabbits with some enlargement of Haversian canals. The authors suggested that the differences observed may be due to the different release profiles of the treatment agents. In the implant infection model, not only was bacteria present at the implant surface, but also distal to the implant. Antibiotic-loaded bone cements were able to act both at the implant surface and distal to it. In contrast, AgNP-PMMA bone cements contained nanoparticles that were not released from the cements and needed to be ionized to become active. It suggested that AgNP loaded bone cements were able to act locally, but would have decreased efficacy in preventing infection at distal locations [60].
6 Summary
Ag and AgNPs have found some applications in orthopedics. These include Ag dressings, Ag-coated prosthetic implants, and Ag-based bone cements. Most studies seem to show strong antimicrobial properties of Ag along with limited toxicity, while inconsistencies exist among various studies, and among in vitro, in vivo, and clinical studies. Some applications have been heavily explored, such as Ag dressings and Ag-coated megaprostheses , and there have been quite a few clinical studies reported which support their antimicrobial efficacy and limited toxicity . However, other applications, including Ag-coated external fixation pins and Ag-based bone cements have either limited or no reported clinical studies. There remains a need for more clinical studies to better understand the uses of Ag and AgNPs before implementing these applications into daily practice. Some of the main concerns are their toxicity and potential accumulation in the body. In addition, the long-term clinical effects of Ag and AgNPs are not well known; future research may need to reduce their toxicity, which may likely broaden their clinical applications.
References
Lansdown AB. Silver in health care: antimicrobial effects and safety in use. Curr Probl Dermatol. 2006;33:17–34.
Li B, Webster TJ. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J Orthop Res 2017. https://doi.org/10.1002/jor.23656.
Chaloupka K, Malam Y, Seifalian AM. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28(11):580–8.
Chernousova S, Epple M. Silver as antibacterial agent: ion, nanoparticle, and metal. Angew Chem Int Ed Engl. 2013;52(6):1636–53.
Drake PL, Hazelwood KJ. Exposure-related health effects of silver and silver compounds: a review. Ann Occup Hyg. 2005;49(7):575–85.
Gunawan C, Marquis CP, Amal R, Sotiriou GA, Rice SA, Harry EJ. Widespread and indiscriminate nanosilver use: genuine potential for microbial resistance. ACS Nano. 2017;11(4):3438–45.
Walker M, Parsons D. The biological fate of silver ions following the use of silver-containing wound care products—a review. Int Wound J. 2014;11(5):496–504.
National Collaborating Centre for Ws, Children’s H. National Institute for Health and Clinical Excellence: guidance. Surgical site infection: prevention and treatment of surgical site infection. London: RCOG Press National Collaborating Centre for Women’s and Children’s Health; 2008.
Leaper D. Appropriate use of silver dressings in wounds: an expert working group consensus. Wounds Int. 2012;9(5):461–4.
Burd A, Kwok CH, Hung SC, Chan HS, Gu H, Lam WK, Huang L. A comparative study of the cytotoxicity of silver-based dressings in monolayer cell, tissue explant, and animal models. Wound Repair Regen. 2007;15(1):94–104.
Parsons D, Meredith K, Rowlands VJ, Short D, Metcalf DG, Bowler PG. Enhanced performance and mode of action of a novel antibiofilm hydrofiber(R) wound dressing. Biomed Res Int. 2016;2016:7616471. PMCID:PMC5136405
Ip M, Lui SL, Poon VKM, Lung I, Burd A. Antimicrobial activities of silver dressings: an in vitro comparison. J Med Microbiol. 2006;55(1):59–63.
Bourdillon KA, Delury CP, Cullen BM. Biofilms and delayed healing—an in vitro evaluation of silver- and iodine-containing dressings and their effect on bacterial and human cells. Int Wound J 2017. https://doi.org/10.1111/iwj.12761.
Mohseni M, Shamloo A, Aghababaei Z, Vossoughi M, Moravvej H. Antimicrobial wound dressing containing silver sulfadiazine with high biocompatibility: in vitro study. Artif Organs. 2016;40(8):765–73.
Lin YH, Hsu WS, Chung WY, Ko TH, Lin JH. Silver-based wound dressings reduce bacterial burden and promote wound healing. Int Wound J. 2016;13(4):505–11.
Paddle-Ledinek JE, Nasa Z, Cleland HJ. Effect of different wound dressings on cell viability and proliferation. Plast Reconstr Surg. 2006;117(7 Suppl):110S–8S. discussion 9S–20S
Hiro ME, Pierpont YN, Ko F, Wright TE, Robson MC, Payne WG. Comparative evaluation of silver-containing antimicrobial dressings on in vitro and in vivo processes of wound healing. Eplasty. 2012;12:e48. PMCID:PMC3471607
Gee Kee E, Stockton K, Kimble RM, Cuttle L, McPhail SM. Cost-effectiveness of silver dressings for paediatric partial thickness burns: an economic evaluation from a randomized controlled trial. Burns. 2017;43(4):724–32.
Munteanu A, Florescu IP, Nitescu C. A modern method of treatment: the role of silver dressings in promoting healing and preventing pathological scarring in patients with burn wounds. J Med Life. 2016;9(3):306–15. PMCID:PMC5154321
Jude EB, Apelqvist J, Spraul M, Martini J. Prospective randomized controlled study of Hydrofiber dressing containing ionic silver or calcium alginate dressings in non-ischaemic diabetic foot ulcers. Diabet Med. 2007;24(3):280–8.
Jemec GB, Kerihuel JC, Ousey K, Lauemoller SL, Leaper DJ. Cost-effective use of silver dressings for the treatment of hard-to-heal chronic venous leg ulcers. PLoS One. 2014;9(6):e100582. PMCID:PMC4063949
Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplast. 2008;23(7):984–91.
Lamagni T. Epidemiology and burden of prosthetic joint infections. J Antimicrob Chemother. 2014;69(Suppl 1):i5–10.
Grosso MJ, Berg A, LaRussa S, Murtaugh T, Trofa DP, Geller JA. Silver-impregnated occlusive dressing reduces rates of acute periprosthetic joint infection after total joint arthroplasty. J Arthroplast. 2017;32(3):929–32.
Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case-control study. J Arthroplast. 2014;29(6):1098–100.
Springer BD, Beaver WB, Griffin WL, Mason JB, Odum SM. Role of surgical dressings in total joint arthroplasty: a randomized controlled trial. Am J Orthop (Belle Mead NJ). 2015;44(9):415–20.
Ravenscroft MJ, Harker J, Buch KA. A prospective, randomised, controlled trial comparing wound dressings used in hip and knee surgery: aquacel and tegaderm versus cutiplast. Ann R Coll Surg Engl. 2006;88(1):18–22.
Jurczak F, Dugre T, Johnstone A, Offori T, Vujovic Z, Hollander D. Randomised clinical trial of Hydrofiber dressing with silver versus povidone-iodine gauze in the management of open surgical and traumatic wounds. Int Wound J. 2007;4(1):66–76.
Keen JS, Desai PP, Smith CS, Suk M. Efficacy of hydrosurgical debridement and nanocrystalline silver dressings for infection prevention in type II and III open injuries. Int Wound J. 2012;9(1):7–13.
Kadar A, Eisenberg G, Yahav E, Drexler M, Salai M, Steinberg EL. Surgical site infection in elderly patients with hip fractures, silver-coated versus regular dressings: a randomised prospective trial. J Wound Care. 2015;24(10):441–2. 4–5
Fries CA, Ayalew Y, Penn-Barwell JG, Porter K, Jeffery SL, Midwinter MJ. Prospective randomised controlled trial of nanocrystalline silver dressing versus plain gauze as the initial post-debridement management of military wounds on wound microbiology and healing. Injury. 2014;45(7):1111–6.
Muller ME. Internal fixation for fresh fractures and for non-union. Proc R Soc Med. 1963;56:455–60. PMCID:PMC1897011
Harris WH, Sledge CB. Total hip and total knee replacement. N Engl J Med. 1990;323(11):725–31.
Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422–9.
Trampuz A, Widmer AF. Infections associated with orthopedic implants. Curr Opin Infect Dis. 2006;19(4):349–56.
Ribeiro M, Monteiro FJ, Ferraz MP. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter. 2012;2(4):176–94.
Coester LM, Nepola JV, Allen J, Marsh JL. The effects of silver coated external fixation pins. Iowa Orthop J. 2006;26:48–53. PMCID:PMC1888577
Masse A, Bruno A, Bosetti M, Biasibetti A, Cannas M, Gallinaro P. Prevention of pin track infection in external fixation with silver coated pins: clinical and microbiological results. J Biomed Mater Res. 2000;53(5):600–4.
Wassall MA, Santin M, Isalberti C, Cannas M, Denyer SP. Adhesion of bacteria to stainless steel and silver-coated orthopedic external fixation pins. J Biomed Mater Res. 1997;36(3):325–30.
Collinge CA, Goll G, Seligson D, Easley KJ. Pin tract infections: silver vs uncoated pins. Orthopedics. 1994;17(5):445–8.
Bosetti M, Masse A, Tobin E, Cannas M. Silver coated materials for external fixation devices: in vitro biocompatibility and genotoxicity. Biomaterials. 2002;23(3):887–92.
Gkavardina A, Tsagozis P. The use of megaprostheses for reconstruction of large skeletal defects in the extremities: a critical review. Open Orthop J. 2014;8:384–9.
Schmidt-Braekling T, Streitbuerger A, Gosheger G, Boettner F, Nottrott M, Ahrens H, Dieckmann R, Guder W, Andreou D, Hauschild G, Moellenbeck B, Waldstein W, Hardes J. Silver-coated megaprostheses: review of the literature. Eur J Orthop Surg Traumatol. 2017;27(4):483–9.
Ewald A, Glückermann SK, Thull R, Gbureck U. Antimicrobial titanium/silver PVD coatings on titanium. BioMed Eng Online. 2006;5:22.
Hardes J, Streitburger A, Ahrens H, Nusselt T, Gebert C, Winkelmann W, Battmann A, Gosheger G. The influence of elementary silver versus titanium on osteoblasts behaviour in vitro using human osteosarcoma cell lines. Sarcoma. 2007;2007:26539. PMCID:PMC1920591
Gosheger G, Hardes J, Ahrens H, Streitburger A, Buerger H, Erren M, Gunsel A, Kemper FH, Winkelmann W, Von Eiff C. Silver-coated megaendoprostheses in a rabbit model—an analysis of the infection rate and toxicological side effects. Biomaterials. 2004;25(24):5547–56.
Wafa H, Grimer RJ, Reddy K, Jeys L, Abudu A, Carter SR, Tillman RM. Retrospective evaluation of the incidence of early periprosthetic infection with silver-treated endoprostheses in high-risk patients: case-control study. Bone Joint J. 2015;97-b(2):252–7.
Hardes J, von Eiff C, Streitbuerger A, Balke M, Budny T, Henrichs MP, Hauschild G, Ahrens H. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J Surg Oncol. 2010;101(5):389–95.
Scoccianti G, Frenos F, Beltrami G, Campanacci DA, Capanna R. Levels of silver ions in body fluids and clinical results in silver-coated megaprostheses after tumour, trauma or failed arthroplasty. Injury. 2016;47(Suppl 4):S11–S6.
Glehr M, Leithner A, Friesenbichler J, Goessler W, Avian A, Andreou D, Maurer-Ertl W, Windhager R, Tunn PU. Argyria following the use of silver-coated megaprostheses: no association between the development of local argyria and elevated silver levels. Bone Joint J. 2013;95-b(7):988–92.
Kose N, Caylak R, Peksen C, Kiremitci A, Burukoglu D, Koparal S, Dogan A. Silver ion doped ceramic nano-powder coated nails prevent infection in open fractures: In vivo study. Injury. 2016;47(2):320–4.
Secinti KD, Ozalp H, Attar A, Sargon MF. Nanoparticle silver ion coatings inhibit biofilm formation on titanium implants. J Clin Neurosci. 2011;18(3):391–5.
Vaishya R, Chauhan M, Vaish A. Bone cement. J Clin Orthop Trauma. 2013;4(4):157–63.
Spadaro JA, Webster DA, Becker RO. Silver polymethyl methacrylate antibacterial bone cement. Clin Orthop Relat Res. 1979;(143):266–70.
Dueland R, Spadaro JA, Rahn BA. Silver antibacterial bone cement. Comparison with gentamicin in experimental osteomyelitis. Clin Orthop Relat Res. 1982;169:264–8.
Alt V, Bechert T, Steinrucke P, Wagener M, Seidel P, Dingeldein E, Domann E, Schnettler R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials. 2004;25(18):4383–91.
Oei JD, Zhao WW, Chu L, DeSilva MN, Ghimire A, Rawls HR, Whang K. Antimicrobial acrylic materials with in situ generated silver nanoparticles. J Biomed Mater Res B Appl Biomater. 2012;100(2):409–15.
Prokopovich P, Leech R, Carmalt CJ, Parkin IP, Perni S. A novel bone cement impregnated with silver-tiopronin nanoparticles: its antimicrobial, cytotoxic, and mechanical properties. Int J Nanomed. 2013;8:2227–37. PMCID:PMC3693822
Slane J, Vivanco J, Rose W, Ploeg HL, Squire M. Mechanical, material, and antimicrobial properties of acrylic bone cement impregnated with silver nanoparticles. Mater Sci Eng C Mater Biol Appl. 2015;48:188–96.
Moojen DJ, Vogely HC, Fleer A, Verbout AJ, Castelein RM, Dhert WJ. No efficacy of silver bone cement in the prevention of methicillin-sensitive Staphylococcal infections in a rabbit contaminated implant bed model. J Orthop Res. 2009;27(8):1002–7.
Acknowledgement
We acknowledge financial support from AO Foundation (Project S-13-15L was supported by the AO Foundation), Osteosynthesis & Trauma Care Foundation, the West Virginia National Aeronautics and Space Administration Experimental Program to Stimulate Competitive Research (WV NASA EPSCoR), NIH Grant P20GM103434, and the National Institute of General Medical Sciences of the National Institutes of Health under Award Number 2U54GM104942-02. This work was also supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Peer Reviewed Medical Research Program, Discovery Award under Award No. W81XWH-17-1-0603. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the funding agencies. We thank Suzanne Danley for proofreading.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Kang, J., Hughes, K., Xing, M., Li, B. (2017). Orthopedic Applications of Silver and Silver Nanoparticles. In: Li, B., Webster, T. (eds) Orthopedic Biomaterials. Springer, Cham. https://doi.org/10.1007/978-3-319-73664-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-73664-8_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-73663-1
Online ISBN: 978-3-319-73664-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)