Abstract
The enormous growing problem with antibiotic resistance in pathogenic microbes is one of the greatest threats we are facing today. In the context of orthopedic applications, infections also lead to the limited healing ability of infected and defected bone. Generally, these problems are treated with a load of antibiotics or surgical intervention. Therefore, having antibacterial properties integrated with a biomaterial would reduce the time of healing and treatment, amount of antibiotic needed, and total cost. Currently, there exists several strategies and materials with the potential of tackling these challenges. Some materials with antibacterial properties currently employed are silver nanoparticles (AgNPs), cerium oxide nanoparticles (CeO2NPs), selenium nanoparticles (SeNPs), copper nanoparticles (CuNPs), antimicrobial peptides (AMPs), biopolymers (such as chitosan), and carbon nanostructures. On the other hand, osteoinductive and osteoconductive materials are important to promote bone healing and regeneration. Within this framework, materials which have been employed widely are bioactive glasses (BG), calcium phosphates (CaPs) (e.g., hydroxyapatite (HA), tricalcium β-phosphate (β-TCP), and biphasic calcium phosphate (BCP)), peptides, growth factors, and other elements (e.g., magnesium (Mg), zinc (Zn), strontium (Sr), silicon (Si), selenium (Se), and Cu, to name a few). Some of the current technological solutions that have been employed are, for instance, the use of a co-delivery system, where both the antibacterial and the osteoinducing agents are delivered from the same delivery system. However, this approach requires overcoming challenges with local delivery in a sustained and prolonged way, thus avoiding tissue toxicity. To address these challenges and promote novel biomaterials with dual action, sophisticated thinking and approaches have to be employed. For this, it is of the utmost importance to have a solid fundamental understanding of current technologies, bacteria behavior and response to treatments, and also a correlation between the material of use, the host tissue and bacteria. We hope by highlighting these aspects, we will promote the invention of the next generation of smart biomaterials with dual action ability to both inhibit infection and promote tissue growth.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Antibacterial
- Osteoinduction
- Osteoconduction
- Biomaterials
- Orthopedic treatment
- Tissue engineering
- Defect
- Infection
- Antibiotic resistant
- Dentistry
Introduction
Currently, there is no doubt that the grand challenging problem with the prevalence of multi-antibiotic resistant microbes due to the overuse of antibiotics is among the greatest threats to society and the healthcare system. With respect to orthopedic challenges, infection also plays an important role in negatively impacting the treatment and healing process significantly [1]. For instance, defected or damaged bone can be treated with osteoinductive biomaterials in order to promote healing and regeneration; however, these materials does not prevent infection. There are several challenges in orthopedic problems associated with infections, foremost, they could be difficult to detect at an early stage, as vide supra stated an increased challenge to treat multidrug-resistant organisms, and persistence and recurrence of infection, particularly associated with implants [2, 3]. In the context of implants associated with a risk of microbial infection, the general approaches are, for instance, implant replacement, or in worst case amputation or mortality [4]. Hence, integrating antimicrobial properties with the implant would provide huge advantages [4]. Furthermore, a great solution to the vide supra mentioned challenges would be the development of dual functional biomaterials with the ability to promote the healing of the bone by displaying osteoinductive properties, and simultaneously inherent antibacterial properties, without the use of antibiotics [5]. This could enable the treatment or prevention of future conceivable infections [6, 7]. Here, over the years, a plethora of biomaterials with antibacterial or osteoinductive properties have been reported. Examples of the latter, in particularly, in their nanoparticle forms are silver nanoparticles (AgNPs) [8], cerium oxide nanoparticles (CeO2NPs) [9] selenium nanoparticles (SeNPs) [10], and polymers or materials such as carbon nanostructures [11], chitosan [12], natural-based polyphenols [13,14,15], and antimicrobial peptides (AMPs) [16] (Fig. 1). Examples of biomaterials with osteoinductive properties are those which include osteogenic growth factors (OGF), fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF), bioactive glass (BG), bone morphogenic proteins (BMP), hydroxyapatite (HA), elements (e.g., magnesium (Mg), zinc (Zn), silicon (Si), selenium (Se), and copper (Cu)), and peptides such as those in parathyroid hormone (PTH) and arginine-glycine-aspartic acid (RGD) [17,18,19,20,21] (Fig. 1). In this chapter, the current challenges with bone repair/regeneration and antibacterial infection will be highlighted. Furthermore, fundamental aspects of antimicrobial and osteoinductive properties will be discussed providing the reader an essential platform information within this topic and then some examples of antimicrobial and osteoinductive biomaterials. Subsequently biomaterials displaying dual functions or dual delivery systems with both antimicrobial and osteoinductive properties in various orthopedic applications will be presented.
Current Challenges
Reviewing the historical development of bone tissue engineering and its advancements, it is clear to see that a large impact has been mainly made due to the integration of multidisciplinary fields such as biology, material science and engineering, and clinical avenues [22]. Starting from the first example of prosthetics employed in humans in the 1960s, and then further through a more sophisticated design and development of biomaterials by 1984 [23]. Here, the dogma transformed from designing a bioinert tissue responsive material to a bioactive one, which more resembles the host bone and also with similar mechanical properties. These types of biomaterials mainly consisted of ceramics, glasses, and glass-ceramics, and were thought to have better performance due to their ability to promote cellular functions such as colonization, proliferation, and differentiation within the surrounding environment of the implant. Therefore, this class of materials was further implemented into various orthopedics and dentistry applications [24]. The incorporation of various bioactive components (such as HA) onto implants and prostheses improved their performance and osteoinduction properties [25, 26]. Further, important features of this new class of biomaterials, besides resembling the native bone, both structurally and mechanically, were that they were also resorbable [27]. This property allowed for the chemical breakdown of the material, thus, eventually being completely replaced by newly formed tissue. In this regard, an important study by Hench on the impact of time on the resorption of the Dexon sutures in vivo promoted the employment of resorbable polymers as implants [27]. To date, a wide range of implant products have been approved by the Food and Drug Administration (FDA) for a wide range of applications and are available for clinical use, displaying properties such as bioinertness, bioactivity, and resorption [22, 23]. Nevertheless, great research efforts have been devoted to further improve biomaterial properties and performance, thus overcoming some current limitations leading to imperfect implant function and survival (Fig. 2a). One of the greatest challenges to be addressed is the precise control of the biomaterial features such as material composition, surface chemistry, pore size, porosity, morphology, degradation rate, and mechanical performance (Fig. 2b) [28]. In order to tackle these problems, a fundamental understanding of the vital processes such as osteogenesis, biomaterial and bone interactions, a mechanistic interfacial interaction between the host tissue and the biomaterial impact at the cellular level are important aspects. All of these features would highly promote the invention of the next generation of materials. Furthermore, the more sophisticated challenge of designing a material which displays both osteoconductive and antimicrobial properties functioning for a wide range of applications is an important research topic. This would allow for the design of biomaterials that are resistant to infections, prevent drug resistance and at the same time promote bone healing and regeneration. To pursue this vision, the right optimization between its fabrication (sustainable and eco-friendly technologies), safety, and performance need to be included, without impeding one another [29]. As vide supra mentioned, an optimal biomaterial for promoting the healing and regeneration of bone defects should not only possess the right mechanical and degradation properties, but also the right surface chemistry to endorse cellular processes such as cell attachment, proliferation, migration, differentiation, and remodeling leading to vascularization and eventually the formation of new bone tissue [30]. Some of the central challenges and desired properties in such a devised biomaterial for orthopedic engineering are highlighted in Fig. 2.
Nowadays, the concept of taking biomaterial features to the next level is paramount, where it should not only function as a replacement, but rather regenerate the damaged/defected tissue [23]. This paradigm shift is highly dependent on the biomaterial design, hence due to the general lack of synthetic biomaterials responding to physiological stimuli [31], naturally or biologically derived materials can be employed. For instance, decellularization of biological structures has proven to be able to function as vascularized scaffolds [32]. The aim here is to promote vascularization, allowing the transport of oxygen and nutrients to cells and simultaneously removal of waste products [33]. Biomaterials for bone tissue regeneration are generally more challenging to design due to the lack of proximal blood supply, thus, less access to existing blood supply comparable for instance to cartilage tissues [34]. However, several strategies have been developed to address these challenges, such as the employment of prevascularized scaffolds and/or the use of growth factors [35, 36]. Another important parameter of the scaffolds is the porosity, since this will play an important role in the interaction with the localized blood vessels, which indirectly impact vascularization [37]. Here, the design of the scaffolds which smoothly integrate with the host vasculature is another challenge and necessity [38]. To date, several biomaterials with various properties have been developed for improving vascularization (Fig. 3) [39].
Furthermore, features on the implant surface play a crucial role since they have the ability to direct protein adsorption or cellular attachments. This mechanism can start a cascade reaction where it promotes the vascularization and subsequently endorses the proliferation of osteoblasts. In this context, Bielby et al. demonstrated the differentiation of murine embryonic stem cells into osteogenic cells which further enhanced proliferation through the incorporation of soluble ions onto the scaffold [40, 41]. Several reports have disclosed various strategies for improving the interface between the implant and host bone, for instance through the employment of interconnected porous biomaterials [42], which also can be loaded with cells [43]. Here, in the context of direct cell transplantation in implants, it is vital that the right cell type is employed, considering, its accessibility, generated in high yield, and efficiently to promote the repair of the tissue and with a high survival rate [44]. These will overcome the limitations due to poor adjacent vascularization and cell–cell interactions, causing cell death due to insufficient nutrient and oxygen uptake. The overall goal is to obtain a smooth host-tissue cell scaffold interphase, which eventually will allow for incorporation into the surrounding host bone and endure a normal bone remodeling processes [38, 45]. Recently, we have seen blossom advances and interest in the employment of stem cells in various regenerative and tissue engineering applications. However, despite the great promise of stem cell technology and their potential, several contemplations have to be made such as developing a solid controlled approach for stem cell differentiation to the desired phenotype, acceptable purities and negligible carcinogenic latent [23, 46]. Over the years, several types of stem cells have been distinguished starting from the earliest embryonically derived stem cells to stem cells from the bone marrow, gut, liver, brain, and the circulatory system [47, 48]. In the context of orthopedic applications, the induced pluripotent stem cells (iPS cells) have been shown to be good candidates [46]. However, despite the improvement of implant scaffolds through surface tailoring or the addition of cells into the scaffolds, other challenges remain, in order to devise a material with high performance and with no limitations. Here, orthopedic implants can also promote bacterial adhesion and growth ensuing a negative impact on clinical outcomes and increase healthcare expense [2]. This is also one of the major factors leading to orthopedic implant failure. There are several mechanisms triggering this failure, for instance, lapses in surgical hygiene, contact with microbial flora, or the invasion of microorganisms due to implant failure [49]. Moreover, the bacterial adhesion on the implant causes several problems, firstly it promotes colonization leading to biofilm formation and this in turn can hinder tissue integration and thereby block various cellular functions and regeneration processes [50]. Furthermore, these also result in a prodigious negative impact on the patient leading to pain, surgical intervention for removal or replacement of the implant, and continuation of antibiotic treatment. Nevertheless, we have witnessed the problem with the frequent use of antibiotics promoting drug-resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) [51]. Over the years, several strategies and technologies have been invented to overcome these grand challenges, such as implant coatings with/without releasing bactericidal agents, but with the ability to prevent or reduce bacterial adhesion [52,53,54]. In this regard, a bactericidal agent frequently employed within such a coating is silver or their respective nanoparticle system (AgNPs) [55]. Nevertheless, they have to be engineered to provide a sustained, controlled and prolonged release preferable for at least 1 year [51, 55]. Interestingly, common food ingredients or natural medicinal components, such as garlic extracts and Aloe Vera, have been successfully demonstrated for their incorporation with implants promoting bone repair and preventing bacterial infections [56,57,58]. Additionally, bactericidal agents or strategies that have been employed to reduce bacteria adhesion and prevent plausible biofilm formation are the employment of nitric oxide [59] or the use of self-assembled monolayers (SAMs) to block bacterial attachment [60]. Furthermore, these strategies can be designed to provide both bacterial protective and at the same time promote bone healing/repair processes through the incorporation of osteoinductive components, such as BMP [61] and transforming growth factor beta (TGF-β) [62]. There are several challenges encountered when designing a system and materials with dual functions, such as the precise control of the delivery of each component, longstanding over the desired time frame, early and long-term osseointegration, and controlled resorbability [28]. Here resorbability, allowing the material to degrade into non-toxic components, is an important feature of the biomaterials; however, it requires a sophisticated design, in particular when it comes to the precise control of the in vivo degradation rate [63, 64]. Figure 4 highlights some of the most common bone grafting materials, including their resorptions mechanism [65]. Ideally, the material should degrade at the same rate as the tissue ingrowth and healing process [51]. However, this is highly influenced and dependent on several factors such as the site of implantation, the in vivo conditions, and the nature and degree of the infected/defected site, which make it a great challenge in devising biomaterials with the desired resorbability.
Selected bone graft substitutes and their resorption mechanisms. Reproduced with permission [65]. Copyright 2010, Elsevier Ltd. (CC BY) license
Despite all the current challenges, the vast advancement and innovations in the field of biomaterial technology will, most probably, successively promote inventions of grand solutions. For instance, the advancement of wireless technologies has the potential of providing tools to monitor or remotely control the healing process, the delivery of drugs in situ, or even indirectly stimulate the formation of bone tissue [51]. In this context, a great complementary would be the development of personalized medicine, this would allow overcoming current limitations, such as mechanical variations arising from the biomaterial and the treated person, and irregularities in various procedures [66, 67].
The Fundamental Basics of Antimicrobial and Osteoinductive Properties
One of the ideal approaches solving the problems posed by the grand challenges is not only to identify the fundamental mechanism leading to the problem, but also the fundamentals behind the solution. In the context of orthopedics, the biomaterial employed should display an ability to support the adhesion and localization of proteins, osseous cells, and growth factors in the region of the bone defect in order to promote the repair and regeneration of bone [68]. The process of successful bone formation promoted through biomaterials is depicted in Fig. 5. In step 1, osteogenesis starts, where mature osteoblasts are differentiated into progenitor cells, followed by osteoconduction, where bone starts growing on the surface of the biomaterial and simultaneously osseointegration takes place, meaning the direct contact between bone and biomaterial [69, 70]. Next, the osteoinduction process takes place, where cells are developed into bone forming cells (osteoprogenitor cells), and the progression of of osteogenesis is induced. Subsequently, the process of angiogenesis is promoted and cells are recruited and afterward the bone is fruitfully formed (Fig. 5) [71].
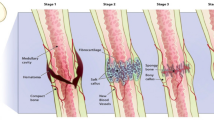
Additionally, alongside with promoting bone healing and repair, a secondary fundamental pursuit in biomaterial design is the prevention of bacterial infections and biofilm formation on the biomaterial employed [70]. In this regard, what makes it difficult is to predict any possible infection due to challenges of early detection. Here, when biomaterials, for instance titanium, are implanted in vivo, several mechanisms are triggered in the process of integration with the microenvironment promoting osteointegration and the prevention of bacterial infections [72]. Initially (Phase I), negatively charged biomolecules are adsorbed onto the positively charged titanium surface and further interact through various non-covalent forces such as hydrophobic, electrostatic, hydrogen bonding, and Van der Waals forces . This process proceeds within seconds. Next (Phase II), cells and bacterial attachment starts (minutes), followed by (Phase III) non-specific cellular adhesion resulting in fixing the cells with the aid of extracellular matrix (ECM) attaching proteins to the surface (can take hours). In the next stage (Phase IV) (days to weeks) migration, proliferation and differentiation proceeds, and lasts (V) the entire process of mineralization and bone remodeling which starts (Weeks) (Fig. 6) [72]. Therefore, tailoring the surface of the biomaterial is an important strategy [73]. For instance, introducing nanotopographies on the surface has shown to successfully promote the detachment of bacteria [74,75,76]. Other approaches regarding surface fabrication are coating of the surface to provide different properties or altering the surface roughness and surface energy [73, 77, 78]. Figure 7 presents the various antibacterial arsenals and therapeutics available to combat against infections and biofilm formation .
Antimicrobial Biomaterials
To date, we are entering an era where antimicrobial diseases are on the rise and predicted to cause enormous of death, even more than all cancers together [80]. Therefore, the development of novel materials with antimicrobial properties would provide an alternative to traditional antibiotics for various biomedical applications [81]. In this context, nanotechnology presents a potential approach to antimicrobial resistance, which could stimulate innovation and create a new generation of antibiotic treatment for future medicines [82]. Within this topic, AgNPs are some of the most employed antimicrobial agents in the biomedical field due to its wide antibacterial activity [83]. Additionally, other materials and elements, and their respective nanoparticle (such as Se, cerium (Ce), gold (Au), titanium (Ti), Cu, iron (Fe), carbon (carbon nanotubes (CNT), fullerene, graphene, etc.)) have been proven to display antimicrobial properties [84]. Noteworthy, while some of these materials, for instance, Ag and Cu, are intrinsically antibacterial even in their bulk state, others such as iron oxide need to be transformed to their respective nanoform in order to display antibacterial properties. The success of these strategies and nanotechnologies have led to several commercialized products for applications in bacterial diagnosis, antibiotic delivery, and medical devices [83]. Several strategies have been developed in order to enhance the antibacterial properties of these nanomaterials. Some of these strategies are: their incorporation into biomaterials thus controlling the release, protection from aggregation, improved solubility, and engineering their size and shape. All of these aspects are also important for providing low toxicity for in vivo applications [83, 85].
Elements
In this section, various elements with antibacterial properties demonstrated in the literature will be presented (Fig. 8a). In this context, AgNPs constitute a very promising approach for the development of new antimicrobial technologies [86]. Nanoparticle formulations can add significant improvements to the antibacterial activity of elements through specific actions, such as improved adsorption at the bacterial surface [8, 87, 88]. AgNPs have attracted increasing interest due to their chemical stability, catalytic activity, localized surface plasma resonance, and high conductivity. In addition, previous reports have shown that the reactive oxygen species (ROS) formed at the surface of the AgNPs, or the release of free silver ions under certain conditions may induce cell death of either mammalian cells or microbial cells, which endows the AgNPs with unique antibacterial and antifungal effects [89, 90]. Based on these effects, AgNPs hold great potential in preventing wound inflammation and hence promoting wound healing in the form of topical administration. Here, for topical use, skin penetration ability and safety of AgNPs should be assessed [88, 91]. Small silver particles (e.g., 4–122 nm) with lower toxicity to humans have been developed, but must be released in a controlled manner to minimize side effects and maximize antimicrobial activity [84, 88]. To date, a vast number of reports have been disclosed demonstrating AgNPs and combinations as an efficient antimicrobial agent for a wide range of bacteria [8, 92, 93]. Prominently, despite the promising potential of AgNPs, some reports have demonstrated bacterial resistance against silver [94]. However, which the high antibacterial effect of AgNPs has been widely described the exact mechanism of their action has yet to be fully elucidated. In fact, their potent antibacterial and broad-spectrum activity against morphologically and metabolically different microorganisms seems to be correlated with a multifaceted mechanism by which the nanoparticles interact with the microbes. As depicted in Fig. 8b, the mechanism may proceed via various pathways, such as an interaction with the bacterial cell wall to cause leakage, interaction with various metabolic pathways, inhibited protein synthesis and promoted ROS triggering DNA damage and degradation [8, 79, 95, 96]. Furthermore, elemental Se has also shown to be a good antimicrobial candidate, particularly in its nanoform (SeNPs) [10, 97]. Interestingly, despite its broad and high antibacterial performance, an in vitro study demonstrated a safe toxicity profile [10, 98]. Several groups have demonstrated the successful employment of SeNPs and its derivative against various bacteria [99,100,101]. For instance, they have been employed as a medical device coating for preventing biofilm formation [98, 102]. Moreover, Ce in its oxide form (CeO2NPs) also displays antibacterial properties through a similar mechanism as the AgNPs [9, 83, 103].
(a) Various elements with antibacterial activity. (b) The proposed mechanism of the antibacterial activity of AgNPs. Reproduced with permission [79]. Copyright 2014, Springer Science Business Media New York
Polymers and Miscellaneous
Polymers have also been shown to have characteristic antimicrobial activity, but here it is well established that polymers with cationic components interact better with the negatively charge bacteria membrane and promote damage and cell lysis [104]. A good example within the subject is nitrogen containing compounds such as chitosan, poly-ε-lysine, polyethyleneimine, and polyguanidines [105]. A careful material design will allow for the tailoring of the antibacterial properties of the polymers, for instance, addition of a hydrophobic group will endorse infiltration into the hydrophobic bacterial membrane [85, 106, 107]. Here, chitosan is probably one of the most known and widely employed nitrogen containing polymer with antimicrobial properties [108]. As depicted in Fig. 9a, chitosan is obtained after deacetylation from chitin, a polysaccharide extracted from the exoskeletons from insects, cell walls of fungi, and from invertebrates [109]. It is well known that materials with a quaternary amine moiety displayed increased antibacterial properties [110]; here several studies have confirmed increased antibacterial performance of quaternary chitosan compared to pure chitosan [111,112,113,114]. Interestingly, the antibacterial property of chitosan can be improved through several strategies, by the length of the alkyl moiety on the amine generating quaternary amine, where generally an increased alkyl chain promotes higher performance, molecular weight, and degree of acetylation, where lower molecular weight and lower degree of acetylation result in improved performance [115]. The plausible antibacterial mechanism of action of chitosan is depicted in Fig. 9b, which proceeds through several pathways [108]. As vide supra mentioned, the positive charge interferes with the negatively charge bacteria surface, or through inhibition of the mRNA and protein synthesis, chelation of important metals and nutrients and thereby changing cell permeability or preventing nutrients from entering the cell through electrostatic interaction with the cell wall [115,116,117].
(a) The generation of chitosan through a deacetylation step from chitin and their chemical structures. (b) The plausible antibacterial mechanisms of chitosan. Reproduced with permission [108]. Copyright 2019, Elsevier B.V
Furthermore, antimicrobial peptides (AMPs) , widely found in nature, have also shown the potential for being a source of antibacterial material for a wide range of microbials [16, 85, 118]. These AMPs can be categorized based on their structure as following: α-helical AMPs, cysteine-rich AMPs, β-sheet AMPs, AMPs rich in regular amino acids, and AMPs with rare modified amino acids [16]. Moreover, some modes of action of these AMPs are, for instance, bacteria membrane disruption and ion channel formation leading to leakage of potassium ions and other components [16]. Going further, carbon nanostructure such as fullerene [11, 119], carbon nanotubes (CNTs), and graphene all have inherent antibacterial properties and proceed with a wide range of mode of actions [11, 120,121,122,123]. Some of the antibacterial mechanisms are, for instance, reduction of biofilm formation and cell attachment, generation of oxidative stress and ROS, and promoted the loss of the cellular integrity [11, 124, 125]. Nevertheless, the antibacterial efficiency and mode of action depend on several factors such as the composition of the material, size, type of microbe, etc., and can also be tailored through surface modification [11, 125]. Here, in 2007, Kang et al. disclosed a seminal work demonstrating the evidence of the antimicrobial activity of single-walled carbon nanotubes (SWCNT) . The authors concluded that the SWCNT promoted membrane damage causing cell inactivation and bacterial cell death. Considering the high cost of a pure carbon nanostructure material, a good alternative could be the merging with other materials. In this regard, Aslan et al. incorporated the polymer poly(lactic-co-glycolic acid) (PLGA) with SWCNT and employed the constructs against Escherichia coli (E. coli) and Staphylococcus epidermidis (S. epidermidis), providing up to 98% bacteria death [124]. Furthermore, graphene in various forms, such as graphene oxide (GO) [126], graphene oxide nanoribbons (O-GNR) [127], and graphene-wrapped silver nanowires (AgNWs) [128] have all been reported to be good candidates for eliminating or reducing various bacterial types. There have also been reports where several types of carbon nanostructures have been merged in order to provide for higher antibacterial performance [129]. Nevertheless, in order for the carbon materials to find translational applications, limitations such as high cost and also in some cases relatively low solubility or insolubility in water must not be overlooked. Furthermore, another class of natural based omnipresent materials with antimicrobial properties are polyphenols [130] such as lignin [131,132,133] and tannin [134, 135], which are widely found in nature [13, 136]. Besides displaying antimicrobial properties, these components have several additional advantages such as being cheap, readily available and renewable, and valorization of these products is of great interest and importance, and therefore they are good candidates, particularly in the quest for fighting microbial resistance challenges [137,138,139]. It is believed that the hydroxyl (OH) groups in the polyphenol structure are inherent for its antibacterial properties [140]. However, the unique structure of polyphenols allows for a wide range of interaction possibilities such as covalent and physical interactions, e.g., hydrogen bonding, metal coordination, hydrophobic, imine and amine formation through a Schiff base reaction, and ionic based interaction [141]. All these interaction possibilities will promote the interaction with the bacterial cell wall and membrane, inhibition of biofilm formation, inhibition of bacterial enzymes and substrate deprivation, protein regulation, and metal iron deprivation due to chelating ability [140].
Osteoinductive Biomaterials
Biomaterials employed for various orthopedic applications should possess the ability to function as a scaffold and induced new bone formation (osteoinductive) [142]. Nevertheless, little is known about the exact mechanism for how these processes proceed, despite that several materials identified and implemented with an osteoinductive ability [143, 144]. One good strategy for the invention of a new biomaterial for bone repair and regeneration could be to mimic the composition of native bone, where it mainly consists of collagen (type 1) fibers combined with inorganic minerals such as HA and other important materials such as osteogenic factors [143, 145, 146]. In this context, materials such as calcium phosphates (CaPs) have shown osteoinductive and osteoconductive properties due to their resembling of the minerals in native bone [17, 147]. Examples of CaPs are HA, β-tricalcium phosphate (β-TCP) (both started to be employed in 1980), and biphasic calcium phosphates (BCP) (started being employed in 1990) [143]. Some differences between these materials are their mechanical properties, solubility, and resorbability [17, 148, 149]. Therefore, it is important in selecting the appropriate material with suitable properties for their intended application without compromising any other properties. For instance, employing a material with high resistance could also mean increased brittleness [149]. Alternative approaches surmounting these limitations could be through merging with other materials such as polymeric based biomaterials, which also would provide an ECM like composition [150,151,152,153,154,155]. Enduring inorganic materials, silica-based material bioglasses with an ability to easily form bonds with bone and stimulate new bond formation are other types of materials widely employed for various orthopedic applications [19, 25]. The conventional BG (45S5Bioglass®) is comprised of SiO2 (45 wt%), Na2O (24.5 wt%), CaO (6 wt%), and P2O5 (6 wt%) [148, 156]. Advantages with BG except being non-toxic, biocompatible, osteoconductive, and osteoinductive are their fast reaction with tissue and the ability to bind both hard tissues like bone and also soft tissues [155, 157]. Lately, BG based on borate and borosilicate have demonstrated an improved performance compared to the silica-based materials, due to their controllable degradation; however, one limitation is the concern of toxicity due to boron released [148].
Moreover, the mechanism of BG bioactivity has been well studied and starts with the formation of silanol (SiOH) bonds and the release of silicic acid (Si(OH)4) , and then a polycondensation step of the SiOH takes place generating hydrated silica gel. Subsequently, adsorption of an amorphous formed carbonate film composed of CaO-P2O5, and then crystallization of hydroxyl carbonate apatite (HCA). Afterwards, adsorption of biological moieties in the HCA layer occurs, then BG reacts with macrophages, osteoblast stem cells attach, and differentiation and proliferation of osteoblasts leading to matrix formation ensues. Next, crystallization of the matrix and growth of bone ensues, and finally bone formation takes place [148].
To date, several different BG have been developed and even commercialized; for more detailed information within this subject, the readers are referred to other beautiful reviews and articles found in the literature [148, 158,159,160,161,162]. Beside BGs, other inorganic biomaterials with osteoinductive properties employed in orthopedics are for instance elements such as Mg, Zn, Sr, Si, Se and Cu [20], and also the silicate nanoplatelets Laponite® [163]. Interestingly, Si as an element has also proven to be an important agent promoting various fundamental processes such as metabolism, formation and calcification of bone tissue, increasing bone mineral density and stimulating the formation of collagen and osteoblastic differentiation [164,165,166]. Here, each element contributes differently, for instance, Mg plays a vital role in the structure, density, and mechanical properties of bone. However, it also promotes ECM interactions and the activation of alkaline phosphatase (ALP) and integrins [167]. On the other hand, Zn is important for various cellular processes such as formation, mineralization, development, and maintenance of healthy bones [17, 148, 168]. Moreover, the element Sr not only stimulates bone formation, but also inhibits bone resorption and promotes the death of osteoclasts [17, 169,170,171]. Several reports have demonstrated the use of Sr as an additive in combination with other osteoinductive biomaterials [172,173,174]. Different from the other elements, Se functions as a protecting agent and aids in immune defenses, and antioxidant protection against ROS, reactive nitrogen species (RNS) and oxidative damage. However, it also promotes collagen expression, calcium (Ca) deposition, and osteoblastic differentiation [175,176,177]. Lastly, the element Cu has proven several functions, such as promoting the synthesis of bone and connective tissues, inhibiting bone resorption, and enhancing angiogenesis through functioning as a hypoxia-mimicking material [158, 178,179,180].
Other strategies stimulating bone production could be through the addition of OGF with the potential of stimulating osteoinduction, osteoconduction, and osseointegration. Some examples are FGF, TGF, VEGF, BMPs [181, 182], and EGF [18, 21]. De facto, these materials have been employed by native bone during bone formation (osteogenesis). In order to stimulate several processes simultaneously, within this framework, Zhan et al. disclosed the combination of both VEGF and BMP-2 in a silk scaffold. Here, VEGF promoted angiogenesis and BMP-2 enhanced bone formation [18]. Moreover, in addition to OGF, certain type of peptides have also shown important applications in orthopedic challenges, such as RGD peptides (promoting the osseointegration), PTH [183], thrombin peptide 508 (TP508), PepGen (P-15), calcitonin gene-related peptides (CGRP) [21], osteogenic growth peptide (OGP), and ECM-derived peptides. All these agents play different roles in the final quest of improving regeneration and repair of bone tissue. For instance, the PTH influences the regulation of calcium phosphate metabolism and activates osteoblasts through several processes such as promoting osteoblast proliferation and differentiation, and reducing osteoblast apoptosis and peroxisome activator receptor [18, 21, 184]. On the other hand, OGP plays an important role to increase bone formation through promoting ALP activity, and regulation of osteoprogenitor cell proliferation, differentiation, osteocalcin secretion, and collagen and matrix mineralization [21].
Dual Functional Biomaterials
Biomaterial-associated infection in various orthopedic applications is a great challenge and an increasing problem. Prominently, since the current golden standard of treatment includes high doses of antibiotics, or in some cases, additional surgical debridement of the infected tissue, such advances are badly needed [185]. Consequently, advances in the development of novel technologies for this overwhelming problem are of great interest. There are several strategies disclosed to address this problem, which will be highlighted in this section. One approach could be through the use of a co-delivery system, where both osteoinductive and antibacterial agents are delivered from a scaffold. However, there are several challenges with this strategy. Here, some of the challenges with a co-delivery system are difficulties with precise control of the delivery rate (sustained), sequential or simultaneously delivery, site (locally), and avoiding interference between the two agents [6, 186]. Therefore, the next generation of materials in this context would be a material that possesses dual function inherently without the need of adding any antibacterial or osteoinductive agents [6]. Nevertheless, designing and inventing these kinds of materials requires a sophisticated strategy including rational design. Moreover, the material also needs to display other vital properties, such as biocompatibility and adequate mechanical and degradation properties [31]. From the perspective of dual functional biomaterials, Lobo and coworkers very recently disclosed the fabrication and generation of nanofibers based on the combination of polycaprolactone (PCL), polyethylene glycol (PEG), and gelatin methacryloyl (GelMA) with the potential to promote bone regeneration and repair through stimulating ALP activity and Ca deposition [187]. Interestingly, the same material also showed antibacterial activity against S. aureus, P. aeruginosa and MRSA [188]. Based on these studies, the material could be a potential candidate with dual function for orthopedic applications. Very recently, Wang and coauthors designed core shell nanofibers as a co-delivery system, where the shell was comprised of PCL and the core of gelatin [189]. The nanofibers were designed as a bone regeneration and anti-infective membrane; therefore, the core was loaded with the antibiotic metronidazole, while the shell with nano-HA. A prolonged release of the antibiotic was observed for more than 20 days and showed significant improvement compared to nanofibers without core shell structure. There are still several limitations with the presented technology, besides employing an antibiotic, with the potential of promoting antibiotic resistance; for example, about 55% of the drug was already released at just day 1. Moreover, Shi et al. employed different strategies for inducing both antibacterial and osteoinductive properties through the design of a bio-interface consisting of the cationic polymer (polyhexamethylene biguanide (PHMB)) [190]. Primarily, the material was coated with polydopamine and then further through cation–π interactions with the PHMB bioelectrical environment that could be generated. Surface modification or coating is a powerful and facile approach allowing for tailoring of the properties of biomaterials and providing osteogenic and antibacterial properties [191]. This strategy has been employed by Kumar et al. for the addition of amine and carboxylic functionalities onto PCL and multiwall CNT (MWCNT) [192]. The material did not only exhibit osteoinductive but also antibacterial properties, nevertheless it also improved mechanical properties (increased tensile strength and elastic modulus) and polymer crystallinity. Other groups have also employed the surface coating strategy in order to induce dual functionality on titanium implants [193, 194].
Moreover, chitosan has been widely employed in various orthopedic applications due to its favorable antimicrobial property [114, 195]. In the context of dual functional biomaterials for orthopedic applications, it has been merged with BG-poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) for the generation of microspheres [196]. The membrane was designed as a multidrug delivery scaffold against bacterial infection and osteoporosis for periodontal repair, therefore, the antibiotic drug tetracycline hydrochloride and the antiosteoporosis agent daidzein were selected as the model drugs. Nevertheless, no bacterial test was demonstrated, which could be interesting to see if the antibacterial properties could be inherent from the chitosan within the membrane, thus avoiding the use of antibiotics [196]. Overall, the material displayed multifunctionality, besides antibacterial and antiosteoporosis properties, it also displayed enhanced osteoblast activity, increased surface roughness, improved hydrophilicity, decreased swelling ratio, and decreased degradation. Moreover, the quaternary chitosan hydroxypropyltrimethyl ammonium chloride chitosan (HACC) has been integrated with BMP-2 for inducing dual function within zein-based materials [197]. Here, the BMP-2 was incorporated into the porous silica material SBA-15 in order to provide sustained and localized release. The multicomponent scaffold displayed prolonged antibacterial activity for up to 5 days and the BMP-2 could be released for more than 27 days. The performance of the material was demonstrated using an in vivo rabbit model of a critical-sized radius bone defect, which showed efficient bone formation [197].
Alternative to the fibers for periodontal applications, recently, dual functional PCL based electrospun materials loaded with zinc oxide nanoparticles (ZnO-NPs) have been disclosed [198]. The authors demonstrated the translational application of their devised material through an in vivo experiment by implanting the material in a rat periodontal defect model. The successful performance of the material could be observed in the decreased distance between the cementoenamel junction (CEJ) and the bone crest [198]. Moreover, a bone implant composed of calcium silicate-gelatin (CSG) coated with chitosan or chitosan oligosaccharide has been demonstrated as a dual functional biomaterial [199]. The authors demonstrate that having 0.2% of chitosan or 0.4% of chitosan oligosaccharide displayed comparable antibacterial properties against S. aureus and E. coli as to Ag coating. Nevertheless, the Ag showed significant toxicity even at a low concentration (0.004%), while the chitosan-based material did not show any cytotoxicity at <0.4% concentration. In parallel, osteogenic properties were also demonstrated showing an ability to promote cell attachment, proliferation, ALP activity, and osteocalcin and Ca deposition.
Furthermore, Bari et al. designed a Cu containing mesoporous BG (Cu-MBG) nanoparticle composed of SiO2-CaO as a multifunctional biomaterial for bone regeneration. As stated above, Cu has the ability of inducing both antibacterial and osteoinductive properties through various mechanisms [200, 201]. The biomaterial released copper ions (Cu2+) in a sustained manner for up to 7 days. However, no proper biological study was performed on its osteogenic ability, rather through analyzing the HA-forming ability of the biomaterial. The biomaterial showed improved antibacterial properties tested against E. coli, S. aureus, and S. epidermidis compared to the material without Cu (e.g., a 50% reduction against S. epidermidis at day 3, while in the absence of Cu no reduction was observed). Moreover, the osteoinductive component BMP-2 was merged with AgNPs within a scaffold made from PLGA and was successfully demonstrated for the repair of rat femoral infected segmental defects [202]. A similar combination with PLGA as the base polymer was also demonstrated by other research groups [203, 204]. Additionally, Sun et al. employed the same combinations; however, the scaffold employed in this study was a collagen composite [205]. Several reports have demonstrated the use of BMP-2 in combination with vancomycin employing different scaffolds such as a silica calcium phosphate nanocomposite [206], a calcium sulfate composite [207], poly(2-hydroxyethyl methacrylate)-nanocrystalline HA (pHEMA-nHA) [208], and polyurethane [209]. Additionally, an interesting strategy and dual combination was fruitfully demonstrated for bone regeneration using Sr and Ag combination within NTs [210, 211]. Furthermore, Fig. 10 depicts additional antimicrobial and osteoinductive biomaterials, their delivery methods and approaches employed for the incorporation of the agents [212].
Future Perspective and Remarks
A wide range of biomaterials and technologies displaying both antimicrobial and osteoinductive functionalities have been presented and developed over the years. Despite this fruitful progress, no ideal biomaterials providing dual functionalities with high efficiency, controllable triggering mechanisms, avoiding the use of antibiotic and long-lasting have been invented. All these should also be integrated with facile, scalable and sustainable fabrication and preparation technologies. Organic chemistry can play a crucial role in the quest of designing novel dual functional biomaterials allowing for the employment of green chemistry parameters such as atom economy, waste reduction, reduced toxicity, and the use of renewable resources [213, 214]. This would also allow tailoring the surface of the biomaterials and in that way introducing antimicrobial and osteoinductive functionalities [215]. Therefore, further advancements within this field are important for not only future perspectives, but also the fundamental understanding of various cellular mechanism, material and tissue interactions, long-term performance of such materials, etc. These will provide for the development and invention of a more solid technological platform which will be easier to translate into the clinic and find useful and suitable applications. A co-delivery system is one strategy extensively employed allowing the delivery of both agents consecutively without interfering with one and other, nevertheless, some of the limitations and challenges that need to be overcome in the future are, for instance, controlled local and sustained delivery, thus avoiding systemic toxicity, delivery over the desired time point and avoiding the use of antibiotics, thus circumventing the risk and promotion of the development of antibiotic resistance organisms. Moreover, having biomaterials that prevent future infection will also promote the healing and repair of bone since infections have the ability to decline or hinder the healing process. Besides being dual functional biomaterials, they should also display vital properties such as biocompatibility, biodegradability, support tissue attachment, regeneration, proliferation, optimal mechanical properties, and good integration with the host tissue. Another option for future advanced biomaterial within the discussed topic could be the employment of smart materials [31]. Adding properties to existing biomaterials, such as the ability of triggering itself in case of any treatment or future damages or infections, is progressing. These are some of the future materials we hope will boost this endeavor and advance the current topic.
References
Thomas MV, Puleo DA (2011) Infection, inflammation, and bone regeneration: a paradoxical relationship. J Dent Res 90(9):1052–1061
Moriarty TF, Kuehl R, Coenye T, Metsemakers W-J, Morgenstern M, Schwarz EM et al (2016) Orthopaedic device-related infection: current and future interventions for improved prevention and treatment. EFORT Open Rev 1(4):89–99
Winkler H (2017) Treatment of chronic orthopaedic infection. EFORT Open Rev 2(5):110–116
Ribeiro M, Monteiro FJ, Ferraz MP (2012) Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2(4):176–194
Zhang K, Wang S, Zhou C, Cheng L, Gao X, Xie X et al (2018) Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res 6(1):31
Lu H, Liu Y, Guo J, Wu H, Wang J, Wu G (2016) Biomaterials with antibacterial and osteoinductive properties to repair infected bone defects. Int J Mol Sci 17(3):334
Raphel J, Holodniy M, Goodman SB, Heilshorn SC (2016) Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 84:301–314
Franci G, Falanga A, Galdiero S, Palomba L, Rai M, Morelli G et al (2015) Silver nanoparticles as potential antibacterial agents. Molecules 20(5):8856–8874
Pelletier DA, Suresh AK, Holton GA, McKeown CK, Wang W, Gu B et al (2010) Effects of engineered cerium oxide nanoparticles on bacterial growth and viability. Appl Environ Microbiol 76(24):7981–7989
Guisbiers G, Wang Q, Khachatryan E, Mimun L, Mendoza-Cruz R, Larese-Casanova P et al (2016) Inhibition of E. coli and S. aureus with selenium nanoparticles synthesized by pulsed laser ablation in deionized water. Int J Nanomedicine 11:3731–3736
Al-Jumaili A, Alancherry S, Bazaka K, Jacob MV (2017) Review on the antimicrobial properties of carbon nanostructures. Materials 10(9):1066
Tan H, Ma R, Lin C, Liu Z, Tang T (2013) Quaternized chitosan as an antimicrobial agent: antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int J Mol Sci 14(1):1854–1869
Coppo E, Marchese A (2014) Antibacterial activity of polyphenols. Curr Pharm Biotechnol 15(4):380–390
Lee E, Song Y, Lee S (2014) Antimicrobial property and biodegradability of lignin nanofibers. Master’s thesis, Yonsei University, Republic of Korea
Scalbert A (1991) Antimicrobial properties of tannins. Phytochemistry 30(12):3875–3883
Reddy K, Yedery R, Aranha C (2004) Antimicrobial peptides: premises and promises. Int J Antimicrob Agents 24(6):536–547
Habibovic P, Barralet J (2011) Bioinorganics and biomaterials: bone repair. Acta Biomater 7(8):3013–3026
Zhang W, Zhu C, Wu Y, Ye D, Wang S, Zou D et al (2014) VEGF and BMP-2 promote bone regeneration by facilitating bone marrow stem cell homing and differentiation. Eur Cell Mater 27(12):1–11
Hench LL, Splinter RJ, Allen W, Greenlee T (1971) Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res 5(6):117–141
Tomás H, Alves CS, Rodrigues J (2018) Laponite®: a key nanoplatform for biomedical applications? Nanomedicine 14(7):2407–2420
Pountos I, Panteli M, Lampropoulos A, Jones E, Calori GM, Giannoudis PV (2016) The role of peptides in bone healing and regeneration: a systematic review. BMC Med 14(1):103
Ratner BD, Hoffman AS, Schoen FJ, Lemons JE (2004) Biomaterials science—an introduction to materials in medicine, 2nd edn. Elsevier, New York
Hench LL, Thompson I (2010) Twenty-first century challenges for biomaterials. J R Soc Interface 7(Suppl 4):S379–SS91
Campana V, Milano G, Pagano E, Barba M, Cicione C, Salonna G et al (2014) Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med 25(10):2445–2461
Hench LL (1998) Bioceram J Am Ceram Soc 81(7):1705–1728
Rea S, Bonfield W (2004) Biocomposites for medical applications. J Australas Ceram Soc 40(1):43–57
Hench L (1980) Biomaterials. Science 208(4446):826–831
Ratner BD, Hoffman AS, Yaszemski MJ, Lemons JE, Schoen FJ (2012) Biomaterials science : an introduction to materials in medicine. Elsevier Science & Technology, San Diego
Uludağ H (2014) Grand challenges in biomaterials. Front Bioeng Biotechnol 2:43
Muschler GF, Nakamoto C, Griffith LG (2004) Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am 86(7):1541–1558
Kowalski PS, Bhattacharya C, Afewerki S, Langer R (2018) Smart biomaterials: recent advances and future directions. ACS Biomater Sci Eng 4(11):3809–3817
Novosel EC, Kleinhans C, Kluger PJ (2011) Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev 63(4):300–311
Soker S, Machado M, Atala A (2000) Systems for therapeutic angiogenesis in tissue engineering. World J Urol 18(1):10–18
Rouwkema J, Rivron NC, van Blitterswijk CA (2008) Vascularization in tissue engineering. Trends Biotechnol 26(8):434–441
Mikos AG, Sarakinos G, Lyman MD, Ingber DE, Vacanti JP, Langer R (1993) Prevascularization of porous biodegradable polymers. Biotechnol Bioeng 42(6):716–723
Patel ZS, Young S, Tabata Y, Jansen JA, Wong ME, Mikos AG (2008) Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 43(5):931–940
Whang K, Healy K, Elenz D, Nam E, Tsai D, Thomas C et al (1999) Engineering bone regeneration with bioabsorbable scaffolds with novel microarchitecture. Tissue Eng 5(1):35–51
Petite H, Viateau V, Bensaid W, Meunier A, de Pollak C, Bourguignon M et al (2000) Tissue-engineered bone regeneration. Nat Biotechnol 18(9):959–963
García JR, García AJ (2016) Biomaterial-mediated strategies targeting vascularization for bone repair. Drug Delivery Transl Res 6(2):77–95
Bielby RC, Christodoulou IS, Pryce RS, Radford WJ, Hench LL, Polak JM (2004) Time- and concentration-dependent effects of dissolution products of 58S Sol–gel bioactive glass on proliferation and differentiation of murine and human osteoblasts. Tissue Eng 10:1018–1026
Bielby RC, Pryce RS, Hench LL, Polak JM (2005) Enhanced derivation of osteogenic cells from murine embryonic stem cells after treatment with ionic dissolution products of 58S bioactive sol–gel glass. Tissue Eng 11(3–4):479–488
Gao J, Dennis JE, Solchaga LA, Awadallah AS, Goldberg VM, Caplan AI (2001) Tissue-engineered fabrication of an osteochondral composite graft using rat bone marrow-derived mesenchymal stem cells. Tissue Eng 7(4):363–371
Karp JM, Shoichet MS, Davies JE (2003) Bone formation on two-dimensional poly (DL-lactide-co-glycolide)(PLGA) films and three-dimensional PLGA tissue engineering scaffolds in vitro. J Biomed Mater Res A 64(2):388–396
Grande DA, Breitbart AS, Mason J, Paulino C, Laser J, Schwartz RE (1999) Cartilage tissue engineering: current limitations and solutions. Clin Orthop Relat Res 367:S176–SS85
Hutmacher DW, Sittinger M (2003) Periosteal cells in bone tissue engineering. Tissue Eng 9(Suppl 1):S45–S64
De Miguel MP, Fuentes-Julián S, Alcaina Y (2010) Pluripotent stem cells: origin, maintenance and induction. Stem Cell Rev 6(4):633–649
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS et al (1998) Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–1147
Hubbell JA, Thomas SN, Swartz MA (2009) Materials engineering for immunomodulation. Nature 462(7272):449–460
Quaile A (2012) Infections associated with spinal implants. Int Orthop 36(2):451–456
Arciola CR, Campoccia D, Montanaro L (2018) Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol 16(7):397–409
Bose S, Bandyopadhyay A (2017) Materials and devices for bone disorders, 1st edn. Elsevier, Amsterdam, pp 1–560
Romanò CL, Scarponi S, Gallazzi E, Romanò D, Drago L (2015) Antibacterial coating of implants in orthopaedics and trauma: a classification proposal in an evolving panorama. J Orthop Surg Res 10(1):157
Aranya AK, Pushalkar S, Zhao M, LeGeros RZ, Zhang Y, Saxena D (2017) Antibacterial and bioactive coatings on titanium implant surfaces. J Biomed Mater Res A 105(8):2218–2227
Orapiriyakul W, Young PS, Damiati L, Tsimbouri PM (2018) Antibacterial surface modification of titanium implants in orthopaedics. J Tissue Eng 9:2041731418789838
Kim JS, Kuk E, Yu KN, Kim J-H, Park SJ, Lee HJ et al (2007) Antimicrobial effects of silver nanoparticles. Nanomedicine 3(1):95–101
Rahman S, Carter P, Bhattarai N (2017) Aloe vera for tissue engineering applications. J Funct Biomater 8(1):6
Bansal SS, Kausar H, Vadhanam MV, Ravoori S, Pan J, Rai SN et al (2014) Curcumin implants, not curcumin diet, inhibit estrogen-induced mammary carcinogenesis in ACI rats. Cancer Prev Res (Phila) 7(4):456–465
Pakdel F, Ghasemi S, Babaloo A, Javadzadeh Y, Momeni R, Ghanizadeh M et al (2017) Antibacterial effects of garlic extracts and ziziphora essential oil on bacteria associated with peri-implantitis. J Clin Diagn Res 11(4):ZC16–ZC19
Nichols SP, Schoenfisch MH (2013) Nitric oxide flux-dependent bacterial adhesion and viability at fibrinogen-coated surfaces. Biomater Sci 1(11):1151–1159
Freitas SC, Correa-Uribe A, Cristina L, Martins M, Pelaez-Vargas A (2018) Self-assembled monolayers for dental implants. Int J Dent 2018:4395460
Bessa PC, Casal M, Reis RL (2008) Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery). J Tissue Eng Regen Med 2(2–3):81–96
Simmons CA, Alsberg E, Hsiong S, Kim WJ, Mooney DJ (2004) Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone 35(2):562–569
Zhao L, Chu PK, Zhang Y, Wu Z (2009) Antibacterial coatings on titanium implants. J Biomed Mater Res B Appl Biomater 91(1):470–480
Modjarrad K, Ebnesajjad S (2013) Handbook of polymer applications in medicine and medical devices. Elsevier, Amsterdam, pp 1–354
Bohner M (2010) Resorbable biomaterials as bone graft substitutes. Mater Today 13(1):24–30
Wilhelmi M, Haverich A (2013) Functionalized medical implants in the era of personalized medicine. Clin Pract 10(2):119–121
Qin M, Liu Y, Wang L, Li D, Jin Z, Liu Y et al (2017) Laser metal direct forming of the customized titanium implants. Rare Metal Mater Eng 46(2017):1924–1928
Bosetti M, Fusaro L, Nicolì E, Borrone A, Aprile S, Cannas M (2014) Poly-L-lactide acid-modified scaffolds for osteoinduction and osteoconduction. J Biomed Mater Res Part A 102(10):3531–3539
Goonoo N, Bhaw-Luximon A (2018) Regenerative medicine: induced pluripotent stem cells and their benefits on accelerated bone tissue reconstruction using scaffolds. J Mater Res 33(11):1573–1591
Algburi A, Comito N, Kashtanov D, Dicks LMT, Chikindas ML (2017) Control of biofilm formation: antibiotics and beyond. Appl Environ Microbiol 83(3):e02508–e02516
Albrektsson T, Johansson C (2001) Osteoinduction, osteoconduction and osseointegration. Eur Spine J 10(Suppl 2):S96–S101
Jäger M, Jennissen HP, Dittrich F, Fischer A, Köhling HL (2017) Antimicrobial and osseointegration properties of nanostructured titanium orthopaedic implants. Materials (Basel) 10(11):1302
Hatton BD (2015) Antimicrobial coatings for metallic biomaterials. In: Wen C (ed) Surface coating and modification of metallic biomaterials. Woodhead Publishing, Sawston, pp 379–391
Betancourt T, Brannon-Peppas L (2006) Micro- and nanofabrication methods in nanotechnological medical and pharmaceutical devices. Int J Nanomed 1(4):483–495
Besinis A, Hadi SD, Le HR, Tredwin C, Handy RD (2017) Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology 11(3):327–338
Tripathy A, Pahal S, Mudakavi RJ, Raichur AM, Varma MM, Sen P (2018) Impact of bioinspired nanotopography on the antibacterial and antibiofilm efficacy of chitosan. Biomacromolecules 19(4):1340–1346
Ercan B, Khang D, Carpenter J, Webster TJ (2013) Using mathematical models to understand the effect of nanoscale roughness on protein adsorption for improving medical devices. Int J Nanomedicine 8(Suppl 1):75–81
Slavin YN, Asnis J, Häfeli UO, Bach H (2017) Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnol 15(1):65
Pandey JK, Swarnkar R, Soumya K, Dwivedi P, Singh MK, Sundaram S et al (2014) Silver nanoparticles synthesized by pulsed laser ablation: as a potent antibacterial agent for human enteropathogenic gram-positive and gram-negative bacterial strains. Appl Biochem Biotechnol 174(3):1021–1031
O’Neill J (2016) The review on antimicrobial resistance. Tackling drug-resistant infections globally: final report and recommendations
Gupta A, Mumtaz S, Li C-H, Hussain I, Rotello VM (2019) Combatting antibiotic-resistant bacteria using nanomaterials. Chem Soc Rev 48(2):415–427
Kumar M, Curtis A, Hoskins C (2018) Application of nanoparticle technologies in the combat against anti-microbial resistance. Pharmaceutics 10(1):11
Alpaslan E, Geilich BM, Yazici H, Webster TJ (2017) pH-controlled cerium oxide nanoparticle inhibition of both gram-positive and gram-negative bacteria growth. Sci Rep 7:45859
Zhang BG, Myers DE, Wallace GG, Brandt M, Choong PF (2014) Bioactive coatings for orthopaedic implants-recent trends in development of implant coatings. Int J Mol Sci 15(7):11878–11921
Gupta A, Landis RF, Li C-H, Schnurr M, Das R, Lee Y-W et al (2018) Engineered polymer nanoparticles with unprecedented antimicrobial efficacy and therapeutic indices against multidrug-resistant bacteria and biofilms. J Am Chem Soc 140(38):12137–12143
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27(1):76–83
Le Ouay B, Stellacci F (2015) Antibacterial activity of silver nanoparticles: a surface science insight. Nano Today 10(3):339–354
Chaloupka K, Malam Y, Seifalian AM (2010) Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol 28(11):580–588
Qin H, Zhu C, An Z, Jiang Y, Zhao Y, Wang J et al (2014) Silver nanoparticles promote osteogenic differentiation of human urine-derived stem cells at noncytotoxic concentrations. Int J Nanomedicine 9:2469–2478
Mahmood M, Li Z, Casciano D, Khodakovskaya MV, Chen T, Karmakar A et al (2011) Nanostructural materials increase mineralization in bone cells and affect gene expression through miRNA regulation. J Cell Mol Med 15(11):2297–2306
Sportelli MC, Izzi M, Volpe A, Clemente M, Picca RA, Ancona A et al (2018) The pros and cons of the use of laser ablation synthesis for the production of silver nano-antimicrobials. Antibiotics (Basel) 7(3):67
Loo YY, Rukayadi Y, Nor-Khaizura M-A-R, Kuan CH, Chieng BW, Nishibuchi M, Radu S (2018) In vitro antimicrobial activity of green synthesized silver nanoparticles against selected gram-negative foodborne pathogens. Front Microbiol 9:1555
Huma Z-e, Gupta A, Javed I, Das R, Hussain SZ, Mumtaz S et al (2018) Cationic silver nanoclusters as potent antimicrobials against multidrug-resistant bacteria. ACS Omega 3(12):16721–16727
Panáček A, Kvítek L, Smékalová M, Večeřová R, Kolář M, Röderová M et al (2018) Bacterial resistance to silver nanoparticles and how to overcome it. Nat Nanotechnol 13(1):65–71
Pareek V, Gupta R, Panwar J (2018) Do physico-chemical properties of silver nanoparticles decide their interaction with biological media and bactericidal action? A review. Mater Sci Eng C 90(11):739–749
Patil MP, Kim G-D (2017) Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl Microbiol Biotechnol 101(1):79–92
Hosnedlova B, Kepinska M, Skalickova S, Fernandez C, Ruttkay-Nedecky B, Peng Q et al (2018) Nano-selenium and its nanomedicine applications: a critical review. Int J Nanomedicine 13:2107–2128
Stolzoff M, Wang S, Webster T (eds) (2016) Efficacy and mechanism of selenium nanoparticles as antibacterial agents. In: Front. bioeng. biotechnol. conference abstract: 10th world biomaterials congress. https://doi.org/10.3389/conf.FBIOE.2016.01.01826
Srivastava N, Mukhopadhyay M (2015) Green synthesis and structural characterization of selenium nanoparticles and assessment of their antimicrobial property. Bioprocess Biosyst Eng 38(9):1723–1730
Shoeibi S, Mashreghi M (2017) Biosynthesis of selenium nanoparticles using Enterococcus faecalis and evaluation of their antibacterial activities. J Trace Elem Med Biol 39:135–139
Huang X, Chen X, Chen Q, Yu Q, Sun D, Liu J (2016) Investigation of functional selenium nanoparticles as potent antimicrobial agents against superbugs. Acta Biomater 30:397–407
Wang Q, Webster TJ (2012) Nanostructured selenium for preventing biofilm formation on polycarbonate medical devices. J Biomed Mater Res Part A 100(12):3205–3210
Farias IAP, dos Santos CCL, Sampaio FC (2018) Antimicrobial activity of cerium oxide nanoparticles on opportunistic microorganisms: a systematic review. Biomed Res Int 2018:1
Li S, Dong S, Xu W, Tu S, Yan L, Zhao C et al (2018) Antibacterial hydrogels. Adv Sci 5(5):1700527
Jain A, Duvvuri LS, Farah S, Beyth N, Domb AJ, Khan W (2014) Antimicrobial polymers. Adv Healthc Mater 3(12):1969–1985
Yang Y, Cai Z, Huang Z, Tang X, Zhang X (2018) Antimicrobial cationic polymers: from structural design to functional control. Polym J 50(1):33–44
Du H, Wang Y, Yao X, Luo Q, Zhu W, Li X et al (2016) Injectable cationic hydrogels with high antibacterial activity and low toxicity. Polym Chem 7(36):5620–5624
Hosseinnejad M, Jafari SM (2016) Evaluation of different factors affecting antimicrobial properties of chitosan. Int J Biol Macromol 85:467–475
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31(7):603–632
Muñoz-Bonilla A, Fernández-García M (2012) Polymeric materials with antimicrobial activity. Prog Polym Sci 37(2):281–339
Cheah WY, Show P-L, Ng IS, Lin G-Y, Chiu C-Y, Chang Y-K (2019) Antibacterial activity of quaternized chitosan modified nanofiber membrane. Int J Biol Macromol 126:569–577
Ignatova M, Starbova K, Markova N, Manolova N, Rashkov I (2006) Electrospun nano-fibre mats with antibacterial properties from quaternised chitosan and poly(vinyl alcohol). Carbohydr Res 341(12):2098–2107
Sajomsang W, Gonil P, Tantayanon S (2009) Antibacterial activity of quaternary ammonium chitosan containing mono or disaccharide moieties: preparation and characterization. Int J Biol Macromol 44(5):419–427
Wang D (2016) Osteoinductive and antibacterial biomaterials for bone tissue engineering. Dissertation, Vrije Universiteit Amsterdam
Goy RC, Britto D, Assis OBG (2009) A review of the antimicrobial activity of chitosan. Polímeros 19(3):241–247
Rabea EI, Badawy MET, Stevens CV, Smagghe G, Steurbaut W (2003) Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 4(6):1457–1465
Raafat D, Sahl HG (2009) Chitosan and its antimicrobial potential—a critical literature survey. Microb Biotechnol 2(2):186–201
Eckhard LH, Sol A, Abtew E, Shai Y, Domb AJ, Bachrach G et al (2014) Biohybrid polymer-antimicrobial peptide medium against Enterococcus faecalis. PLoS One 9(10):e109413
Chen Q, Ma Z, Liu G, Wei H, Xie X (2016) Antibacterial activity of cationic cyclen-functionalized fullerene derivatives: membrane stress. Dig J Nanomater Bios 11:753–761
Medeiros SJ, Oliveira AM, de Carvalho JO, Ricci R, Martins MCC, Rodrigues BV et al (2018) Nanohydroxyapatite/graphene nanoribbons nanocomposites induce in vitro osteogenesis and promote in vivo bone neoformation. ACS Biomater Sci Eng 4(5):1580–1590
Siqueira IA, Corat MAF, Cavalcanti BN, Neto WAR, Martin AA, Bretas RES et al (2015) In vitro and in vivo studies of novel poly (D, L-lactic acid), superhydrophilic carbon nanotubes, and nanohydroxyapatite scaffolds for bone regeneration. ACS Appl Mater Interfaces 7(18):9385–9398
Van Noorden R (2011) Chemistry: the trials of new carbon. Nature 469(7328):14–16
Mocan T, Matea CT, Pop T, Mosteanu O, Buzoianu AD, Suciu S et al (2017) Carbon nanotubes as anti-bacterial agents. Cell Mol Life Sci 74(19):3467–3479
Aslan S, Loebick CZ, Kang S, Elimelech M, Pfefferle LD, Van Tassel PR (2010) Antimicrobial biomaterials based on carbon nanotubes dispersed in poly (lactic-co-glycolic acid). Nanoscale 2(9):1789–1794
Dizaj SM, Mennati A, Jafari S, Khezri K, Adibkia K (2015) Antimicrobial activity of carbon-based nanoparticles. Adv Pharm Bull 5(1):19–23
Gurunathan S, Han JW, Dayem AA, Eppakayala V, Kim J-H (2012) Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int J Nanomed 7:5901–5914
Ricci R, Leite N, Da-Silva N, Pacheco-Soares C, Canevari R, Marciano F et al (2017) Graphene oxide nanoribbons as nanomaterial for bone regeneration: effects on cytotoxicity, gene expression and bactericidal effect. Mater Sci Eng C 78:341–348
Zhao C, Deng B, Chen G, Lei B, Hua H, Peng H et al (2016) Large-area chemical vapor deposition-grown monolayer graphene-wrapped silver nanowires for broad-spectrum and robust antimicrobial coating. Nano Res 9(4):963–973
Rodrigues BV, Leite NC, das Neves Cavalcanti B, da Silva NS, Marciano FR, Corat EJ et al (2016) Graphene oxide/multi-walled carbon nanotubes as nanofeatured scaffolds for the assisted deposition of nanohydroxyapatite: characterization and biological evaluation. Int J Nanomedicine 11:2569–2585
Lochab B, Shukla S, Varma IK (2014) Naturally occurring phenolic sources: monomers and polymers. RSC Adv 4(42):21712–21752
Upton BM, Kasko AM (2016) Strategies for the conversion of lignin to high-value polymeric materials: review and perspective. Chem Rev 116(4):2275–2306
Dong X, Dong M, Lu Y, Turley A, Jin T, Wu C (2011) Antimicrobial and antioxidant activities of lignin from residue of corn stover to ethanol production. Ind Crop Prod 34(3):1629–1634
Erakovic S, Jankovic A, Tsui GCP, Tang C-Y, Miskovic-Stankovic V, Stevanovic T (2014) Novel bioactive antimicrobial lignin containing coatings on titanium obtained by electrophoretic deposition. Int J Mol Sci 15(7):12294–12322
Chung K-T, Wong TY, Wei C-I, Huang Y-W, Lin Y (1998) Tannins and human health: a review. Crit Rev Food Sci Nutr 38(6):421–464
Park JH, Choi S, Moon HC, Seo H, Kim JY, Hong S-P et al (2017) Antimicrobial spray nanocoating of supramolecular Fe(III)-tannic acid metal-organic coordination complex: applications to shoe insoles and fruits. Sci Rep 7(1):6980
Sarjit A, Wang Y, Dykes GA (2015) Antimicrobial activity of gallic acid against thermophilic campylobacter is strain specific and associated with a loss of calcium ions. Food Microbiol 46:227–233
Arbenz A, Averous L (2015) Chemical modification of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem 17(5):2626–2646
Redondo LM, Chacana PA, Dominguez JE, Fernandez Miyakawa ME (2014) Perspectives in the use of tannins as alternative to antimicrobial growth promoter factors in poultry. Front Microbiol 5:118
Daglia M (2012) Polyphenols as antimicrobial agents. Curr Opin Biotechnol 23(2):174–181
Papuc C, Goran GV, Predescu CN, Nicorescu V, Stefan G (2017) Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: classification, structures, sources, and action mechanisms. Compr Rev Food Sci Food Saf 16(6):1243–1268
Ahn BK (2017) Perspectives on mussel-inspired wet adhesion. J Am Chem Soc 139(30):10166–10171
Habibovic P, de Groot K (2007) Osteoinductive biomaterials—properties and relevance in bone repair. J Tissue Eng Regen Med 1(1):25–32
Zhu Y, Zhang K, Zhao R, Ye X, Chen X, Xiao Z et al (2017) Bone regeneration with micro/nano hybrid-structured biphasic calcium phosphate bioceramics at segmental bone defect and the induced immunoregulation of MSCs. Biomaterials 147:133–144
Barradas A, Yuan H, van Blitterswijk CA, Habibovic P (2011) Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms. Eur Cell Mater 21:407–429
Holzwarth JM, Ma PX (2011) Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 32(36):9622–9629
Webster TJ, Massa-Schlueter EA, Smith JL, Slamovich EB (2004) Osteoblast response to hydroxyapatite doped with divalent and trivalent cations. Biomaterials 25(11):2111–2121
LeGeros RZ (2008) Calcium phosphate-based osteoinductive materials. Chem Rev 108(11):4742–4753
Rahaman MN, Day DE, Bal BS, Fu Q, Jung SB, Bonewald LF et al (2011) Bioactive glass in tissue engineering. Acta Biomater 7(6):2355–2373
Roseti L, Parisi V, Petretta M, Cavallo C, Desando G, Bartolotti I et al (2017) Scaffolds for bone tissue engineering: state of the art and new perspectives. Mater Sci Eng C 78:1246–1262
Caballero SSR, Saiz E, Montembault A, Tadier S, Maire E, David L et al (2019) 3-D printing of chitosan-calcium phosphate inks: rheology, interactions and characterization. J Mater Sci Mater Med 30(1):6
Iviglia G, Morra M, Cassinelli C, Torre E, Rodriguez Y, Baena R (2018) New collagen-coated calcium phosphate synthetic bone filler (Synergoss®): a comparative surface analysis. Int J Appl Ceram Technol 15(4):910–920
Inzana JA, Olvera D, Fuller SM, Kelly JP, Graeve OA, Schwarz EM et al (2014) 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 35(13):4026–4034
Gao X, Song J, Ji P, Zhang X, Li X, Xu X et al (2016) Polydopamine-templated hydroxyapatite reinforced polycaprolactone composite nanofibers with enhanced cytocompatibility and osteogenesis for bone tissue engineering. ACS Appl Mater Interfaces 8(5):3499–3515
Li H, Chang J (2005) pH-compensation effect of bioactive inorganic fillers on the degradation of PLGA. Compos Sci Technol 65(14):2226–2232
Stevanović M, Filipović N, Djurdjević J, Lukić M, Milenković M, Boccaccini A (2015) 45S5Bioglass®-based scaffolds coated with selenium nanoparticles or with poly (lactide-co-glycolide)/selenium particles: processing, evaluation and antibacterial activity. Colloids Surf B Biointerfaces 132:208–215
Li A, Ren H, Cui Y, Wang C, Zhou X, Lin H et al (2017) Detailed structure of a new bioactive glass composition for the design of bone repair materials. J Non-Cryst Solids 475:10–14
Sarin S, Rekhi A (2016) Bioactive glass: a potential next generation biomaterial. SRM J Res Dent Sci 7(1):27–32
El-Rashidy AA, Roether JA, Harhaus L, Kneser U, Boccaccini AR (2017) Regenerating bone with bioactive glass scaffolds: a review of in vivo studies in bone defect models. Acta Biomater 62:1–28
Jones JR (2013) Review of bioactive glass: from Hench to hybrids. Acta Biomater 9(1):4457–4486
Chen QZ, Thompson ID, Boccaccini AR (2006) 45S5 Bioglass®-derived glass–ceramic scaffolds for bone tissue engineering. Biomaterials 27(11):2414–2425
Kargozar S, Baino F, Hamzehlou S, Hill RG, Mozafari M (2018) Bioactive glasses: sprouting angiogenesis in tissue engineering. Trends Biotechnol 36(4):430–444
Jones JR, Brauer DS, Hupa L, Greenspan DC (2016) Bioglass and bioactive glasses and their impact on healthcare. Int J Appl Glas Sci 7(4):423–434
Sheikhi A, Afewerki S, Oklu R, Gaharwar AK, Khademhosseini A (2018) Effect of ionic strength on shear-thinning nanoclay–polymer composite hydrogels. Biomater Sci 6:2073–2083
Hoppe A, Güldal NS, Boccaccini AR (2011) A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 32(11):2757–2774
Valliant EM, Romer F, Wang D, McPhail DS, Smith ME, Hanna JV et al (2013) Bioactivity in silica/poly (γ-glutamic acid) sol–gel hybrids through calcium chelation. Acta Biomater 9(8):7662–7671
Catauro M, Bollino F, Papale F (2018) Surface modifications of titanium implants by coating with bioactive and biocompatible poly (ε-caprolactone)/SiO2 hybrids synthesized via sol–gel. Arab J Chem 11(7):1126–1133
Hickey DJ, Ercan B, Sun L, Webster TJ (2015) Adding MgO nanoparticles to hydroxyapatite–PLLA nanocomposites for improved bone tissue engineering applications. Acta Biomater 14:175–184
Webster TJ, Ergun C, Doremus RH, Bizios R (2002) Hydroxylapatite with substituted magnesium, zinc, cadmium, and yttrium. II. Mechanisms of osteoblast adhesion. J Biomed Mater Res 59(2):312–317
Querido W, Rossi AL, Farina M (2016) The effects of strontium on bone mineral: a review on current knowledge and microanalytical approaches. Micron 80:122–134
Yang F, Yang D, Tu J, Zheng Q, Cai L, Wang L (2011) Strontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating Wnt/catenin signaling. Stem Cells 29(6):981–991
Lemaire-Hurtel A-S, Mentaverri R, Caudrillier A, Cournarie F, Wattel A, Kamel S et al (2009) The calcium-sensing receptor is involved in strontium ranelate-induced osteoclast apoptosis. New insights into the associated signaling pathways. J Biol Chem 284(1):575–584
Fiorilli S, Molino G, Pontremoli C, Iviglia G, Torre E, Cassinelli C et al (2018) The incorporation of strontium to improve bone-regeneration ability of mesoporous bioactive glasses. Materials 11(5):678
Kannan S, Pina S, Ferreira JMF (2006) Formation of strontium-stabilized β-tricalcium phosphate from calcium-deficient apatite. J Am Ceram Soc 89(10):3277–3280
Oryan A, Baghaban Eslaminejad M, Kamali A, Hosseini S, Sayahpour FA, Baharvand H (2019) Synergistic effect of strontium, bioactive glass and nano-hydroxyapatite promotes bone regeneration of critical-sized radial bone defects. J Biomed Mater Res B Appl Biomater 107(1):50–64
Zeng H, Cao J (2013) Selenium in bone health: roles in antioxidant protection and cell proliferation. Nutrients 5:97–110
Ebert R, Ulmer M, Zeck S, Meissner-Weigl J, Schneider D, Stopper H et al (2006) Selenium supplementation restores the antioxidative capacity and prevents cell damage in bone marrow stromal cells in vitro. Stem Cells 24(5):1226–1235
Liu H, Bian W, Liu S, Huang K (2012) Selenium protects bone marrow stromal cells against hydrogen peroxide-induced inhibition of osteoblastic differentiation by suppressing oxidative stress and ERK signaling pathway. Biol Trace Elem Res 150(1):441–450
Dollwet HHA, Sorenson JRJ (1988) Roles of copper in bone maintenance and healing. Biol Trace Elem Res 18(1):39–48
Shi M, Chen Z, Farnaghi S, Friis T, Mao X, Xiao Y et al (2016) Copper-doped mesoporous silica nanospheres, a promising immunomodulatory agent for inducing osteogenesis. Acta Biomater 30:334–344
Li X, He Q, Shi J (2014) Global gene expression analysis of cellular death mechanisms induced by mesoporous silica nanoparticle-based drug delivery system. ACS Nano 8(2):1309–1320
Vukicevic S, Sampath KT (2002) Bone morphogenetic proteins: from laboratory to clinical practice, 1st edn. Birkhäuser, Basel
Ho-Shui-Ling A, Bolander J, Rustom LE, Johnson AW, Luyten FP, Picart C (2018) Bone regeneration strategies: engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 180:143–162
Lombardi G, Di CS, Rubino M, Faggiano A, Vuolo L, Guerra E et al (2011) The roles of parathyroid hormone in bone remodeling: prospects for novel therapeutics. J Endocrinol Investig 34(7 Suppl):18–22
Jung RE, Hämmerle CH, Kokovic V, Weber FE (2007) Bone regeneration using a synthetic matrix containing a parathyroid hormone peptide combined with a grafting material. Int J Oral Maxillofac Implants 22(2):258–266
Johnson CT, García AJ (2015) Scaffold-based anti-infection strategies in bone repair. Ann Biomed Eng 43(3):515–528
Salles GN, Calió ML, Afewerki S, Pacheco-Soares C, Porcionatto M, Hölscher C et al (2018) Prolonged drug-releasing fibers attenuate Alzheimer’s disease-like pathogenesis. ACS Appl Mater Interfaces 10(43):36693–36702
Lobo AO, Afewerki S, de Paula MMM, Ghannadian P, Marciano FR, Zhang YS et al (2018) Electrospun nanofiber blend with improved mechanical and biological performance. Int J Nanomedicine 13:7891–7903
De Paula MMM, Bassous NJ, Afewerki S, Harb SV, Ghannadian P, Marciano FR et al (2018) Understanding the impact of crosslinked PCL/PEG/GelMA electrospun nanofibers on bactericidal activity. PLoS One 13(12):e0209386
Wang Y, Jiang Y, Zhang Y, Wen S, Wang Y, Zhang H (2019) Dual functional electrospun core-shell nanofibers for anti-infective guided bone regeneration membranes. Mater Sci Eng C 98:134–139
Shi L, Zhang W, Yang K, Shi H, Li D, Liu J et al (2015) Antibacterial and osteoinductive capability of orthopedic materials via cation–π interaction mediated positive charge. J Mater Chem B 3(5):733–737
Yang G, Yang H, Shi L, Wang T, Zhou W, Zhou T et al (2018) Enhancing corrosion resistance, osteoinduction, and antibacterial properties by Zn/Sr additional surface modification of magnesium alloy. ACS Biomater Sci Eng 4(12):4289–4298
Kumar S, Bose S, Chatterjee K (2014) Amine-functionalized multiwall carbon nanotubes impart osteoinductive and bactericidal properties in poly(ε-caprolactone) composites. RSC Adv 4(37):19086–19098
Zhang Y, Dong C, Yang S, Chiu T-W, Wu J, Xiao K et al (2018) Enhanced silver loaded antibacterial titanium implant coating with novel hierarchical effect. J Biomater Appl 32(9):1289–1299
Qian X, Qing F, Jun O, Hong S (2014) Construction of drug-loaded titanium implants via layer-by-layer electrostatic self-assembly. West China J Stomatol 32:537–541
Xu C, Lei C, Meng L, Wang C, Song Y (2012) Chitosan as a barrier membrane material in periodontal tissue regeneration. J Biomed Mater Res B Appl Biomater 100(5):1435–1443
Li W, Ding Y, Yu S, Yao Q, Boccaccini AR (2015) Multifunctional chitosan-45S5 bioactive glass-poly(3-hydroxybutyrate-co-3-hydroxyvalerate) microsphere composite membranes for guided tissue/bone regeneration. ACS Appl Mater Interfaces 7(37):20845–20854
Zhou P, Xia Y, Cheng X, Wang P, Xie Y, Xu S (2014) Enhanced bone tissue regeneration by antibacterial and osteoinductive silica-HACC-zein composite scaffolds loaded with rhBMP-2. Biomaterials 35(38):10033–10045
Nasajpour A, Ansari S, Rinoldi C, Shahrokhi Rad A, Aghaloo T, Ryon Shin S et al (2018) A multifunctional polymeric periodontal membrane with osteogenic and antibacterial characteristics. Adv Funct Mater 28:1703437
Wei C-K, Ding S-J (2017) Dual-functional bone implants with antibacterial ability and osteogenic activity. J Mater Chem B 5(10):1943–1953
Bergemann C, Zaatreh S, Wegner K, Arndt K, Podbielski A, Bader R et al (2017) Copper as an alternative antimicrobial coating for implants—an in vitro study. World J Transplant 7(3):193–202
Lemire JA, Harrison JJ, Turner RJ (2013) Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol 11(6):371–384
Zheng Z, Yin W, Zara JN, Li W, Kwak J, Mamidi R et al (2010) The use of BMP-2 coupled—nanosilver-PLGA composite grafts to induce bone repair in grossly infected segmental defects. Biomaterials 31(35):9293–9300
Liu Y, Zheng Z, Zara JN, Hsu C, Soofer DE, Lee KS et al (2012) The antimicrobial and osteoinductive properties of silver nanoparticle/poly (DL-lactic-co-glycolic acid)-coated stainless steel. Biomaterials 33(34):8745–8756
Stevanović M, Uskoković V, Filipović M, Škapin SD, Uskoković D (2013) Composite PLGA/AgNpPGA/AscH nanospheres with combined osteoinductive, antioxidative, and antimicrobial activities. ACS Appl Mater Interfaces 5(18):9034–9042
Sun CY, Che YJ, Lu SJ (2015) Preparation and application of collagen scaffold-encapsulated silver nanoparticles and bone morphogenetic protein 2 for enhancing the repair of infected bone. Biotechnol Lett 37(2):467–473
Pacheco H, Vedantham K, Aniket, Young A, Marriott I, El-Ghannam A (2014) Tissue engineering scaffold for sequential release of vancomycin and rhBMP2 to treat bone infections. J Biomed Mater Res A 102(12):4213–4223
Wang Y, Wang X, Li H, Xue D, Shi Z, Qi Y et al (2011) Assessing the character of the rhBMP-2- and vancomycin-loaded calcium sulphate composites in vitro and in vivo. Arch Orthop Trauma Surg 131(7):991–1001
Li X, Xu J, Filion TM, Ayers DC, Song J (2013) pHEMA-nHA encapsulation and delivery of vancomycin and rhBMP-2 enhances its role as a bone graft substitute. Clin Orthop Relat Res 471(8):2540–2547
Guelcher SA, Brown KV, Li B, Guda T, Lee B-H, Wenke JC (2011) Dual-purpose bone grafts improve healing and reduce infection. J Orthop Trauma 25(8):477–482
Neoh KG, Hu X, Zheng D, Kang ET (2012) Balancing osteoblast functions and bacterial adhesion on functionalized titanium surfaces. Biomaterials 33(10):2813–2822
Cheng H, Xiong W, Fang Z, Guan H, Wu W, Li Y et al (2016) Strontium (Sr) and silver (Ag) loaded nanotubular structures with combined osteoinductive and antimicrobial activities. Acta Biomater 31:388–400
Bose S, Banerjee D, Bandyopadhyay A (2017) Chapter 1—Introduction to biomaterials and devices for bone disorders. In: Bose S, Bandyopadhyay A (eds) Materials for bone disorders. Academic, New York, pp 1–27
Anastas PT, Warner JC (1998) Green chemistry: theory and practice. Oxford University Press, Oxford
Anastas P, Eghbali N (2010) Green chemistry: principles and practice. Chem Soc Rev 39(1):301–312
Kiran A, Kumar T, Sanghavi R, Doble M, Ramakrishna S (2018) Antibacterial and bioactive surface modifications of titanium implants by PCL/TiO2 nanocomposite coatings. Nanomaterials. 8(10):860
Acknowledgements
Dr. Afewerki gratefully acknowledges the financial support from the Sweden-America Foundation (The Family Mix Entrepreneur Foundation) and the Olle Engkvist Byggmästare Foundation. Professor Lobo and Professor Marciano acknowledge the National Council for Scientific and Technological Development for support (CNPq, #303752/2017-3 to AOL and #304133/2017-5 to FRM).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Afewerki, S. et al. (2020). Advances in Antimicrobial and Osteoinductive Biomaterials. In: Li, B., Moriarty, T., Webster, T., Xing, M. (eds) Racing for the Surface. Springer, Cham. https://doi.org/10.1007/978-3-030-34471-9_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-34471-9_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-34470-2
Online ISBN: 978-3-030-34471-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)