Abstract
Vascular hemodynamics are an important part of many medical and engineering applications. The advances in imaging technologies, modeling methods and the increasing computational power allow for sophisticated in-depth studies of the fluid dynamical behavior of blood. The integration of patient-specific geometries, the consideration of dynamical boundary conditions and the interaction between fluid and solid structures play the major role in all numerical modeling frameworks. The following chapter gives a brief summary on each of these topics providing a concise guideline for the interested modeler. In the end three applications are provided. Studies on Fluid-Structure-Interaction in the aortic arch, multiscale CFD simulations of heart support and the vascular hemodynamics in the cerebral arteries (Circle of Willis) are shown. The cases are chosen to emphasize the emerging complexity of fluid dynamics in biological systems and the methods to tackle these obstacles.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
The fluid mechanics of blood play a major role in many problems of biomedical engineering. Medical devices such as stents, heart valves and pumps are in constant contact with blood and therefore need to be carefully designed with respect to their influence on the flow field. Another important question is the blood flow through parts of the human vascular system. Hereby the interaction with these devices may be of interest. Further on, investigations of various physiological and pathological scenarios increases the understanding of the underlying mechanisms and ultimately help improving the clinical outcome.
From the numerical modeling viewpoint there are several aspects which need to be considered. First of all, the investigated geometries are rather complex and exhibit high variability. As patient specific considerations gain more and more importance, (non-invasive) imaging techniques need to be incorporated in the model creation process. Secondly, boundary conditions need to be defined carefully with respect to the cardiovascular system as a whole. Finally, vessel elasticity and wall movement address the need of fluid-structure interaction (FSI) simulations.
This book chapter gives a concise overview of the current methods and challenges in the field of vascular hemodynamics with a focus on clinical application. It should be seen as a starting point for a numerical modeler interested in these scientific questions. The explanations do not claim to be exhaustive. In the second part, some examples of these methods are presented.
2 Geometry Creation and General Simulation Settings
As stated in the introduction, patient specificity is of course defined by the input data of the geometric anatomy. Non-invasive imaging techniques such as magnetic resonance imaging (MRI) and computer tomography (CT) are the common data sources. The geometry is created by surface reconstruction from these grayscale images. Semi-automated processes as region growing algorithms increase the model creation speed. Some exemplary applications can be found in [1, 7,8,9]. However the error probability can be quiet high in low-resolution imaging or the visualization of moving objects like heart valves. Feedback from clinicians at this stage can omit unrealistic geometrical representations and improve the meaningfulness of the acquired results.
After model creation the meshing is usually done by unstructured tetrahedral elements. As wall shear stress plays a major role in almost all clinical questions, the boundary layer needs to be carefully represented by prismatic elements. The shear-thinning behavior of blood can be expressed by the appropriate material models (Power-law, Carreau-model etc.). However, these phenomenological models are based on experimental data. Especially in the low shear regime <5 s−1 the model predictions show limitations. An a priori estimation of the shear rates can help to judge whether a non-Newtonian blood model is necessary. At last, turbulent effects need to be considered in the simulations. Comparison of a peak Reynolds number with a critical Reynolds number (Eq. 1) is a possible approach Peacock et al. [14]
With α as the Womersley number and St as the Strouhal number. In case of turbulence the Shear Stress Transport model is a common RANS description.
3 Boundary Conditions
As we are only able to consider a selected region of interest of the cardiovascular system, regarding its pathological-anatomical specific features, (mostly a piece of the bigger sized arteries or veins), the question arises how to include the remaining part of the circulation.
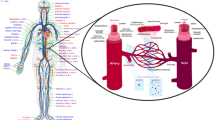
Figure 1 shows the 3-D model of the aortic arch embedded in the entire cardiovascular system. It is evident, that one should try and model the system as a closed-loop to get a realistic simulation. With a multiscale description it is possible to solve this problem. At first, we model the entire cardiovascular circulation as a 0-D lumped parameter (LP) network leading to a system of ordinary differential equations (ODE). Then the ODEs are coupled to the fluid solver to combine the high resolution of computational fluid dynamics (CFD) with the overall dynamical behavior. The final model is adaptable to patient specific measurements of pressures and flows in the various parts of the cardiovascular system. Further on pathologies like valve insufficiencies, hypertension or diminished autoregulation capabilities can be represented by adjustments of the lumped parameters.
3.1 Lumped Parameter Modeling
Basically LP modeling uses elements of hydraulic circuits such as resistances, capacitors and inductances to express the flow resistance, the vessel compliance and the inertance of the blood. The so-called Windkessel models describe the combination of these elements to express the pressure/flow relationship of an elastic vessel segment. There has been extensive research on Windkessel modeling and on LP modeling of the cardiovascular system [4, 10, 13, 17]. The reader is referred to these works for more information.
A very simple case, a resistance with a capacitor (two-element Windkessel), is presented in Eqs. 2a and 2b:
Qin and Qout are the incoming and outgoing flows into the compartment i (Ri and Ci). pi and pi+1 are the pressures of the corresponding and the downstream compartment, respectively. This procedure is repeated for every block of the cardiovascular circulation, until there is a coupled system of ODEs. Elements as valves, cardiac chambers and coronary arteries have more sophisticated descriptions than Eqs. 2a and 2b. Again [10] give a thorough explanation.
3.2 0-D/3-D Coupling
The coupling between LP and CFD is achieved with the consolidated approach from [12] by an interchange of flows (CFD) and pressures (LP) at the boundary faces. At each timestep the flows Qi across the boundaries are calculated by the CFD solver and serve as the input for the LP model. The ODE system is solved and the interface pressures are imposed as uniformly distributed pressure boundary conditions in the CFD solver.
4 Fluid-Structure-Interaction
There are mainly two approaches to perform a two-way coupled FSI simulation, the monolithic and the partitioned approach. The monolithic approach solves the fluid and solid domain simultaneously within the same solver and thus by using one set of equations, resulting in relatively stable simulations. The downside is the rather complicated formulation of the equations.
The second approach for FSI simulations is the partitioned approach, in which the fluid and solid domain are solved separately with two different solvers and the information between both systems is transferred through a domain interface. The CFD solver calculates the solution of the fluid domain and thereby also the load on the fluid-solid interface. The structural solver receives the load and calculates the displacement of the vessel wall, which is transferred back to the fluid domain. This procedure is one iteration loop and is repeated until the loading and the displacement are converged. The main challenge is the instability of the simulations due to the explicit nature of the fluid and structural iterations.
This means that displacements of the interface are not included in the fluid domain and the fluid pressures are not considered in the structural domain during each stagger iteration [3, 16]. After each iteration loop the differences of the calculated interface displacements lead to large pressure gradients in the incompressible fluid domain and destabilize the computations. This is known as the “artificial added-mass instability” [2, 6, 11] and can occur in fluid-structure interactions of an incompressible fluid with a slender flexible structure.
To overcome this obstacle the interface artificial compressibility method, which has been introduced by Degroote et al. [3] can be applied. Hereby, an additional source term in the continuity equations for elements adjacent to the interface imitates the structural displacement and dampens out the arising disturbances. With progressing iteration loops the displacement differences vanish and both solvers converge. The choice of an appropriate time step is critical to achieve a stable coupling scheme and two contrasting factors need to be considered. First of all, the time step has to be small enough to limit the fluid pressure changes. Secondly, and counterintuitively, the “artificial added-mass instability” increases with a decreasing time step.
As the displacements of the interface deform the elements in the fluid domain, changes in mesh quality need to be considered. A possibility to maintain high mesh quality and prevent folding of mesh elements is to adapt the mesh stiffness (or mesh diffusivity) such that the deformation is compensated by less skewed elements. Another approach is adaptive remeshing of highly deformed mesh regions. This numerically expensive option should only be considered if the deformations are too high to be compensated by an increase of mesh stiffness.
4.1 0-D/3-D Coupling of FSI Simulations
The coupling of an LP network to the FSI domain works with the same procedure as for the CFD domain. One additional aspect is the reflection of pressure waves and the unrealistic structural deformations [5, 15]. As a solution an additional resistance representing the characteristic impedance of the truncated artery can be included in the LP network. With this modification the incoming pressure wave is absorbed at the 3-D/0-D interface. The characteristic impedance can be calculated from geometrical dimensions (inertance L) and material properties (compliance C) according to Eq. 3:
5 Examples
5.1 An FSI Model of Cardiopulmonary Bypass with Cerebral Autoregulation
Neurological complications often occur during cardiopulmonary bypass (CPB). Hypoperfusion of brain tissue due to diminished cerebral autoregulation (CA), atherosclerotic disease and consecutive thromboembolism reduce the cerebral oxygen supply and increase the risk of perioperative stroke. To improve the outcome of cardiac surgery, patient-specific FSI models can be used to investigate the blood flow during CPB. In this study, a computational model of CPB which includes cerebral autoregulation and movement of aortic walls on the basis of in vivo measurements is established.
First, the Baroreflex mechanism, which plays a leading role in CA, is represented with a 0-D control circuit. The model parameters are assessed with respect to their physiological meaning and their influence on the cerebral blood flow (CBF). Pathologies like hypertension or impaired autoregulation can be expressed by the adjustment of the parameters. Further on, the effect of drugs on the static and dynamic behavior of the Baroreflex can be reproduced.
Additionally a two-way coupled fluid-structure interaction (FSI) model with CA is set up. The wall shear stress (WSS) distribution is computed for the whole FSI domain and a comparison to rigid wall CFD is made. Constant flow and pulsatile flow CPB is considered.
Rigid wall CFD delivers higher wall shear stress values than FSI simulations, especially during pulsatile perfusion. The flow rates through the supraaortic vessels are almost not affected, if considered as percentages of total cannula output. The developed multiphysic multiscale framework (Fig. 2) allows deeper insights into the underlying mechanisms during CPB on a patient-specific basis.
5.2 A CFD Model of VAD Support Using Closed-Loop Multiscale Simulations to Evaluate Various Cannulation Strategies
Left ventricular assist devices (LVADs) are a common therapy for end-stage heart failure patients. As the heart cannot supply enough blood into the systemic circulation, a blood pump is put in parallel to the left ventricle to unload the heart and increase the blood flow. One open question investigates the positioning of the outflow cannula in the aortic arch. Several techniques as ascending or descending aortic grafting exist, which have an influence on the hemodynamics in the aortic arch and on the therapy outcome. A CFD model which takes the patient specific variability and the interaction between cardiac function and pump speed needs an addition of a lumped parameter model to the 3-D domain.
The cardiovascular system is divided into twelve compartments describing the systemic and pulmonary circulation, the four heart chambers and the coronary arteries. Further on, the VAD is modeled as a pressure and rotational speed dependent flow source. The entire model with exemplary results is shown in Fig. 3.
Physiological non-heart failure (NHF) and pathological heart failure (HF) conditions can be set by decreasing the elastance of the left ventricle. The effect is seen in the left ventricular pressure-volume loop. Reduced elastance decreases the stroke volume and the aortic pressure from 120/80 mmHg to 80/60 mmHg. VAD support (in constant flow full support conditions) reduces the ventricular afterload and pulsatility and increases the blood flow from 3 l/min to 5 l/min (results not shown). This behavior has also been observed in several in vitro and in vivo studies. With this numerical framework the position of the outflow cannula can be varied to see its effect on the hemodynamics in the aortic arch.
5.3 A Numerical Framework to Investigate Hemodynamics During Endovascular Mechanical Recanalization in Acute Stroke
Ischemic stroke, caused by embolism of cerebral vessels is accompanied by high morbidity and mortality. Endovascular aspiration of the blood clot is an interventional technique for the recanalization of the occluded arteries. However, the hemodynamics in the Circle of Willis (CoW) is not completely understood, which results in medical misjudgment and complications during surgical intervention. In this study a multiscale description of cerebral hemodynamics during aspiration thrombectomy is established.
First, the CoW is modeled as a 1-D pipe network on the basis of CT angiography (CTA) scans and different vascular configurations are investigated regarding the overall flow distribution in the arterial network (Fig. 4, center)
Afterwards, a vascular occlusion is placed in the middle cerebral artery and the relevant section of the CoW is transferred to a 3-D computational fluid dynamic (CFD) domain. A suction catheter in different positions is included in the CFD simulations. The boundary conditions of the 3-D domain are taken from the 1-D domain to ensure system coupling. An Eulerian-Eulerian multiphase simulation describes the process of thrombus aspiration (Fig. 4, right).
The physiological blood flow in the 1-D and 3-D domain is validated with literature data. Further on, it is proven that domain reduction and pressure coupling at the boundaries is an appropriate method to reduce computational costs.
6 Conclusion
Vascular hemodynamics play an important role in many research areas of biomedical engineering. The presented methodology can of course be extended to other problems. As patient-specific models play an important role in current investigations, the behavior of the entire system should be taken into account. With a combination of lumped parameter and 3-D models it is now not only possible to look beyond one patient condition (geometry A with boundary conditions B), but to cover several scenarios which include different pathologies, the effect of medication and the remodeling of the system. Multiple projects which deal with patient cohort measurements can serve as a valuable source to combine the different scales of numerical modeling to draw to new and insightful conclusions. As computational power increases multiphysic approaches, which are right now still numerically expensive, will become increasingly important.
References
A. Assmann, A.C. Benim, F. Guel, P. Lux, P. Akhyari, U. Boeken, F. Joos, P. Feindt, A. Lichtenberg, Pulsatile extracorporeal circulation during on-pump cardiac surgery enhances aortic wall shear stress. J. Biomech. 45(1), 156–163 (2012)
P. Causin, J. Gerbeau, F. Nobile, Added-mass effect in the design of partitioned algorithms for fluid structure problems. Comput. Methods Appl. Mech. Eng. 194(42), 4506–4527 (2005)
J. Degroote, P. Bruggeman, R. Haelterman, J. Vierendeels, Stability of a coupling technique for partitioned solvers in FSI applications. Comput. Struct. 86(23), 2224–2234 (2008)
G. Ferrari, M. Kozarski, K. Zielinski, L. Fresiello, A. Di Molfetta, K. Górczynska, K.J. Pako, M. Darowski, A modular computational circulatory model applicable to vad testing and training. J. Artif. Org. 15(1), 32–43 (2012)
L. Formaggia, A. Moura, F. Nobile, On the stability of the coupling of 3D and 1D fluid-structure interaction models for blood flow simulations ESAIM. Math. Modell. Numer. Anal. 41(04), 743–769 (2007)
C. Förster, W.A. Wall, E. Ramm, The artificial added mass effect in sequential staggered fluid-structure interaction algorithms, in Prooceedings European Conference on Computational Fluid Dynamics ECCOMAS CFD, 2006
I. Fukuda, S. Osanai, M. Shirota, T. Inamura, H. Yanaoka, M. Minakawa, K. Fukui, Computer-simulated fluid dynamics of arterial perfusion in extracorporeal circulation: from reality to virtual simulation. Int. J. Artif. Org. 32(6), 362–370 (2009)
B. Ji, A. Undar, An evaluation of the benefits of pulsatile versus nonpulsatile perfusion during cardiopulmonary bypass procedures in pediatric and adult cardiac patients. ASAIO J. 52(4), 357–361 (2006)
T.A.S. Kaufmann, M. Hormes, M. Laumen, D.L. Timms, T. Schmitz-Rode, A. Moritz, O. Dzemali, U. Steinseifer, Flow distribution during cardiopulmonary bypass in dependency on the outflow cannula positioning. Artif. Org. 33(11), 988–992 (2009)
T. Korakianitis, Y. Shi, A concentrated parameter model for the human cardiovascular system including heart valve dynamics and atrioventricular interaction. Med. Eng. Phys. 28(7), 613–628 (2006)
P. Le Tallec, J. Mouro, Fluid structure interaction with large structural displacements. Comput. Methods Appl. Mech. Eng. 190(24), 3039–3067 (2001)
F. Migliavacca, R. Balossino, G. Pennati, G. Dubini, T.-Y. Hsia, M.R. de Leval, E.L. Bove, Multiscale modelling in biofluidynamics: application to reconstructive paediatric cardiac surgery. J. Biomech. 39(6), 1010–1020 (2006)
J.T Ottesen, M.S. Olufsen, J.K. Larsen, Applied Mathematical Models in Human Physiology, Society for Industrial and Applied Mathematics (2004)
J. Peacock, T. Jones, C. Tock, R. Lutz, The onset of turbulence in physiological pulsatile flow in a straight tube. Exp. Fluids 24(1), 1–9 (1998)
P. Reymond, P. Crosetto, S. Deparis, A. Quarteroni, N. Stergiopulos, Physiological simulation of blood flow in the aorta: comparison of hemodynamic indices as predicted by 3-D FSI, 3-D rigid wall and 1-D models. Med. Eng. Phys. 35(6), 784–791 (2013)
S.J. Sonntag, T.A. Kaufmann, M.R. Büsen, M. Laumen, T. Linde, T. Schmitz-Rode, U. Steinseifer, Simulation of a pulsatile total artificial heart: development of a partitioned fluid structure interaction model. J. Fluids Struct. 38, 187–204 (2013)
N. Westerhof, J.-W. Lankhaar, B.E. Westerhof, The arterial windkessel. Med. Biol. Eng. Comput. 47(2), 131–141 (2009)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Neidlin, M., Kaufmann, T.A.S., Steinseifer, U., Schmitz-Rode, T. (2018). Multiscale Multiphysic Approaches in Vascular Hemodynamics. In: Wriggers, P., Lenarz, T. (eds) Biomedical Technology. Lecture Notes in Applied and Computational Mechanics, vol 84. Springer, Cham. https://doi.org/10.1007/978-3-319-59548-1_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-59548-1_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-59547-4
Online ISBN: 978-3-319-59548-1
eBook Packages: EngineeringEngineering (R0)