Abstract
The partitioning of biological particles in aqueous two-phase systems has been extensively studied due to the multiple advantages this liquid–liquid extraction technique possesses. In this chapter, special attention is given to the following bioparticles: whole cells, stem cells, and cell components from animals, bacterial cells and components, viral particles, and nucleic acids. The major advantages of employing aqueous two-phase systems for the recovery of these studied products are highlighted, in addition to the multiple applications and variant modes that can be exploited with this extraction technique. The principal partitioning mechanism and the experimental variables governing the partition behavior of the studied bioproducts in aqueous two-phase systems are also discussed for each particular case. Reported examples for each category of bioparticles are presented in each section. Finally, the challenges and future trends for the separation of macroparticles in aqueous two-phase system are addressed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Aqueous two-phase systems
- ATPS
- Cells
- Stem cells
- Cell components
- Viral particles
- Nucleic acids
- Particle partitioning
4.1 Introduction
Fractionation and purification techniques represent one of the most important areas for research and development of novel technologies involved in the recovery and effective isolation of high-added value compounds (Ruiz-Ruiz et al. 2013). Within these techniques, protocols or unit operations that allow concentration and selective partitioning of a particle or molecule to a specific recovery phase within the employed methodology are preferred (Asenjo and Andrews 2008; Benavides et al. 2008). Considering that during the past five decades, research comprising the deep study of cell physiology has taken place in order to understand its biochemistry for industrial and scientific purposes, a very important number of research groups have devoted time, effort, and resources to develop highly efficient and selective methods for whole cell and cell particle isolation (Albertsson 1961). Up to date, the correct separation and concentration of cell nuclei, mitochondria, microsomes, chloroplasts, cell walls, and cell membranes constitute one of the primary cores of whole-particle isolation procedures (Albertsson 1961; Silva et al. 2014). In this tenor, novel strategies intended for macroparticles’ recovery and purification are one of the current trends in bioprocessing development, specifically within the design of new downstream processing trains for the pharmaceutical, cosmetic, and food industries.
Particle isolation procedures can be grouped within several categories, the most studied being the ones that consider the physical properties of the particle or molecule in order to design an effective methodology for its recovery, purification, or concentration. In this sense, isopycnic centrifugation techniques employing density gradients are the current workhorse for particle isolation offering high reproducibility and relatively low processing times (González-González et al. 2012). The separation process within these protocols considers the density and size of the target particle. The traditional process consists of a sample that is loaded on top of a centrifugation tube which is filled with a solution of active components (e.g., Ficoll, Percoll, cesium chloride) that will form a density gradient when subjected to high centrifugal speeds. The complex mixture loaded into the system travels different lengths according to the physical characteristics of each particle in the blend, until each component of the sample is retained in its isopycnic position. Numerous researches have employed this technique for the effective isolation of whole cells (red blood cells, stem cells, tumor cells), cell fragments or organelles (chloroplasts), or cell membranes (Albertsson 1961; González-González et al. 2012). Nevertheless, this technique has important limitations that may hinder its application during industrial-based processes. Reduced sample loading, nonspecific product concentration or separation, intensive labor required for proper technique evaluation, and low resolution are the main drawbacks for this physical separation of target products in biotechnological approaches. In addition, the overall potential for scale-up is practically null since batch procedures are preferred for isopycnic centrifugation and continuous centrifuges dominate the industrial environment nowadays.
Considering these situations, the application and characterization of alternative and effective technologies for the recovery and purification of macroparticles have been a major challenge in the past two decades. In this context, liquid–liquid extraction technologies based on aqueous two-phase systems (ATPS) have been proposed as a novel and biocompatible alternative for selective product partitioning (Mayolo-Deloisa et al. 2011; Ruiz-Ruiz et al. 2012). These systems form when specific components (e.g., two polymers, a polymer and a salt, alcohol and a salt, ionic liquid and a salt, detergents) are mixed over certain critical conditions, giving a two-phase system with immiscible phases and particular biochemical characteristics in each phase. ATPS have several advantages when compared to traditional downstream processing techniques since they represent economic approaches for product concentration and purification, they provide increased scale-up potential, the requirement of temperature shifts is avoided in almost every case, systems are easy to set up, and finally they offer a biocompatible environment for target products because of the aqueous nature of the system employed (Benavides et al. 2006). Albertsson employed this technique for the first time in 1955 by mistake in an attempt to extract pyrenoids from the algae Chlorella pyrenoidosa. He determined that a polyethylene glycol–potassium phosphate system was efficient in correctly isolating chloroplasts in the polymer-rich phase and other components in the bottom salt phase (Albertsson 1985). From this experience, a very important number of research proposals and publications have been made worldwide in order to exploit ATPS advantages in biotechnological applications ranging from the food industry to cosmetics, point of care diagnostics, and pharmaceutical and environmental uses.
In terms of particle partitioning, the most important characteristic that ATPS offer is the possibility of recovering or concentrating a target molecule or particle by means of a biological and biochemical selectivity, in addition to the traditional physical approaches (size, shape, and/or density) of several sedimentation-based operations currently employed (Asenjo and Andrews 2012). One simple example is that of cells, since ATPS can be designed to work at physiological pH levels, thus working with isotonic conditions in order to work with these biological particles (Sousa et al. 2011). Considering the outer structure of a typical cell, which includes a well-defined membrane with phospholipids, transmembrane proteins, and specific carbohydrates, the set of interactions that may be present in a two-phase system can be primarily electrostatic and hydrophobic, depending on the active groups or residues involved in the behavior. In addition, inherent physical properties such as size and density also play a vital role in order to determine the final partitioning behavior of the molecule in the system. In this regard, the use of ATPS has been studied not only for recovery of particles but also for molecular characterization (Mayolo-Deloisa et al. 2011; González-González et al. 2012). One particular research niche in this area is that of integral proteins in prokaryotic and eukaryotic expression systems, since ATPS studies of cell lysates allow a deep characterization of the intracellular and transmembrane components based on the partitioning behavior of the molecules involved, thus providing important conclusions for research in terms of molecular structures, stability, and superficial net charge and hydrophobicity, among others (Schindler and Nothwang 2006).
Considering the potential of ATPS for whole-particle recovery for diverse purposes, this chapter aims to present and discuss the principal areas of study of this liquid–liquid extraction technology for the proper study of cells, cellular components, and current bioactive compounds such as viral particles or nucleic acids. The impact of these components on their partition in selected ATPS is discussed, as well as the principal challenges and trends in regard to the design of novel downstream processing alternatives aimed to provide additional options for the biotechnology industry in terms of particle characterization and new product development.
4.2 Partitioning of Whole Cells, Stem Cells, and Cellular Components in ATPS
Whole cells have been the separation target of a wide range of technologies, including density gradient, cell culture, fluorescent-activated cell sorter (FACS), magnetic-activated cell sorter (MACS), quadrupole magnetic cell sorter (QMS), cryogels, membranes, pre-plating, dielectrophoretic microdevices, panning, affinity columns, field-flow fractionation (FFF), and aqueous two-phase systems (ATPS). The isolation of a specific purified type of cells has endless applications, from basic science to clinical. Moreover, there are a variety of cell sources, all of them having a complex biological background. Another key feature about cells as the targeting recovery product is how extremely fragile and susceptible they can be to abrupt environmental changes (i.e., pH, osmolarity, temperature, available nutrients, etc.). With all this into account, it is predictable to conclude that ATPS is a suitable separation approach for cells.
ATPS present the following advantages for the recovery and purification of different types of whole cells:
-
A simple, low energy input, fast, and highly reproducible technique that does not require sophisticated and costly equipment or specialized personnel.
-
Their high water content confers a mild and biocompatible environment for cells. Furthermore, they can be adjusted to provide an isotonic and physiological media suitable for the separation of viable cells.
-
Cells are exposed to identical conditions throughout the separation process (pH, temperature, osmolarity, and ion concentration), avoiding abrupt changes that may have a detrimental effect in viability and functionality.
-
ATPS are easily miniaturized via microdevices, which require small amounts of sample and reagents plus are highly portable. On the other hand, ATPS are well-known for their scale-up capacity and possibility of continuous operation.
-
Polymers provide a protective surrounding for cells, which helps to retain their viability and biological functions.
All these benefits support the idea of employing ATPS as a feasible technique for whole cell separation or their fractionation into subpopulations. Additionally, the gentleness of ATPS for cells has also been exploited in other applications, including extractive bioconversion, studying surface properties (i.e., charge, hydrophobic) or alterations occurring as a result of both normal and abnormal in vivo processes (i.e., differentiation, maturation, aging, etc.), and as an analytical and preparative technique (Hatti-Kaul 2001; Cabral 2007).
Another relevant point to mention regarding ATPS for cell isolation is the multiple factors that enter into play during sample partitioning. These can be divided into three main categories: (1) the intrinsic polymeric and ionic properties of the two hydrophilic aqueous phase-forming solutions and additives (density, viscosity, and interfacial tension); (2) the selected system parameters of volume ratio (V R), tie-line length (TLL), pH, and temperature; and (3) the surface properties of the sample (i.e., net surface charge, hydrophobicity, affinity interaction, etc.). As it can be expected, the complexity of the factors has prevented researchers to propose a generic guideline for the separation of any kind of cells employing ATPS.
Throughout scientific literature, the following types of polymers forming ATPS have been employed for the separation of cells from a variety of biological sources: polyethylene glycol (PEG), dextran (DEX), Ficoll, polyvinyl alcohol (PVA), polyvinylpyrrolidone (PVP), hydroxypropyl starch, and methoxypolyethylene glycol. Stimuli-responsive soluble–insoluble polymers (temperature or pH), such as ethylene oxide–propylene oxide (UCON), poly-N-isopropylacrylamide [poly(NIPAM)], and methacrylic acid–methyl methacrylate (Eudragit), have also been reported for cell partitioning in ATPS. In polymer–polymer ATPS, the phase separation is attributed to small repulsive interactions between the two types of monomers in the solution. Given that each of the two polymers is composed of several monomers, the total interaction between the polymers is large (Cabral 2007). Polymer–polymer ATPS are preferred over polymer–salt systems in the case of cell separation because biospecific interaction is usually obstructed by high salt concentration (Cabral 2007) and because of the hypertonicity of the salt component, which affects the viability of cells. Regarding two-polymer systems, the PEG–dextran systems are much less susceptible to salt effect when compared to PVP–dextran or Ficoll–dextran systems (Cabral 2007). Furthermore, the most employed solvent for this type of separation is phosphate-buffered saline (PBS, pH 7.4, 150 mM NaCl), which provides a suitable media for the separation of viable cells (González-González and Rito-Palomares 2013). The use of “charged” ATPS is also commonly employed for the isolation of this kind of products, as the interfacial potential between phases may affect their partitioning (Fisher 1981). These “charged” systems are constructed by adding anions, cations, or salts to the systems. In other cases, as, for example, when employing ATPS as extractive bioconversion, authors have also reported the specific cell growing media as a solvent (Zijlstra et al. 1996; Atefi et al. 2015).
The main goal in ATPS is to determine the partition behavior of cells to the top, interface, and bottom phases of the studied system. This can be performed by determining the number of cells and their viability in the top and bottom and attributing the difference from the total number in the system to the interface. Furthermore, phase fractionation has not only been employed to separate the cell product from the contaminants but also to give information on the charge, receptors, and hydrophobic properties of the surfaces of the product cell (Fisher 1981). Ideally, one single partition is necessary to separate the cell of interest from the complex mixture if the product of interest fractionates entirely one of the phases and the contaminants (cell particles, debris, RNA, carbohydrates, lipids, etc.) into the opposite phase. Nevertheless, it is common to use a sequential partition strategy named countercurrent distribution (CCD) to separate cells. In CCD experiments, the top phase of the ATPS is transferred sequentially to a fresh bottom phase with the same polymer composition as the initial system.
Another common strategy employed for the isolation of cells exploiting ATPS is the addition of affinity ligands into the system to enhance the selectivity of the target product to the phase to which the ligand has more affinity. Examples of ligand types include dyes, metal ions, enzymes, and antibodies. The ligands could be added freely in the ATPS or coupled reversibly to one of the phase-forming polymers. In the case of PEG–DEX systems, the dextran-rich phase is the most suitable for the conjugation of the ligand because of the following reasons: (1) dextran concentration in the PEG-rich phase is lower than the PEG concentration in the dextran-rich phase; (2) dextran has a higher molecular weight than PEG, thus is less affected by the ligand; and (3) dextran molecule has more coupling potential sites.
Diverse types of cells have been reported to be separated employing ATPS, including animal, plant, bacteria, and viruses. More specifically, different types of animal cells such as cancer cells (leukemia), stem cells (CD34+, CD133+), blood cells (erythrocytes, leukocytes, lymphocytes, T lymphoma), and hybridomas have been documented. Table 4.1 presents a summary of the different kinds of cells, their source, and the type of ATPS employed for their isolation.
4.2.1 Blood Components
Since almost 40 decades ago, different blood species of cells have been reported to be separated employing ATPS, such as leukocytes, platelets, lymphocytes, and erythrocytes. For this, diverse sources of blood have been studied, highlighting human, rat, rabbit, and sheep blood. Moreover, authors have tested varying modes of ATPS including CCD, microfluidic devices, affinity ligands, and charged ATPS. PEG–DEX polymer–polymer ATPS are the most exploited systems for this particular application.
ATPS have been reported to selectively fractionate and thus detect difference in erythrocytes from different species, maturation age, and cell membrane abnormalities. For example, ATPS containing DEX, PEG, and PEG fatty acid esters were able to distinguish between erythrocytes from multiple sclerosis patients and the healthy control (Van Alstine and Brooks 1984). In a charge-sensitive DEX–PEG ATPS, human and rat erythrocytes were separated by CCD (Jimeno et al. 1991). Rabbit and human erythrocytes were fractionated in PEG–DEX immunoaffinity ATPS by CCD (Sharp et al. 1986). Likewise, the separation by CCD of small number of cells (~106) from rabbit, rat, and sheep blood was reported to be successful with the incorporation of fetal bovine serum in PEG–DEX systems (Walter and Krob 1984). A charged PEG–DEX CCD systems containing 0.05 M NaPB and 0.09 M NaCl were also able to partition erythrocytes from rat bone marrow cells as a function of charge-associated cell surface properties (García-Pérez et al. 1987).
A number of studies on the separation of leukocyte cells have been performed in PEG–DEX ATPS. Leukocytes from single-cell suspensions from rat spleen, lymph nodes, and thoracic duct lymph were reported to be fractionated by CCD in ATPS containing PEG–DEX added with 0.094 M sodium phosphate buffer and fetal calf serum (Malmström et al. 1978). Microfluidic PEG–DEX ATPS devices have also been employed to separate leukocyte from whole blood samples (SooHoo and Walker 2009; Tsukamoto et al. 2009). The subfractionation of lymphocytes from human peripheral blood by CCD in a charged PEG–DEX ATPS has been well documented in multiple studies (Walter et al. 1979; Malmström et al. 1980a, b).
4.2.2 Stem Cells
Stem cells have lately been an attractive target of application for ATPS due to the well-known and documented advantages of this technique. The major sources of cells for this type of studies are bone marrow, peripheral blood, umbilical cord blood, and model stem cell lines. The most reported stem cell types are CD133+ and CD34+, as their purified forms have been used as a therapeutic alternative for several incurable and chronic degenerative diseases. Immunoaffinity ATPS have been the most popular mode with the addition of the free antibody or its conjugation to a polymer forming ATPS. For example, Kumar and coworkers reported the separation of CD34+ human acute myeloid leukemia cells from T-lymphoma cells employing a PEG 8000–DEX T-500 additioned with anti-CD34 conjugated with the temperature-sensitive polymer, [poly(NIPAM)] (Kumar et al. 2001). Another report for the purification of CD34+ stem cells was conducted by Sousa and colleagues, where a monoclonal antibody against the CD34 antigen was used for the direct partitioning of the stem cell of interest from human umbilical cord blood to the PEG-rich phase of a PEG 8000–DEX 500,000 ATPS (Sousa et al. 2011). In the case of CD133+ stem cells, the following work has been conducted: traditional partitioning of CD133+ stem cells from human umbilical sample in the following polymer–polymer systems: UCON–DEX 75,000, PEG 8000–DEX 500,000, and Ficoll 400,000–DEX 70,000 (González-González et al. 2014) and the addition of anti-CD133 to the previous studied systems to compare the selectivity of the immunoaffinity ATPS (González-González and Rito-Palomares 2015).
4.2.3 Other Cell Types
The fractionation of hybridoma cells from their culture media has also been studied in PEG–DEX ATPS. One example employs PEG 8000–DEX T-500 additioned with an antigen coupled to Eudragit (a copolymer of methacrylic acid–methyl methacrylate with a reversible soluble–insoluble feature dependent on pH) to separate the mouse–mouse hybridoma 16-3F cells from NS-1 cells. Without the antigen’s presence, the hybridoma cells and contaminants are distributed to the bottom and interface. After the addition of the ligand (which has a favorable partition to the top phase), the target product could be recovered in the top phase (Hamamoto et al. 1996). In another investigation, the mouse–mouse hybridoma BIF6A7 cells partitioned almost completely to the bottom phase of a PEG 35,000–DEX 40,000 system. Moreover, when this system was constructed with culture media, the hybridoma cells were successfully cultured over a period of 2 months (Zijlstra et al. 1996). This scenario elucidates the feasibility of achieving extractive bioconversion using ATPS, where the integration of fermentation and downstream processing is possible.
Other types of cells have also been studied in ATPS with the objective of characterizing specific properties (surface properties or alterations). The physicochemical properties (isoelectric point, surface net, and local hydrophobicity) of Saccharomyces cerevisiae and Escherichia coli were determined by studying their partitioning behavior in ATPS by using the same method that has been previously employed for the characterization of the surface properties of proteins in ATPS (Umakoshi et al. 1997). In another example, A431.H9 human skin cancer cells were employed to study the effect of interfacial tension on cell partitioning in a PEG 35,000–DEX 500,000 ATPS (Atefi et al. 2015).
4.3 Cell Components
Different cell components have also been isolated using ATPS, including plasma membranes, endosomes, mitochondria, lysosomes, endoplasmic reticulum, Golgi apparatus, small inclusion bodies, and extracellular vesicles, among others. Table 4.2 presents a list of examples of cell components, their source, and the type of ATPS employed for their fractionation. The mechanism of action for the separation of the cell components exploits the differences of charge and hydrophobicity of plasma membranes in order to fractionate cell organelles (Benavides et al. 2008). ATPS present several advantages for the isolation of cell organelles, including high yield and purity in a short processing time without requiring expensive equipment or large amount of tissue sample. Furthermore, the low interfacial tension joined to the non-denaturing conditions and high water content guarantee the conservation of structure and biological activity. The most studied factors for optimizing ATPS for cell organelles are the molecular weight and concentrations of the polymers and salts. Furthermore, affinity ligands (lectins, antibodies, or receptor agonists and antagonist) can also be additioned to increase the selectivity of the systems, as well as countercurrent distribution could be applied in order to rise the yield and purity of the recovered cell organelle.
The isolation of animal and plant plasma membranes has been popularly investigated with ATPS given to their intrinsic membrane surface characteristics, including the hydrophilic/hydrophobic properties and net surface charge. Most applications to membrane purification have centered on plasma membranes, plasma membrane domains, and separation of right-side-out and inside-out plasma membrane vesicles (Morré and Morre 2000). In general, plasma membranes show a higher affinity for the more hydrophobic phase, and salt (composition and concentration) and temperature have a strong influence in the partition of membranes. CCD experiments are often employed for the purification of this type of cell component, as their recovery cannot be achieved in a single-step extraction. Another option is to combine ATPS with another technique in order to obtain the desired fractionation of the sample. In summary, the reported order of affinity of animal cell membranes for the upper phase is plasma membranes > lysosomes and endosomes > Golgi apparatus > mitochondria > endoplasmic reticulum (Morré and Morre 2000).
Plasma membrane vesicles from human peripheral blood mononuclear cells were recovered from the interface of a PEG–DEX system additioned with 90 mM sodium phosphate buffer and 0.1 mM NaCl for further proteomic analysis (Everberg et al. 2006). Another study reports the isolation of plasma membranes vesicles from the liver, hepatomas, and cultured cells in the upper phase of a PEG–DEX ATPS containing 0.25 M sucrose and 5 mM potassium phosphate in a CCD mode. Furthermore, the authors report that they could further fractionate the enriched membranes into mitochondria-, endoplasmic reticulum-, or Golgi apparatus-enriched fractions employing preparative free-flow electrophoresis or sucrose gradient centrifugation (Navas et al. 1989). Plasma membrane protein from HeLa cells was recovered on the top phase of a PEG–DEX ATPS additioned with 0.2 M potassium phosphate; meanwhile, a 3.5-fold relative enrichment of Golgi apparatus fragments was detected in the lower phase (Morré and Morre 2000).
Extracellular vesicles such as exosomes and microvesicles have also been partitioned in PEG–DEX ATPS. For example, extracellular vesicles from tumor interstitial fluid of a C57BL6/j strain mice were isolated in the DEX-rich phase of a PEG–DEX system in less than 15 min (Shin et al. 2015). In another case, extracellular vesicles from tumor intestinal fluid of C57BL6/j mice were isolated from a complex mixture of extracellular vesicles and serum proteins in the bottom phase of a PEG–DEX system dissolved in PBS (Kim et al. 2015).
Isolation of endosomes from fresh or frozen HeLa and bovine kidney cells was conducted in a PEG–DEX multistep ATPS. Afterward, the recovered endosomes were subjected to preparative free-flow electrophoresis to fractionate early and late endosomes (Morré et al. 1998).
4.4 Partition of Viral Particles: Toward New Therapeutic Strategies
The mass study of viral components has presented a marked growth within academic and industrial research with a final aim of employing and designing new technologies using these particles as active elements. The most common viral molecules used are viruslike particles (VLPs), which are macromolecular structures directly derived from the expression of viral proteins that exhibit a spontaneous behavior for reassembly into their active viral form without the nucleic acid content (Jacinto et al. 2015). Since these particles cannot replicate, they can serve as important drug delivery vehicles and immunogens for vaccination procedures. Currently present in the international market, five VLP vaccines have been approved: Gardasil (Merck & Co.), Cervarix (GlaxoSmithKline), Recombivax HB (Merck & Co.), Engerix-B (GlaxoSmithKline), and Hecolin (Xiamen Innovax Biotech Co., Ltd) (Ladd Effio et al. 2015). Nevertheless, the major problems for VLP production schemes reside in downstream processing operations within industrial processes (Benavides et al. 2006; Ladd Effio et al. 2015). In this tenor, the study and implementation of alternative unit operations to reduce purification steps and increase recovery and purity yields are constantly being studied. In this section, successful examples of recovery of viral particles with ATPS will be discussed in order to establish potential trends and benefits of this liquid–liquid extraction technique for these macromolecules at industrial and research levels.
Nowadays, the scale-up of bioprocesses is one of the major trends in biotech engineering in order to design novel strategies that can satisfy the increased demand of selected bioproducts. Despite this, another important trend and constant challenge in process design is the scale-down of protocols to minimize reagents and time-consuming procedures. As presented by Jacinto et al. (Jacinto et al. 2015), the necessity of novel recovery procedures for human immunodeficiency virus–VLPs (HIV–VLPs) is crucial, since traditional extraction procedures (e.g., ultracentrifugation or polymer precipitation) constantly interfere with the particle final activity or infectivity. In this context, a miniature ATPS-based approach was developed to effectively recover HIV–VLPs covalently coupled to a GFP protein and thus facilitate particle detection in the mini extraction system. PEG–DEX and PEG/potassium phosphate or ammonium sulfate systems were selected to maximize VLP partitioning toward one of the phases in the system and thus optimize the extraction step. The best system for particle recovery was composed of PEG 1500 and (NH4)2SO4 since a partition coefficient of 3.9 was achieved in the continuous mini system. The results were analyzed, and the authors concluded that the primary variables involved in particle partitioning within the system were size (macrostructure, hence volume exclusion profiles are evident) and the hydrophobic character of the studied VLPs. In order to determine system robustness, batch ATPS were also constructed, and a K p of 4.4 was achieved, giving important information to the authors and thus determine optimization strategies through geometry changes and mixing protocols for their mini continuous system. It was also concluded that these improvements may potentially aid issues regarding mass transfer and phase component concentrations within the developed device. The major contribution of the authors in this research was the use of a miniature ATPS system that proved effective recovery of HIV–VLPs which maintained infectivity (>55%) and were processed in just a couple of minutes (10 min or less per partitioning analysis). Processing time is an important concern in current biotech applications, and with this research, it was concluded that the employment of micro-ATPS may enhance rapid partition characterization of selected systems and thus provide a wide array of feasible scale-up options for VLP recovery at pilot plant and industrial scales without time- and resource-consuming protocols.
As presented by Effio and others (Ladd Effio et al. 2015), in the past decade more than ten VLP therapeutics have entered clinical phases II or III for indications against malaria, gastroenteritis, and influenza. For these molecules, ultracentrifugation techniques are the most employed in order to generate purified samples of VLPs for further study and characterization. In this research, an effective methodology for human B19 parvo-VLPs (parvovirus) recovery from a complex sample was developed to propose an ATPS extraction in a single- or multistage process with scale-up potential. PEG 400/PEG1450 and sodium/potassium phosphate were constructed to maximize viral particle partition toward the polymer phase. One of the most important factors was the influence of pH levels in the selected systems. Considering the macrostructures comprised by the B19 VLPs, electrostatic interactions determined the direction of particle partitioning within the ATPS tested. When a low molecular weight PEG was used, a 100% recovery of VLPs in the top phase was obtained when an untreated sample was loaded into the separation system. This effect was also achieved by the selective addition of sodium chloride (0.01–0.1 M) in order to stabilize the partitioning of the B19 particles. The main challenges of this research were oriented toward the polishing steps needed to further process the VLP-rich phases of the optimal ATPS employed, since protein contaminants from the crude extracts loaded posed an important set of impurities for the molecules recovered. In this sense, the authors proposed a centrifugal partition chromatography step in order to enhance particle isolation; nevertheless, it was determined that a marked decrease in VLP concentration was obtained after this partition strategy. Since particle purity is a vital step in industrial bioprocesses, important research opportunities can be envisioned and derived from the work presented by Effio and collaborators, specifically those steps involving polishing operations with ATPS.
The recovery of bacteriophage particles in ATPS has been done in recent years to concentrate these particles and employ them as marker particles for specific diseases. One of the most promising applications of phage particles is their potential to improve point-of-care diagnostic assays, since they can be used as ultrasensitive viral reporter molecules and thus enhance the limit of detection of lateral flow assays (LFAs) (Adhikari et al. 2013). In this respect, a vital step consists in pre-concentration of a phage sample with a simple and effective technology in order to further employ the viral molecules in novel diagnostic protocols. In 2015, Jue et al. (Jue et al. 2014) successfully designed an ATPS strategy as a sample prep stage to concentrate M13 phage particles before an LFA procedure. An experimental design involving systems composed of PEG 8000 and potassium phosphate, pH 7.4, and V R = 1, 3, 6, and 9 was developed. Considering size particle and hydrophilic nature, the authors suspected that concentration of the target product in salt phases would be favored. With an optimal procedure which included a 30 min extraction time, 37 °C, pH 7.4, and V R 0.9, a partition coefficient of 0.0001 was obtained allowing >90% recovery of M13 particles and thus improved tenfold the detection limit of the proposed LFA assay when compared to another lateral flow test without the ATPS pretreatment. The most important challenge discussed by the authors and that poses an interesting research opportunity area is regarding phage quantification, since actual protocols involve particle determination by the plaque assay which is considered to be very time- and resource-consuming, with high variation of results in each batch. In this tenor, technologies involving immunodetection (e.g., ELISA) could be positively enhanced in the near future to design time-saving protocols in this area and thus provide feasible and validated methods to facilitate phage studies within ATPS applications.
One of the actual challenges of pharmaceutical companies is the constant and increasing need of rapid and feasible immunization programs, which by definition require high-quality vaccination products, with competent production yields and affordable prices for the human population. Within this aspect, reengineering of classic-established bioprocesses for biological productions needs to be done in order to reduce the actual financial burdens and hence allow product availability worldwide. Typical unit operations involved in downstream processing (DSP) of vaccine-derived products involve precipitation, centrifugation, and chromatographic separation techniques to achieve selected purity and recovery yields. A recent study presented by Vijayaragavan et al. (Vijayaragavan et al. 2014) presented the recovery of a surrogate molecule for hepatitis A and poliovirus, porcine parvovirus particles (PPV), considering also their small size and structural similarity to adeno-associated virus which are commonly used in gene therapy. After an elaborated experimental design, an effective ATPS strategy for infectious PPV in a serum-containing media was designed to discuss industrial potential for vaccine production. A PEG 12,000 and citrate system allowed 63% PPV recovery in the polymer-rich phase. The authors concluded that due to the molecular nature of PPV particles, hydrophobic forces induced viral molecules to partition into the more hydrophobic PEG phase. In addition, the difference in ionic force and electrochemical forces allowed the majority of contaminant proteins from the serum media (>85%) to be recovered in the bottom, salt-rich phase. The major challenge in this research was the importance of understanding and controlling the surface tension of the system in order to prevent the viral particles to be concentrated at the interface. This was achieved with strict control in the citrate or NaCl concentration in the employed systems, and the authors concluded that this variable could be determinant in future research comprising viral particle recovery in two-phase systems in order to design feasible protocols for industrial applications.
Analyzing the above-presented examples and the information described in Table 4.3, it can be concluded that the viral particle recovery has been a strong research niche for ATPS strategies in the past decade. If it is considered that typical recovery yields in liquid–liquid extraction methodologies are above 60%, thus this simple technology might suppose an interesting option for actual industrial processes that commonly exhibit recovery rates that range from 20% to 35%. One of the constant challenges that needs to be studied in the future is the polishing of virus-rich phases in ATPS. Adequate removing of phase components and contaminants (e.g., PEG molecules, high salt concentrations, contaminant products such as proteins) is a key step in bioprocess development, since common unoptimized procedures have a direct impact in particle activity/infectivity. In addition, a major disadvantage of ATPS for viral particles recovery is the employed dilution factors. New approaches of protocols must be adequately studied and implemented in order to concentrate target products in small volumes without compromising particle structure. The last elements to be considered are the analytical methodologies employed for VLP or phage quantification, since plaque assays tend to be labor- and resource-consuming. In this subject, more research is needed in terms of more robust immunodetection and ELISA tests in order to reduce analytical times in the process. It must be considered that phase components may hamper chemical interactions in these analytical procedures, reason that constitutes the primary challenge in this area nowadays.
4.5 Nucleic Acids Recovery in ATPS: Current Innovations
Improved healthcare advances have been possible in the past four decades primarily because of novel and efficient biotech-derived efforts in both academic and industrial research facilities. One of the most studied areas in current medicine is that of human gene therapy. These therapies actually require efficient DSP for the obtention of high-quality vectors (Bhambure et al. 2013; Tateishi-Karimta et al. 2014). Nevertheless, production titers and recovery yields for these molecules, typically nucleic acids, are modest and thus represent one of the limiting steps for bioprocess engineering strategies. In addition, it must be taken into account that the scale-up potential of traditional purification techniques for nucleic acid isolation is limited, hence the importance of considering alternative technologies such as ATPS (Jungbauer 2013). The following section explores selected examples to emphasize the potential of two-phase extraction for nucleic acid-derived molecules.
The spread of viral-related tropical diseases, such as dengue and its lethal hemorrhagic fever, has been a target for development of new treatments around the world. In this context, an improved interest in gene therapy and vaccines composed of nucleic acids (e.g., DNA principally) as active ingredients has arisen, situation that has increased the demand for production and purification of plasmidic DNA (pDNA). An urgent topic in pDNA production schemes is the vital need of novel and efficient DSP technologies that may offer minimal or null final product contamination. An interesting approach for purifying a dengue 2 plasmid DNA vaccine was developed to determine the potential of ATPS as a scale-up technique for product recovery (Moreira et al. 2007). A simple experimental design using PEG/potassium phosphate systems was designed. After several experimental runs, it was determined that a PEG 1000/PO4 system allowed almost a 40% total recovery of the plasmid in the top phase. The authors hypothesized that because of the disrupting interactions between PEG and salt molecules, the partition of plasmid molecules would be favored to the polymer-rich phase. Nevertheless, plasmid stability and high recovery yields are the major challenges, since high ionic strengths disrupt the plasmidic structure in high TLL systems. In addition, nucleic acid structure should be considered when designing an ATPS strategy, since selected systems tested by Moreira clearly concentrated the target molecule in the interface but its recovery from this phase was not further studied (Moreira 2007). Despite this, a 40% plasmid recovery was considered as successful, since traditional pDNA recovery kits have efficiencies ranging from 20% to 30%.
Partitioning of complex nucleic acid samples has been carried in the past, considering several variables that might prove a statistical difference regarding macromolecule partitioning behavior. A deep study presented by Luechau et al. (Luechau et al. 2009a, b) identified the effects of neutral salts onto the behavior of different types of nucleic acids being loaded from a complex crude sample. Considering that pDNA is commonly recovered from a blend of DNA, RNA, and pDNA, this research emphasized the importance of understanding the effect posed by excluded volumes in selected ATPS formed by PEG (300, 1450, and 6000) and potassium phosphate with NaCl addition. The main vision of the authors was to design an extraction system that could concentrate pDNA in one phase and contaminant RNA and DNA in the opposite one. Because of the particle size and electrostatic interactions with the surrounding media, a PEG 300–PO4 system was able to concentrate pDNA in the salt-rich phase (K p > 0.020) and contaminant RNA in the PEG phase (K p > 3). Even though the results do not include a graphical response of the partitioning behavior of the nucleic acids versus the changes in TLL, the authors discussed the fact that increasing values of TLLs may enhance pDNA partition toward salt-rich phases following their theoretical results based on volume exclusion. In addition, a response surface methodology (RSM) analysis could be developed to accurately predict partitioning behaviors considering several target products and experimental variables. These studies are not common for ATPS, but they could prove important advances in terms of operation design and further industrial feasibility analysis.
As previously mentioned in this chapter, an important variant within ATPS is their potential to accept a chemical or biological modification in order to enhance partition selectivity by the coupling of affinity ligands into the phase-forming chemicals or adding them free in solution, as defined by Ruiz-Ruiz et al. (Ruiz-Ruiz et al. 2012). For example, liquid–liquid affinity partitioning (ATPAP) of pDNA using a PEGylated zinc finger–GST (glutathione-S-transferase) fusion protein as affinity ligand was studied in PEG–DEX ATPAP by Barbosa et al. (Barbosa et al. 2008). Purified pDNA-based therapies emphasize the importance of scalable and cost-effective process development. The coupling of a fusion protein to a polymeric matrix reduces steric hindrance commonly present in resin-coupled proteins. PEG 600–DEX 40 and PEG 1000–DEX 500 systems were used for pDNA isolation. Comparative experiments determined that when the fusion protein was first administered in the system in its unPEGylated form, its K p was ~38 and the K p of the plasmid added to the system was calculated as 0.005. Nevertheless, pDNA partition coefficient increased 5900-fold (from K p 0.005 to 29.42) when adding the PEGylated form of the proposed affinity ligand to system PEG 1000–DEX 500. The authors confirmed that a strong interaction between the PEGylated fusion protein and the pDNA allows the complex to concentrate in a specific phase (PEG-rich phase). PEG 600–DEX 40 systems presented a lower increase in K p (from 0.0003 to 2.42). However, when increasing the PEGylated fusion ligand concentration to 293 μg/ml, pDNA partitioned completely (100%) toward the PEG-rich phase, thus representing an efficient strategy for its recovery. The authors demonstrated the potential of affinity partitioning of pDNA in ATPAP, emphasizing the effect of polymer characteristics such as concentration and molecular weight on ligand distribution through the system, which can be exploited to develop new concentration and purification strategies.
Nucleic acids have been recovered since ATPS were first proposed by Albertsson as an interesting recovery technique for macromolecules and particles. As presented in Table 4.4, the most recent studies of nucleic acid recovery are referred to plasmid DNA variants of this type of molecules. Considering the important increase in vector-based therapies, the constant study and development of novel and alternative technologies that may enhance particle recovery, without substantially increasing overall processing cost, are an actual constant trend in the bioprocess engineering research environment. Regarding ATPS strategies, structured studies are needed in which relations between pH, volume ratios, TLLs, and ionic force shifts and the use of naturally occurring phase-forming chemicals might positively trigger the particle partition of nucleic acid-derived structures. In addition, no scale-up procedures for these molecules (i.e., nucleic acids) have been carried out, and this is of extreme importance considering that almost all of the actual published research papers state that ATPS technologies could be positively coupled in actual industrial bioprocessing DSP trains. In this same line, no economic projections have been presented for this type of particle recovery using ATPS. This is an important opportunity area since the economic dimension of a bioprocess tends to be considered a valid source of information for well-established pharmaceutical companies that may look into alternative technologies in order to expand their current production schemes into a more robust manufacturing plan within their capabilities. In this tenor, software-aided economic projections for nucleic acid–ATPS could pose an important advantage for future research that seek to exploit the relation between this recovery technique and macromolecular structures.
4.6 Macroparticle Recovery with ATPS: Challenges and Future Trends
Considering the information presented in the past sections, interesting trends regarding the use of aqueous two-phase systems for the recovery and purification of high-added value particles can be envisioned.
As discussed by several authors, one of the major advantages of liquid–liquid extraction consists in the ease of setup, robustness, and scale-up feasibility. In this sense, ATPS strategies for macroparticles may be easily coupled to fermentation, filtration, precipitation, or centrifugation steps and hence significantly increase the recovery and/or purity yields of novel designed bioprocesses with important potential in industrial applications. Taking this into account, one of the major trends in ATPS protocols in terms of cell recovery is the engineering of in situ and continuous extraction systems. The first advances in this field started in South America (Professor Juan Asenjo in Chile) and Europe (Professor Joaquim Cabral in Portugal), and now a solid research line in continuous extraction of high-added value biologicals has also been developed in our research group (Professor Marco Rito-Palomares in Mexico). An important aspect that represents an important advantage of continuous processing, as opposed to a batch-based protocol, is that regulatory aspects of the designed strategies can be more easily fulfilled at industrial scales, providing ATPS-based approaches a theoretical easier inclusion into the biotech environment (i.e., pharmaceutical applications).
Within continuous operation, two additional aspects that constitute another marked trend within recovery of particles in ATPS are the manufacturing and design of miniaturized and fully automated lab-on-a-chip devices capable of mixing and separating in real-time complex samples. In this context, new designs based on microfluidics keep to prove the positive potential of two-phase extraction of whole cells and proteins. Despite this fact, no commercial applications with this technology have been developed since system selectivity has not been optimal in these devices. It has been suggested by several authors that a microscale separation platform constructed with polydimethylsiloxane (PDMS) devices covalently coupling antibodies targeted to specific cell epitopes may prove to be a successful strategy for highly specific stem cell separations. Nevertheless, because of the challenges and physical limitations of this technology, this approach has not been truly proved, and hence an interesting opportunity area for cell recovery in miniature-scale ATPS still remains.
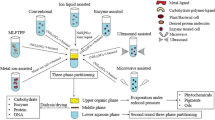
Bioprocess engineering strategies and novel designs based on ATPS technology remain to be applied at industrial scales because of several constant challenges this technology poses. One of the most discussed opportunity areas for two-phase extraction of biomolecules resides in the understanding of the precise molecular mechanisms involved in product/particle partitioning. Even though several authors, such as Aires-Barros (Silva et al. 2014; Jacinto et al. 2015), Asenjo (Asenjo and Andrews 2012), Benavides and Rito-Palomares (Benavides and Rito-Palomares 2008), Zaslavsky (Zaslavsky 1992), and Albertsson (Albertsson 1961, 1985), have thoroughly discussed the molecular and thermodynamic mechanisms of solutes partitioning in ATPS, up to date no general mathematical models and rules can be generally applied to fractionate random biological products. Considering the partitioning of macroparticles, as presented in this chapter, Fig. 4.1 presents the governing interactions that enable selective recovery of whole cells, viral particles, cell fragments/organelles, and nucleic acids. Viral particles and DNA variants, for example, exhibit a marked partition behavior based on their physical size, hence supporting the theory of excluded volumes within the systems. Nevertheless, research analyzing complex biological samples has proved that depending on the nature of the added components to the selected ATPS, ionic force shifts, and hence electrochemical charge potential, can dramatically improve partition of double-stranded DNA molecules toward a polymer (typically PEG)-rich phase. This is a constant challenge that must be overcome and constantly discussed in order to favor ATPS implementation at pilot plant and industrial scales.
One of the most important aspects of biotechnology applications is that of analytical techniques. Considering the research done in the past decades in ATPS design, characterization, and implementation, efficient and reliable identification techniques for target molecules in these liquid–liquid systems have constituted one of the primary challenges. Even though the most studied molecules in ATPS are proteins, traditional detection methods (e.g., Bradford assay, Lowry method, ninhydrin assay, or Smith assay [BCA]) are severely affected by the high concentration of phase-forming components, such as PEG or salts (e.g., phosphate, citrate, ammonium sulfate). Regarding aliquot analysis, phase samples need to be highly diluted up to 1% PEG or 10% salt (final concentration) in order to favor protein detection without compromising more than 10% the final sensitivity of the assay (González-González et al. 2011). In this same subject, detection of whole cells and viral particles represents an important opportunity area since in most of the studied cases, the analytical techniques involved in particle quantification take more time than the actual ATPS processing lapse. Besides this important fact, the complex protocols or equipment (i.e., flow cytometry or plaque assays for phage quantification) can significantly decrease the technical or economic industrial feasibility for a proposed two-phase strategy from the analytical point of view.
Further into the separation process, the polishing steps required in this liquid–liquid technology can be a limiting agent. Since a particular product is to be recovered in a specific phase regarding its physicochemical and biochemical properties, effective protocols for polymer/salt removal and concentration are very common. For viral particles, it has been reported that ultrafiltration, centrifugal partition chromatography, or precipitation steps can dramatically destabilize the particle, and thus up to 50% of the recovered infectivity in the ATPS can be lost. Despite the fact that polishing steps might reduce the final isolated quantity of active particles, the overall benefits of employing a simple and scalable ATPS might overcompensate this and might prove to be a feasible adaptation to actual industrial applications.
The above-discussed challenges should be carefully considered in order to design novel downstream processing methodologies that could possibly trigger the application of ATPS at industrial levels within specific industries, especially in the pharmaceutical sector.
4.7 Concluding Remarks
ATPS have demonstrated to be a promising technique for the recovery of cells, cell organelles, viruses, and nucleic acids, thanks to the multiple advantages this method has demonstrated. Furthermore, a better understanding of the principle parameters that drive ATPS partitioning for this type of biomolecules has resulted in the development of more robust strategies for their recovery. However, existing challenges such as the development of reliable methods for the quantification and polishing of the target biomolecules in these systems must be attended to promote the implementation of ATPS at industrial scale.
Abbreviations
- ATPAP:
-
Aqueous two-phase affinity partitioning
- ATPS:
-
Aqueous two-phase systems
- CCD:
-
Countercurrent distribution
- CD:
-
Cluster of differentiation
- DEX:
-
Dextran
- FACS:
-
Fluorescent-activated cell sorter
- FFF:
-
Field-flow fractionation
- LFA:
-
Lateral flow assay
- MACS:
-
Magnetic-activated cell sorter
- PBS:
-
Phosphate-buffered saline
- pDNA:
-
Plasmid DNA
- PEG:
-
Polyethylene glycol
- Poly(NIPAM):
-
Poly-N-isopropylacrylamide
- PVA:
-
Polyvinyl alcohol
- PVP:
-
polyvinylpyrrolidone
- QMS:
-
Quadrupole magnetic cell sorter
- RSM:
-
Response surface methodology
- TLL:
-
Tie-line length
- VLPs:
-
Viruslike particles
- V R :
-
Volume ratio
References
Adhikari M, Dhamane S, Hagström AEV, Garvey G, Chen WH, Kourentzi K, Strych U, Willson RC. Functionalized viral nanoparticles as ultrasensitive reporters in lateral-flow assays. Analyst. 2013;138(19):5584–7.
Albertsson PÅ. Fractionation of particles and macromolecules in aqueous two-phase systems. Biochem Pharmacol. 1961;5(4):351–8.
Albertsson PÅ. History of aqueous polymer two-phase partition. In: Walter H, Brooks DE, Fisher D, editors. Partitioning in aqueous two-phase system. Orlando: Academic; 1985. p. 1–10.
Asenjo JA, Andrews BA. Challenges and trends in bioseparations. J Chem Technol Biotechnol. 2008;83(2):117–20.
Asenjo JA, Andrews BA. Aqueous two-phase systems for protein separation: phase separation and applications. J Chromatogr A. 2012;1238:1–10.
Atefi E, Joshi R, Mann JA, Tavana H. Interfacial tension effect on cell partition in aqueous two-phase systems. ACS Appl Mater Interfaces. 2015;7(38):21305–14.
Barbosa H, Hine AV, Brocchini S, Slater NKH, Marcos JC. Affinity partitioning of plasmid DNA with a zinc finger protein. J Chromatogr A. 2008;1206(2):105–12.
Benavides J, Rito-Palomares M. Practical experiences from the development of aqueous two-phase processes for the recovery of high value biological products. J Chem Technol Biotechnol. 2008;83:133–42.
Benavides J, Mena JA, Cisneros-Ruiz M, Ramírez OT, Palomares LA, Rito-Palomares M. Rotavirus-like particles primary recovery from insect cells in aqueous two-phase systems. J Chromatogr B. 2006;842(1):48–57.
Benavides J, Aguilar O, Lapizco-Encinas BH, Rito-Palomares M. Extraction and purification of bioproducts and nanoparticles using aqueous two-phase systems strategies. Chem Eng Technol. 2008;31(6):838–45.
Bhambure R, Sharma R, Gupta D, Rathore AS. A novel aqueous two phase assisted platform for efficient removal of process related impurities associated with E. coli based biotherapeutic protein products. J Chromatogr A. 2013;1307:49–57.
Braas GMF, Walker SG, Lyddiatt A. Recovery in aqueous two-phase systems of nanoparticulates applied as surrogate mimics for viral gene therapy vectors. J Chromatogr B. 2000;743(1–2):409–19.
Cabral J. Cell partitioning in aqueous two-phase polymer systems. In: Kumar A, Galaev I, Mattiasson B, editors. Cell separation. Berlin/Heidelberg: Springer; 2007. p. 151–71.
Duarte SP, Fortes AG, Prazeres DMF, Marcos JC. Preparation of plasmid DNA polyplexes from alkaline lysates by a two-step aqueous two-phase extraction process. J Chromatogr A. 2007;1164(1–2):105–12.
Everberg H, Peterson R, Rak S, Tjerneld P, Emanuelsson C. Aqueous two-phase partitioning for proteomic monitoring of cell surface biomarkers in human peripheral blood mononuclear cells. J Proteome Res. 2006;5(5):1168–75.
Fisher D. The separation of cells and organelles by partitioning in two-polymer aqueous phases. Biochem J. 1981;196(1):1–10.
García-Pérez AI, Recio MN, Sancho P, Luque J. Partitioning behaviour of rat bone marrow cells in aqueous two-phase systems. Dependence of cell partition on the interfacial tension and electrical potential difference between the phases. J Chromatogr A. 1987;403(C):131–43.
González-González M, Rito-Palomares M. Aqueous two-phase systems strategies to establish novel bioprocesses for stem cells recovery. Crit Rev Biotechnol. 2013;34(4):318–27.
González-González M, Rito-Palomares M. Application of affinity aqueous two-phase systems for the fractionation of CD133+ stem cells from human umbilical cord blood. J Mol Recognit. 2015;28(3):142–7.
González-González M, Mayolo-Deloisa K, Rito-Palomares M, Winkler R. Colorimetric protein quantification in aqueous two-phase systems. Process Biochem. 2011;46(1):413–7.
González-González M, Vázquez-Villegas P, García-Salinas C, Rito-Palomares M. Current strategies and challenges for the purification of stem cells. J Chem Technol Biotechnol. 2012;87(1):2–10.
González-González M, Rito-Palomares M, Méndez Quintero O. Partition behavior of CD133+ stem cells from human umbilical cord blood in aqueous two-phase systems: in route to establish novel stem cell primary recovery strategies. Biotechnol Prog. 2014;30(3):700–7.
Hamamoto R, Kamihira M, Iijima S. Specific separation of animal cells using aqueous two-phase systems. J Ferment Bioeng. 1996;82(1):73–6.
Hammar L, Gilljam G. Extraction of HIV-1 in aqueous two-phase systems to obtain a high yield of gp120. Aids Res Hum Retroviruses. 1990;6(12):1379–88.
Hatti-Kaul R. Aqueous two-phase systems – a general overview. Mol Biotechnol. 2001;19(3):269–77.
Jacinto MJ, Soares RRG, Azevedo AM, Chu V, Tover A, Conde JP, Aires-Barros MR. Optimization and miniaturization of aqueous two phase systems for the purification of recombinant human immunodeficiency virus-like particles from a CHO cell supernatant. Sep Purif Technol. 2015;154:27–35.
Jimeno P, Garcia-Perez AI, Luque J, Pinilla M. Changes in glycolytic enzyme activities in aging erythrocytes fractionated by counter-current distribution in aqueous polymer two-phase systems. Biochem J. 1991;279(1):237–43.
Jue E, Yamanishi CD, Chiu RYT, Wu BM, Kamei DT. Using an aqueous two-phase polymer-salt system to rapidly concentrate viruses for improving the detection limit of the lateral-flow immunoassay. Biotechnol Bioeng. 2014;111(12):2499–507.
Jungbauer A. Continuous downstream processing of biopharmaceuticals. Trends Biotechnol. 2013;31(8):479–92.
Kamei DT, King JA, Wang DIC, Blankschtein D. Understanding viral partitioning in two-phase aqueous nonionic micellar systems: 2. Effect of entrained micelle-poor domains. Biotechnol Bioeng. 2002a;78(2):203–16.
Kamei DT, Liu C-L, Haase-Pettingell C, King JA, Wang DIC, Blankschtein D. Understanding viral partitioning in two-phase aqueous nonionic micellar systems: 1. Role of attractive interactions between viruses and micelles. Biotechnol Bioeng. 2002b;78(2):190–202.
Kim J, Shin H, Kim J, Kim J, Park J. Isolation of high-purity extracellular vesicles by extracting proteins using aqueous two-phase system. PLoS One. 2015;10(6):e0129760.
Kumar A, Kamihira M, Galaev IY, Mattiasson B, Iijima S. Type-specific separation of animal cells in aqueous two-phase systems using antibody conjugates with temperature-sensitive polymers. Biotechnol Bioeng. 2001;75(5):570–80.
Ladd Effio C, Wenger L, Ötes O, Oelmeier SA, Kneusel R, Hubbuch J. Downstream processing of virus-like particles: single-stage and multi-stage aqueous two-phase extraction. J Chromatogr A. 2015;1383:35–46.
Luechau F, Ling TC, Lyddiatt A. Partition of plasmid DNA in polymer-salt aqueous two-phase systems. Sep Purif Technol. 2009a;66(2):397–404.
Luechau F, Ling TC, Lyddiatt A. Selective partition of plasmid DNA and RNA in aqueous two-phase systems by the addition of neutral salt. Sep Purif Technol. 2009b;68(1):114–8.
Luechau F, Ling TC, Lyddiatt A. Recovery of B19 virus-like particles by aqueous two-phase systems. Food Bioprod Process. 2011;89(4):322–7.
Malmström P, Nelson K, Jönsson Å, Sjögren HO, Walter H, Albertsson PÅ. Separation of rat leukocytes by countercurrent distribution in aqueous two-phase systems. Cell Immunol. 1978;37(2):409–21.
Malmström P, Jonsson A, Hallberg T, Sjogren HO. Countercurrent distribution of lymphocytes from human peripheral blood in an aqueous two-phase system. I. Separation into subsets of lymphocytes bearing distinctive markers. Cell Immunol. 1980a;53(1):39–50.
Malmström P, Jonsson A, Sjogren HO. Countercurrent distribution of lymphocytes from human peripheral blood in an aqueous 2-phase system. II. Separation into subsets of lymphocytes with distinctive functions. Cell Immunol. 1980b;53(1):51–64.
Mashayekhi F, Chiu RYT, Le AM, Chao FC, Wu BM, Kamei DT. Enhancing the lateral-flow immunoassay for viral detection using an aqueous two-phase micellar system. Anal Bioanal Chem. 2010;398(7–8):2955–61.
Mayolo-Deloisa K, Gonzalez-Valdez J, Guajardo-Flores D, Aguilar O, Benavides J, Rito-Palomares M. Current advances in the non-chromatographic fractionation and characterization of PEGylated proteins. J Chem Technol Biotechnol. 2011;86(1):18–25.
Moreira KA, Chaves AC, Marques ET, Prazeres DMF, De Azevedo WM, Porto ALF, De Filho JLL. Extraction of dengue 2 plasmid DNA vaccine (pD2) from cell lysates by aqueous two-phase systems. Biotechnol. 2007;6(4):520–6.
Morré DM, Morre DJ. Aqueous two-phase partition applied to the isolation of plasma membranes and Golgi apparatus from cultured mammalian cells. J Chromatogr B. 2000;743(1–2):377–87.
Morré DJ, Morré DM, Van Alstine JM. Separation of endosomes by aqueous two-phase partition and free-flow electrophoresis. J Chromatogr B. 1998;711(1–2):203–15.
Navas P, Nowack DD, Morré DJ. Isolation of purified plasma membranes from cultured cells and hepatomas by two-phase partition and preparative free-flow electrophoresis. Cancer Res. 1989;49(8):2147–56.
Ribeiro SC, Monteiro GA, Cabral JMS, Prazeres DMF. Isolation of plasmid DNA from cell lysates by aqueous two-phase systems. Biotechnol Bioeng. 2002;78(4):376–84.
Rodrigues T, Carrondo MJT, Alves PM, Cruz PE. Purification of retroviral vectors for clinical application: biological implications and technological challenges. J Biotechnol. 2007;127(3):520–41.
Ruiz-Ruiz F, Benavides J, Aguilar O, Rito-Palomares M. Aqueous two-phase affinity partitioning systems: current applications and trends. J Chromatogr A. 2012;1244:1–13.
Ruiz-Ruiz F, Benavides J, Rito-Palomares M. Scaling-up of a B-phycoerythrin production and purification bioprocess involving aqueous two-phase systems: practical experiences. Process Biochem. 2013;48(4):738–45.
Schindler J, Nothwang HG. Aqueous polymer two-phase systems: effective tools for plasma membrane proteomics. Proteomics. 2006;6(20):5409–17.
Sharp KA, Yalpani M, Howard SJ, Brooks DE. Synthesis and application of a poly(ethylene glycol)-antibody affinity ligand for cell separations in aqueous polymer two-phase systems. Anal Biochem. 1986;154(1):110–7.
Shin H, Han C, Labuz JM, Kim J, Kim J, Cho S, Gho YS, Takayama S, Park J. High-yield isolation of extracellular vesicles using aqueous two-phase system. Sci Rep. 2015;5:13103.
Silva DFC, Azevedo AM, Fernandes P, Chu V, Conde JP, Aires-Barros MR. Determination of aqueous two phase system binodal curves using a microfluidic device. J Chromatogr A. 2014;1370:115–20.
SooHoo J, Walker G. Microfluidic aqueous two phase system for leukocyte concentration from whole blood. Biomed Microdevices. 2009;11(2):323–9.
Sousa A, Andrade P, Pirzgalska R, Galhoz T, Azevedo A, da Silva C, Raquel Aires-Barros M, Cabral J. A novel method for human hematopoietic stem/progenitor cell isolation from umbilical cord blood based on immunoaffinity aqueous two-phase partitioning. Biotechnol Lett. 2011;33(12):2373–7.
Tateishi-Karimta H, Sugimoto N. Control of stability and structure of nucleic acids using cosolutes. Methods. 2014;67(2):151–8.
Tsukamoto M, Taira S, Yamamura S, Morita Y, Nagatani N, Takamura Y, Tamiya E. Cell separation by an aqueous two-phase system in a microfluidic device. Analyst. 2009;134(10):1994–8.
Umakoshi H, Kuboi R, Komasawa I. Control of partitioning of bacterial cells and characterization of their surface properties in aqueous two-phase systems. J Ferment Bioeng. 1997;84(6):572–8.
Van Alstine JM, Brooks DE. Cell membrane abnormality detected in erythrocytes from patients with multiple sclerosis by partition in two-polymer aqueous-phase systems. Clin Chem. 1984;30(3):441–3.
Vijayaragavan KS, Zahid A, Young JW, Heldt CL. Separation of porcine parvovirus from bovine serum albumin using PEG–salt aqueous two-phase system. J Chromatogr B. 2014;967:118–26.
Walker SG, Lyddiatt A. Aqueous two-phase systems as an alternative process route for the fractionation of small inclusion bodies. J Chromatogr B. 1998;711(1–2):185–94.
Walter H, Krob EJ. Separation and subfractionation of small numbers of cells (∼106) by countercurrent distribution in dextran-poly(ethylene glycol) aqueous-phase systems. Cell Biophys. 1984;6(4):253–62.
Walter H, Webber TJ, Michalski JP, McCombs CC, Moncla BJ, Krob EJ, Graham LL. Subfractionation of human peripheral blood lymphocytes on the basis of their surface properties by partitioning in two-polymer aqueous phase systems. J Immunol. 1979;123(4):1687–95.
Zaslavsky BY. Bioanalytical applications of partitioning in aqueous polymer two-phase systems. Anal Chem. 1992;64(15):765A–73A.
Zijlstra GM, de Gooijer CD, van der Pol LA, Tramper J. Design of aqueous two-phase systems supporting animal cell growth: a first step toward extractive bioconversions. Enzyme Microb Technol. 1996;19(1):2–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
González-González, M., Ruiz-Ruiz, F. (2017). Aqueous Two-Phase Systems for the Recovery of Bioparticles. In: Rito-Palomares, M., Benavides, J. (eds) Aqueous Two-Phase Systems for Bioprocess Development for the Recovery of Biological Products. Food Engineering Series. Springer, Cham. https://doi.org/10.1007/978-3-319-59309-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-59309-8_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-59308-1
Online ISBN: 978-3-319-59309-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)