Abstract
A novel cell separation process based on immunoaffinity aqueous two phase systems is presented to isolate and purify CD34+ stem/progenitor cells directly from the whole umbilical cord blood (UCB). A system, composed of polyethylene glycol and dextran, was evaluated for the selective recovery of CD34+ cells from UCB. A monoclonal antibody against the CD34 surface antigen was used for the direct partitioning of CD34+ cells in UCB to the PEG-rich phase. The initial population of CD34+ cells (0.2% of the initial sample) was enriched to values up to 42% in a single partitioning step, while the majority of contaminant cells were partitioned to the dextran-rich phase (1.37 × 10−2 < KP < 2.76 × 10−2). This novel selection method allowed a recovery yield of 95% of CD34+ cells with a purification factor of 245 and is expected to pave a new way to purify hematopoietic stem/progenitor cells for use in a variety of clinical settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of an effective separation system for large-scale cell separation, with high throughput, yield and purity, while featuring potential compliancy with good manufacturing practices (GMP) standards, has remained a challenging goal for process engineering. This is particularly relevant for human stem cells, which have long been the focus of interest for both scientific and clinical communities due to their vast potential. The hematopoietic system, in particular, is one of the most intensively studied systems in the body. A very rare population of hematopoietic stem/progenitor cells (HSC) is responsible for the generation of all hematopoietic cell lineages, including mature blood cells such as erythrocytes, leukocytes or platelets. These primitive cells (approx. 1–3% within the mononuclear cell fraction of adult bone marrow (BM) and <1% in umbilical cord blood (UCB)) are characterized by their self-renewal capacity, multilineage differentiation ability and, more importantly, the capacity of generating long-term hematopoiesis when transplanted into immunocompromised hosts. In order to obtain efficient HSC expanded grafts, pre-isolation of a subpopulation of UCB or BM cells prior to cell culture is highly recommended (Weissman and Shizuru 2008). In the clinical setting, the final number of HSC in the graft to be transplanted is the essential parameter. Thus, one isolation/purification process to be used should maximize the number of HSC to be transplanted, avoiding significant cell losses. On the other hand, considering the feasibility and safety of transplanting HSC ex vivo expanded grafts, pre-isolation of a subpopulation of UCB or BM cells prior to cultivation is highly recommended to get efficient cell expansion (Chou et al. 2010). Nonetheless, this might result in significant cell loss (as low as 50%) that may impair a successful cellular therapy procedure (Andrade et al. 2011).
The isolation and purification of human HSC have been difficult due to their low frequency in the hematopoietic sources namely BM and UCB, the fact that they divide infrequently and the lack of specific cell markers related to HSC function (Mayani and Lansdorp 1998). CD34 expression, in particular, became the hallmark for selection of primitive hematopoietic cells. Despite the demand for effective methods for cell separation, the existing techniques have major limitations when the desired degree of performance on a preparative scale is required. Fluorescence activated cell sorting (FACS) and magnetic activated cell separation sorting (MACS), both using specific antibodies either fluorescently or magnetically labeled, respectively, are the methods typically used for HSC separation. However, FACS is an expensive analytical technique, not suitable at process scale, and MACS is limited at isolation and purification yields, thus aqueous two-phase systems (ATPS) combined with immunoaffinity ligands could be advantageous as an alternative process for HSC separation.
ATPS is an aqueous liquid–liquid biphasic system that is obtained either by the mixture of two incompatible hydrophilic polymers or a polymer and an inorganic salt solution, above a certain critical concentration (Albertsson et al. 1990). ATPS has great potential and versatility for the downstream processing of biopharmaceuticals such as monoclonal antibodies (Azevedo et al. 2009), high density lipoproteins (Wiegel et al. 1994), cytokines (Delgado et al. 1994), and plasmid DNA (Ribeiro et al. 2002). These systems combine a high biocompatibility, as both phases consists mainly of water, with an easy scale-up and continuous mode of operation (Albertsson et al. 1990).
One of the characteristic of ATPS is also the ability to handle not only soluble biomolecules but also particulate structures, such as human cells (Cabral 2007). An ATPS containing a macroligand carrier was already developed for the specific separation of animal cells (KG1 leukemic cell line) (Kumar et al. 2001). However, to our best knowledge, no reports are found in the literature concerning the selection of human HSC from UCB using ATPS.
In this work, an immunoaffinity ATPS composed of polyethylene glycol (PEG) and dextran was used for the selective recovery of CD34+ cells from UCB. With the purpose of obtaining the specific partitioning of CD34+ cells, an immunoaffinity ligand was introduced into the two-phase system, consisting of a monoclonal antibody against the CD34 antigen marker (anti-CD34).
Materials and methods
Materials
The monoclonal antibody (mAb) against human CD34 antigen (anti-CD34, IgG1) was produced by murine hybridoma cells, AC133.1, from ATCC (Manassas, VA, USA) in our laboratory according to cell-line supplier. Anti-CD34 was then purified from culture supernatants using protein G affinity chromatography on a hitrap protein G column (GE Healthcare, Uppsala, Sweden) to yield 200 μg/ml. Alexa Fluor 488 goat anti-mouse IgG used in flow cytometry was purchased from Invitrogen (Eugene, OR, USA).
Dextran with a molecular weight of 500,000 Da and PEG with molecular weight of 8,000 Da were purchased from Fluka (Buchs, Switzerland).
Human donor cell preparation
The UCB sample was kindly provided by Serviço de Obstetrícia, Centro Hospitalar Lisboa Norte, Lisboa, Portugal and were collected after maternal donor consent. The sample was processed within 24 h after collection.
UCB partition studies
ATPS were prepared in 15 ml graduated conical centrifuge tubes with a total mass of 5×g for whole UCB partition. Prior to adding cells to each two-phases system, three solutions were prepared in which the UCB samples were incubated with different antibody solutions. Samples (200 μl) containing 1.2 × 109 cells were incubated with (1) 100 μl 10 mM phosphate buffered saline (PBS, pH 7.4, 150 mM NaCl) (blank), (2) 5 μg of anti-CD34 mAb in 100 μl 10 mM PBS, and (3) 10 μg of anti-CD34 mAb in 100 μl 10 mM PBS, in individual tubes and left to incubate for 30 min at room temperature. The cell extract was then added to each conical tube. ATPS were left to settle for 1.5 h and centrifuged at 180×g for 5 min. Due to the affinity to the top phase, CD34+-enriched populations were layered just above the interface. Cells were then collected, and prepared for flow cytometry analysis using a secondary antibody anti-IgG (Fig. 1). Samples from top and bottom phases were also collected after the centrifugation step. The number of cells was assessed using the Trypan Blue (Gibco, City, Germany) exclusion method.
The partitioning of cells is characterized by the term, partition coefficient, KP, which is a ratio between the concentration of cells in the top phase (Ct) and the one in the bottom phase (Cb).
Flow cytometry analysis
After the recovery of the cells from each phase of the ATPS, these were then analysed by flow cytometry (FACSCalibur, Becton–Dickinson) using goat anti-mouse IgG (Alexa Fluor 488-conjugated) (indirect labeling of CD34+ cells) or mouse anti-human CD34 (direct labeling) (Invitrogen). A minimum of 10,000 gated events was collected for each sample.
The recovery of cells is given by the ratio between the number of CD34+ cells obtained after partitioning, and the initial number of CD34+ cells.
The purification factor was calculated by comparing the initial percentage of CD34+ cells in the whole UCB samples and after the partitioning in the ATPS, by the given formula:
Results and discussion
Whole umbilical cord blood partitioning studies
Cell partitioning
By using an ATPS composed by 5.6% PEG, 7.5% dextran and supplemented with 0.15 M NaCl, most of cells in UCB samples, namely the erythrocytes, partitioned into the more hydrophilic dextran-rich phase (1.37 × 102 < KP < 2.76 × 10−2). In addition to the hydrophobicity difference between both phases, and the polymers inherent characteristics, this preferential partitioning behavior can also be due to the uneven partition of the charged ions Na+ and Cl− in the ATPS balancing the electrostatic potential difference between phases, interacting with the cells membrane. In an ATPS, anions and cations distribute unequally across the interface. Furthermore, water structure making ions (e.g. Li+, Na+, NH4+, Ca2+, Mg2+, Fˆ) favour the more hydrophilic phase (Dextran), whereas water structure breaking ions (e.g. K+, Rb+, Cs+, Clˆ, Brˆ, Iˆ, SCNˆ) favour the more hydrophobic phase.
The anti-CD34 mAb partitions preferentially to the PEG-rich phase, under the experimental conditions used, inducing thus the partition of CD34+ cells to the top phase. These cells were further recovered at the interface after a centrifugation step.
CD34+ cell quantification
After counting the number of cells in the top and bottom phases and interface, the specific partitioning of the cells to one of the phases was evaluated by flow cytometry analysis.
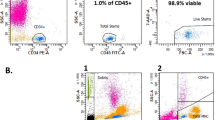
By using target-specific antibodies, this should increase the purity of cell populations in the interface after the first centrifugation step. Figure 2 shows an enrichment of CD34+ cells on the interface of the ATPS when anti-CD34 was used as an immunoaffinity ligand. Starting from a population of 0.2% of CD34+ cells (Fig. 2a) it was possible to reach values up to 42% (Fig. 2c) of CD34 expression with a single step partitioning in the ATPS. The selective targeting of the mAb to the cells and its major affinity to the top phase avoided the partition of these cells to the bottom phase. Also, by increasing the amount of antibody (5 and 10 μg) there was an increase of both the purification and the recovery yields (Table 1).
A control run was also performed where cells were added to the ATPS in a PBS solution in the absence of the anti-CD34 mAb. The percentage of CD34+ cells in the interface after partitioning increased only to 2.3% (Table 1). Although some purification was achieved (13-fold), the percentage of stem/progenitor cells recovery was very low (2.3%) when comparing to the experiments, where mAb was used (81–95%). The specificity of the immunoaffinity ATPS demonstrated that purification was only effective in the presence of anti-CD34. A major achievement of this system is its easiness of recovering the desired cells after partitioning. The highest recovery yields were obtained using 10 μg anti-CD34 mAb and reached 95%, which is comparable to the most common techniques for HSC purification upon mononuclear cell fraction as MACS (Andrade et al. 2011). Considering the expression of CD34 in the initial cell sample (0.2%, non-fractioned, whole UCB), purification factors higher than 245 have been achieved (Table 1), highlighting the efficiency and selectivity of the designed ATPS.
Conclusions
An ATPS composed of two polymers, PEG 8000 and dextran, was successfully used for the specific partitioning and recovery of CD34+ stem/progenitor cells from UCB in the presence of 0.15 M NaCl. Purification factors up to 245 were achieved with a single step partitioning experiment, which demonstrate that aqueous two-phase processing besides being easy to implement, cost-effective and biologically compatible method can constitute a major option to traditional techniques for UCB processing namely the classical Ficoll density gradient centrifugation and magnetic sorting.
Overall, this novel selection method is expected to contribute for the development of stem cell engineering, eliminating time- and labor-consuming procedures to be able to purify and expand target populations in order to increase the number of cells available for use in a variety of clinical settings.
References
Albertsson PA, Johansson G, Tjerneld F (1990) Separation processes in biotechnology. Marcel Dekker, New York
Andrade PZ, da Silva CL, dos Santos F, Almeida-Porada G, Cabral JMS (2011) Initial CD34(+) cell-enrichment of cord blood determines hematopoietic stem/progenitor cell yield upon ex vivo expansion. J Cell Biochem. 112:1822–1831
Azevedo AM, Rosa PAJ, Ferreira IF, Aires-Barros MR (2009) Chromatography-free recovery of biopharmaceuticals through aqueous two-phase processing. Trends Biotechnol 27:240–247
Cabral JMS (2007) Cell partitioning in aqueous two-phase polymer systems. In: Kumar A, Galaev IY, Mattiasson B (eds) Cell separation—fundamentals, analytical and preparative methods. Springer-Verlag, Berlin, pp 151–171
Chou S, Chu P, Hwang W, Lodish H (2010) Expansion of human cord blood hematopoietic stem cells for transplantation. Stem Cells. 7:427–428
Delgado C, Malik F, Selisko B, Fischer D, Francis GE (1994) Quantitative analysis of polyethylene glycol (PEG) in PEG-modified proteins/cytokines by aqueous two-phase systems. J Biochem Biophys Meth 29:237–250
Kumar A, Kamihira M, Galaev IY, Mattiasson B, Iijima S (2001) Type-specific separation of animal cells in aqueous two-phase systems using antibody conjugates with temperature-sensitive polymers. Biotechnol Bioeng 75:570–580
Mayani H, Lansdorp PM (1998) Biology of human umbilical cord blood-derived hematopoietic stem/progenitor cells. Stem Cells. 16:153–165
Ribeiro SC, Monteiro GA, Cabral JMS, Prazeres DMF (2002) Isolation of plasmid DNA from cell lysates by aqueous two-phase systems. Biotechnol Bioeng 78:376–384
Weissman IL, Shizuru JA (2008) The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood 112:3543–3553
Wiegel D, Meyer C, Arnold K (1994) Partitioning of high-density lipoproteins in charge-sensitive two-phase systems. J Chrom B Biomed Sci Appl. 661:159–164
Acknowledgments
This work was carried out within the frame of the Project StemSelect (contract no.PTDC/EQU-EPR/104663/2008) granted by Fundação para a Ciência e Tecnologia – FCT. António F. Sousa and Pedro Z. Andrade also acknowledge FCT for grants 3386/2010 and SFRH/BD/38719/2007, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sousa, A.F., Andrade, P.Z., Pirzgalska, R.M. et al. A novel method for human hematopoietic stem/progenitor cell isolation from umbilical cord blood based on immunoaffinity aqueous two-phase partitioning. Biotechnol Lett 33, 2373–2377 (2011). https://doi.org/10.1007/s10529-011-0727-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-011-0727-0