Abstract
Over the past years, there has been an increasing trend in research on the extraction and purification of proteins using aqueous biphasic systems (ABS) formed by polymers, e.g., polyethylene glycol (PEG). In general, when dealing with protein purification processes, it is essential to maintain their native structure and functional stability. In this context, ABS, liquid-liquid systems where both phases are water-rich, provide a biocompatible medium for such attempts. More recently, it was shown that the versatility offered by ABS is further enhanced by the introduction of ionic liquids (ILs) as alternative phase-forming components. This chapter describes and highlights the current progress on the field of protein extraction and purification using IL-based ABS. The general approach for protein extraction using IL-based ABS and factors influencing the partitioning are discussed. In addition, the challenges to overcome the use of IL-based ABS for protein extraction are also presented.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Proteins are an integral part of all living systems and have various applications in food and feed (both relatively low value) and pharmaceuticals (high value). Purification of proteins involves various unit operations, using low to high resolution techniques, to obtain proteins with desired purity and quality. Proteins, being fragile molecules, can be easily denatured by acid/base solutions, salts, and high temperature. Therefore, mild operation conditions for their recovery and purification are required, in order to maintain their nativity and functionality. With the current advances in biotechnology, a large increase in the titers of protein production was already observed; yet, the development of cost-effective purification methods is still required. The high cost of protein purification continues to remain a bottleneck in downstream processing of proteins and mainly for protein value-added biopharmaceuticals. On the other hand, in the field of food and feed, proteins are obtained from, e.g., soya, and also there has been a growing interest in third-generation biofuels from microalgae. For instance, in fuel production processes, large amounts of proteins are generated which could be used for feed and food [1–3]. In fact, to make these processes economically feasible, it is necessary to refine other components from biomass. Proteins are a major fraction of algae biomass and are normally denatured by the solvents used for lipids extraction. The main challenge therefore lies in separating the proteins in their native form without affecting their functionality. Thus, depending on the biomass or initial medium, protein purification protocols vary and drive the development of more specific, robust, and cost-effective methods [4].
Aqueous biphasic systems (ABS) based on polymers were first proposed by Albertsson [5], who studied their applicability in protein extraction and purification. ABS allow the integration of concentration and purification processing steps and serve as an alternative approach to the traditional processes. Typical ABS are formed by mixing polymer-polymer and polymer-salt combinations above given concentrations to form two distinct aqueous phases, each one enriched in one of the phase-forming components. Both phases are water-rich (~80–90 % w/w), and thus ABS can provide a mild and gentle environment for protein separation without affecting their native structure and stability [6, 7]. In addition to the largely investigated polymer-based ABS, in the last decade, ionic liquids (ILs) were proposed as alternative phase-forming components of ABS [8]. And because of the inherent properties of ILs, this possibility allowed the use of ABS in a new range of applications.
The interest on ILs as extractive solvents increased primarily because of their nonvolatile nature, which is the major advantage over traditional organic volatile solvents. In addition to their nonvolatility, ILs, being composed of cations and anions, can be more easily tuned to achieve specific properties, such as a tunable polarity, viscosity, and solvent miscibility. Their tunable polarity enabled them to be used in biotransformations to increase substrate solubility, to dissolve enzymes, and to tailor the reaction rate [9]. Moreover, due to their tailoring ability, ILs are also able to form ABS not only with inorganic salts but also with polymers [10], carbohydrates [11], and amino acids [12]. The main advantage of IL-based ABS over the conventional systems comprises their ability to tune and tailor the properties of the coexisting phases by permutation and combination of different cations and anions, thereby improving the selectivity of these systems for a wide variety of solutes [13].
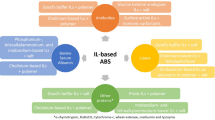
Based on the advantages and large recent interest on IL-based ABS for separation purposes, this chapter describes the general approaches of protein purification described in the literature using IL-based ABS and factors that influence the partitioning of proteins in these systems. The challenges in developing a successful ABS for extraction of proteins are also discussed. Finally, this chapter aims a better understanding on the mechanisms ruling protein extraction using IL-based ABS. Figure 6.1. depicts a scheme on the approach required to use IL-based ABS for the extraction of proteins.
6.2 Extraction of Proteins/Enzymes Using IL-Based ABS
The extraction of proteins using IL-based ABS has been studied by different research groups and for which a summary is given in Table 6.1. This table was adapted from [14] and updated to include more recent studies.
As a first point, only water-miscible ILs are able to form ABS since water-immiscible ILs do not form two aqueous-rich phases (see Table 6.2). IL-based ABS are formed by mixing water-miscible ILs with salts, carbohydrates, amino acids, and polymers [8, 10, 12, 15]. The ability to form ABS with solutes other than salts has indeed been studied [8, 10, 12, 15], but their efficiency in extracting proteins is however scarcely studied. Although more promising than inorganic salts in what concerns the use of more biocompatible systems, these alternative systems suffer the drawback of only being able to form ABS with a limited number of ILs due to their low salting-out ability (carbohydrates, amino acids, and polymers versus salts).
In general, most of the studies reported in the literature deal with imidazolium-based ILs composed of halogens or [BF4]− anions. Recently, ABS based on guanidinium-based ILs have been studied for protein extraction [16, 17] and where it was shown that model proteins, such as BSA, could be extracted with high efficiency for the IL-rich phase without losing its native structure and maintain its stability. IL-based ABS could thus serve as potential platforms for protein extraction if the stability of proteins at the IL-rich phase is maintained. While most of the studies focused on the extraction efficiency of model proteins/enzymes, the studies carried out by Dreyer [18] and Pei [19] made an attempt to understand the mechanisms responsible for the high extractions attained.
6.2.1 Stability of Proteins in IL-Rich Phases
Proteins are complex macromolecules and require a gentle environment to maintain their structural and functional integrity. Changes in this environment, such as solvent concentration, pH, ionic strength, and temperature, could result in denaturation of proteins. Thus, the primary criterion for any protein purification process is the ability to maintain the proteins’ structural integrity and functionality. In this context, when using IL-based ABS for the purification of proteins, it is necessary to understand their stability in aqueous solutions of ILs. There are some studies carried out to infer on protein-IL interactions [20, 21] and where model proteins have been used, namely, BSA, lysozyme, and cytochrome c. However, there are other proteins with higher commercial value, such as monoclonal antibodies, RuBisCo (ribulose-1,5-bisphosphate carboxylase/oxygenase), etc., that should be studied in what concerns their stability in aqueous solutions of ILs and their feasibility to be extracted by ABS. Although some hydrophobic ILs are able to stabilize enzymes [22, 23], they are not discussed in this chapter since these do not form ABS.
In the studies regarding the stability of proteins in aqueous solutions of ILs, the techniques employed to monitor the proteins’ structural and thermal stability include UV spectroscopy, fluorescence, circular dichroism (CD),small-angle neutron scattering (SANS), differential scanning calorimetry (DSC), dynamic light scattering (DLS), and size exclusion chromatography (SEC). The stability studies were designed to address the factors that influence the formation of ABS and the stability of proteins, such as (i) type of IL; (ii) concentration of IL; (iii) other process conditions, such as pH, ionic strength, and temperature; and (iv) protein properties, such as size, charge, and surface hydrophobicity.
ABS consist of two aqueous-rich phases: an IL-rich phase and a phase rich in salt, polymer, amino acid, or carbohydrate. The concentration of IL in the IL-rich phase of ABS can vary from 1.5 to 3.0 mol/kg [24], and thus it is prudent to study IL-protein interactions in aqueous solutions. Moreover, it was already shown that the concentration of IL has a strong influence on the protein stability [21, 25]. In our recent study, we have shown that the protein’s stability in aqueous solutions of ILs is influenced by the concentration of IL and by the protein properties, such as size and complexity of the molecule [25]. In this study, the stability of BSA, IgG, and RuBisCo was studied in aqueous solutions of two ILs, Iolilyte 221 PG and Cyphos 108, at different concentrations [25]. It was found that as the concentration of the IL increases (0–50 %, v/v), the proteins start forming aggregates. RuBisCo (~540 kDa), being a large complex protein/enzyme that consists of eight large and small subunits, begins to aggregate at lower IL concentrations (~30 %, v/v), while BSA (~67 kDa), a smaller protein, forms no aggregates or only negligible aggregates at 50 % v/v of IL(Iolilyte 221 PG). IgG (~150 kDa), with an intermediate size, forms aggregates at 50 % (v/v) of Iolilyte 221 PG. In this study [25], the aggregate formation was monitored using SEC and DLS studies. In an additional study, SANS results showed that human serum albumin and cytochrome c form aggregates at high concentrations (50 %, v/v) of [C4mim]Cl and retain their high-order structure at lower IL concentrations (25 %, v/v) [26]. Lysozyme and interleukin-2 (IL-2) showed increased thermal stability in aqueous solutions up to 40 % (w/w) of IL, although it is dependent on the pH [27], indicating thus that the charge of the protein also influences its stability in IL aqueous solutions. Different ILs with varying “kosmotropicity” were also investigated for their effect on protein structure and long-term stability [28]. In this study, cytochrome c showed no significant changes in its structure when dissolved in hydrated choline dihydrogen phosphate (containing 20 % w/w of water). Cytochrome c additionally showed a higher thermal and long-term stability, leading the authors to conclude that the “kosmotropicity” of ILs has strong implications on the proteins’ stability [28].
The influence of inorganic salts and ion-specific-induced precipitation of proteins is well described by the Hofmeister series [29]. Since ILs are also composed of ions, their influence on protein stability in aqueous solutions can also be explained, to some extent, by the Hofmeister series [20, 28, 30, 31]. Ions can be classified as kosmotropes (water structure makers) which stabilize proteins and chaotropes (water structure breakers) which destabilize proteins. The rank of kosmotrope-chaotrope ions according to the Hofmeister series is shown in Fig. 6.2. The most suitable combination to enhance protein stability comprises a kosmotropic anion and a chaotropic cation [32–34]. According to the example described above, the cytochrome c stability in hydrated choline dihydrogen phosphate is a result of this type of ion combination. Though the stability of proteins in ILs can be explained by the Hofmeister series, some deviations were also found while following a reverse trend [31]. In fact, a large array of factors is responsible for the protein stability in aqueous solutions of ILs, such as the ability to establish hydrogen bond, electrostatic, and dispersive interactions and hydrophobicity.
6.2.2 Partitioning Behavior of Proteins in IL-Based ABS
It has been shown that the partitioning of solutes in typical PEG-based ABS is primarily governed by the system properties, such as type and concentration of phase-forming components, pH, and temperature and the solute properties, such as hydrophobicity, charge, molecular weight, etc. [35]. Thus, partition coefficients and selectivity can be tuned by modifying these parameters. The extraction of proteins using IL-based ABS has been studied by several authors (see Table 6.1), revealing that protein partition preferentially to the IL-rich phase. Most of these studies are however empirical, and to be able to use IL-based ABS as a separation tool on a preparative scale, it is mandatory to understand the mechanisms and factors influencing the partitioning of proteins in these systems.
Different authors have studied the influence of the phase-forming components, concentration, pH, and temperature on the partitioning of proteins in IL-based ABS [25, 36–38]. Protein distribution in ABS depends on their ability to interact with the phase-forming components and extraction conditions, so that the separation could be protein specific. Cao et.al. [39] studied the extraction of horseradish peroxidase in four alkylimidazolium-based ABS. The enzyme partitioned to the IL-rich phase, but its activity decreases with the increase in the alkyl side chain length of the IL. In the same study, increasing the IL concentration favors the maintenance of the enzyme activity. Dreyer et al. [13] studied the feasibility of ABS formation with ammonium-based ILs and showed that Ammoeng 110 forms ABS more easily than Ammoeng 100 and Ammoeng 101. Ammoeng ILs contain an oligo-ethylene side chain in the cation which was expected to have a stabilizing effect on the enzyme (alcohol dehydrogenase) extracted. A low temperature for ABS formation together with ILs with oligo-ethylene side chains demonstrated to provide a gentle environment for protein extractions [13]. Desai et al. [25] showed that the partition coefficient of RuBisCo increases as the IL and salt concentration increases; however, a decrease in the enzyme activity was observed with higher concentrations (>20 %, w/w) of IL. In summary, all these results indicate that the chaotropicity of the IL and its concentration influence the stability of the protein to be extracted.
The system parameters (pH and temperature) also influence the partitioning of proteins to the IL-rich phase through the modification on the protein charge and surface properties. Protein properties contributing to their partitioning in ABS can be summarized as follows [5]:
where K is the partition coefficient and el, hphob, biosp, and conf are, respectively, electrostatic, hydrophobicity, biospecificity, and configuration which contribute to the partition coefficient value, while K0 represents additional factors.
Partitioning of proteins is governed, in a large extent, by the pH of the system. Depending on their isoelectric point, proteins carry a net positive or net negative charge at a given pH. The extraction of BSA, myoglobin, lysozyme, and trypsin using IL-based ABS at different pH values showed that proteins are preferentially transferred to the IL-rich phase as the pH increases [18]. On the other hand, the molecular weight of the protein also influences its partitioning in the biphasic system. Dreyer et al. [18] showed that larger proteins, such as BSA, are better extracted in the IL-rich phase, while smaller proteins, like myoglobin, remain in the salt-buffer-rich phase. In a separate study, RuBisCo, which is large protein (540 kDa), is also extracted into the IL-rich phase [25].
Du et al. [40] studied the extraction of BSA from biological fluids using imidazolium-based ABS and observed that the electrostatic interactions and salting-out effect are the driving forces in protein partitioning. In summary, both research groups [18, 40] have shown that there is a strong correlation between the protein charge and its partitioning in IL-based ABS. Thus, indicating electrostatic interaction between the amino acids on the protein surface and IL cations to be the main driving force. On the other hand, Pei et al. [41] have shown that hydrophobic interactions are the main driving force for protein extraction in IL-based ABS. In the same study, the influence of temperature on the extraction of BSA was evaluated demonstrating that higher temperatures favor the partitioning of proteins to the IL-rich phase.
It could be summarized that the partitioning of proteins in IL-based ABS can be tuned by changing the phase components and the composition, pH, and temperature of the system. Nevertheless, partitioning in IL-based ABS is a quite complex phenomenon not influenced by a single factor, yet it is a result of a combined effect of these factors.
6.3 Recovery of Proteins from the IL-Rich Phase
Like conventional ABS, protein extraction using IL-based ABS involves two main steps: (i) forward extraction, i.e., extraction of the protein from the initial source/matrix into one of the phases (here, IL-rich phase), and (ii) recovery of the (purified) protein from the IL-rich phase.
In conventional ABS, proteins can be recovered by modification of system parameters, such as pH, change in salt concentration, or addition of other salts. The main goal is to achieve a high recovery of a protein with a high purity level without affecting the functionality of the protein. This is indeed one of the major lacunas in the literature since there are almost no attempts on the literature to this end. An isolated work was recently published by Pereira et al. [42] where the protein (BSA) was recovered by dialysis from the IL-rich phase, allowing the further use of the ABS in a new extraction step. The authors [42] demonstrated the recovery of the protein and the IL reusability in three-step consecutive extractions, concluding that IL-based ABS can be adequately reused without losses on their extraction performance.
ILs being salts and proteins being macromolecules, their separation can be achieved by ultrafiltration and/or nanofiltration, induced precipitation, and chromatographic techniques, such as size exclusion chromatography and by the use of affinity tags (HisTags) able to help in recovering the protein from the IL-rich phase by Immobilized Metal Affinity Chromatography (IMAC). Protein recovery studies are thus one of the major lacunas in the IL-based ABS field and must be investigated in the near future.
6.4 Conclusions and Future Perspectives
IL-based ABS are a promising platform for the extraction and purification of proteins. However, there are still some issues which need to be addressed to be able to use IL-based ABS on a commercial scale, namely:
-
1.
Currently, studies on proteins of commercial importance are scarce; only few studies were performed, for instance, for RuBisCo and alcohol dehydrogenases. Most studies in the literature address model proteins (BSA, lysozyme, etc.).
-
2.
With a plethora of ILs available and the complex and variable nature of proteins, it is difficult to generalize or to predict the behavior of proteins in IL-based ABS. However, the setup of a well-defined guideline with respect to some protein classes would be useful. A mechanistic modeling approach still seems to be far off.
-
3.
Stability of proteins in ILs is the prime requirement to guarantee the viability of IL-based ABS for protein separation. Most studies on this line are focused on model proteins, such as BSA and lysozyme. A pragmatic approach would be to create a public and free available (online) database with respect to the functional stability of commercial proteins in IL-based systems.
-
4.
More sophisticated analytical methods to quantify proteins in the IL-rich phase should be attempted to avoid interferences from the IL. Also for preparative chromatography, the stability and functionality of currently available resins need to be determined.
-
5.
The high costs of ILs are one of the major drawbacks when envisaging the large-scale application of IL-based ABS. The reuse of ILs in large-scale applications is essential to guarantee the economic viability.
-
6.
The ILs used for ABS formation are water-soluble and hence can enter into the ecosystem. Thus, toxicity and biodegradation of ILs pose another concern and must be considered while designing protein extraction and separation processes.
All these points require not only extra efforts to study different IL-based ABS but more focused studies on the use of biodegradable and biocompatible ILs and efficient IL recycling processes. Since polymers, such as PEG, are able to maintain and even increase the stability of some proteins, IL-PEG ABS seem as an interesting option for protein extraction. Progress in IL-based ABS would open up new applications on their use, especially in biorefinery of third-generation biomass feedstocks (e.g., microalgae), where proteins could be separated from more hydrophobic components. IL-based ABS are novel systems and their use for protein extraction is still in an early stage. Thus, there is ample scope for improvement in protein extractions using IL-based ABS and a strong requirement for further in-depth investigations.
References
Kiron V, Phromkunthong W, Huntley M, Archibald I, De Scheemaker G (2012) Marine microalgae from biorefinery as a potential feed protein source for Atlantic salmon, common carp and whiteleg shrimp. Aquac Nutr 18(5):521–531. doi:10.1111/j.1365-2095.2011.00923.x
Schwenzfeier A, Wierenga PA, Gruppen H (2011) Isolation and characterization of soluble protein from the green microalgae Tetraselmis sp. Bioresour Technol 102(19):9121–9127. doi:http://dx.doi.org/10.1016/j.biortech.2011.07.046
Wijffels RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science 329(5993):796–799. doi:10.1126/science.1189003
Labrou NE (2014) Protein downstream processing: design, development and application of high and low-resolution methods. Humana Press, Totowa
Albertsson P-A (1958) Partition of proteins in liquid polymer-polymer two-phase systems. Nature 182(4637):709–711
Rosa PAJ, Azevedo AM, Sommerfeld S, Bäcker W, Aires-Barros MR (2011) Aqueous two-phase extraction as a platform in the biomanufacturing industry: economical and environmental sustainability. Biotechnol Adv 29(6):559–567. doi:http://dx.doi.org/10.1016/j.biotechadv.2011.03.006
Rosa PAJ, Ferreira IF, Azevedo AM, Aires-Barros MR (2010) Aqueous two-phase systems: a viable platform in the manufacturing of biopharmaceuticals (extraction techniques). J Chromatogr A 1217(16):2296–2305. doi:http://dx.doi.org/10.1016/j.chroma.2009.11.034
Gutowski KE, Broker GA, Willauer HD, Huddleston JG, Swatloski RP, Holbrey JD, Rogers RD (2003) Controlling the aqueous miscibility of ionic liquids: aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J Am Chem Soc 125(22):6632–6633. doi:10.1021/ja0351802
Yang Z (2012) Ionic liquids and proteins: academic and some practical interactions. In: Ionic liquids in biotransformations and organocatalysis. Wiley, pp 15–71. doi:10.1002/9781118158753.ch2
Freire MG, Pereira JFB, Francisco M, Rodríguez H, Rebelo LPN, Rogers RD, Coutinho JAP (2012) Insight into the interactions that control the phase behaviour of new aqueous biphasic systems composed of polyethylene glycol polymers and ionic liquids. Chem Eur J 18(6):1831–1839. doi:10.1002/chem.201101780
Wu B, Zhang YM, Wang HP (2008) Aqueous biphasic systems of hydrophilic ionic liquids + sucrose for separation. J Chem Eng Data 53(4):983–985. doi:10.1021/je700729p
Zhang J, Zhang Y, Chen Y, Zhang S (2007) Mutual coexistence curve measurement of aqueous biphasic systems composed of [bmim][BF4] and glycine, l-serine, and l-proline, respectively. J Chem Eng Data 52(6):2488–2490. doi:10.1021/je0601053
Dreyer S, Kragl U (2008) Ionic liquids for aqueous two-phase extraction and stabilization of enzymes. Biotechnol Bioeng 99(6):1416–1424. doi:10.1002/bit.21720
Freire MG, Cláudio AFM, Araújo JMM, Coutinho JAP, Marrucho IM, Lopes JNC, Rebelo LPN (2012) Aqueous biphasic systems: a boost brought about by using ionic liquids. Chem Soc Rev 41(14):4966–4995
Zhang Y, Zhang S, Chen Y, Zhang J (2007) Aqueous biphasic systems composed of ionic liquid and fructose (4th MTMS 4th international symposium on molecular thermodynamics and molecular simulation). Fluid Phase Equilib 257(2):173–176. doi:http://dx.doi.org/10.1016/j.fluid.2007.01.027
Ding X, Wang Y, Zeng Q, Chen J, Huang Y, Xu K (2014) Design of functional guanidinium ionic liquid aqueous two-phase systems for the efficient purification of protein. Analytica Chimica Acta 815(0):22–32. doi:http://dx.doi.org/10.1016/j.aca.2014.01.030
Zeng Q, Wang Y, Li N, Huang X, Ding X, Lin X, Huang S, Liu X (2013) Extraction of proteins with ionic liquid aqueous two-phase system based on guanidine ionic liquid. Talanta 116(0):409–416. doi:http://dx.doi.org/10.1016/j.talanta.2013.06.011
Dreyer S, Salim P, Kragl U (2009) Driving forces of protein partitioning in an ionic liquid-based aqueous two-phase system. Biochem Eng J 46(2):176–185
Pei Y, Wang J, Wu K, Xuan X, Lu X (2009) Ionic liquid-based aqueous two-phase extraction of selected proteins. Sep Purif Technol 64(3):288–295
Constantinescu D, Weingärtner H, Herrmann C (2007) Protein denaturation by ionic liquids and the Hofmeister series: a case study of aqueous solutions of ribonuclease A. Angew Chem Int Ed 46(46):8887–8889. doi:10.1002/anie.200702295
Takekiyo T, Yamazaki K, Yamaguchi E, Abe H, Yoshimura Y (2012) High ionic liquid concentration-induced structural change of protein in aqueous solution: a case study of lysozyme. J Phys Chem B 116(36):11092–11097. doi:10.1021/jp3057064
Zhang W-G, Wei D-Z, Yang X-P, Song Q-X (2006) Penicillin acylase catalysis in the presence of ionic liquids. Bioprocess Biosyst Eng 29(5–6):379–383. doi:10.1007/s00449-006-0085-9
Nara SJ, Harjani JR, Salunkhe MM (2002) Lipase-catalysed transesterification in ionic liquids and organic solvents: a comparative study. Tetrahedron Letters 43(16):2979–2982. doi:http://dx.doi.org/10.1016/S0040-4039(02)00420-3
Li Z, Pei Y, Wang H, Fan J, Wang J (2010) Ionic liquid-based aqueous two-phase systems and their applications in green separation processes (bioCop – monitoring chemical contaminants in foods). TrAC Trends Anal Chem 29(11):1336–1346. doi:http://dx.doi.org/10.1016/j.trac.2010.07.014
Desai R, Streefland M, Wijffels RH, Eppink M (2014) Extraction and stability of selected proteins in ionic liquid based aqueous two phase systems. Green Chem 16:2670. doi:10.1039/c3gc42631a
Baker GA, Heller WT (2009) Small-angle neutron scattering studies of model protein denaturation in aqueous solutions of the ionic liquid 1-butyl-3-methylimidazolium chloride (application of ionic liquids in chemical and environmental engineering). Chem Eng J 147(1):6–12. doi:http://dx.doi.org/10.1016/j.cej.2008.11.033
Weaver KD, Vrikkis RM, Van Vorst MP, Trullinger J, Vijayaraghavan R, Foureau DM, McKillop IH, MacFarlane DR, Krueger JK, Elliott GD (2012) Structure and function of proteins in hydrated choline dihydrogen phosphate ionic liquid. Phys Chem Chem Phys 14(2):790–801
Fujita K, MacFarlane DR, Forsyth M, Yoshizawa-Fujita M, Murata K, Nakamura N, Ohno H (2007) Solubility and stability of cytochrome c in hydrated ionic liquids: effect of oxo acid residues and kosmotropicity. Biomacromolecules 8(7):2080–2086. doi:10.1021/bm070041o
Fukaya Y, Hayashi K, Wada M, Ohno H (2008) Cellulose dissolution with polar ionic liquids under mild conditions: required factors for anions. Green Chem 10(1):44–46. doi:10.1039/b713289a
Kilpeläinen I, Xie H, King A, Granstrom M, Heikkinen S, Argyropoulos DS (2007) Dissolution of wood in ionic liquids. J Agric Food Chem 55(22):9142–9148. doi:10.1021/jf071692e
Debeljuh N, Barrow CJ, Byrne N (2011) The impact of ionic liquids on amyloid fibrilization of A[small beta]16-22: tuning the rate of fibrilization using a reverse Hofmeister strategy. Phys Chem Chem Phys 13(37):16534–16536. doi:10.1039/c1cp22256b
Zhao H (2005) Effect of ions and other compatible solutes on enzyme activity, and its implication for biocatalysis using ionic liquids. J Mol Catal B Enzym 37(1–6):16–25. doi:10.1016/j.molcatb.2005.08.007
Baldwin RL (1996) How Hofmeister ion interactions affect protein stability. Biophys J 71(4):2056–2063. doi:S0006-3495(96)79404-3 [pii] 10.1016/S0006-3495(96)79404-3
Collins KD, Washabaugh MW (1985) The Hofmeister effect and the behaviour of water at interfaces. Q Rev Biophys 18(4):323–422
Albertsson PÅ (1986) Partition of cell particles and macromolecules: separation and purification of biomolecules, cell organelles, membranes, and cells in aqueous polymer two-phase systems and their use in biochemical analysis and biotechnology. Wiley, New York [etc.]
Lin X, Wang Y, Zeng Q, Ding X, Chen J (2013) Extraction and separation of proteins by ionic liquid aqueous two-phase system. Analyst 138(21):6445–6453. doi:10.1039/c3an01301d
Chen J, Wang Y, Zeng Q, Ding X, Huang Y (2014) Partition of proteins with extraction in aqueous two-phase system by hydroxyl ammonium-based ionic liquid. Anal Methods 6(12):4067–4076. doi:10.1039/c4ay00233d
Dreyer SE (2008) Aqueous two phase extraction of proteins and enzymes using tetraalkylammonium-based ionic liquids. PhD thesis, University of Rostock
Cao Q, Quan L, He C, Li N, Li K, Liu F (2008) Partition of horseradish peroxidase with maintained activity in aqueous biphasic system based on ionic liquid. Talanta 77(1):160–165. doi:http://dx.doi.org/10.1016/j.talanta.2008.05.055
Du Z, Yu Y-L, Wang J-H (2007) Extraction of proteins from biological fluids by use of an ionic liquid/aqueous two-phase system. Chem Eur J 13(7):2130–2137. doi:10.1002/chem.200601234
Pei YC, Liu L, Li ZY, Wang JJ, Wang HY (2010) Selective separation of protein and saccharides by ionic liquids aqueous two-phase systems. SCIENCE CHINA Chem 53(7):1554–1560. doi:10.1007/s11426-010-4025-9
Pereira MM, Pedro SN, Quental MV, Lima AS, Coutinho JAP, Freire MG (2015) Enhanced extraction of bovine serum albumin with aqueous biphasic systems of phosphonium- and ammonium-based ionic liquids. J Biotechnol 206:17–25
Ruiz-Angel MJ, Pino V, Carda-Broch S, Berthod A (2007) Solvent systems for countercurrent chromatography: an aqueous two phase liquid system based on a room temperature ionic liquid (4th international conference on countercurrent chromatography). J Chromatogr A 1151(1–2):65–73
Wu C, Wang J, Wang H, Pei Y, Li Z (2011) Effect of anionic structure on the phase formation and hydrophobicity of amino acid ionic liquids aqueous two-phase systems. J Chromatogr A 1218(48):8587–8593. doi:http://dx.doi.org/10.1016/j.chroma.2011.10.003
Wu C, Wang J, Li Z, Jing J, Wang H (2013) Relative hydrophobicity between the phases and partition of cytochrome-c in glycine ionic liquids aqueous two-phase systems. J Chromatogr A 1305(0):1–6. doi:http://dx.doi.org/10.1016/j.chroma.2013.06.066
Yan J-K, Ma H-L, Pei J-J, Wang Z-B, Wu J-Y (2014) Facile and effective separation of polysaccharides and proteins from Cordyceps sinensis mycelia by ionic liquid aqueous two-phase system. Sep Purif Technol. doi:http://dx.doi.org/10.1016/j.seppur.2014.03.020
Deive FJ, Rodríguez A, Rebelo LPN, Marrucho IM (2012) Extraction of Candida antarctica lipase A from aqueous solutions using imidazolium-based ionic liquids (ILSEPT2011 special issue). Sep Purif Technol 97(0):205–210. doi:http://dx.doi.org/10.1016/j.seppur.2011.12.013
Ventura SPM, Sousa SG, Freire MG, Serafim LS, Lima ÁS, Coutinho JAP (2011) Design of ionic liquids for lipase purification. J Chromatogr B 879(26):2679–2687. doi:http://dx.doi.org/10.1016/j.jchromb.2011.07.022
Deive FJ, Rodriguez A, Pereiro AB, Araujo JMM, Longo MA, Coelho MAZ, Lopes JNC, Esperanca JMSS, Rebelo LPN, Marrucho IM (2011) Ionic liquid-based aqueous biphasic system for lipase extraction. Green Chem 13(2):390–396. doi:10.1039/c0gc00075b
Jiang B, Feng Z, Liu C, Xu Y, Li D, Ji G (2014) Extraction and purification of wheat-esterase using aqueous two-phase systems of ionic liquid and salt. J Food Sci Technol 1–8. doi:10.1007/s13197-014-1319-5
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Desai, R.K., Streefland, M., Wijffels, R.H., Eppink, M.H.M. (2016). Extraction of Proteins with ABS. In: Freire, M. (eds) Ionic-Liquid-Based Aqueous Biphasic Systems. Green Chemistry and Sustainable Technology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-52875-4_6
Download citation
DOI: https://doi.org/10.1007/978-3-662-52875-4_6
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-52873-0
Online ISBN: 978-3-662-52875-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)