Abstract
This study aims to investigate if neural oscillations can play a role as a bridge between GABAergic systems and emotional behaviors. Mice were divided into streptozotocin-induced diabetes mellitus (DM) group and control (CON) group. After 8 weeks of successfully modeling, the diabetic mice exhibited depression and anxiety, while the GABAAR \( \alpha_{1} \) subunit expression and the GABA level were significantly changed as well. There were increase power percent at gamma (50–80 Hz) band in the power spectrum between these two groups. However, DM attenuated the identical-frequency strength of phase synchronization and information flow at both theta (8–13 Hz) and gamma rhythms, and reduced the theta-gamma phase-amplitude coupling strength either in the PP & DG regions or on PP-DG pathway. It suggests that DM changes the pattern of neural oscillations by modulating the GABAAR \( \alpha_{1} \) subunit expression and the GABA level in depression and anxiety in diabetic mice.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Neural oscillations are generated by interaction and influence of neurons with each other through excitatory and inhibitory synaptic connections. As known, the classification for oscillations is usually identified with different frequencies, such as theta (3–13 Hz) and gamma (30–100 Hz) [1]. Each frequency has its own distinctive effect on corresponding to different physiological functions. GABA and its receptors, play an key role in neural oscillations and fundamentally implicate in modulating all of frequency bands [2]. However, the role of neural oscillations, in which depressive-like behaviors are induced by GABAergic deficits in diabetic rodents, is yet to be elucidated. In the study, a hypothesis was raised that neural synchronization as the critical “middle ground” connecting GABAergic system with emotional behaviors could play a role of bridge between behaviors and molecular biology. Accordingly, the diabetic mouse-model was established and the alterations of emotional behaviors were examined. The protein expression of GABAAR \( \alpha_{1} \) subunit and the concentration of GABA were determined. In order to represent a potential neural mechanism, the functional role of neural oscillations was extensively investigated.
2 Materials and Methods
2.1 Animals and Treatment
In the study, eighteen male C57BL/6 J mice (22–24 g, around 7–8 week-old) were used, which were purchased from the Laboratory Animal Center, Academy of Military Medical Science of People’s Liberation Army. Mice were reared in a specific pathogen free house with a 12 h light-dark cycle (8 am–8 pm), and they were free to food and water under a constant temperature (22 ± 1 ℃) and about 50% humidity. Protocols were approved by the Animal Research Ethics Committee, School of Medicine, Nankai University. And all efforts were made to minimize the number of animals used and their suffering. Eighteen mice were randomly divided into control group (CON group, n = 10) and diabetes mellitus group (DM group, n = 8). Mice in the DM group were intraperitoneally injected 55 mg/kg/day streptozotocin (STZ) for consecutive five days. At the same time, mice in the CON group were intraperitoneally injected the same volume of sterile citrate buffer. On the 8th day, the glucose concentration of blood was detected. If the glucose level was not lower than 16.7 mM, mice were identified as hyperglycemia.
2.2 Biological Experiments
Behavioral tests including elevated plus maze, tail suspension and forced swimming were performed. Then, electrophysiological experiments were carried out at the end of the behavioral experiments. The signals of local field potential (LFPs) were recorded from both the hippocampal perforant path (PP) and dentate gyrus (DG) regions. At last, molecular biological tests including Western and HPLC were performed. The more details cold be seen.
2.3 Neurodynamic Analysis
The following approaches provide the neural oscillations assessment at theta frequency band (8–13 Hz) and gamma frequency band (50–80 Hz).
Power Spectrum Density (PSD) Analysis.
PSD analysis was used to test the power changes of different frequency in both the hippocampal PP and DG areas. Multitaper Spectral Estimation is one of the most classical methods to perform PSD analysis. Its advantage is that sequence data analysis can be performed by using an optimal set of orthogonal localization windows (Slepian windows) to obtain direct spectrum and average spectral estimation, thereby achieving a smaller variance and bias properties for the estimation result.
Phase Locking Value (PLV).
Phase synchronization of oscillations at an identical frequency could promote neural communication [3]. PLV is a great identical frequency algorithm to measure the strength of phase synchronization by the mean value of a composite of phase difference between two brain areas. Before extracting the phase of two signals, the original LFPs were filtered into theta and gamma frequency bands by the mean of eegfilt.m from EEGLAB toolbox [4]. The bandwidth was set to 1 Hz incremented in 1 Hz steps. Afterward, the instantaneous phases of filtered LFPs (theta, gamma) were obtained by Hilbert transform from the hippocampal PP and DG areas in all these frequency bands. N was for the length of the signal. Then PLV was given as
Generalized Partial Directed Coherence (gPDC).
The gPDC algorithm has been developed to assess the directional coupling in an identical frequency based on multivariate vector autoregressive model [5]. Actually, its meaning is the Granger causality in the frequency domain. The method is characterized by analysis based on the signal itself and direction recognition of information flow.
Mean Vector Length (MVL).
Phase-amplitude coupling (PAC) has been by far the most commonly reported in recent years as one of cross-frequency coupling types. In order to assess the strength of PAC, the MVL method was employed in both the hippocampal PP and DG areas as well as PP-DG pathway [6].
3 Results
3.1 DM Induced Depression and Changed GABAAR \( \alpha_{1} \) and GABA
There was significant difference of FST immobility between these two groups (t 8.43 = −4.804, P = 0.001). Moreover, the time of immobility in the DM group was higher than that in the CON group (t 8.84 = −3.810, P = 0.004), which was consistent with the results obtained from the FST. DM markedly decreased the expression of GABAAR \( \alpha_{1} \) subunit to 0.390 ± 0.028. The level of GABA in the hippocampus showed that there was significant difference between the CON group and the DM group (Z = −39.273, P < 0.001). The level of GABA in the CON group was 1.28 ± 0.23 μg/mg protein. However, the level of GABA was increased to 5.63 ± 0.13 μg/mg protein in the DM group.
3.2 Power Percent Increased at Gamma Band but Not at Theta Band
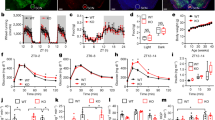
PSD analysis showed that DM decreased the power percent of gamma (t 16 = −2.787, P = 0.013) bands while there were no statistical changes of power percent at theta (t 16 = 1.857, P = 0.082) bands in the hippocampal PP area (Fig. 1C Left). The similar results were also obtained from PSD estimating in the DG area at theta rhythm (t 8.85 = 0.174, P = 0.866) and gamma rhythm (Z = −2.488, P = 0.012) frequency bands (Fig. 1C Right).
There were significant changes of power at gamma frequency bands but not at theta bands between the CON group and the DM group. (A) and (B) were representative power spectrum in the CON and DM groups resulting from 40 s window at 1 Hz to 80 Hz, respectively. (C) The statistical results of power spectrum distribution in PP (left) and DG (right) areas. The n.s. represents no significant differences of power between the CON group and the DM group.
3.3 Strength of Phase Synchronization Was Impaired by DM on PP-DG Pathway
PLV measurement showed that the strength of phase synchronization was slightly decreased at theta frequency band in the DM group compared to that in the CON group (t 9.02 = 2.105, P = 0.065). However, there was significant difference of the strength of phase synchronization between these two groups in gamma band (t 16 = 2.468, P = 0.025, Fig. 2).
3.4 Direction of Coupling Was Significantly Altered by DM on PP-DG Pathway
Figure 3 showed the gPDC analysis of PP-DG pathway in the CON and DM groups over theta, gamma bands. The group values for the unidirectional influence from the PP to DG were presented in Fig. 3A. It was found that the unidirectional influence indexes in all three frequency bands were considerable decreased in the diabetic state, and the statistical differences between the CON group and the DM group were found in the theta band (Z = −3.554, P < 0.001) and gamma band (t 16 = 7.825, P < 0.001), respectively (Fig. 3A). Interestingly, bidirectional coupling indexes between PP and the DG were greatly altered from positive values to negative values at the same bands (theta: t 7.79 = 9.168, P < 0.001; gamma: t 16 = 7.935, P < 0.001, Fig. 3B). Together with above indexes, it could be inferred that DM significantly attenuated unidirectional information flow from PP to DG, and further altered the direction of bidirectional information flow.
The gPDC index represented the changes of coupling direction in an identical frequency band on PP-DG pathway in DM mice. (A) Decreased unidirectional coupling gPDC index from PP to DG in DM mice. (B) Altered bidirectional gPDC index in DM mice at these three frequency bands. **P < 0.01 and ***P < 0.001 represent significant differences between the CON group and the DM group.
3.5 Strength of Phase-Amplitude Coupling Was Significantly Impaired by DM
In order to measure cross-frequency coupling between PP and DG areas, MVL algorithm was employed. There was a clear and strong theta-gamma PAC in the CON group. (Figure 4A). Moreover, the strength of PAC was obviously decreased in the DM group (Fig. 4A-right column). Again the similar changes of PAC were in either PP or DG area. Statistical analysis showed that the coupling strength between theta and gamma was significantly reduced in the PP area (Z = −2.132, P = 0.033, Fig. 4B), and the DG area (Z = −2.932, P = 0.003, Fig. 4D). Most importantly, the strength of PAC between PP and DG areas was also considerably decreased (Z = −3.465, P = 0.001, Fig. 4C).
Reduced MVL index at cross-frequency (theta-gamma) between the hippocampal PP and DG regions as well as PP-DG pathway in DM mice. (A) Representative PAC of PP-DG pathway between PP low frequency phase (6–14 Hz) and DG gamma band amplitude (30–80 Hz) in a 40 s window of one mouse in the CON group (left) and another in the DM group (right). (B–D) The statistical results of theta and gamma cross-frequency measured by MVL in PP (B), PP-DG pathway (C), and DG (D), respectively. *P < 0.05, **P < 0.01 and ***P < 0.001 represent significant differences of the indexes between the CON group and the DM group.
4 Discussion
4.1 Diabetes and Depression
The deleterious effects of diabetes on the CNS could cause the impairment of cognitive functions, including learning, memory, problem solving, informative proposal, mental and psychomotor speed in the patients [7]. In particular, the risk of depression incident is twice in diabetes than that of counterparts, and the rates of depression in diabetes are in the range of 11% to 13.5% a share. A battery of neuropsychological and neurobehavioral changes in diabetic subjects have been discovered on the experimental animals. In the present study, it was found that mice spend less time in the open arms, presented less enteries into open arms, and had a lower percent of enteries into open arms of EPM in the DM group compared to that in the CON group. It indicated that mice became higher anxiety in DM group, which was consistent with Tang’s results. Moreover, our data showed that mice in the DM group spent much longer time of immobility in FST and TST, which was in accordance with the results of other research groups [8].
4.2 GABAergic System and Diabetic Encephalopathy-Related Depression
GABA, as one of the most important inhibitory amino acid neurotransmitters of CNS, almost affects all neuronal activities, and about 1/3 of the neurons employ GABA as their primary neurotransmitter. The relationship between GABA and depression is widely investigated in both human and animals. In comparable animal models, rats exposed to CMS had lower hippocampal GABA levels accompanied by depression-like symptoms [9]. A previous study reported that there was lower basal striatal GABA levels in the experiment of STZ-induced diabetic rats [10]. GABAergic drugs were able to reverse these depressive-like behaviors, suggesting that GABA treatment protected against the development of diabetic complications in STZ-induced diabetic rats [11]. On the contrary, GABA level is obviously increased in the cerebrospinal fluid of diabetic rats. Consequently, it is speculated that DM and depression induced the species-specific, cerebral area-specific, and tissues-specific abnormal concentration of GABA. As we known, most ionotropic GABAARs are comprised of two \( \alpha \), one \( \beta \), and two \( \gamma \) subunits. GABAergic effects are mediated via ionotropic GABAARs, which are functionally defined by their \( \alpha \) subunits (\( \alpha_{1} { - }\alpha_{6} \)). Repeated swim-stress reduces GABAAR \( \alpha \) subunit mRNAs in the mouse hippocampus [12]. Furthermore, decreased GABAAR clustering results in enhanced anxiety in mice heterozygous for the \( \gamma_{2} \) subunit. But how about the protein expression of GABAAR \( \alpha_{1} \) subunit in diabetic mice still poorly understood. In this study, the protein expression of GABAAR \( \alpha_{1} \) subunit was dramatically reduced in hippocampus of diabetic mice compared to that of the non-diabetic mice.
4.3 A Role of Neural Oscillations as a Bridge Between GABAergic System and Emotional Behaviors
If the well-studied of GABAergic system was viewed as a “small ground” with molecular level, and emotional behavior was viewed as a “large ground” with behavioral level, then neural oscillations may be as a bridge between them. Based on this hypothesis, both theta and gamma frequencies in the hippocampus were chosen to be the bonds investigating mechanism in diabetic encephalopathy. Neural oscillations can be measured at different levels or scales, such as large-scale electroencephalographic (EEG), electrocorticogram (ECoG), and magnetoencephalography (MEG) recordings, medium-scale LFPs, and small-scale action potential recordings. All of them are viewed as the critical “middle ground” linking molecule to behavior. In the study, the data showed that both the strength of phase coupling (Fig. 2) and the strength of unidirectional coupling (Fig. 3A) on gamma between the hippocampal PP and DG was considerably decreased by DM, suggesting that both low and high gamma rhythms were implicated in anxiety-like and depression-like emotional behavior in DM mice. Moreover, gamma-band rhythmogenesis is a synchronous activity of fast-spiking inhibitory interneurons, with the resulting rhythmic inhibition producing neural ensemble synchrony by generating a narrow window for effective excitation. When applying the GABAAR antagonist bicuculline to the hippocampal slice in vitro, gamma rhythmic would be blocked. Our study also provided an evidence that the decreased expression of GABAAR \( \alpha_{1} \) subunit and the compensative increased GABA level were able to generate above kind of change at gamma frequency band in the hippocampus of DM mice.
References
Lakatos, P., et al.: An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J. Neurophysiol. 94, 1904–1911 (2005)
Vijayan, S., et al.: Thalamocortical mechanisms for the anteriorization of alpha rhythms during propofol-induced unconsciousness. J. Neurosci. Off. J. Soc. Neurosci. 33, 11070–11075 (2013)
Fell, J., Axmacher, N.: The role of phase synchronization in memory processes. Nat. Rev. Neurosci. 12, 105–118 (2011)
Delorme, A., Makeig, S.: EEGLAB: an open-source toolbox for analysis of EEG dynamics. J. Neurosci. Methods 134, 9–21 (2004)
Mi, X.C., et al.: Performance comparison between gPDC and PCMI for measuring directionality of neural information flow. J. Neurosci. Methods 227, 57–64 (2014)
Canolty, R.T., et al.: High gamma power is phase-locked to theta oscillations in human neocortex. Science 313, 1626–1628 (2006)
Wayhs, C.A.Y., et al.: GABAergic modulation in diabetic encephalopathy-related depression. Curr. Pharm. Des. 21, 4980–4988 (2015)
Tang, Z.J., et al.: Antidepressant-like and anxiolytic-like effects of hydrogen sulfide in streptozotocin-induced diabetic rats through inhibition of hippocampal oxidative stress. Behav. Pharmacol. 26, 427–435 (2015)
Grønli, J., et al.: Extracellular levels of serotonin and GABA in the hippocampus after chronic mild stress in rats. A microdialysis study in an animal model of depression. Behav. Brain Res. 181, 42–51 (2007)
Gomez, R., et al.: Lower in vivo brain extracellular GABA concentration in diabetic rats during forced swimming. Brain Res. 968, 281–284 (2003)
Nakagawa, T., et al.: Protective effects of γ-aminobutyric acid in rats with streptozotocin-induced diabetes. J. Nutr. Sci. Vitaminol. 51, 278–282 (2005)
Montpied, P., et al.: Repeated swim-stress reduces GABAA receptor α subunit mRNAs in the mouse hippocampus. Mol. Brain Res. 18, 267–272 (1993)
Acknowledgement
This work was supported by grants from the National Natural Science Foundation of China (11232005, 31171053).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this paper
Cite this paper
Zhang, T., Li, Q., Yang, Z. (2017). Neural Oscillations as a Bridge Between GABAergic System and Emotional Behaviors. In: Cong, F., Leung, A., Wei, Q. (eds) Advances in Neural Networks - ISNN 2017. ISNN 2017. Lecture Notes in Computer Science(), vol 10262. Springer, Cham. https://doi.org/10.1007/978-3-319-59081-3_66
Download citation
DOI: https://doi.org/10.1007/978-3-319-59081-3_66
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-59080-6
Online ISBN: 978-3-319-59081-3
eBook Packages: Computer ScienceComputer Science (R0)