Abstract
Since childbirth presents a significant risk factor for pathology occurrence of the pelvic floor, analysis of the phenomena involved during a vaginal delivery is a major issue in obstetrics and gynecology researches. Computational biomechanics tool dedicated to the delivery could help to understand the causes of injuries and predict the perineal lesion. From MRI images of four women at different terms of pregnancy, a parametric FE model is generated and allows to analyze the potential damage areas during childbirth, related to strain rate of anatomical structures. The influence of the geometry of levator ani muscle, head size, terms, and cephalic presentations are investigated. The geometrical refinement of anatomical structures influences the strain levels and helps to localized more precisely the most injured areas. Posterior cephalic presentation presents higher injury risk than the anterior one. Maternal geometry at different terms brings equivalent results contrary to the fetal head sizes that have an influence on the strain level and the potential damage induced. This multi-parametric investigation allows us to have a customizable and predictive tool evaluating the potential damages on the pelvis during delivery.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The pelvic floor mobility is linked to a complex equilibrium related to the mechanical properties of tissues and the geometry of the related organs [1]. Different anatomical structures seem to play a major contribution in such balance and life events, such as aging, pregnancy, or delivery that could affect the physiologic mobility and induces pathology such as genital prolapse (POP). As POP concerns 30% of the women population [2], understanding of these diseases is a major health issue. Multiparity related to vaginal delivery is now identified as a significant risk factor [3]. In addition, modification of the pelvic floor during pregnancy is also reported as a change of organs equilibrium, leading to an alteration of POP-Q (POP quantification system) [4]. The main hypothesis of the changes highlighted previously was the change in biomechanical properties of the pelvic tissues [5]. These observations bring us to study the sustainable structures such as muscles and ligaments for a better understanding of the involved phenomena.
Furthermore, many studies are interested in perineal avulsion that could be classified into four categories, with first-degree corresponding to laceration on superficial perineal skin and fourth-degree is used for the most critical form [6]. These different forms of perineal trauma occurred on 85% of women having vaginal birth and 60% receive stitches [7]. Literature reveals also a link between occurrence of levator ani muscle (LAM) trauma and POP pathology [8, 9]. It is interesting to notice also significant differences on LAM defects between nulliparous patient and primiparous women with vaginal delivery [10]. No LAM defects are observed on nulliparous against 20% on primiparous women with vaginal delivery (71% of women with LAM trauma present incontinence).
Finite element method, coupled with mechanical behavior models of biological structures, is commonly used in biomechanics to investigate strains and stresses occurred on human body. In gynecology/obstetric, FE simulation allows us to analyze the mobility of the pelvic system in physiological and pathological conditions [1, 11]. Further studies are also reported in the literature concerning the pelvic system during childbirth and more precisely the deformations that occurred on muscle of the pelvic floor [12, 13]. However, these studies are often represented by simplified models of anatomy and rarely focused on the local geometry of pelvic structures. It would be interesting to develop an FE model of the whole pelvic system with refined geometry on sustainable structure such as LAM to access to precise quantifiable measurements. Such a tool will help to evaluate the behavior of anatomical structures under different loading conditions and appears to be relevant to assess the damages and predict the risk of perineal lesions during delivery following different scenarios.
2 Materials and Methods
The approach engaged in this study follows a classical protocol involving the data acquisition from pregnant woman through clinical MRI, modeling the anatomical structures involved and generating an FE model. We have focused on several relevant parameters to better understand the strain levels induced on the pelvic floor during childbirth; this model is set to match different delivery scenarios or morphology of the patient and fetus.
2.1 MRI Protocol to 3D Representation
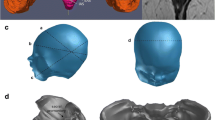
Different MRI sequences were performed on four pregnant primiparous women (institutional ethical approval CEROG OBS 2012-05-01 R1), without noticeable medical history and presenting a normal gynecologic examination (Fig. 1a). The MRI are performed on T1, T2, and proton density sequences under medical supervision. For each patient, five MRI are performed, distributed at different weeks of gestation (e.g., 16, 32, and 38) and 2 months and 1 year after delivery. These sequences allow us to obtain images in different incidences (axial, coronal, and sagittal). Thanks to the AVIZO Standard edition 7® (Visualization Sciences Group VSG, SAS) software, a manual thresholding is applied on these data to generate a 3D pelvic models composed by bone structures, organs, muscles, ligaments, and fetus (Fig. 1b).
(a) MRI at 38 weeks, (b) 3D reconstruction at 38 weeks with principal anatomic structure: (1) uterus, (2) fetal head, (3) bladder, (4) sacrum, (5) rectum, (6) uterosacral ligaments, (7) levator ani muscle, (8) vagina, (c) Comparison between LAM data from MRI and fitted surface, deviation analysis (black surface represents the interpolated geometry)
Eighteen structures are identified individually for each term in order to have representative models of pelvic system during pregnancy and on post-partum. Based on the four women, this applied protocol brings us to the opportunity to create 20 3D reconstruction of the entire pelvic to analyze the geometrical evolution during pregnancy and after, and to take into account the geometrical modification close to delivery time.
2.2 Geometrical Surfaces to FE Model
A protocol is developed to represent the geometrical changes such as cervical dilation which must be taken into consideration for FE simulation. Using a reconstruction software from the MRI is not sufficiently adapted to use the geometries in FE simulations. Manual work is needed to make geometric reconstructions compatible with the FE method. This intermediate step is performed using CATIA software (Dassault Systèmes) and consists in transforming each anatomical structure into a viable surface model for the FE meshing (Fig. 2a).
(a) Surface model reconstruction, (b) mapping of the LAM thicknesses at 38 weeks, with constant value and refinement with eight groups, (c) illustration of the points of reference on the LAM (R ul and R lr , related to the upper/lower and left/right directions, respectively) and boundary conditions on LAM (A—link with the pubic symphysis, B–link with obturator muscle, C—link with sacrospinous ligament, and D—link with the coccyx)
This step involves B-Spline curves on which surfaces can be interpolated to match with the geometry of the organs or muscles. An analysis of the deviation between these surfaces and reconstructions resulting from AVIZO was established to validate the process and guarantee the representativeness of the patient. The average deviation is about −0.06 mm, which allows us to have a relevant precision, in accordance with the tolerance induced by the voxel size (0.53 × 0.53 × 4.8 mm). Some geometries with thin structures, difficult to detect on MRI, are interpolated to avoid the holes (Fig. 1c). Imperfections from operator-dependent segmentation, reconstruction algorithms or software, are removed especially the stair-step artifact induced by the voxel size, a phenomenon commonly observed in such type of reconstruction method.
In order to develop an FE model, we used the 38 weeks models corresponding to the geometry 2 or 3 weeks before childbirth. Analysis of the geometry of each patient was made according to the term as well as to the fetal head. No significant differences have been observed on the bone structure. For muscle structures, the levator ani muscle (LAM) was individualized to consider each muscle structure (puborectalis, pubococcygeus, and iliococcygeus). During segmentation, observations on the MRI images revealed a change in the size of LAM. This observation was confirmed during the reconstruction and analysis of the geometrical model with an increase in thickness close to the delivery followed by a decrease during post-partum. As LAM is one of the anatomical structures impacted during delivery [14], this structure could highly damage and represents a priority analysis area to evaluate the risk of lesion. The thickness of the structures has been investigated during surface model definition. Special attention is paid to the creation of LAM geometry (Fig. 2b). In literature, many studies use surfaces with a constant thickness [12, 13] and few used a thickness variation [15, 16]. Since the principal aim is to analyze the areas where deformations are critical, consequently strongly influenced by the geometry of the model, it was necessary to develop a method to refine the geometry locally by increasing the mesh definition. A Python routine has been developed in order to attribute the real thickness in a shell formulation by computing the distance between each gravity center of element and external surface of muscle (Fig. 2b).
2.3 FE Simulation and Scenario
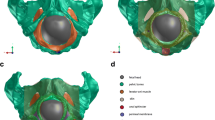
These models lead us to perform simulations with FE method into Abaqus/CAE 6.12–2 software (Dassault Systèmes Simulia Corp.). We generate an FE mesh of the geometrical model derived from MRI to 38 weeks. This model is established with 15,000 elements and a convergence study was performed to guarantee the quality of the mesh (Fig. 3a). We assign mechanical properties from preliminary works to organs and ligaments [17, 18]. One of the conclusions of these publications is to show that despite the intra and inter dispersions, the mechanical properties can be differentiated in the old women but remain statistically equivalent among young people. So we assume that the ligament properties were similar in a parturient. We favored a hyperelastic behavior on our simulation to compute strain more accurately than with linear elastic models [1]. In accordance with the mechanical properties of pelvic cavity, a C0 (0.24) and C1 (0.19) coefficients are employed to defined a hyperelastic behavior with large strain into ABAQUS (second order Yeoh model [19]). The bone structures are considered as a stiff deformable part (E = 10 GPa).
(a) FE model with anatomical sustainable structures with boundary conditions; (1) fetal head, (2) sacrum, (3) obturator internus muscle, (4) LAM iliococcygeus muscle, (5) sacrospinous ligament, (6) LAM pubococcygeus muscle, (7) skin limit, (b) illustration of the reference trajectory corresponding to a left occipitoanterior cephalic presentation (R s ratio related to the time of simulation)
Boundary conditions (Figs. 2c and 3a) for each structure suspension are equivalent to previous works already published [1]. During delivery, uterine contraction occurred but such a phenomenon is “poorly” described in literature and hard to quantify with experimental test. This lack of information leads to difficulties to consider muscle activation in our FE method approach. We decided to impose trajectory of the fetal head descent and not focus on the uterine contractions. The advantage of this approach is to employ parameter path to correspond to different scenarios and cephalic presentations.
The imposed trajectories allow to compare to one to each other and investigate their influence on the pelvic floor. The most common cephalic presentation is the left occipitoanterior (LOA), which occurred in 57% of delivery scenarios and is related to a longitudinal vertex presentation where the occiput is close to the pubis and faces to the left (Fig. 3b). A first internal rotation is performed at the beginning of the simulation to align the occiput to the pubic symphysis. The head then performs an important rotation (axis normal to the sagittal plane) during passage into the LAM. We decided to study this trajectory as a reference in the following parts. This presentation will be compared with a posterior position (occiput faces posteriorly) and more precisely the right occipitoposterior (ROP), occurred in 33% of delivery.
3 Result
3.1 Strain Analysis on Levator Ani Muscle
First part evaluates the influence of the muscle thickness on the computed strain level. Since a relationship between strain and material damage has been observed on experimental tests on pelvic organs [20], strain level is a relevant criterion to evaluate the avulsion risk. The first simulation is performed with a constant thickness on the LAM. The protocol of thickness allocation is applied to increase the representativeness of the model. To optimize the computing time, we chose to gather the elements with closed thicknesses by groups. Refinement groups used in this study correspond to 5, 8, and 12. Variable thicknesses influence the strain levels locally and are relevant to access to critical strain level (Fig. 4a). Maximal strain with constant thickness is about 81%, on the right side of muscle (R lr = 0.67), while 109% with variable model with 12 groups of refinement, located on the other side (R lr = 0.24). In addition with this result, we may notice that the zones mostly impacted change with the descent of fetal head in the birth canal. Obviously, the upper part (R ul <0.2) is more impacted at the beginning of the simulation than during the extraction of fetal head where lower part (R ul >0.6) suffers largest strain. This argument brings us to identify, thanks to the R ul , R lr , and R s ratio (Fig. 2c), the location of the critical position of the head which is configuration and time dependent.
Strain distribution on LAM depends on the thickness mapping. The comparison between constant and different groups of thickness shows that the simulation results converge in function of the number of employed groups. A configuration with eight groups of refinement is sufficient to take into account the precise level of strain (Fig. 4b). Since the global geometry and local distribution are not symmetric, taking account of the LAM thickness shows that some complications may occur rather on one side than another (R lr ratio). As expected, the higher strain occurs in the thinner regions (Figs. 2b and 4a). The first conclusion reveals the necessity of FE refinement of the thickness to evaluate the lesion risk with a better precision. The application of this mapping in patient-specific is strongly advised. The following simulations take this configuration as reference to assess the influence of head size, parturient morphology at different terms or descent scenario (Fig. 5).
3.2 Fetal Head and Term
Secondly, we investigated morphological parameters with the FE parametric model such as the size of fetal head. To estimate the impact on the strain levels, the cranial perimeter varies from ±5%, corresponding to the percentile classes. In accordance with the LAM refinement study, some differences of the maximal strain location are observed (Fig. 6a). The critical areas are also positioned on the lower part of LAM but with few differences of location between the three configurations (1 mm2 zone). The simulation allows us to predict that the most important damage area depends on the duration of the head descend in the LAM. Since the trajectory is unchanged in this study, it helps to show that strain levels follow the same trend at the beginning of simulation but become higher with large head before the delivery, when the fetal head arrives at the vulva. Maximum strain levels observed for the 0.95, 1, and 1.05 head ratios rise to 103%, 109%, and 128% respectively. In Fig. 6, the box-plot representation is used by taking the principal strain value of the eight elements close to the most impacted one.
MRI reconstructions on the four pregnant women at different weeks allow us to show that the thickness on LAM increases during pregnancy with a mean difference about 12% between 33 (5.4 mm) and 38 weeks (6.1 mm). This difference in LAM thickness has been implemented on the model to correspond to a premature scenario. In order to separate the effect of the geometry of the parturient from the geometry of the fetal head that evolved also during the term, we chose to keep the same head size for this simulation. At equivalent head size, we can see that the strain levels are quite similar between 33 and 38 weeks (Fig. 6b). The individualization of each muscle structure reveals that this trend matches with the localizations, dispersions, and maximum strain values. The modification of the fetal head size has a stronger impact than the morphological change of mother local geometry. The acquisition of patient geometry near the second trimester seems sufficient to predict the high strain location.
3.3 Cephalic Presentation
Comparison between left occipitoanterior and right occipitoposterior configurations has a last motivating result to evaluate the strain levels. An increase in maximal strain is observed with a posterior cephalic presentation (Fig. 6c). The maximum value changes from 109 to 142%. For the occiput posterior configuration, FE simulation shows that this level occurred later in time with R s ratio above 0.92 against 0.78 previously. This result is caused by the rotation of the head that happens later on the kinematic. Since the face is closer to the pubis in the ROP presentation, it is necessary to consider a higher displacement magnitude on the back before the head deflexion, contrary to an anterior presentation where occipital bone is leaned on the pubic symphysis, leading to a more progressive rotation during head deflexion. Since such phenomena occur later, the critical area is located on the lower position of LAM, near the external anal sphincter that represents an area commonly injured during laceration of perineum. Similarly, the maximal strain location is more symmetrical on the LAM with that configuration since it is centered on the puborectal muscle with R lr ratio about 0.47. On our fetal head model, occipital bone presents a more rounded shape compared to the frontal bone and could help to explain this difference on the quasi-symmetrical strain distribution. To conclude, the posterior presentation has higher strain levels, essentially caused by the rotation of the head that appeared later and the shape of the occipital bone affecting the LAM.
4 Discussion
Thanks to this study, we can notice that prediction of lesion risk during childbirth through a simulation tool involves several parameters related to numerical modeling, patient-specific morphology, and scenario type.
The damage evaluation on anatomical structures depends firstly on the quality of the anatomical representation. At a global scale, the reconstruction of a specific-patient follows a standard approach. The geometrical model is strongly conditioned by the MRI resolution. Moreover, the muscles structures, as well as ligaments, are difficult to segment and the 3D model needs substantial manual work to achieve characteristic simulation. The sustainable structures close to the LAM are also important to have a representative kinematic of the pelvic during delivery. At the local scale, the definition of muscle thicknesses provides also an essential step to predict the lesion risk. Taking thickness into consideration at any point of LAM allows us to identify the lower part of the muscle (puborectalis) as the most impacted area. These geometrical variations allow us to have a non-uniform distribution of strain over the LAM to offer a precise location of the damage at the most critical moment that corresponds to the appearance of the fetal head at the vaginal orifice.
In literature, the most critical cases are identified at the superficial level (skin), corresponding to the third and fourth degree perineal tears and are equivalent to an avulsion of the external anal sphincter [6]. However, on the second degree, lesions appear in a deep level and more precisely on the puborectalis muscle. Our tool helps to predict this grade level as it gives strain mapping over the LAM. In this study, the inclusion of MRI at several months of pregnancy also brings interesting results in warning about risk of lesion. Considering no evolution of fetal head between 33 and 38 weeks of pregnancy, results are significantly equivalent when we compare the influence of LAM geometry. The maximal strain area is located on the same zone and we do not have a significant difference in strain level. The most influential factor considering the term is the size of the fetal head. To develop the refined FE model of a specific patient, we could consider an analysis of the pelvic floor at the second trimester to predict the risks. Simulation could be performed according to different sizes, shapes, and positions of fetal head before childbirth and thus prevent eventual damage to ensure a better medical follow-up.
Regarding the cephalic presentation, posterior position is more critical than the anterior position. This comparison is already observed in literature but the risk rate is not significant and corresponds to a trend [21]. Severe perineal lacerations may occur on the patient with higher rates with a posterior presentation than anterior one [22]. Third and fourth degree laceration occurs with 6.7% on anterior presentation and 18.2% on posterior one [23]. The simulations performed in this work allow us to quantify the injury risk in function of the general kinematic and the shape of the head. Thanks to the imposed trajectory, we could modify displacement magnitude to identify more accurately the influence of the rotation of the head and better understand the origin of the lesions.
In this study, some topics are not addressed such as the individualization of each fontanelle allowing the movement of skull to facilitate the fetal head descent. It was assumed that the simulations are performed with a rigid head with prescribed path that could cause an overestimation of strain levels. A comparison with no path could be used in future study to quantify the difference between the two approaches. Coccyx mobility observed during childbirth was not integrated but need to be investigated in future study as it has a significant role in pelvic floor mobility [24]. Muscle nonlinearity and anisotropy [15, 16, 25] could be also integrated in the model to identify more precisely the critical strains.
5 Conclusion
The knowledges on the occurrence of injury criteria are referenced but few personalized means exist to study the damage induced during delivery for a prediction of lesion risks. The FE method helps us to identify the most influential parameters. The local geometry is a major factor influencing the damage evaluation. Taking thickness variation into consideration is needed to better quantify risk areas on the LAM. Variations of strain levels depend also on the size of the head or cephalic presentation. All these results are in agreement with statistical and epidemiological data and could help to explain some clinical differences such as the perineal lacerations induced by an anterior or posterior presentation of fetal head. Since the modeling of the parturient at the second trimester of pregnancy is sufficient to observe the maximal strain level, this developed approach could be used like a predictive tool to assess potential risks and adapt medical follow-up to the patient. The simulation protocol could be done on a semi-automated way to be a new tool evaluating damages on the pelvis during delivery and predicting patient risks.
References
Mayeur O, Witz JF, Lecomte-Grosbras P, Brieu M, Cosson M, Miller K (2016) Influence of geometry and mechanical properties on the accuracy of patient-specific simulation of women pelvic floor. Ann Biomed Eng 44(1):202–212
Samuelsson EC, Arne Victor FT, Tibblin G, Svardsudd KF (1999) Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol 180(2):299–305
Rortveit G, Brown JS, Thom DH, Van Den Eeden SK, Creasman JM, Subak LL (2007) Symptomatic pelvic organ prolapse: prevalence and risk factors in a population-based, racially diverse cohort. Obstet Gynecol 109(6):1396–1403
O’Boyle AL, O’Boyle JD, Ricks RE, Patience TH, Calhoun B, Davis G (2003) The natural history of pelvic organ support in pregnancy. Int Urogynecol J Pelvic Floor Dysfunct 14(1): 46–49
Rahn DD, Ruff MD, Brown SA, Tibbals HF, Word RA (2008) Biomechanical properties of the vaginal wall: effect of pregnancy, elastic fiber deficiency, and pelvic organ prolapse. Am J Obstet Gynecol 198(5):590–596
Sultan AH (1999) Clinical focus: obstetric perineal injury and faecal incontinence after childbirth – editorial: obstetrical perineal injury and anal incontinence. Clin Risk 5(6): 193–196
Kettle C, Tohill S (2008) Perineal care. BMJ Clin Evidence 2008:1–18
Dietz HP, Lanzarone V (2005) Levator trauma after vaginal delivery. Obstet Gynecol 106(4):707–712
Dietz HP, Gillespie AV, Phadke P (2007) Avulsion of the pubovisceral muscle associated with large vaginal tear after normal vaginal delivery at term. ANZJOG 47(4):341–344
DeLancey JOL, Kearney R, Chou Q, Speights S, Binno S (2003) The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol 101(1):46–53
Chen L, Ashton-Miller JA, DeLancey JOL (2009) A 3d finite element model of anterior vaginal wall support to evaluate mechanisms underlying cystocele formation. J Biomech 42(10): 1371–1377
Parente MPL, Natal Jorge RM, Mascarenhas T, Fernandes AA, Martins JAC (2009) The influence of the material properties on the biomechanical behavior of the pelvic floor muscles during vaginal delivery. J Biomech 42(9):1301–1306
Ashton-Miller JA, Delancey JOL (2009) On the biomechanics of vaginal birth and common sequelae. Annu Rev Biomed Eng 11:163–176
van Delft K, Thakar R, Sultan AH, Schwertner-Tiepelmann N, Kluivers K (2014) Levator ani muscle avulsion during childbirth: a risk prediction model. BJOG 121(9):1155–1163
Li X, Kruger JA, Nash MP, Nielsen PMF (2010) Anisotropic effects of the levator ani muscle during childbirth. Biomech Model Mechanobiol 10(4):485–494
Li X, Kruger JA, Nash MP, Nielsen PMF (2010) Effects of nonlinear muscle elasticity on pelvic floor mechanics during vaginal childbirth. J Biomech Eng 132(11):111010–111015
Rubod C, Boukerrou M, Brieu M, Jean-Charles C, Dubois P, Cosson M (2008) Biomechanical properties of vaginal tissue: preliminary results. Int Urogynecol J Pelvic Floor Dysfunct 121(9):811–816
Chantereau P, Brieu M, Kammal M, Farthmann J, Gabriel B, Cosson M (2014) Mechanical properties of pelvic soft tissue of young women and impact of aging. Int Urogynecol J 25(11):1547–1553
Yeoh OH (1993) Some forms of the strain energy function for rubber. Rubber Chem Technol 66(5):754–771
Rubod C, Brieu M, Cosson M, Rivaux G, Clay JC, Gabriel B (2012) Biomechanical properties of human pelvic organs. J Urol 79(4):1346–1354
Salameh C, Canoui-Poitrine F, Cortet M, Lafon A, Rudigoz RC, Huissoud C (2011) Does persistent occiput posterior position increase the risk of severe perineal laceration? Gynecol Obstet Fertil 39(10):545–548
Pergialiotis V, Vlachos D, Protopapas A, Pappa K, Vlachos G (2014) Risk factors for severe perineal lacerations during childbirth. Int J Gynaecol Obstet 125(1):6–14
Ponkey SE, Cohen AP, Heffner LJ, Lieberman E (2003) Persistent fetal occiput posterior position: obstetric outcomes. Obstet Gynecol 101(5):915–920
Berardi M, Martinez-Romero O, Elías-Zúñiga A, Rodríguez M, Ceretti E, Fiorentino A, Donzella G, Avanzini A (2014) Levator ani deformation during the second stage of labour. Proc Inst Mech Eng 228:501–508
Dejun J, Ashton-Miller JA, DeLancey JOL (2012) A subject specific anisotropic visco-hyperelastic finite element model of female pelvic floor stress and strain during the second stage of labor. J Biomech 45(3):455–460
Acknowledgments
This research is a part of the project « MAMAN » financially supported by the University of Lille 2.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this paper
Cite this paper
Mayeur, O. et al. (2017). Evaluation of Strains on Levator Ani Muscle: Damage Induced During Delivery for a Prediction of Patient Risks. In: Wittek, A., Joldes, G., Nielsen, P., Doyle, B., Miller, K. (eds) Computational Biomechanics for Medicine. Springer, Cham. https://doi.org/10.1007/978-3-319-54481-6_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-54481-6_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-54480-9
Online ISBN: 978-3-319-54481-6
eBook Packages: EngineeringEngineering (R0)