Abstract
The evolution of surgical oncologic technology has moved toward reducing patient morbidity without compromising oncologic resection. In head and neck surgery, organ-preserving techniques have paved the way for the development of transoral approaches that remove tumors of the upper aerodigestive tract without external incisions and potentially spare patients adjuvant treatment. The introduction of robotic-assisted surgery and transoral robotic surgery (TORS) reflects progression of the current transoral techniques to the upper aerodigestive tract. This chapter reviews the evolution of robotic-assisted surgery. We discuss its applications in head and neck surgery and early oncologic and functional outcomes, training of surgeons, costs, and future directions.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1.1 Introduction
Over the last two decades, robotic-assisted surgery has revolutionized minimally invasive surgery in multiple surgical specialties. The first robotic surgery system, the PUMA 560, was developed in 1985 to provide greater precision in performing image-guided intracranial biopsies. Further refinement in the early 1990s led to ROBODOC, which was the first robotic system to receive FDA approval for arthroscopic hip surgery in 1994 [1]. Interest in medical robots led to collaborative efforts between the National Aeronautics and Space Administration (NASA) and Stanford Research Institute (SRI) in the early 1980s, to develop telepresence surgery, the virtual placement of a remotely located surgeon in the operative field.
Experience with minimally invasive laparoscopic procedures has helped surgeons understand the limitations of rigid equipment and two-dimensional views. This has resulted in the development of semirigid robotic equipment with three-dimensional views for the operative setting. Combining these tools with telepresence surgery led to the development of the Automated Endoscopic System for Optimal Positioning (AESOP), a robotic arm (controlled by a surgeon’s voice commands) that manipulates an endoscopic camera [2]. The first robotic system that enabled surgery over a large distance consisted of two separate subsystems, i.e., “surgeon-side” and “patient-side” (ZEUS, Computer Motion, California). The operator site was located in New York and the animals were in Strasbourg. The two sites were connected through a high-speed terrestrial optical-fiber network that transports data through dedicated connections using asynchronous transfer mode (ATM) technology [3].
Shortly thereafter, Intuitive Systems (Sunnyvale, CA) released the SRI Telepresence Surgery System that was recently updated to the current da Vinci Surgical System, the most common robotic system in use today [4].
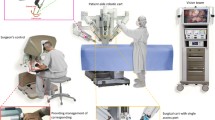
In short, the current da Vinci system functions as a master-slave robot, with the surgeon manipulating instruments connected by a cable network to the robotic cart. The system comprises three arms (one for the 12 mm 0° or 30° camera and two accommodate 8 mm and 5 mm instruments). The camera not only enables magnification but also three-dimensional viewing of the surgical field. Robot-assisted surgery enables excellent visualization and the capacity to manipulate and resect tumors due to the six degrees of freedom offered by the robotic arms and by the camera.
1.2 Applications in Fields Other Than Otolaryngology
Robot-assisted surgery is currently utilized in almost every surgical field. In general surgery, there is an abundance of reports on its use in cholecystectomy, Heller myotomy, Nissen fundoplication, bowel resection with reanastomosis, splenectomy, and Whipple and hepatobiliary surgery [5]. These reports endorse the benefits of stable visualization and improved dexterity of the robotic arms with suturing and dissection. Cardiothoracic surgeons used robotic surgery first in 1998 to perform coronary revascularization procedures and mitral valve replacements [6]. Numerous additional case series have since been published, describing esophagectomy, lung resection, tumor resections, atrial fibrillation ablations, and congenital cardiac anomalies. Results have been encouraging, with evidence demonstrating fewer blood transfusions, shorter hospital stays, faster returns to preoperative function levels, and improved quality of life compared to patient series of sternotomy [7]. Multiple pediatric surgery robotic-assisted procedures include tracheoesophageal fistula repair, cholecystectomy, Nissen fundoplication, Morgagni’s hernia repair, Kasai portoenterostomy, and congenital diaphragmatic hernia repair.
Gynecologists utilize robotic surgery in hysterectomies, myomectomies, and tubal reanastomoses and achieve similarly positive results as in laparoscopic and open procedures. However, a recent Cochrane review showed an uncertain benefit for robotic surgery in gynecology because it is unclear if it affects rates of complications [8]. Oncologic outcomes were similar to laparoscopic and open methods. The setup time for both exposure and docking of the robotic arms is longer with robot-assisted surgery but may be associated with a shorter hospital stay following hysterectomy. In addition, gynecologic surgeons observed another major disadvantage; the lack of haptic feedback, which is a virtual tactile feedback technology that provides mechanical feedback to the surgeon. Currently, in the United States, robotic-assisted hysterectomy is mainly used for benign conditions and has been shown to be more expensive than conventional laparoscopic hysterectomy, with no difference in overall rates of complications [9].
The development of robotic technology has paved the way for the performance of highly complex procedures such as transplant surgery, in a minimally invasive fashion. The first fully robotic kidney transplantations were performed in the late 2000s. The use of the robotic-assisted approach has enabled transplantation of kidneys with minimal complications and has significantly shortened the recovery period. This has made possible kidney transplantation in obese patients, who were frequently denied access to transplantation.
The field of urologic surgery has perhaps seen the greatest incorporation of robotic surgery: To date, more than two-thirds of prostatectomies are performed with robotic assistance [10]. Positive margin status and PSA levels achieved by the robotic technique are comparable to those achieved by open procedures [11]. However, surgeons noted significantly lower blood loss and transfusion rates, less pain, and shorter hospital stays for robotic techniques than open prostatectomies; erectile and urinary functional outcomes were found to be equivalent among open, laparoscopic, and robotic prostatectomies [12].
1.3 Evolution of Robotic Applications in Otolaryngology
The first TORS procedure was reported in Washington by McLeod et al. only a little more than one decade ago [13]. Since then, surgeons have laid infrastructure for its use, and it has been successfully incorporated into routine practice in the field of otolaryngology. Incorporation of robotic-assisted surgery in otolaryngology can be attributed to three main driving forces: (1) technological advancements that improved visualization and instrumentation, (2) fast learning curve, and (3) better understanding of head and neck cancer biology while exploring organ conservation treatment protocols.
Traditionally, surgical removal of oropharyngeal cancer required mandibulotomy with or without free flap reconstruction in most cases. Unfortunately, this approach results in significant morbidity. Mandibulotomy patients often require tracheotomies and feeding tubes. In addition, postoperative recovery, including rehabilitation, might further be slowed by adjuvant chemotherapy and/or radiation [14]. The pendulum started to shift in the late 1980s when multiple institutions investigated alternative treatment protocols based on organ preservation. The VA trial and RTOG 91-11 showed that survival rates following chemotherapy and radiation protocols were equivalent to those for patients who underwent surgery followed by radiation. By preserving the functional laryngopharyngeal complex, these protocols became the standard of care in the treatment of squamous cell carcinoma of the larynx [15, 16]. Alongside the highly conformal radiation delivery techniques (e.g., IMRT), molecular targeted therapies (e.g., cetuximab) were successfully introduced and represent an evolutionary advancement in head and neck cancer management. Nonetheless, survival and quality of life are still poor for some patients [17].
Over the last decade, we encountered an increase in oropharyngeal squamous cell carcinoma (OPSCC) caused by the human papilloma virus (HPV). HPV was recognized as a powerful prognostic biomarker for responsiveness to radiotherapy; however, HPV-positive patients tend to be younger, and thus the potential is greater for long-term sequelae from radiation, such as radiation-induced malignancy [18]. The development of successful minimally invasive surgical techniques has assisted in achieving sound oncological resection with local control and possibly sparing patients from undergoing concurrent chemoradiation.
First attempts to control OPSCC with minimally invasive techniques in the modern radiotherapy era used transoral laser microsurgery (TLM). While no randomized trials have compared surgery and radiation, small series from various institutions have shown success at achieving local control by using TLM as the primary modality for OPSCC [19]. However, rigid narrow field exposure through laryngoscopes is very limited and challenging to maneuver within the complex anatomy of the oropharynx.
Robotic surgery overcomes some of these limitations and provides a unique advantage by introducing angled optics and instrumentation with multiple degrees of rotation, which allows access to the entire upper aerodigestive tract surface. In addition, superior optics enable a precise three-dimensional assessment of resection margins, less collateral tissue damage, and an excellent view of the surgical bed.
1.4 Feasibility
Robotic-assisted salivary gland excision and neck dissection in a porcine model were the first applications of robotics in otolaryngology, as documented at Stanford University in 2003 [20]. Among the advantages claimed were the elimination of hand tremor and superior visualization without tactile sensation. Next, Hockstein and O’Malley reported gaining wide access to the laryngopharynx using mouth gag retractors in an airway mannequin and cadaver [21]. Later, Weinstein performed a supraglottic laryngectomy in a canine model [21]. The authors reported increased exposure with the mouth gag, yielding adjustable visualization of the larynx [22]. The final step before attempts on live human surgery was the technological increment achieved by coupling of 5-mm instruments and other mouth retractors to the robotic system at Cleveland Clinic by Solares [23]. The latter incorporated the CO2 laser with the robotic arm for robotic-assisted supraglottic laryngectomy and demonstrated the importance of evaluating variable patient factors such as oral opening and neck extension.
Weinstein and O’Malley first reported the efficacy of robotic-assisted head and neck surgery. They described a series of patients with early-stage, base of tongue squamous cell carcinomas who underwent complete en bloc resection of their tumors with negative margins. No immediate complications were noted, and patients were able to return to a full diet within 6 weeks of surgery [24]. With the feasibility of TORS established in OPSCC, institutions have begun recruiting patients for clinical trials such as ECOG3311 and RTOG1221 to assess treatment de-escalation of HPV+ patients with surgery and surgical intensification of treatment in HPV patients. Currently, robotic-assisted surgery has a wide range of applications in otorhinolaryngology. These include transoral surgery for sleep disorders, malignant and benign tumor resection from the upper aerodigestive tract, and skull base surgery. In addition, various approaches have been utilized for neck surgery, i.e., the transaxillary approach for thyroid and parathyroid surgery, and the retroauricular approach for neck dissection, congenital lesion resection, and salivary gland surgery. Table 1.1 summarizes published applications of robotic-assisted surgery in otorhinolaryngology.
1.5 Oncologic and Functional Outcomes
The effectiveness of a therapeutic modality appears to be strongly inversely related to the number of clinical trials that investigate the modality. While most head and neck cancers are surgically treated, only few clinical trials isolate any given surgical question.
Long-term survival outcomes of TORS are not currently available. Still, several institutions have published promising small cohort short-term data. A phase I study of 27 patients with early-stage tonsillar squamous cell carcinoma undergoing TORS revealed a 92 % negative margin rate. Population-based analysis revealed that TORS is associated with a lower rate of positive margins than non-robotic surgery and that high-volume centers have the lowest rates of positive margins and unplanned readmissions [28]. After achieving resection with negative margins, adjuvant treatment may be administered. However, even if the patient requires adjuvant therapy, the toxicity from the lower dose of radiation, with possible sparing of concurrent chemoradiation, tends to be significantly less following adequate robotic surgery and to result in better functional outcomes [94]. In addition, most patients do not need a tracheotomy or extended hospitalization.
From a functional standpoint, many clinical studies have shown improved post-TORS swallowing function compared with other surgical modalities and compared with primary chemoradiation therapy, along with shorter hospital stay and faster recovery, as well as a more efficient return to work after completion of therapy [29]. Most patients after TORS for OPSCC maintain full oral feeding and eventually acceptable to normal physiological swallowing. In a negligible minority of patients, elective temporary tracheotomy (1–2 weeks) is performed at the discretion of the surgeon, based on the estimated risk of postoperative upper airway obstruction due to mucosal swelling and the risk of postoperative bleeding. Faster recovery means that adjuvant therapy, if indicated, may start sooner, which improves locoregional control [30, 31].
Favorable oncological and functional outcomes of TORS, which permit resection of the tumor en bloc while preserving patients’ swallowing ability, led the FDA to approve, in December 2009, TORS for use in selected benign and malignant tumors of the head and neck. Using TORS, a mandibulotomy and/or pharyngotomy is avoided. As evidence accumulates regarding survival implications of HPV status in patients undergoing primary surgical therapy, TORS may play a significant role in the application of surgery to escalate or de-escalate first-line treatment for select patients with OPSCC.
1.6 Cost
High costs are a significant concern and a potential disadvantage of the implementation of a robotic program solely for TORS. With an initial cost of 1.5 million US dollars and annual maintenance fees of 100,000 US dollars, most programs rely on sharing the robotic facility with other departments. Disposable equipment such as graspers, cautery arms, and other surgical instruments total approximately 200 dollars per case. A nationwide cross-sectional analysis of more than 9,000 patients showed that after controlling for all other variables, TORS patients had lower rates of gastrostomy tube placement and tracheotomy tube placement, shorter length of hospitalization (mean, −1.5 days), and lower hospital-related costs (mean, −$4,285) [95].
1.7 Training
Naturally, as the popularity of robotic surgery is growing, practitioners are seeking training and certification in this area. The pitfall of such market-driven health care is the possibility that adverse outcomes may decrease positive results of surgery when less-experienced surgeons perform oncologic resections simply because TORS is a new and marketable procedure [96]. Intuitive surgical provides a training curriculum on their website, which includes didactic lectures on the da Vinci console, cadaver dissections, and live case observation. Nearly 1,500 surgical clips of TORS can be viewed on YouTube, and representatives for the company provide surgeon tutoring during practitioners’ initial procedures.
Robust outcomes data are not yet available, but potentially, robot-assisted surgery will become a standardized integral part of treatment protocols such as the National Comprehensive Cancer Network (NCCN). Once integrated, the implementation of a standardized curriculum for robotic surgery into residency and fellowship education will be vital. Current data indicate that the performance of simple tasks such as grasping inanimate objects and suturing on latex is highly intuitive, and introducing residents to basic robotic surgical skills eases their transition to live patient cases [97]. As a result, many training programs now provide cadaver dissection courses using the robot as part of their training. Training is discussed in more depth in Chapter 4.
1.8 Future Directions
To date, available data on head and neck robotic surgery, mainly TORS, indicate that it is a safe efficacious procedure for benign conditions such as obstructive sleep apnea. As stated, current efforts are being directed to implement TORS in oncology treatment protocols. Attempts are also being made to extend the applications of robot-assisted surgery and to use TORS in innovative ways and in other areas in the head and neck. An example is the field of skull base surgery, which requires precise motions with a steady hand. Surgeons have illustrated an approach to the midline and anterior skull base using two trocars inserted transcervically and placing the camera head in the oral cavity [98]. Anterior skull base and sella were accessed and dissected via bilateral Caldwell Luc incisions and maxillary antrostomies [99].
Robotic-assisted surgery is also being utilized in reconstructive surgery [100]. Microvascular anastomosis in narrow and deep spaces such as the oropharynx has been shown to be fast and effective, in a tremor-free manner. TORS free flap oropharyngeal reconstruction provides improved functional recovery and avoids the need for long-term healing by secondary intention of the oropharyngeal defect.
As current instrumentation is bulky, rigid, and passive, access is limited to narrow 3D complex spaces such as the larynx and skull base. Approaches to such areas will become possible as finer analytical instrumentation such as flexible lasers and Doppler probes will emerge. To overcome some of these obstacles, a flexible nonlinear robot was designed based on the experience gained by the use of the da Vinci system. This robot was further customized and transformed into the Medrobotics(®) Flex(®) System (Medrobotics Corp., Raynham, MA, USA), which was developed specifically for use in surgical applications requiring nonlinear maneuverability such as transoral surgery. The Medrobotics® Flex(®) System is an operator-controlled flexible endoscope system that includes rigid chip-on-tip endoscope and computer-assisted controllers, with two external channels for use with compatible, 3.5 mm flexible instruments. In 2015, the FDA approved the use of the Flex System for transoral resections of head and neck tumors.
Conclusion
Head and neck applications of robotic surgery are an evolutionary increment in surgical capabilities. While robotic-assisted head and neck surgery confers significant advantages, its limitations should be acknowledged. Patients can benefit from en bloc removal of their tumors via minimally invasive surgery without a cervical incision while preserving function and potentially avoiding adjuvant radiation and long-term sequelae. While long-term oncologic and functional data are needed to fully validate its use, early results are promising.
References
Bargar WL, Bauer A, Borner M. Primary and revision total hip replacement using the Robodoc system. Clin Orthop Relat Res. 1998;354:82–91.
Nguyen NT, Hinojosa MW, Finley D, Stevens M, Paya M. Application of robotics in general surgery: initial experience. Am Surg. 2004;70(10):914–7.
Marescaux J, Leroy J, Gagner M, Rubino F, Mutter D, Vix M, et al. Transatlantic robot-assisted telesurgery. Nature. 2001;413(6854):379–80.
http://www.intuitivesurgical.com. Intuitive Surgical Inc.
Toro JP, Lin E, Patel AD. Review of robotics in foregut and bariatric surgery. Surg Endosc. 2015;29(1):1–8.
Bush B, Nifong LW, Chitwood Jr WR. Robotics in cardiac surgery: past, present, and future. Rambam Maimonides Med J. 2013;4(3):e0017.
Rodriguez E, Chitwood WR. Robotics in cardiac surgery. Scand J Surg: SJS: Off Organ Finn Surg Soc Scand Surg Soc. 2009;98(2):120–4.
Liu H, Lawrie TA, Lu D, Song H, Wang L, Shi G. Robot-assisted surgery in gynaecology. Cochrane Database Syst Rev. 2014;12:CD011422.
Committee opinion no. 628: robotic surgery in gynecology. Obstet Gynecol. 2015;125(3):760–7.
Jain S, Gautam G. Robotics in urologic oncology. J Minim Access Surg. 2015;11(1):40–4.
Barocas DA, Salem S, Kordan Y, Herrell SD, Chang SS, Clark PE, et al. Robotic assisted laparoscopic prostatectomy versus radical retropubic prostatectomy for clinically localized prostate cancer: comparison of short-term biochemical recurrence-free survival. J Urol. 2010;183(3):990–6.
Eifler JB, Cookson MS. Best evidence regarding the superiority or inferiority of robot-assisted radical prostatectomy. Urol Clin N Am. 2014;41(4):493–502.
McLeod IK, Melder PC. Da Vinci robot-assisted excision of a vallecular cyst: a case report. Ear Nose Throat J. 2005;84(3):170–2.
Oliveira CM, Nguyen HT, Ferraz AR, Watters K, Rosman B, Rahbar R. Robotic surgery in otolaryngology and head and neck surgery: a review. Minim Invasive Surg. 2012;2012:286563.
Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–8.
The Department of Veterans Affairs Laryngeal Cancer Study Group. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324(24):1685–90.
Chen AY, Halpern M. Factors predictive of survival in advanced laryngeal cancer. Arch Otolaryngol Head Neck Surg. 2007;133(12):1270–6.
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35.
Grant DG, Hinni ML, Salassa JR, Perry WC, Hayden RE, Casler JD. Oropharyngeal cancer: a case for single modality treatment with transoral laser microsurgery. Arch Otolaryngol Head Neck Surg. 2009;135(12):1225–30.
Haus BM, Kambham N, Le D, Moll FM, Gourin C, Terris DJ. Surgical robotic applications in otolaryngology. Laryngoscope. 2003;113(7):1139–44.
Hockstein NG, Nolan JP, O’Malley Jr BW, Woo YJ. Robotic microlaryngeal surgery: a technical feasibility study using the daVinci surgical robot and an airway mannequin. Laryngoscope. 2005;115(5):780–5.
Hockstein NG, Nolan JP, O’Malley Jr BW, Woo YJ. Robot-assisted pharyngeal and laryngeal microsurgery: results of robotic cadaver dissections. Laryngoscope. 2005;115(6):1003–8.
Solares CA, Strome M. Transoral robot-assisted CO2 laser supraglottic laryngectomy: experimental and clinical data. Laryngoscope. 2007;117(5):817–20.
O’Malley Jr BW, Weinstein GS, Snyder W, Hockstein NG. Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope. 2006;116(8):1465–72.
Lawson G, Matar N, Remacle M, Jamart J, Bachy V. Transoral robotic surgery for the management of head and neck tumors: learning curve. Eur Arch Otorhinolaryngol: Off J Eur Fed Otorhinolaryngol Soc. 2011;268(12):1795–801.
Boudreaux BA, Rosenthal EL, Magnuson JS, Newman JR, Desmond RA, Clemons L, et al. Robot-assisted surgery for upper aerodigestive tract neoplasms. Arch Otolaryngol Head Neck Surg. 2009;135(4):397–401.
Byeon HK, Holsinger FC, Kim DH, Kim JW, Park JH, Koh YW, et al. Feasibility of robot-assisted neck dissection followed by transoral robotic surgery. Br J Oral Maxillofac Surg. 2015;53(1):68–73.
Chen MM, Roman SA, Kraus DH, Sosa JA, Judson BL. Transoral robotic surgery: a population-level analysis. Otolaryngol Head Neck Surg: Off J Am Acad Otolaryngol Head Neck Surg. 2014;150(6):968–75.
Sinclair CF, McColloch NL, Carroll WR, Rosenthal EL, Desmond RA, Magnuson JS. Patient-perceived and objective functional outcomes following transoral robotic surgery for early oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2011;137(11):1112–6.
Lorincz BB, Mockelmann N, Busch CJ, Knecht R. Functional outcomes, feasibility, and safety of resection of transoral robotic surgery: single-institution series of 35 consecutive cases of transoral robotic surgery for oropharyngeal squamous cell carcinoma. Head Neck. 2015;37(11):1618–24.
Lee SY, Park YM, Byeon HK, Choi EC, Kim SH. Comparison of oncologic and functional outcomes after transoral robotic lateral oropharyngectomy versus conventional surgery for T1 to T3 tonsillar cancer. Head Neck. 2014;36(8):1138–45.
Weinstein GS, O’Malley Jr BW, Snyder W, Sherman E, Quon H. Transoral robotic surgery: radical tonsillectomy. Arch Otolaryngol Head Neck Surg. 2007;133(12):1220–6.
Moore EJ, Olsen KD, Kasperbauer JL. Transoral robotic surgery for oropharyngeal squamous cell carcinoma: a prospective study of feasibility and functional outcomes. Laryngoscope. 2009;119(11):2156–64.
Iseli TA, Kulbersh BD, Iseli CE, Carroll WR, Rosenthal EL, Magnuson JS. Functional outcomes after transoral robotic surgery for head and neck cancer. Otolaryngol Head Neck Surg: Off J Am Acad Otolaryngol Head Neck Surg. 2009;141(2):166–71.
Weinstein GS, O’Malley Jr BW, Cohen MA, Quon H. Transoral robotic surgery for advanced oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2010;136(11):1079–85.
Moore EJ, Olsen KD, Martin EJ. Concurrent neck dissection and transoral robotic surgery. Laryngoscope. 2011;121(3):541–4.
Pellini R, Mercante G, Ruscito P, Cristalli G, Spriano G. Ectopic lingual goiter treated by transoral robotic surgery. Acta Otorhinolaryngol Ital: Organo Ufficiale Soc Ital Otorinolaringol Chir Cervicofac. 2013;33(5):343–6.
Leonardis RL, Duvvuri U, Mehta D. Transoral robotic-assisted lingual tonsillectomy in the pediatric population. JAMA Otolaryngol Head Neck Surg. 2013;139(10):1032–6.
Kimple AJ, Eliades SJ, Richmon JD. Transoral robotic resection of a lingual thyroglossal duct cyst. J Robot Surg. 2012;6(4):367–9.
Vicini C, Dallan I, Canzi P, Frassineti S, La Pietra MG, Montevecchi F. Transoral robotic tongue base resection in obstructive sleep apnoea-hypopnoea syndrome: a preliminary report. ORL J Otorhinolaryngol Relat Spec. 2010;72(1):22–7.
Vicini C, Dallan I, Canzi P, Frassineti S, Nacci A, Seccia V, et al. Transoral robotic surgery of the tongue base in obstructive sleep Apnea-Hypopnea syndrome: anatomic considerations and clinical experience. Head Neck. 2012;34(1):15–22.
Arora A, Chaidas K, Garas G, Amlani A, Darzi A, Kotecha B, et al. Outcome of TORS to tongue base and epiglottis in patients with OSA intolerant of conventional treatment. Sleep Breathing = Schlaf Atmung. 2015;8:636–643.
Chiffer RC, Schwab RJ, Keenan BT, Borek RC, Thaler ER. Volumetric MRI analysis pre- and post-transoral robotic surgery for obstructive sleep apnea. Laryngoscope. 2015;125(8):1988–95.
Eesa M, Montevecchi F, Hendawy E, D’Agostino G, Meccariello G, Vicini C. Swallowing outcome after TORS for sleep apnea: short- and long-term evaluation. Eur Arch Otorhinolaryngol: Off J Eur Fed Otorhinolaryngol Soc. 2015;272(6):1537–41.
Lin HS, Rowley JA, Badr MS, Folbe AJ, Yoo GH, Victor L, et al. Transoral robotic surgery for treatment of obstructive sleep apnea-hypopnea syndrome. Laryngoscope. 2013;123(7):1811–6.
Lin HS, Rowley JA, Folbe AJ, Yoo GH, Badr MS, Chen W. Transoral robotic surgery for treatment of obstructive sleep apnea: factors predicting surgical response. Laryngoscope. 2015;125(4):1013–20.
Glazer TA, Hoff PT, Spector ME. Transoral robotic surgery for obstructive sleep apnea: perioperative management and postoperative complications. JAMA Otolaryngol Head Neck Surg. 2014;140(12):1207–12.
Toh ST, Han HJ, Tay HN, Kiong KL. Transoral robotic surgery for obstructive sleep apnea in Asian patients: a Singapore sleep centre experience. JAMA Otolaryngol Head Neck Surg. 2014;140(7):624–9.
Vicini C, Montevecchi F, Campanini A, Dallan I, Hoff PT, Spector ME, et al. Clinical outcomes and complications associated with TORS for OSAHS: a benchmark for evaluating an emerging surgical technology in a targeted application for benign disease. ORL J Otorhinolaryngol Relat Spec. 2014;76(2):63–9.
Friedman M, Hamilton C, Samuelson CG, Kelley K, Taylor D, Pearson-Chauhan K, et al. Transoral robotic glossectomy for the treatment of obstructive sleep apnea-hypopnea syndrome. Otolaryngol Head Neck Surg: Off J Am Acad Otolaryngol Head Neck Surg. 2012;146(5):854–62.
Park YM, Lee WJ, Lee JG, Lee WS, Choi EC, Chung SM, et al. Transoral robotic surgery (TORS) in laryngeal and hypopharyngeal cancer. J Laparoendosc Adv Surg Tech A. 2009;19(3):361–8.
Park YM, Kim WS, Byeon HK, De Virgilio A, Jung JS, Kim SH. Feasibility of transoral robotic hypopharyngectomy for early-stage hypopharyngeal carcinoma. Oral Oncol. 2010;46(8):597–602.
Kucur C, Durmus K, Dziegielewski PT, Ozer E. Transoral robot-assisted carbon dioxide laser surgery for hypopharyngeal cancer. Head Neck. 2015;37(5):743–5.
Alon EE, Kasperbauer JL, Olsen KD, Moore EJ. Feasibility of transoral robotic-assisted supraglottic laryngectomy. Head Neck. 2012;34(2):225–9.
Dowthwaite S, Nichols AC, Yoo J, Smith RV, Dhaliwal S, Basmaji J, et al. Transoral robotic total laryngectomy: report of 3 cases. Head Neck. 2013;35(11):E338–42.
Kayhan FT, Kaya KH, Sayin I. Transoral robotic cordectomy for early glottic carcinoma. Ann Otol Rhinol Laryngol. 2012;121(8):497–502.
Rahbar R, Ferrari LR, Borer JG, Peters CA. Robotic surgery in the pediatric airway: application and safety. Arch Otolaryngol Head Neck Surg. 2007;133(1):46–50. discussion
Muderris T, Bercin S, Sevil E, Acar B, Kiris M. Transoral robotic surgery for atypical carcinoid tumor of the larynx. J Craniofac Surg. 2013;24(6):1996–9.
Chan JY, Tsang RK, Eisele DW, Richmon JD. Transoral robotic surgery of the parapharyngeal space: a case series and systematic review. Head Neck. 2015;37(2):293–8.
O’Malley Jr BW, Quon H, Leonhardt FD, Chalian AA, Weinstein GS. Transoral robotic surgery for parapharyngeal space tumors. ORL J Otorhinolaryngol Relat Spec. 2010;72(6):332–6.
De Virgilio A, Park YM, Kim WS, Byeon HK, Lee SY, Kim SH. Transoral robotic surgery for the resection of parapharyngeal tumour: our experience in ten patients. Clin Otolaryngol: Off J ENT-UK; Off J Neth Soc Otorhinolaryngol Cervicofac Surg. 2012;37(6):483–8.
Park YM, De Virgilio A, Kim WS, Chung HP, Kim SH. Parapharyngeal space surgery via a transoral approach using a robotic surgical system: transoral robotic surgery. J Laparoendosc Adv Surg Tech A. 2013;23(3):231–6.
Desai SC, Sung CK, Genden EM. Transoral robotic surgery using an image guidance system. Laryngoscope. 2008;118(11):2003–5.
O’Malley Jr BW, Weinstein GS. Robotic skull base surgery: preclinical investigations to human clinical application. Arch Otolaryngol Head Neck Surg. 2007;133(12):1215–9.
Kim GG, Zanation AM. Transoral robotic surgery to resect skull base tumors via transpalatal and lateral pharyngeal approaches. Laryngoscope. 2012;122(7):1575–8.
Arshad H, Durmus K, Ozer E. Transoral robotic resection of selected parapharyngeal space tumors. Eur Arch Otorhinolaryngol: Off J Eur Fed Otorhinolaryngol Soc. 2013;270(5):1737–40.
Amit M, Gil Z. Transoral robotic resection of parapharyngeal space tumors. Oper Tech Otolaryngol Head Neck Surg. 2014;25(3):293–8.
Lobe TE, Wright SK, Irish MS. Novel uses of surgical robotics in head and neck surgery. J Laparoendosc Adv Surg Tech Part A. 2005;15(6):647–52.
Byeon HK, Ban MJ, Lee JM, Ha JG, Kim ES, Koh YW, et al. Robot-assisted Sistrunk’s operation, total thyroidectomy, and neck dissection via a transaxillary and retroauricular (TARA) approach in papillary carcinoma arising in thyroglossal duct cyst and thyroid gland. Ann Surg Oncol. 2012;19(13):4259–61.
Miyano G, Lobe TE, Wright SK. Bilateral transaxillary endoscopic total thyroidectomy. J Pediatr Surg. 2008;43(2):299–303.
Kang SW, Jeong JJ, Yun JS, Sung TY, Lee SC, Lee YS, et al. Robot-assisted endoscopic surgery for thyroid cancer: experience with the first 100 patients. Surg Endosc. 2009;23(11):2399–406.
Kang SW, Lee SC, Lee SH, Lee KY, Jeong JJ, Lee YS, et al. Robotic thyroid surgery using a gasless, transaxillary approach and the da Vinci S system: the operative outcomes of 338 consecutive patients. Surgery. 2009;146(6):1048–55.
Lee J, Nah KY, Kim RM, Ahn YH, Soh EY, Chung WY. Differences in postoperative outcomes, function, and cosmesis: open versus robotic thyroidectomy. Surg Endosc. 2010;24(12):3186–94.
Lee J, Yun JH, Nam KH, Choi UJ, Chung WY, Soh EY. Perioperative clinical outcomes after robotic thyroidectomy for thyroid carcinoma: a multicenter study. Surg Endosc. 2011;25(3):906–12.
Tae K, Ji YB, Jeong JH, Lee SH, Jeong MA, Park CW. Robotic thyroidectomy by a gasless unilateral axillo-breast or axillary approach: our early experiences. Surg Endosc. 2011;25(1):221–8.
Lee KE, Rao J, Youn YK. Endoscopic thyroidectomy with the da Vinci robot system using the bilateral axillary breast approach (BABA) technique: our initial experience. Surg Laparosc Endosc Percutan Tech. 2009;19(3):e71–5.
Lee S, Ryu HR, Park JH, Kim KH, Kang SW, Jeong JJ, et al. Excellence in robotic thyroid surgery: a comparative study of robot-assisted versus conventional endoscopic thyroidectomy in papillary thyroid microcarcinoma patients. Ann Surg. 2011;253(6):1060–6.
Landry CS, Grubbs EG, Morris GS, Turner NS, Holsinger FC, Lee JE, et al. Robot assisted transaxillary surgery (RATS) for the removal of thyroid and parathyroid glands. Surgery. 2011;149(4):549–55.
Lee KE, Kim HY, Park WS, Choe JH, Kwon MR, Oh SK, et al. Postauricular and axillary approach endoscopic neck surgery: a new technique. World J Surg. 2009;33(4):767–72.
Katz L, Abdel Khalek M, Crawford B, Kandil E. Robotic-assisted transaxillary parathyroidectomy of an atypical adenoma. Minim Invasive Ther Allied Technol: MITAT: Off J Soc Minim Invasive Ther. 2012;21(3):201–5.
Tolley N, Arora A, Palazzo F, Garas G, Dhawan R, Cox J, et al. Robotic-assisted parathyroidectomy: a feasibility study. Otolaryngol Head Neck Surg: Off J Am Acad Otolaryngol Head Neck Surg. 2011;144(6):859–66.
Profanter C, Schmid T, Prommegger R, Bale R, Sauper T, Bodner J. Robot-assisted mediastinal parathyroidectomy. Surg Endosc. 2004;18(5):868–70.
Brunaud L, Ayav A, Bresler L, Schjott B. Da Vinci robot-assisted thoracoscopy for primary hyperparathyroidism: a new application in endocrine surgery. J Chir. 2008;145(2):165–7.
Chan AP, Wan IY, Wong RH, Hsin MK, Underwood MJ. Robot-assisted excision of ectopic mediastinal parathyroid adenoma. Asian Cardiovasc Thorac Ann. 2010;18(1):65–7.
Harvey A, Bohacek L, Neumann D, Mihaljevic T, Berber E. Robotic thoracoscopic mediastinal parathyroidectomy for persistent hyperparathyroidism: case report and review of the literature. Surg Laparosc Endosc Percutan Tech. 2011;21(1):e24–7.
Ismail M, Maza S, Swierzy M, Tsilimparis N, Rogalla P, Sandrock D, et al. Resection of ectopic mediastinal parathyroid glands with the da Vinci robotic system. Br J Surg. 2010;97(3):337–43.
Timmerman GL, Allard B, Lovrien F, Hickey D. Hyperparathyroidism: robotic-assisted thoracoscopic resection of a supernumary anterior mediastinal parathyroid tumor. J Laparoendosc Adv Surg Tech A. 2008;18(1):76–9.
Byeon HK, Holsinger FC, Tufano RP, Chung HJ, Kim WS, Koh YW, et al. Robotic total thyroidectomy with modified radical neck dissection via unilateral retroauricular approach. Ann Surg Oncol. 2014;21(12):3872–5.
Tae K, Ji YB, Song CM, Jeong JH, Cho SH, Lee SH. Robotic selective neck dissection by a postauricular facelift approach: comparison with conventional neck dissection. Otolaryngol Head Neck Surg: Off J Am Acad Otolaryngol Head Neck Surg. 2014;150(3):394–400.
Song CM, Ji YB, Kim KR, Tae K. Robot-assisted excision of branchial cleft cysts using a postauricular facelift approach. Auris Nasus Larynx. 2015;42(5):424–7.
Lee HS, Kim D, Lee SY, Byeon HK, Kim WS, Hong HJ, et al. Robot-assisted versus endoscopic submandibular gland resection via retroauricular approach: a prospective nonrandomized study. Br J Oral Maxillofac Surg. 2014;52(2):179–84.
Kim CH, Byeon HK, Shin YS, Koh YW, Choi EC. Robot-assisted sistrunk’s operation via a retroauricular approach for thyroglossal duct cyst. Head Neck. 2013;36:456–8.
Albergotti WG, Byrd JK, Nance M, Choi EC, Koh YW, Kim S, et al. Robot-assisted neck dissection through a modified facelift incision. Ann Otol Rhinol Laryngol. 2016;125(2):123–9.
Weinstein GS, Quon H, O’Malley Jr BW, Kim GG, Cohen MA. Selective neck dissection and deintensified postoperative radiation and chemotherapy for oropharyngeal cancer: a subset analysis of the University of Pennsylvania transoral robotic surgery trial. Laryngoscope. 2010;120(9):1749–55.
Richmon JD, Quon H, Gourin CG. The effect of transoral robotic surgery on short-term outcomes and cost of care after oropharyngeal cancer surgery. Laryngoscope. 2014;124(1):165–71.
Nickel JC. Are we being seduced by a robot? Can Urol Assoc J = J Assoc Urol Can. 2009;3(5):359–63.
Moles JJ, Connelly PE, Sarti EE, Baredes S. Establishing a training program for residents in robotic surgery. Laryngoscope. 2009;119(10):1927–31.
O’Malley Jr BW, Weinstein GS. Robotic anterior and midline skull base surgery: preclinical investigations. Int J Radiat Oncol Biol Phys. 2007;69(2 Suppl):S125–8.
Hanna EY, Holsinger C, DeMonte F, Kupferman M. Robotic endoscopic surgery of the skull base: a novel surgical approach. Arch Otolaryngol Head Neck Surg. 2007;133(12):1209–14.
Selber JC, Sarhane KA, Ibrahim AE, Holsinger FC. Transoral robotic reconstructive surgery. Semin Plast Surg. 2014;28(1):35–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Amit, M., Na’ara, S., Gil, Z. (2017). Robotics in Surgery. In: Gil, Z., Amit, M., Kupferman, M. (eds) Atlas of Head and Neck Robotic Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-49578-1_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-49578-1_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-49576-7
Online ISBN: 978-3-319-49578-1
eBook Packages: MedicineMedicine (R0)