Abstract
Mistakes in the process of cell division can lead to the loss, gain or rearrangement of chromosomes. Significant chromosomal abnormalities are usually lethal to the cells and cause spontaneous miscarriages. However, in some cases, defects in the spindle assembly checkpoint lead to severe diseases, such as cancer and birth and development defects, including Down’s syndrome. The timely and accurate control of chromosome segregation in mitosis relies on the spindle assembly checkpoint (SAC), an evolutionary conserved, self-regulated signalling system present in higher organisms. The spindle assembly checkpoint is orchestrated by dynamic interactions between spindle microtubules and the kinetochore , a multiprotein complex that constitutes the site for attachment of chromosomes to microtubule polymers to pull sister chromatids apart during cell division. This chapter discusses the current molecular understanding of the essential, highly dynamic molecular interactions underpinning spindle assembly checkpoint signalling and how the complex choreography of interactions can be coordinated in time and space to finely regulate the process. The potential of targeting this signalling pathway to interfere with the abnormal segregation of chromosomes, which occurs in diverse malignancies and the new opportunities that recent technological developments are opening up for a deeper understanding of the spindle assembly checkpoint are also discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Spindle assembly checkpoint (SAC)
- Kinetochore
- Genome instability
- Chromosome segregation
- Cancer
- Protein complexes
- Folding upon binding
- Disorder-to-order transitions
- KMN network
16.1 Introduction
16.1.1 The SAC-KMN axis
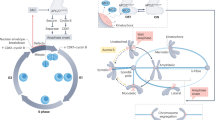
The accurate and timely segregation of chromosomes during mitosis requires the formation of a bipolar mitotic spindle with stably attached chromosomes. Once all of the chromosomes are aligned properly, the connection between the sister chromatids is severed by the action of separase, a cysteine protease. Separase also contributes to centriole disengagement at the end of mitosis. Temporal and spatial coordination of these two activities with the rest of the cell cycle is required for the successful completion of mitosis. The accurate segregation of chromosomes when cells divide is ensured by the spindle assembly checkpoint (SAC) , a highly intricate regulatory mechanism that monitors and corrects defects in chromosome attachment to the metaphase plate. The SAC is controlled by a panel of protein kinases that includes Bub1, BubR1, Mps1 and Aurora B and the non-kinase proteins Mad1, Mad2, Bub3, BuGZ and Cdc20.
16.1.2 Multidomain Protein Kinases Regulate the SAC
Budding uninhibited by benzimidazoles 1 (Bub1), Budding uninhibited by benzimidazoles related 1 (BubR1), dual-specificity kinase Monopolar spindle 1 (Mps1) and Aurora B are multidomain serine/threonine protein kinases with essential roles in the SAC signalling pathway in higher organisms (Krenn and Musacchio 2015; Elowe 2011; Musacchio 2011; Boyarchuk et al. 2007; Abrieu et al. 2001). These SAC proteins have evolutionarily conserved catalytic domains in organisms ranging from budding and fission yeasts to worms to humans (Bavetsias and Linardopoulos 2015; Bolanos-Garcia and Blundell 2011). For instance, Bub1 is required for the proper assembly of the inner centromere (Boyarchuk et al. 2007). Phosphorylation of the kinetochore organiser protein KNL1 by Mps1 is required for the recruitment of Bub1, BubR1 and Bub3 to the kinetochore while Mps1 from fission yeast (known as Mph1 in this specie) phosphorylates Mad3 to inhibit Cdc20 (known as Slp1 in fission yeast) and this post-translational modification appears important to maintain SAC arrest (Zich et al. 2016). It has been proposed that Mps1 compete with microtubules to bind at kinetochores and that such competitive binding contributes to regulate SAC signalling (Hiruma et al. 2015; Ji et al. 2015). Whether additional interactions mediated by Mps1, microtubules and/or the Ndc80 complex are involved in this process is an aspect that remains to be clarified (Aravamudhan et al. 2015; Krenn and Musacchio 2015; Nilsson 2015).
The proteins Mitotic arrest deficient 1 and 2 (Mad1 and Mad2, respectively); Budding uninhibited by benzimidazoles 3 (Bub3); Cell division cycle protein 20 (Cdc20); Bub3-interacting GLEBS-motif-containing ZNF207 (BuGZ); and Rod, Zwilch and ZW10, which define the RZZ-complex (Lara-Gonzalez et al. 2012), are also central components of the SAC. BubR1 (known as Mad3 in yeasts) interacts with Bub3, Mad2 and Cdc20 to form the Mitotic Checkpoint Complex (MCC; see Fig. 16.1), which inhibits the Anaphase Promoting Complex/Cyclosome (APC/C) to prevent metaphase-anaphase transition (Zhang et al. 2016; Musacchio 2011; Chao et al. 2012). Some of the key interactions underpinning SAC signalling are presented in Table 16.1. It has been shown that in both mammalian cells and in the fission yeast Schizosaccharomyces pombe, kinetochores shortened after microtubule severing. However, whereas in fission yeast all kinetochores could relax to a similar length, in human cells the more stretched kinetochores remained more stretched suggesting that the differences are due to the increased structural complexity of the mammalian kinetochore (Cojoc et al. 2016).
A, crystal structure of a ternary heterotrimer of the Mitotic Checkpoint Complex (MCC) from Schizosaccharomyces pombe (pdb id 4AEZ). B, crystal structure of Cdc20 (pdb id 4GGA), another WD 40 fold protein that forms part of the MCC. Figures generated with PyMOL (DeLano 2002)
The protein BuGZ was identified recently as a critical component of the SAC that contributes to the targeting of the Bub1-Bub3 and BubR1-Bub3 heterodimer complexes to the kinetochore . BuGZ is predicted to contain a N-terminal zinc finger domain; a GLEBS motif that is conserved from yeast to human and essential to bind Bub3, and a region of low structural complexity (Jiang et al. 2014; Toledo et al. 2014). BuGZ binds to microtubules and tubulin to regulate the SAC in a process that involves BuGZ phase transition (coacervation) (Jiang et al. 2015). It will be important to clarify if coacervation is a physicochemical feature shared by other proteins that regulate spindle assembly. Ultimately, this knowledge can be used to define new cancer therapies that rely on mitosis inhibition (Herman et al. 2015).
Zeste-white 10 (ZW10) and rough deal (ROD) were initially identified in D. melanogaster. Both proteins are highly conserved among multicellular eukaryotes (Karess 2005; Scaërou et al. 1999, 2001; Williams et al. 1992; Karess and Glover 1989). Null mutations in the zw10 and rod genes and depletion of these proteins in C. elegans and vertebrate cells result in chromosome segregation defects and extensive aneuploidy in mitotic and meiotic cells. Co-immunoprecipitation of ZW10 and ROD in fly and human cell extracts and their immunostaining in mitotic cells suggest an interdependent recruitment of the proteins to the kinetochore (Williams et al. 1992). Zwilch mutations cause a similar mitotic phenotype as ROD and ZW10, thus confirming a role of this protein in the SAC. Zwilch, ROD and ZW10 from human extracts isolated by affinity chromatography methods using ZW10 as bait show the three proteins associate to form a stable complex that seems to contain two copies of each protein (Civril et al., 2010; Kops et al. 2005a; Williams et al. 2003). The recent crystallisation and preliminary X-ray crystallographic analysis of a human ROD-ZW10-Zwilch complex (Altenfeld et al. 2015) further supports the notion of a complex of the three constitutive subunits with a 2:2:2 stoichiometry.
The control of chromosome segregation in higher organisms requires communication of SAC sub-complexes with the KMN (KNL1/Mis12/Ndc80) network, a multiprotein macromolecular assembly that constitutes the structural core of the kinetochore and is essential for the establishment of proper kinetochore-microtubule attachments (Liu et al. 2016; Aravamudhan et al. 2015; Ghongane et al. 2014; Santaguida and Musacchio 2009). Recently considerable progress has been made in understanding the composition of the kinetochore, the recruitment hierarchy of its components, and the principles of its regulation (revised in Agarwal and Varma 2014; Przewloka and Glover 2009). However, structural details of the molecular interactions of the KMN network have proved elusive even though they are clearly indispensable for the mechanism of kinetochore assembly and SAC signalling. The understanding of the function of the individual subunits and the different sub-complexes of the KMN network requires a description of the functional and structural features of its central components, and this is presented below.
16.2 The Kinetochore Null Mutant 1 (KNL1)
The kinetochore protein KNL1 (also known as CASC5, AF15q14 and Blinkin in humans; Spc105 in budding yeast, Spc7 in fission yeast, and KNL1 in humans and C. elegans) acts as a multi-substrate docking platform of the KMN network . KNL1 was initially identified in Saccharomyces cerevisiae as component of the spindle pole body (hence the acronym Spc105) (Nekrasov et al. 2003), which in C. elegans is commonly referred to as KNL1 (kinetochore-null phenotype 1) (Cheeseman et al. 2004) and Spc105R (Spc105-related) in Drosophila (revised in Przewloka and Glover 2009). Spc105R from Drosophila shows considerable sequence divergence compared to other species. This feature of the KMN contributes to define a distinctive structural organisation to the KMN network of the fruit fly. Depletion of KNL1 of higher organisms by RNAi causes severe chromosomal segregation defects that resemble phenotypes observed after depletion of the SAC kinases Bub1 and BubR1, including premature exit from mitosis and early onset of anaphase (Przewloka and Glover 2009). KNL1 is the largest subunit of the KMN network and is required for accurate chromosome segregation during mitosis (Desai et al. 2003). KNL1 integrates SAC kinase and phosphatase activities and contributes to the formation of kinetochore-microtubule attachments (Przewloka and Glover 2009; Santaguida and Musacchio 2009). Evidence of the precise role of functional regions in KNL1, including the motifs SILK, RVSF, MELT, KI, and a domain that adopts the RWD fold is providing new insights how KNL1 coordinates SAC activity. This is an important aspect of the SAC that has been revised recently (Caldas and DeLuca 2014; Ghongane et al. 2014).
16.3 The Ndc80 Complex
Microtubules contribute to key biological processes controlled by cell motility, including the maintenance of cell orientation and the regulation of focal adhesion turnover (Alushin et al. 2010). In human cells the Ndc80 complex is linked to centromeric chromatin to mediate end-on attachment of spindle microtubules in a process that requires Ndc80 binding to the kinetochore proteins CENP-T and CENP-C (Suzuki et al. 2015; Tanaka 2013). In humans, the Ndc80 complex is composed of the proteins Hec1 (a subunit that is also commonly referred to as Ndc80), Nuf2, Spc24 and Spc25 (DeLuca and Musacchio 2012; Tooley and Stukenberg 2011; Varma and Salmon 2012). The Ndc80 complex adopts a dumbbell-shape architecture with the subcomplexes Nuf2-Ndc80 and Spc24-Spc25 located in opposite ends of the molecule (see Fig. 16.2) (Ciferri et al. 2005; Wei et al. 2005). In one hand, Nuf2 binding to Ndc80 is required for the localisation of the Ndc80 complex to microtubules. On the other hand, Spc24-Spc25 heterodimer complex formation appears to be a pre-requisite for the binding of Spc24-Spc25 to KNL1 and the Mis12 complex (Cheeseman et al. 2006; Kiyomitsu et al. 2007; Wei et al. 2007; Ciferri et al. 2008; Wan et al. 2009; Joglekar and DeLuca 2009). The hairpin region of Ndc80 seems to be an important structural requirement for the effective kinetochore recruitment of Mps1 (Mph1), at least in fission yeast (Chmielewska et al. 2016).
Protein depletion assays coupled to quantification of kinetochore protein copy numbers in human cells have provided clues of the extent of the interactions between centromeric chromatin and the microtubule-binding Ndc80 complex. Such studies revealed about 244 Ndc80 complexes per human kinetochore (i.e., approximately 14 per kinetochore microtubule) and 151 Ndc80 complexes are associated to the KMN network . These studies also showed that each CENP-T molecule recruited approximately two copies of the Ndc80 complex (one as part of a KMN network) with nearly 40% of CENP-C recruited exclusively to the KMN network (Suzuki et al. 2015). Undoubtedly, the quantification of kinetochore protein copy numbers in other species should provide important new insights into the evolution and the subtle differences in the mode of regulation of the KMN network.
16.4 Mis12 Complex
In humans, the Mis12 complex (also known as the MIND complex) consists of the proteins Mis12, Dsn1, Nsl1 and Nnf1. The complex is a central component of the KMN network . Nsl1 has been identified as a link between the human Mis12 and Ndc80 complexes (Petrovic et al. 2010) and this protein seems to play a similar important role in yeast kinetochores (Kudalkar et al. 2015). Indeed, recent studies in yeast cells have shown that the interaction between the Mis12 complex (which in yeast is made up of the proteins Mtw1, Nsl1, Nnf1, Dsn1) and the Ndc80 complex (constituted by the proteins Ndc80, Nuf2, Spc24, Spc25) (Biggins 2013) is mediated by an extensive number of contacts (Kudalkar et al. 2015). Mis12 alone does not bind to microtubules but it does bind to the Ndc80 complex, an interaction that enhances Ndc80 binding to microtubules (Kudalkar et al. 2015). In Drosophila, the Mis12 complex localises to the mitotic centromere in a process that implicates the binding of the N-terminal regions of Mis12 and Nnf1 to CENP-C (Richter et al. 2016). Reconstitution of the yeast Mis12-Ndc80 assembly coupled to cross-linking analysis revealed an intricate set of interactions involving five of the eight proteins within the Mis12 and Ndc80 complexes and a direct interaction between the proteins Nsl1 and Spc24/Spc25. The latter interaction defines a unique interface thus suggesting that in different organisms the regulation of Ndc80 functions may be achieved by a distinctive mode of interactions (Kudalkar et al. 2015).
16.5 Centromere-Associated Protein E (CENP-E)
CENP-E is required to maintain a stable genome through the stabilisation of microtubule capture in the kinetochore . CENP-E functions as a highly processive plus end-directed motor that couples chromosome position with microtubule depolymerisation thus linking kinetochores to dynamic spindle microtubules. CENP-E participates in the recruitment of BubR1, Mad1 and Mad2 to attached and newly unattached kinetochores and its binding to the SKAP protein is required for accurate chromosome segregation in mitotic cells (Huang et al. 2012). SKAP seems to form part of the kinetochore corona fibres of mammalian centromeres as judged by immunoelectron microscope imaging. The interaction between CENP-E and SKAP, which involves the C-terminal tail of the former protein, is thought to be essential for kinetochore-microtubule attachment in vivo. Depletion of SKAP or CENP-E by RNA interference drastically reduces inter-kinetochore tension, thus leading to chromosome segregation defects and a prolonged delay to fulfill metaphase alignment (Huang et al. 2012).
In human cells CENP-E kinetochore localisation depends on its binding to Nuf2, an interaction that is mediated by C-terminal regions of both CENP-E and Nuf2 as determined with the yeast two-hybrid system and pulldown assays (Liu et al. 2007). Moreover, depletion of human Nuf2 by small interfering RNA abolished CENP-E kinetochore localisation and resulted in chromosome segregation defects, thus confirming the requirement of Nuf2 for CENP-E localisation to the kinetochore and the essential role of the interaction for the correct segregation of chromosomes in mitosis (Liu et al. 2007).
16.6 The Centromeric Nucleosome-Associated Network (CCAN)
Centromeres are differentiated chromatin domains, present once per chromosome, that direct segregation of the genome in mitosis and meiosis by specifying assembly of the kinetochore . The latter provides an essential link that brings together chromosomes and spindle microtubules (Pesenti et al. 2016; Rago et al. 2015). The specific spatial configuration of the centromere is likely to contribute to the tight regulation of mitosis and the dynamics of kinetochore-microtubule attachments (George and Walworth 2016). The centromeric nucleosome-associated network (CCAN) is a constitutive complex that is assembled onto centromeric CENP-A chromatin and widely considered as the prime candidate for specifying centromere identity (Foltz et al. 2006). The CCAN is composed by the proteins CENP-C, CENP-H/ CENP-I/ CENP-K, CENP-L/ CENP-M/ CENP-N, CENP-O/CENP-P/CENP-Q/CENP-R/CENP-U, CENP-T/CENP-W, and CENP-S/CENP-X (Foltz et al. 2006; revised by Perpelescu and Fukagawa 2011). The CCAN recruits the outer kinetochore components of the KMN network KNL1, the Mis12 complex, and the Ndc80 complex thus bringing together kinetochore proteins and spindle microtubules. Disruption of the interaction between CENP-A and CCAN causes errors of chromosome alignment and segregation that prevent cell survival (Foltz et al. 2006).
CENP-A is a centromere-specific isoform of histone H3 (Perspelescu and Fukugawa 2011; Stoler et al. 1995; Palmer et al. 1991) that contributes to kinetochore formation and centromere-kinetochore assembly thus guiding the movement of chromosomes and cell cycle progression throughout mitosis (Fachinetti et al. 2013; Mendiburo et al. 2011; Barnhart et al. 2011; Wan et al. 2009). The crystal structures of two human centromeric nucleosomes, one containing CENP-A (pdb id 3AN2) and one containing CENP-C in complex with the cognate α-satellite DNA derivative (pdb id 4X23) revealed that the latter molecule wraps around a histone octamer (see Fig. 16.3). Such octameric complex is defined by the assembly of two copies of histones H2A, H2B, H4 and CENP-A (Tachinawa et al. 2011).
In addition to CENP-A, CENP-C and CENP-T contribute to kinetochore assembly in vertebrates as shown by studies in which the DNA-binding regions of CENP-C and CENP-T were replaced with alternate chromosome-targeting domains, thus resulting in the localisation of functional CENP-C and CENP-T to ectopic loci and a CENP-A-independent assembly of the kinetochore (Gascoigne et al. 2011). Furthermore, phosphorylation of CENP-T appears as an important requirement for proper mitotic assembly of both endogenous and ectopic kinetochores (Gascoigne et al. 2011).
CENP-H is an inner kinetochore protein that is highly conserved amongst eukaryotes (Orthaus et al. 2006). CENP-H directly interacts with CENP-K through multiple contacts to form a stable heterodimeric complex. CENP-H and CENP-K are predicted to contain extensive coiled-coil regions that seem to play an important role in the stabilisation of the CENP-H-CENP-K heterocomplex (Qiu et al. 2009). Depletion of CENP-H in human cells led to severe mitotic phenotypes including misaligned chromosomes and multipolar spindles but not mitotic arrest (Orthaus et al. 2006). CENP-H depletion results in reduced levels of CENP-E but only slightly affects the levels of CENP-C bound to the kinetochore while suppression of CENP-H expression has not effect on BubR1 kinetochore localisation and a SAC response (Orthaus et al. 2006).
The CENP-T/W complex assembles in late S and G2 phases of the cell cycle and is required for mitosis. The CENP-T/W complex is integrated with centromeric chromatin in association with Histone H3 nucleosomes (Prendergast et al. 2011) but it does not persist across cell generations. Instead, association of H3 with the CENP-T/W complex seems to be specific for the regulation of kinetochore activity (Prendergast et al. 2011). CENP-T centromere localisation is restricted to the S-phase of the cell cycle. CENP-T directly associates with CENP-A and CENP-B as shown by Förster resonance energy transfer (FRET) studies. Taken together these studies indicate that CENP-T is required for the recruitment of other proteins to the kinetochore (Hellwig et al., 2008). Furthermore, centromeric-bound CENP-T-W and CENP-S-X subcomplexes associate to form a stable CENP-T-W-S-X heterotetramer that binds to DNA to form supercoil structures (Takeuchi et al. 2014; Nishino et al. 2012). High-resolution structural analyses of the individual subcomplexes and the tetramer have revealed important structural similarities with the nucleosome and certain histone fold-containing complexes (Nishino et al. 2012).
In human cells the inner kinetochore components CENP-C and CENP-T function in parallel pathways to recruit the KMN network to the kinetochore (Nishino et al. 2013; Schleiffer et al. 2012) as shown by independent ectopic targeting of these proteins to a chromosomal locus (Rago et al. 2015). For instance, the physical interaction of CENP-C with KNL1 and the Mis12 complex is required for the recruitment of the Ndc80 complex to the kinetochore whereas CENP-T kinetochore recruitment is only dependent of CENP-T binding to the Ndc80 complex. Furthermore, the CENP-T-Ndc80 complex assembly in turn promotes KNL1/Mis12 complex recruitment in a process that implicates a separate region on CENP-T (Rago et al. 2015). The formation of the CENP-C and CENP-T sub-complexes seems to obey different regulatory controls: the recruitment of the KMN network to CENP-C is stimulated by Aurora B kinase while that of CENP-T is regulated by cyclin-dependent kinase (Cdk) (Rago et al. 2015).
A number of additional microtubule plus-end binding proteins that have been associated with the kinetochore include the CLIP-associating protein 1 (CLASP-1) and 2 (CLASP-2), Astrin, Kinastrin, KIF2B, Kif18A and SKAP. The general structural and functional features of these proteins and their roles in SAC signalling are described below.
16.7 CLASP-1 and CLASP-2
The microtubule plus-end binding proteins CLASP-1 and CLASP-2 play important roles in the regulation of the density, length distribution and stability of interphase microtubules thus integrating spindle and kinetochore functions (Pereira et al. 2006; Maiato et al. 2003). In yeast, Drosophila, and Xenopus, one CLASP orthologue is present, whereas in human two proteins have been identified: CLASP-1 and CLASP-2. In all these organisms CLASP proteins are required for mitotic spindle assembly through the regulation of microtubule dynamics at the kinetochore. In mitotic cells both proteins associate with the ends of growing microtubules and with kinetochores in a process that requires the binding of these proteins to EB1 (Mimori-Kiyosue et al. 2005). The interaction of CLASP-1 and CLASP-2 with EB1 implicates the middle region of both CLASPs (Mimori-Kiyosue et al. 2005). At least in HeLa cells CLASP-1 and CLASP-2 show similar and at least partially redundant roles in organising the mitotic apparatus (Pereira et al. 2006). Their simultaneous depletion results in extensive mitotic spindle defects and an abnormal exit from mitosis. Targeting CLASP-1 with specific anti-CLASP-1 antibodies impairs microtubule dynamics in the kinetochore and the mitotic spindle, leading to the formation of abnormal monopolar asters in which the chromosomes are found buried in the interior. Similarly, the expression of a truncated form of CLASP-1 lacking the kinetochore binding domain results in the formation of depolymerisation-resistant microtubule bundles with a radial array (Maiato et al. 2003). Inhibition of glycogen synthase kinase -3 (GSK3) activity by the tyrosine kinase receptor ErbB2 regulates microtubule capture and stabilisation. Inhibition of Glycogen synthase kinase 3 beta (GSK3b) causes relocalisation of CLASP-2 to the plasma membrane and ruffles (Zaoui et al. 2010). All these observations strongly support an important role for CLASP-1 and CLASP-2 in the organisation of the mitotic spindle and the control of microtubules attachments.
It has been suggested that microtubules in vertebrate somatic cells are not only formed by the centrosome but that a significant number of them originate from the Golgi apparatus in a centrosome-independent manner. The process requires CLASPs recruitment to the trans-Golgi network by the protein GCC185 (Efimov et al. 2007; Zhonghua et al. 2007). However, mechanistic details of the regulation of spindle assembly in mitosis by membrane systems remain largely obscure.
16.8 Astrin and Kinastrin
Astrin is a mitotic spindle-associated protein found in most human cell lines and tissues that is required for proper chromosome alignment at the metaphase plate; is essential for progression through mitosis and contributes to the regulation of separase activity (Dunsch et al. 2011; Thein et al. 2007; Gruber et al. 2002). Depletion of this protein by RNA interference delays chromosome alignment, leads to the loss of spindle architecture and sister chromatid cohesion before the onset of anaphase, and ultimately results in apoptosis (Gruber et al. 2002). Amino acid sequence analysis and fold recognition bioinformatics tools suggest that Astrin has an N-terminal globular domain and an extended coiled-coil domain. Electron microscopy studies of recombinant Astrin showed that this protein self-associates to form parallel dimers with head-stalk structures reminiscent of motor proteins. However, the low amino acid sequence identity and structural similarity to known motor proteins requires further investigations to establish to what extent there is a functional correspondence between Astrin and kinesins.
Kinastrin is the major interacting partner of Astrin in mitotic cells and the interaction is required for Astrin targeting to microtubule plus ends. Overexpression or depletion of Kinastrin mislocalise Astrin and causes mitotic defects that resemble those observed in Astrin-depleted cells. Astrin and Kinastrin can form a complex with SKAP, which also co-localises to microtubule plus ends to facilitate chromosome alignment (Dunsch et al. 2011). These observations support the notion that the microtubule plus end targeting activity of Astrin is required to sustain spindle architecture and to ensure chromosome alignment and that perturbation of these interactions delay mitosis and cause the premature activation of separase (Dunsch et al. 2011). Interestingly, Astrin acts as a negative regulator of mTORC1, which seems to be essential to elicit a cellular stress response. Under stress conditions, Astrin blocks mTORC1 self-association and recruits Raptor, a protein component of mTORC1, to stress granules, thus preventing apoptosis caused by the induction of mTORC1 hyperactivation (Thedieck et al. 2013). This is an exciting finding that suggests a potential link between cellular stress response and the control of chromosome segregation. Further studies should aim to clarify this aspect of SAC signaling.
16.9 KIF2B and Kif18A
The human genome has three genes (Kif2a, Kif2b, and MCAK [also known as Kif2c]) that encode for kinesin-13 proteins. Kif2a, Kif2b, and MCAK fulfill distinct functions during mitosis in human cells (Hood et al. 2012; Manning et al. 2007). Human kinesin Kif18A is a kinesin-8 protein and microtubule-depolymerising protein that contributes to stabilise the CENP-E-Bub1 complex at the kinetochores during early mitosis (Mayr et al. 2007). In vitro, Kif18A shows a slow plus-end-directed microtubule depolymerising activity whereas in mitotic cells in vivo Kif18A localises close to the plus ends of kinetochore microtubules. Depletion of Kif18A induces aberrant mitotic spindles and loss of tension across sister kinetochores and activates the SAC (Mayr et al. 2007). During vertebrate cell division, chromosomes oscillate with periods of smooth motion and rapid reversals in direction. These fluctuations must be spatially constrained to ensure the proper alignment and high fidelity segregation of chromosomes. In humans, Kif18A plays an essential role in the control of chromosome oscillations by reducing the amplitude of pre-anaphase oscillations and slowing down poleward movements during anaphase. This manner, Kif18A contributes to the control of kinetochore microtubule dynamics underlying chromosome positioning in mitosis (Gardner et al. 2008; Stumpff et al. 2008). Moreover, Kif18A physically interact with CENP-E and BubR1 during mitosis as revealed by co-immunoprecipitation studies. Kif18A depletion results in mitotic arrest and chromosome missalignment and stimulates CENP-E degradation indicating that chromosome congression defects due to Kif18A depletion are at least in part mediated through destabilisation of CENP-E (Huang et al. 2009).
16.10 SKAP
SKAP is an essential component of the mitotic spindle that associates with kinetochores and is required for chromosome alignment, normal timing of sister chromatid segregation and maintenance of spindle pole architecture (Fang et al. 2009). SKAP also plays a role in the control of kinetochore oscillations and the regulation of microtubule plus-ends dynamics during mitosis (Wang et al. 2012a). Although suppression of SKAP expression does not stimulate the SAC, it substantially increases the duration of metaphase, delays the activation of separase and decreases the fidelity of chromosome segregation (Fang et al. 2009).
SKAP binds to microtubules in vitro, an interaction that is synergised by CENP-E. Thus, CENP-E and SKAP work together to control dynamic kinetochore -microtubule interactions (Huang et al. 2012). SKAP binds to the C-terminal tail of CENP-E in vitro and is essential for an accurate kinetochore-microtubule attachment in vivo. Depletion of SKAP or CENP-E by RNA interference drastically impairs inter-kinetochore tension and causes chromosome missegregation (Wang et al. 2012 b; Huang et al. 2012). SKAP also interacts with Mis13, which seems important for the accurate interaction between kinetochore and dynamic spindle microtubules. SKAP directly binds Mis13 and the interaction specifies the kinetochore localisation of the former protein, an observation that has been confirmed by small interfering RNA studies to suppress Mis13 expression (Wang et al. 2012b). A complex formed between SKAP and Astrin-Kinastrin localises to microtubule plus ends to facilitate proper chromosome alignment (Dunsch et al. 2011). Further studies should aim to clarify the role of these interactions in the control of SAC signalling.
16.11 Disorder-to-Order Transitions in the KMN
It is worth noting that many of the kinetochore proteins described above are predicted to contain large regions of low structural complexity (see Fig. 16.4). A pattern of disorder-to-order transitions in SAC signalling has emerged from the structures of diverse complexes involving the kinetochore organiser protein KNL1, including the N-terminal TPR-containing domains of Bub1 and BubR1 in complex with the KNL1 N-terminal KI motifs; Bub3 bound to KNL1 MELT motifs and the KNL1 RWD domain in complex with a synthetic peptide that mimics the protein Nls1 (Bolanos-Garcia et al. 2011; Krenn et al. 2012; Primorac et al. 2013; Petrovic et al. 2014) (see Fig. 16.5). One distinctive feature that emerges from the analysis of the above mentioned complexes is the predominance of cooperative hydrophobic interactions that stabilise the complexes. With the exception of the C-terminal region which contains a globular RWD domain, multiple regions of low structure complexity that span most of the polypeptide chain occur in KNL1. This is not surprising because multiple regions of low structure complexity occur often in hub proteins that define interactome networks (Babu et al. 2012; Kim et al. 2006; Dosztányi et al. 2006; Dunker et al. 2005, 2008; Haynes et al. 2006). Indeed, in multiple biological systems cooperative interactions involve the recognition of a flexible protein by a globular one, leading to concerted folding and binding (Blundell et al. 2002). This is particularly evident in hub proteins that define interactome networks because such proteins contain intrinsic local disordered regions (Dunker et al., 1998; Gsponer and Babu, 2009) that often associate with interacting partners through concerted binding and folding (Uversky 2015; Dosztányi et al. 2006; Dunker et al. 2005). The general model for concerted folding upon binding appears to be initial binding of a large side chain into a deep pocket, usually followed by interaction at a second and sometimes third pocket, forming a cluster of small pockets (Fuller et al. 2009). Less conserved interactions involving regions N- or C-terminal to the conserved motif then fold cooperatively onto the surface of the globular partner. There are examples of this type of interactions in SAC signalling, including the binding of the KI motif of KNL1 to BubR1 (Bolanos-Garcia et al. 2011) and possibly that of an equivalent KI motif in KNL1 specific to Bub1 (Krenn et al. 2012) and the binding of Bub3 to the MELT motifs of KNL1, a sequential, multisite interaction that is subjected to phospho-regulation (Vleugel et al. 2015). Indeed, the interplay of phosphorylation and dephosphorylation cascades rises as an important mechanism to regulate the SAC (Manic et al. 2017; Funabiki and Wynne 2013; London et al. 2012; Shepperd et al. 2012; Rosenberg et al. 2011; Liu et al. 2010).
Different KNL1 complexes revealed a similar mode of binding underlying disorder-to-order transitions . A, crystal structure of human TPR BubR1 in complex with the KNL1 KI motif (pdb id 3SI5); crystal structure of a Bub3-Bub1 GLEBS motif-KNL1 MELT motif complex (pdb id 4BL0). C, KNL1 RWD domain in complex with a Nsl1 fragment (pdb id 4NF9).
The presence of large segments of disordered regions in multiple kinetochore binding proteins is a common structural feature. The plot shows the disorder predictions based on PONDR-FIT (Xue et al. 2010) analyses.
Furthermore, the reciprocal communication of disorder-to-order transitions on two or more distant functional surfaces of high intrinsic disorder can maximise allosteric coupling between proteins. This mode of molecular recognition and signal amplification may obey the same mechano-chemical principles underlying the interaction of simpler systems such as binding of a biotin repressor to biotin protein ligase (Egington et al. 2015). Also, macromolecular crowding effects (Mourão et al. 2014; Cino et al. 2012; Babu et al. 2012; Wang et al. 2012a) can be anticipated to play a major role in the regulation of the SAC given the prominent role of proteins with multiple regions of low structural complexity in the process, including kinesin motors (Leduc et al. 2012).
In summary, regulation of the rate in which spindle microtubules attach/detach to/from kinetochores plays a central role in the control of chromosome segregation. Multiple mechanisms of assembly and holistic models that take into account the role of protein receptors, signalling networks and regulatory feedback mechanisms have been proposed in an attempt to describe more precisely the role of kinetochore -microtubules interactions for the control mitotic progression in higher organisms (Kim and Yu 2015; Godek et al. 2015). As discussed below, disruption of this balance quickly results in aneuploidy , genome instability , cancer and diverse birth and development defects.
16.12 The SAC-KMN Axis in Disease
Mistakes in the process of cell division can lead to the rearrangement, the loss or gain of chromosomes (aneuploidy). Solid tumors are frequently aneuploid, and many display high rates of chromosome missegregation and chromosomal instability (CIN). The most common cause of CIN is the persistence of aberrant kinetochore -microtubule attachments, which manifest as lagging chromosomes in anaphase. Errors in kinetochore-microtubule attachments during prometaphase can be due to stochastic interactions between kinetochores and microtubules.
Mps1 has been identified in the signature of the top 25 genes overexpressed in CIN and aneuploid tumours (Kops et al. 2005b; Carter et al. 2006) and found to be upregulated in a number of tumours of different origins including bladder, anaplastic thyroid, breast, lung, esophagus, and prostate. In the absence of a functional mitotic checkpoint, as occurs when Mps1 function is lost, cells become rapidly aneuploid and subsequently die (Kops et al. 2005b; Janssen et al. 2009). This feature, together with the observations that inhibiting Mps1 with chemical inhibitors kills cultured tumour cells (Kwiatkowski et al. 2010) and that even its partial inhibition creates tumour cells more sensitive to clinical doses of taxol (Janssen et al. 2009), show that targeting Mps1 with drugs may be beneficial to arrest proliferation of tumour cells. Significant chromosomal abnormalities are the cause of severe diseases such as breast cancer , the most common cancer in the UK. This year alone, 50,000 people in the UK will find out they have breast cancer and 12,000 people will die from it (Cancer Research UK organisation). The fact that Mps1 inhibition in tumour xenograft models significantly reduces tumour growth rates while leaving normal cell growth unaffected (Daniel et al. 2011) makes Mps1 an attractive target for cancer therapy (Kapanidou and Bolanos-Garcia 2014). In addition to Mps1, Aurora B kinase is the cellular target of diverse Medicinal Chemistry campaigns to develop inhibitors that function as adenosine triphosphate (ATP) competitors of these protein kinases. Also important is the search of ubiquitin ligase inhibitors that target the E3 ubiquitin ligase activity of the APC/C complex and APC/C regulators (Zhang et al. 2014, 2016; Zhou et al. 2013, 2016; Fujimitsu et al. 2016).It can be anticipated a steady increase of activity in this area in the coming years.
Defects in centrosome and spindle-associated functionsare the most frequent cause of primary microcephaly syndromes in humans. For example, mutations in CENP-E have defined a novel kinetochore -centromeric mechanism for microcephalic primordial dwarfism (Mirzaa et al. 2014) while centromere protein F (CENP-F) has been implicated in Hutchinson-Gilford progeria syndrome, a rare disorder that leads to premature ageing and death due to myocardial infarction or stroke. The disease is caused by expression of the protein Progerin, which is a truncated version of the protein prelamin A (Eisch et al. 2016). Progerin displaces CENP-F from metaphase chromosome kinetochores, thus increasing chromatin lagging and causing genome instability (Eisch et al. 2016).
The range of malignancies described above indicate that the development of new drugs to interfere with abnormal cell proliferation is urgently required. The development of new drugs to interfere with defective SAC signalling and its communication with the KMN network in human tumours appears as an attractive alternative to prevent the proliferation of cells carrying abnormalities in chromosome structure and number. Multiple protein-protein interactions in regulatory hubs that control chromosome segregation and mitosis progression may constitute an important pool of novel drug targets . Structural insight into the molecular architecture of key interactions that regulate the SAC should pave the way for drug target identification and validation. In the absence of high resolution structural data, definition of the relationship between hub proteins and drug targets based on the combinatorial analysis of intrinsic structural disorder and gene onthology seems particularly attractive (Fu et al. 2015; The Gene Ontology Consortium 2010).
16.13 Emerging Methods in Structural Biology
The dynamic and coordinated assembly and disassembly of protein complexes in time and space follows sequential obligate stages that result in an enhanced selectivity with a low margin for errors in the process. At the same time, the dynamics of protein complex assembly and disassembly represents a great challenge for their structural and functional characterisation and often requires a combinatorial multidisciplinary approach involving a range of biochemical, biophysical, molecular and cellular approaches. Recent advances in Förster resonance energy transfer by fluorescence lifetime imaging microscopy; laser ablation; small angle x-ray scattering in structural biology (SAXS); nuclear magnetic resonance (NMR); serial femtosecond crystallography; and cryo-transmission electron microscopy (TEM) represent new exciting opportunities to understand the complex dynamics and mode of regulation of the SAC-KMN-microtubule signaling axis through the combinatorial use of the techniques.
Advances in SAXS methods allow the study of macromolecular complexes in solution that provide information about the shapes, conformations; oligomeric states of globular, non-globular and disordered macromolecules (Chaudhuri 2015) whereas multinuclear relaxation dispersion NMR methods permit to follow molecular recognition events of intrinsically disordered proteins in solution (Schneider et al. 2015; Parigi et al. 2014). Time-resolved protein crystallography using ultrafast X-ray free-electron lasers (XFELs) make it possible to follow rapid structural changes resulting from photolysis in the crystalline state and to resolve reaction intermediates at impressive high resolution (Barends et al. 2015; Tenboer et al. 2014). More recent improvements in serial femtosecond crystallography allowed the collection of X-ray diffraction patterns using X-ray pulses of 50 femtosecond duration that contained approximately 2 × 1012 photons per pulse to achieve a high-resolution XFEL structure of 1.75 Å (Ginn et al. 2015). Laser ablation has been used recently to separate microtubules attached to a merotelic kinetochore to study the mechanical response of the kinetochore resulting from changes of its length (Cojoc et al. 2016). At the same time, the study shows that the use of merotelic kinetochores emerges as an attractive experimental model for studying the mechanical properties of the kinetochore in live cells (Cojoc et al. 2016).
Equally impressive is the pace of instrumental and computational improvements in TEM where modern electron microscopes can produce images at a resolution higher than 2.0 Å (Glaeser 2016; Nogales 2016). A big gain that TEM offers is the possibility of studying samples of a relatively heterogeneous nature, thus allowing the analysis of multiple structural states that recapitulate the dynamics of complex protein-protein interactions including their mode of regulation and assembly/disassembly under diverse conditions (Weis et al. 2015; Louder et al. 2016). Parallel advances in cryo-electron tomography now allow the visualisation of macromolecular assemblies of irregular shapes; of organelles and even entire cells at the subnanometre resolution scale. An excellent review on this topic has been reported recently by Helen Saibil’s group (2016).
16.14 Closing Remarks
The study of cell division, the mechanism of transmission of the genetic material to descendants and the molecular basis of premature aging and cancer are areas of great interest in the Biomedical Sciences. Spindle assembly checkpoint (SAC) signalling is a truly fundamental cellular process of higher organisms that ensures the faithful segregation of chromosomes each time a cell divides. Undoubtedly, the inhibition of aberrant SAC signalling will benefit a wide range of disciplines, ranging from the cellular and molecular understanding of cell division in health and disease to the study of cell development , genome stability, ageing and comparative genomics.
The synergistic combination of biochemical, biophysical and structural biology methods for the characterisation of dynamic macromolecular complexes together with cellular and systems biology approaches should lead to a more comprehensive understanding of the cell and provide insights into how defects in molecular interactions can lead to the impairment of cellular regulation and function.
As the use of these experimental techniques alongside with molecular and computational methods begin to give insights into the dynamics of protein assembly /disassembly and their architecture, we will learn more mechanistic details of the remarkable complexity of the network of interactions between thousands of protein components that regulate metabolic and signalling pathways essential to all eukaryotes.
Because large multi-protein complexes play critical roles in cell regulation, interfering with the dynamics of their assembly and/or dissociation rises as an attractive strategy for the treatment of diseases. Extending the study of the structure and the dynamics of isolated SAC-KMN-microtubule sub-complexes to the molecular understanding of the mode of organisation of larger assemblies that ensure signal generation and amplification in a narrow spatial-temporal framework continues to represent a major challenge. Recent advances in TEM, electron-free lasers and a range of biophysical methods herald a new and exciting era for the molecular understanding of nuclear complexes that ensure genome stability to an unprecedented level of detail.
Abbreviations
- APC/C:
-
Anaphase Promoting Complex/Cyclosome
- ATP:
-
Adenosine triphosphate
- Bub1:
-
Budding uninhibited by benzimidazoles 1
- BubR1:
-
Budding uninhibited by benzimidazoles related 1
- CCAN:
-
Centromeric nucleosome-associated network
- Cdc20:
-
Cell division cycle protein 20
- CENP-E:
-
Centromere-associated protein E
- CENP-F:
-
Centromere-associated protein F
- CIN:
-
Chromosomal instability
- CLASP-1:
-
CLIP-associating protein 1
- CLASP-2:
-
CLIP-associating protein 2
- FRET:
-
Förster resonance energy transfer
- KMN:
-
KNL1/Mis12/Ndc80 network
- KNL1:
-
Kinetochore-null phenotype 1
- Mad1:
-
Mitotic arrest deficient 1
- Mad2:
-
Mitotic arrest deficient 2
- MIND complex:
-
Mis12 complex
- Mps1:
-
Monopolar spindle 1
- NMR:
-
Nuclear magnetic resonance
- ROD:
-
Rough deal
- RZZ-complex:
-
Rod, Zwilch and ZW10 complex
- SAC:
-
Spindle assembly checkpoint
- SAXS:
-
Small angle x-ray scattering
- Spc105:
-
Spindle pole body 105
- Spc105-related:
-
Spc105R
- TEM:
-
cryo-transmission electron microscopy
- XFEL:
-
Ultrafast X-ray free-electron laser
- ZW10:
-
Zeste-white 10
References
Abrieu A, Magnaghi-Jaulin L, Kahana JA, Peter M, Castro A, Vigneron S, Lorca T, Cleveland DW, Labbé JC (2001) Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell 106:83–93
Agarwal S, Varma D (2014) How the SAC gets the axe: Integrating kinetochore microtubule attachments with spindle assembly checkpoint signaling. BioArchitecture 5:1–12
Altenfeld A, Wohlgemuth S, Wehenkel A, Vetter IR, Musacchio A (2015) Complex assembly, crystallization and preliminary X-ray crystallographic analysis of the human Rod-Zwilch-ZW10 (RZZ) complex. Acta Crystallogr F Struct Biol Commun 71:438–442
Alushin GM, Ramey VH, Pasqualato S, Ball DA, Grigorieff N, Musacchio A, Nogales E (2010) The Ndc80 kinetochore complex forms oligomeric arrays along microtubules. Nature 467:805–810
Aravamudhan P, Goldfarb AA, Joglekar AP (2015) The kinetochore encodes a mechanical switch to disrupt spindle assembly checkpoint signalling. Nat Cell Biol 17:868–879
Babu MM, Kriwacki RW, Pappu RV (2012) Structural biology. Versatility from protein disorder Science 337:1460–1461
Barends TR, Foucar L, Ardevol A, Nass K, Aquila A, Botha S, Doak RB, Falahati K, Hartmann E, Hilpert M, Heinz M, Hoffmann MC, Köfinger J, Koglin JE, Kovacsova G, Liang M, Milathianaki D, Lemke HT, Reinstein J, Roome CM, Shoeman RL, Williams GJ, Burghardt I, Hummer G, Boutet S, Schlichting I (2015) Direct observation of ultrafast collective motions in CO myoglobin upon ligand dissociation. Science 350:445–450
Barnhart MC, Kuich PH, Stellfox ME, Ward JA, Bassett EA, Black BE, Foltz DR (2011) HJURP is a CENP-A chromatin assembly factor sufficient to form a functional de novo kinetochore. J Cell Biol 194:229–243
Bavetsias V, Linardopoulos S (2015) Aurora Kinase Inhibitors: Current Status and Outlook. Front Oncol 5:278
Biggins S (2013) The composition, functions, and regulation of the budding yeast kinetochore. Genetics 194:817–846
Blundell TL, Bolanos-Garcia VM, Chirgadze DY, Harmer NJ, Lo T, Pellegrini L, Sibanda BL (2002) Asymmetry in the multiprotein systems of molecular biology. Struct Chem 13:405–412
Bolanos-Garcia VM, Blundell TL (2011) BUB1 and BUBR1: multifaceted kinases of the cell cycle. Trends Biochem Sci 36:141–150
Bolanos-Garcia VM, Lischetti T, Matak-Vinkovic D, Cota E, Simpson PJ, Chirgadze DY, Spring DR, Robinson CV, Nilsson J, Blundell TL (2011) Structure of a Blinkin-BUBR1 complex reveals an interaction crucial for kinetochore-mitotic checkpoint regulation via an unanticipated binding Site. Structure 19:1691–1700
Bolanos-Garcia VM, Wu Q, Ochi T, Chirgadze DY, Sibanda BL, Blundell TL (2012) Spatial and temporal organisation of multiprotein assemblies: achieving sensitive control in information-rich cell regulatory systems. Philos Transact A Math Phys Eng Sci 370:3023–3039
Boyarchuk Y, Salic A, Dasso M, Arnaoutov A (2007) Bub1 is essential for assembly of the functional inner centromere. J Cell Biol 176:919–928
Caldas GV, DeLuca JG (2014) KNL1: bringing order to the kinetochore. Chromosoma 123:169–181
Carroni M, Saibil HR (2016) Cryo electron microscopy to determine the structure of macromolecular complexes. Methods 95:78–85
Carter SL, Eklund AC, Kohane IS, Harris LN. Szallasi Z (2006) A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat Genet 38:1043–1048.
Chao W, Kulkarni K, Zhang Z, Kong E, Barford D (2012) Structure of the mitotic checkpoint complex. Nature 484:208–213
Chaudhuri BN (2015) Emerging applications of small angle solution scattering in structural biology. Protein Sci 24:267–276
Cheeseman IM, Niessen S, Anderson S, Hyndman F, Yates JR 3rd, Oegema K, Desai A (2004) A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes Dev 18:2255–2268
Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A (2006) The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127:983–997
Chmielewska AE, Tang NH, Toda T (2016) The hairpin region of Ndc80 is important for the kinetochore recruitment of Mph1/MPS1 in fission yeast. Cell Cycle 15:740–747
Ciferri C, De Luca J, Monzani S, Ferrari KJ, Ristic D, Wyman C, Stark H, Kilmartin J, Salmon ED, Musacchio A (2005) Architecture of the human ndc80-hec1 complex, a critical constituent of the outer kinetochore. J Biol Chem 280:29088–29095
Ciferri C, Pasqualato S, Screpanti E, Varetti G, Santaguida S, Dos Reis G, Maiolica A, Polka J, De Luca JG, De Wulf P, Salek M, Rappsilber J, Moores CA, Salmon ED, Musacchio A (2008) Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell 133:427–439
Cino EA, Karttunen M, Choy W-Y (2012) Effects of Molecular Crowding on the Dynamics of Intrinsically Disordered Proteins. PLoS One 7:e49876
Civril F, Wehenkel A, Giorgi FM, Santaguida S, Di Fonzo A, Grigorean G, Ciccarelli FD, Musacchio A (2010) Structural analysis of the RZZ complex reveals common ancestry with multisubunit vesicle tethering machinery. Structure 18:616–626
Cojoc G, Roscioli E, Zhang L, García-Ulloa A, Shah JV, Berns MW, Pavin N, Cimini D, Tolić IM, Gregan J (2016) Laser microsurgery reveals conserved viscoelastic behavior of the kinetochore. J Cell Biol 212:767–776
Daniel J, Coulter J, Woo JH, Wilsbach K, Gabrielson E (2011) High levels of the Mps1 checkpoint protein are protective of aneuploidy in breast cancer cells. Proc Natl Acad Sci U S A 108:5384–5389
DeLano WL (2002) The PyMOL molecular graphics system. DeLano Scientific, San Carlos
DeLuca JG, Musacchio A (2012) Structural organization of the kinetochore-microtubule interface. Curr Opin Cell Biol 24:48–56
Desai A, Rybina S, Müller-Reichert T, Shevchenko A, Shevchenko A, Hyman A, Oegema K (2003) KNL-1 directs assembly of the microtubule-binding interface of the kinetochore in C. elegans. Genes Dev 17:2421–2435
Dosztányi Z, Chen J, Dunker AK, Simon I, Tompa P (2006) Disorder and sequence repeats in hub proteins and their implications for network evolution. J Proteome Res 5:2985–2995
Dunker AK, Garner E, Guilliot S, Romero P, Albrecht K, Hart J, Obradovic Z, Kissinger C, Villafranca JE (1998) Protein disorder and the evolution of molecular recognition: theory, predictions and observations. Pac Symp Biocomput:473–484
Dunker AK, Cortese MS, Romero P, Iakoucheva LM, Uversky VN (2005) Flexible nets: the roles of intrinsic disorder in protein interaction networks. FEBS J 272:5129–5148
Dunker AK, Oldfield CJ, Meng J, Romero P, Yang JY, Chen JW, Vacic V, Obradovic Z, Uversky VN (2008) The unfoldomics decade: an update on intrinsically disordered proteins. BMC Genomics 9:S1
Dunsch AK, Linnane E, Barr FA, Gruneberg U (2011) The astrin-kinastrin/SKAP complex localizes to microtubule plus ends and facilitates chromosome alignment. J Cell Biol 192:959–968
Efimov A, Kharitonov A, Efimova N, Loncarek J, Miller PM, Andreyeva N, Gleeson P, Galjart N, Maia AR, McLeod IX, Yates JR 3rd, Maiato H, Khodjakov A, Akhmanova A, Kaverina I (2007) Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev Cell 12:917–930
Eginton C, Cressman WJ, Bachas S, Wade H, Beckett D (2015) Allosteric Coupling via Distant Disorder-to-Order Transitions. J Mol Biol 427:1695–1704
Eisch V, Lu X, Gabriel D, Djaali K (2016) Progerin impairs chromosome maintenance by depleting CENP-F from metaphase kinetochores in Hutchinson-Gilford progeria fibroblasts. Oncotarget Mar 22. 7(17):24700–24718 doi: 10.18632/oncotarget.8267.
Elowe S (2011) Bub1 and BubR1: at the Interface between Chromosome Attachment and the Spindle Checkpoint. Mol Cell Biol 31:3085–3093
Fachinetti D, Folco HD, Nechemia-Arbely Y, Valente LP, Nguyen K, Wong AJ, Zhu Q, Holland AJ, Desai A, Jansen LE, Cleveland DW (2013) A two-step mechanism for epigenetic specification of centromere identity and function. Nat Cell Biol 15:1056–1066
Fang L, Seki A, Fang G (2009) SKAP associates with kinetochores and promotes the metaphase-to-anaphase transition. Cell Cycle 8:2819–2827
Foltz DR, Jansen LE, Black BE, Bailey AO, Yates JR 3rd, Cleveland DW (2006) The human CENP-A centromeric nucleosome-associated complex. Nat Cell Biol 8:458–469
Fu Y, Guo Y, Wang Y, Luo J, Pu X, Li M, Zhang Z (2015) Exploring the relationship between hub proteins and drug targets based on GO and intrinsic disorder. Comput Biol Chem 56:41–48
Fujimitsu K, Grimaldi M, Yamano H (2016) Cyclin-dependent kinase 1-dependent activation of APC/C ubiquitin ligase. Science 352:1121–1124
Fuller AA, Du D, Liu F, Davoren JE, Bhabha G, Kroon G, Case DA, Dyson HJ, Powers ET, Wipf P, Gruebele M, Kelly JW (2009) Evaluating beta-turn mimics as beta-sheet folding nucleators. Proc Natl Acad Sci U S A 106:11067–11072
Funabiki H, Wynne DJ (2013) Making an effective switch at the kinetochore by phosphorylation and dephosphorylation. Chromosoma 122:135–158
Gardner MK, Odde DJ, Bloom K (2008) Kinesin-8 molecular motors: putting the brakes on chromosome oscillations. Trends Cell Biol 18:307–310
Gascoigne KE, Takeuchi K, Suzuki A, Hori T, Fukagawa T, Cheeseman IM (2011) Induced ectopic kinetochore assembly bypasses the requirement for CENP-A nucleosomes. Cell 145:410–422
George AA, Walworth NC (2016) Microtubule dynamics decoded by the epigenetic state of centromeric chromatin. Curr Genet Mar 14. 62(4):691–695 PMID: 26976145.
Ghongane P, Kapanidou M, Asghar A, Elowe S, Bolanos-Garcia VM (2014) The dynamic protein Knl1- a kinetochore rendezvous. J Cell Sci 127:3415–3423
Ginn HM, Messerschmidt M, Ji X, Zhang H, Axford D, Gildea RJ, Winter G, Brewster AS, Hattne J, Wagner A, Grimes JM, Evans G, Sauter NK, Sutton G, Stuart DI (2015) Structure of CPV17 polyhedrin determined by the improved analysis of serial femtosecond crystallographic data. Nat Commun 6:6435
Glaeser RM (2016) How good can cryo-EM become? Nat Methods 13:28–32
Godek KM, Kabeche L, Compton DA (2015) Regulation of kinetochore-microtubule attachments through homeostatic control during mitosis. Nat Rev Mol Cell Biol 16:57–64
Gruber J, Harborth J, Schnabel J, Weber K, Hatzfeld M (2002) The mitotic-spindle-associated protein astrin is essential for progression through mitosis. J Cell Sci 115:4053–4059
Gsponer J, Babu MM (2009) The rules of disorder or why disorder rules. Prog Biophys Mol Biol 99:94–103
Haynes C, Oldfield CJ, Ji F, Klitgord N, Cusick ME, Radivojac P, Uversky VN, Vidal M, Iakoucheva LM (2006) Intrinsic disorder is a common feature of hub proteins from four eukaryotic interactomes. PLoS Comput Biol 2:e100
Hellwig D, Münch S, Orthaus S, Hoischen C, Hemmerich P, Diekmann S (2008) Live-cell imaging reveals sustained centromere binding of CENP-T via CENP-A and CENP-B. J Biophotonics 1:245–254
Herman JA, Toledo CM, Olson JM, DeLuca JG, Paddison PJ (2015) Molecular pathways: regulation and targeting of kinetochore-microtubule attachment in cancer. Clin Cancer Res 21:233–239
Hiruma Y, Sacristan C, Pachis ST, Adamopoulos A, Kuijt T, Ubbink M, von Castelmur E, Perrakis A, Kops GJ (2015) Competition between MPS1 and microtubules at kinetochores regulates spindle checkpoint signaling. Science 348:1264–1267
Hood EA, Kettenbach AN, Gerber SA, Compton DA (2012) Plk1 regulates the kinesin-13 protein Kif2b to promote faithful chromosome segregation. Mol Biol Cell 23:2264–2274
Huang Y, Yao Y, Xu HZ, Wang ZG, Lu L, Dai W (2009) Defects in chromosome congression and mitotic progression in KIF18A-deficient cells are partly mediated through impaired functions of CENP-E. Cell Cycle 8:2643–2649
Huang Y, Wang W, Yao P, Wang X, Liu X, Zhuang X, Yan F, Zhou J, Du J, Ward T, Zou H, Zhang J, Fang G, Ding X, Dou Z, Yao X (2012) CENP-E kinesin interacts with SKAP protein to orchestrate accurate chromosome segregation in mitosis. J Biol Chem 287:1500–1509
Janssen A, Kops GJ, Medema RH (2009) Elevating the frequency of chromosome mis-segregation as a strategy to kill tumor cells. Proc Natl Acad Sci U S A 106:19108–19113
Ji Z, Gao H, Yu H (2015) Kinetochore attachment sensed by competitive Mps1 and microtubule binding to Ndc80C. Science 48:1260–1264
Jiang H, He X, Wang S, Jia J, Wan Y, Wang Y, Zeng R, Yates J 3rd, Zhu X, Zheng Y (2014) A Microtubule-Associated Zinc Finger Protein, BuGZ, Regulates Mitotic Chromosome Alignment by Ensuring Bub3 Stability and Kinetochore Targeting. Dev Cell 28:268–281
Jiang H, Wang S, Huang Y, He X, Cui H, Zhu X, Zheng Y (2015) Phase transition of spindle-associated protein regulate spindle apparatus assembly. Cell 163:108–122
Joglekar AP, DeLuca JG (2009) Chromosome segregation: Ndc80 can carry the load. Curr Biol 19:R404–R407
Kapanidou M, Bolanos-Garcia VM (2014) Spindle Assembly Checkpoint (SAC): More New Targets for Anti-Cancer Drug Therapies. Adv Cancer Drug Targ 2:54–79
Karess R (2005) Rod-ZW10-ZWILCH: a key player in the spindle checkpoint. Trends Cell Biol 15:386–392
Karess RE, Glover DM (1989) Rough deal: a gene required for proper mitotic segregation in Drosophila. J Cell Biol 109:2951–2961
Kim S, Yu H (2015) Multiple assembly mechanisms anchor the KMN spindle checkpoint platform at human mitotic kinetochores. J Cell Biol 208:181–196
Kim PM, Lu LJ, Xia Y, Gerstein MB (2006) Relating three-dimensional structures to protein networks provides evolutionary insights. Science 314:1938–1941
Kiyomitsu T, Obuse C, Yanagida M (2007) Human Blinkin/AF15q14 is required for chromosome alignment and the mitotic checkpoint through direct interaction with Bub1 and BubR1. Dev Cell 13:663–676
Kops GJ, Kim Y, Weaver BA, Mao Y, McLeod I, Yates JR 3rd, Tagaya M, Cleveland DW (2005a) ZW10 links mitotic checkpoint signaling to the structural kinetochore. J Cell Biol 169:49–60
Kops GJ, Weaver BA, Cleveland DW (2005b) On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer 5:773–785
Krenn V, Musacchio A (2015) The Aurora B Kinase in Chromosome Bi-Orientation and Spindle Checkpoint Signaling. Front Oncol 5:225
Krenn V, Wehenkel A, Li X, Santaguida S, Musacchio A (2012) Structural analysis reveals features of the spindle checkpoint kinase Bub1-kinetochore subunit Knl1 interaction. J Cell Biol 196:451–467
Kudalkar EM, Scarborough EA, Umbreit NT, Zelter A, Gestaut DR, Riffle M, Johnson RS, MacCoss MJ, Asbury CL, Davis TN (2015) Regulation of outer kinetochore Ndc80 complex-based microtubule attachments by the central kinetochore Mis12/MIND complex. Proc Natl Acad Sci U S A 112:E5583–E5589
Kwiatkowski N, Jelluma N, Filippakopoulos P, Soundararajan M, Manak MS, Kwon M, Choi HG, Sim T, Deveraux QL, Rottmann S, Pellman D, Shah JV, Kops GJ, Knapp S, Gray NS (2010) Small-molecule kinase inhibitors provide insight into Mps1 cell cycle function. Nat Chem Biol 6:359–368
Lara-Gonzalez P, Westhorpe F, Taylor S (2012) The Spindle Assembly Checkpoint. Curr Biol 22:966–980
Leduc C, Padberg-Gehle K, Varga V, Helbing D, Diez S, Howard J (2012) Molecular crowding creates traffic jams of kinesin motors on microtubules. Proc Natl Acad Sci U S A 109:6100–6105
Liu D, Ding X, Du J, Cai X, Huang Y, Ward T, Shaw A, Yang Y, Hu R, Jin C, Yao X (2007) Human NUF2 interacts with centromere-associated protein E and is essential for a stable spindle microtubule-kinetochore attachment. J Biol Chem 282:21415–21424
Liu D, Vleugel M, Backer CB, Hori T, Fukagawa T, Cheeseman IM, Lampson LA (2010) Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase. J Cell Biol 188:809–920
Liu Y, Petrovic A, Rombaut P, Mosalaganti S, Keller J, Raunser S, Herzog F, Musacchio A (2016) Insights from the reconstitution of the divergent outer kinetochore of Drosophila melanogaster. Open Biol 6(2) pii: 150236.
London N, Ceto S, Ranish JA, Biggins S (2012) Phosphoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores. Curr Biol 22:900–906
Louder RK, He Y, López-Blanco JR, Fang J, Chacón P, Nogales E (2016) Structure of promoter-bound TFIID and model of human pre-initiation complex assembly. Nature 531:604–609
Maiato H, Fairley EA, Rieder CL, Swedlow JR, Sunkel CE, Earnshaw WC (2003) Human CLASP1 is an outer kinetochore component that regulates spindle microtubule dynamics. Cell 113:891–904
Manic G, Corradi F, Sistigu A, Siteni S, Vitale I (2017) Molecular regulation of the spindle assembly checkpoint by kinases and phosphatases. Int Rev Cell Mol Biol 328:105–161
Manning AL, Ganem NJ, Bakhoum SF, Wagenbach M, Wordeman L, Compton DA (2007) The kinesin-13 proteins Kif2a, Kif2b, and Kif2c/MCAK have distinct roles during mitosis in human cells. Mol Biol Cell 18:2970–2979
Mayr MI, Hümmer S, Bormann J, Grüner T, Adio S, Woehlke G, Mayer TU (2007) The human kinesin Kif18A is a motile microtubule depolymerase essential for chromosome congression. Curr Biol 17:488–498
Mendiburo MJ, Padeken J, Fulop S, Schepers A, Heun P (2011) Drosophila CENH3 is sufficient for centromere formation. Science 334:686–690
Mimori-Kiyosue Y, Grigoriev I, Lansbergen G, Sasaki H, Matsui C, Severin F, Galjart N, Grosveld F, Vorobjev I, Tsukita S, Akhmanova A (2005) CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J Cell Biol 168:141–153
Mirzaa GM, Vitre B, Carpenter G, Abramowicz I, Gleeson JG, Paciorkowski AR, Cleveland DW, Dobyns WB, O’Driscoll M (2014) Mutations in CENPE define a novel kinetochore-centromeric mechanism for microcephalic primordial dwarfism. Hum Genet 133:1023–1039
Mourão MA, Hakim JB, Schnell S (2014) Connecting the dots: the effects of macromolecular crowding on cell physiology. Biophys J 107:2761–2766
Musacchio A (2011) Spindle assembly checkpoint: the third decade. Philos Trans R Soc Lond Ser B Biol Sci 366:3595–3604
Nekrasov VS, Smith MA, Peak-Chew S, Kilmartin JV (2003) Interactions between centromere complexes in Saccharomyces cerevisiae. Mol Biol Cell 14:4931–4946
Nilsson J (2015) Mps1-Ndc80: one interaction to rule them all. Oncotarget 6:16822–16823
Nishino T, Takeuchi K, Gascoigne KE, Suzuki A, Hori T, Oyama T, Morikawa K, Cheeseman IM, Fukagawa T (2012) CENP-T-W-S-X forms a unique centromeric chromatin structure with a histone-like fold. Cell 148:487–501
Nishino T, Rago F, Hori T, Tomii K, Cheeseman IM, Fukagawa T (2013) CENP-T provides a structural platform for outer kinetochore assembly. EMBO J 32:424–436
Nogales E (2016) The development of cryo-EM into a mainstream structural biology technique. Nat Methods 13:24–27
Orthaus S, Ohndorf S, Diekmann S (2006) RNAi knockdown of human kinetochore protein CENP-H. Biochem Biophys Res Commun 348:36–46
Palmer DK, O’Day K, Trong HL, Charbonneau H, Margolis RL (1991) Purification of the centromerespecific protein CENP-A and demonstration that it is a distinctive histone. Proc Natl Acad Sci U S A 88:3734–3738
Parigi G, Rezaei-Ghaleh N, Giachetti A, Becker S, Fernandez C, Blackledge M, Griesinger C, Zweckstetter M, Luchinat C (2014) Long-range correlated dynamics in intrinsically disordered proteins. J Am Chem Soc 136:16201–16209
Pereira AL, Pereira AJ, Maia AR, Drabek K, Sayas CL, Hergert PJ, Lince-Faria M, Matos I, Duque C, Stepanova T, Rieder CL, Earnshaw WC, Galjart N, Maiato H (2006) Mammalian CLASP1 and CLASP2 cooperate to ensure mitotic fidelity by regulating spindle and kinetochore function. Mol Biol Cell 17:4526–4542
Perpelescu M, Fukagawa T (2011) The ABCs of CENPs. Chromosoma 120:425–446
Pesenti ME, Weir JR, Musacchio A (2016) Progress in the structural and functional characterization of kinetochores. Curr Opin Struct Biol 37:152–163
Petrovic A, Pasqualato S, Dube P, Krenn V, Santaguida S, Cittaro D, Monzani S, Massimiliano L, Keller J, Tarricone A, Maiolica A, Stark H, Musacchio A (2010) The MIS12 complex is a protein interaction hub for outer kinetochore assembly. J Cell Biol 190:835–852
Petrovic A, Mosalaganti S, Keller J, Mattiuzzo M, Overlack K, Krenn V, De Antoni A, Wohlgemuth S, Cecatiello V, Pasqualato S, Raunser S, Musacchio A (2014) Modular assembly of RWD domains on the Mis12 complex underlies outer kinetochore organization. Mol Cell 53:591–605
Prendergast L, van Vuuren C, Kaczmarczyk A, Doering V, Hellwig D, Quinn N, Hoischen C, Diekmann S, Sullivan KF (2011) Premitotic assembly of human CENPs -T and -W switches centromeric chromatin to a mitotic state. PLoS Biol 9:e1001082
Primorac I, Weir JR, Chiroli E, Gross F, Hoffmann I, van Gerwen S, Ciliberto A. Musacchio A (2013) Bub3 reads phosphorylated MELT repeats to promote spindle assembly checkpoint signaling. Elife 2:01030.
Przewloka MR, Glover DM (2009) The kinetochore and the centromere: a working long distance relationship. Annu Rev Genet 43:439–465
Qiu S, Wang J, Yu C, He D (2009) CENP-K and CENP-H may form coiled-coils in the kinetochores. Sci China C Life Sci 52:352–359
Rago F, Gascoigne KE, Cheeseman IM (2015) Distinct Organization and Regulation of the Outer Kinetochore KMN Network Downstream of CENP-C and CENP-T. Curr Biol 25:671–677
Richter MM, Poznanski J, Zdziarska A, Czarnocki-Cieciura M, Lipinszki Z, Dadlez M, Glover DM, Przewloka MR (2016) Network of protein interactions within the Drosophila inner kinetochore. Open Biol 6:150238
Rosenberg JS, Cross FR, Funabiki H (2011) KNL1/Spc105 recruits PP1 to silence the spindle assembly checkpoint. Curr Biol 21:942–947
Santaguida S, Musacchio A (2009) The life and miracles of kinetochores. EMBO J 28:2511–2531
Scaërou F, Aguilera I, Saunders R, Kane N, Blottière L, Karess R (1999) The rough deal protein is a new kinetochore component required for accurate chromosome segregation in Drosophila. J Cell Sci 112:3757–3768
Scaërou F, Starr DA, Piano F, Papoulas O, Karess RE, Goldberg ML (2001) The ZW10 and Rough Deal checkpoint proteins function together in a large, evolutionarily conserved complex targeted to the kinetochore. J Cell Sci 114:3103–3114
Schleiffer A, Maier M, Litos G, Lampert F, Hornung P, Mechtler K, Westermann S (2012) CENP-T proteins are conserved centromere receptors of the Ndc80 complex. Nat Cell Biol 14:604–613
Schneider R, Maurin D, Communie G, Kragelj J, Hansen DF, Ruigrok RW, Jensen MR, Blackledge M (2015) Visualizing the molecular recognition trajectory of an intrinsically disordered protein using multinuclear relaxation dispersion NMR. J Am Chem Soc 137:1220–1229
Shepperd LA, Meadows JC, Sochaj AM, Lancaster TC, Zou J, Buttrick GJ, Rappsilber J, Hardwick KG, Millar JB (2012) Phosphodependent recruitment of Bub1 and Bub3 to Spc7/KNL1 by Mph1 kinase maintains the spindle checkpoint. Curr Biol 22:891–899
Stoler S, Keith KC, Curnick KE, Fitzgerald-Hayes M (1995) A mutation in CSE4, an essential gene encoding a novel chromatin-associated protein in yeast, causes chromosome nondisjunction and cell cycle arrest at mitosis. Genes Dev 9:573–586
Stumpff J, von Dassow G, Wagenbach M, Asbury C, Wordeman L (2008) The kinesin-8 motor Kif18A suppresses kinetochore movements to control mitotic chromosome alignment. Dev Cell 14:252–262
Suzuki A, Badger BL, Salmon ED (2015) A quantitative description of Ndc80 complex linkage to human kinetochores. Nat Commun 6:8161
Tachiwana H, Kagawa W, Shiga T, Osakabe A, Miya Y, Saito K, Hayashi-Takanaka Y, Oda T, Sato M, Park SY, Kimura H, Kurumizaka H (2011) Crystal structure of the human centromeric nucleosome containing CENP-A. Nature 476:232–235
Takeuchi K, Nishino T, Mayanagi K, Horikoshi N, Osakabe A, Tachiwana H, Hori T, Kurumizaka H, Fukagawa T (2014) The centromeric nucleosome-like CENP-T-W-S-X complex induces positive supercoils into DNA. Nucleic Acids Res 42:1644–1655
Tanaka K (2013) Regulatory mechanisms of kinetochore-microtubule interaction in mitosis. Cell Mol Life Sci 70:559–579
Tenboer J, Basu S, Zatsepin N, Pande K, Milathianaki D, Frank M, Hunter M, Boutet S, Williams GJ, Koglin JE, Oberthuer D, Heymann M, Kupitz C, Conrad C, Coe J, Roy-Chowdhury S, Weierstall U, James D, Wang D, Grant T, Barty A, Yefanov O, Scales J, Gati C, Seuring C, Srajer V, Henning R, Schwander P, Fromme R, Ourmazd A, Moffat K, Van Thor JJ, Spence JC, Fromme P, Chapman HN, Schmidt M (2014) Time-resolved serial crystallography captures high-resolution intermediates of photoactive yellow protein. Science 346:1242–1246
The Gene Ontology Consortium (2010) The gene ontology in 2010: extensions and refinements. Nucleic Acids Res 38:D331–D335
Thedieck K, Holzwarth B, Prentzell MT, Boehlke C, Kläsener K, Ruf S, Sonntag AG, Maerz L, Grellscheid SN, Kremmer E, Nitschke R, Kuehn EW, Jonker JW, Groen AK, Reth M, Hall MN, Baumeister R (2013) Inhibition of mTORC1 by astrin and stress granules prevents apoptosis in cancer cells. Cell 154:859–874
Thein KH, Kleylein-Sohn J, Nigg EA, Gruneberg U (2007) Astrin is required for the maintenance of sister chromatid cohesion and centrosome integrity. J Cell Biol 178:345–354
Toledo CM, Herman JA, Olsen JB, Ding Y, Corrin P, Girard EJ, Olson JM, Emili A, DeLuca JG, Paddison PJ (2014) BuGZ Is Required for Bub3 Stability, Bub1 Kinetochore Function, and Chromosome Alignment. Dev Cell 28:282–294
Tooley J, Stukenberg PT (2011) The Ndc80 complex: integrating the kinetochore’s many movements. Chromosom Res 19:377–391
Uversky VN (2015) The multifaceted roles of intrinsic disorder in protein complexes. FEBS Lett 589:2498–2506
Varma D, Salmon ED (2012) The KMN protein network--chief conductors of the kinetochore orchestra. J Cell Sci 125:5927–5936
Vleugel M, Omerzu M, Groenewold V, Hadders MA, Lens SM, Kops GJ (2015) Sequential Multisite Phospho-Regulation of KNL1-BUB3 Interfaces at Mitotic Kinetochores. Mol Cell 57:824–835
Wan X, O’Quinn RP, Pierce HL, Joglekar AP, Gall WE, DeLuca JG, Carroll CW, Liu ST, Yen TJ, McEwen BF, Stukenberg PT, Desai A, Salmon ED (2009) Protein architecture of the human kinetochore microtubule attachment site. Cell 137:672–684
Wang X, Zhuang X, Cao D, Chu Y, Yao P, Liu W, Liu L, Adams G, Fang G, Dou Z, Ding X, Huang Y, Wang D, Yao X (2012a) Mitotic regulator SKAP forms a link between kinetochore core complex KMN and dynamic spindle microtubules. J Biol Chem 287:39380–39390
Wang Y, Sarkar M, Smith AE, Krois AS, Pielak GJ (2012b) Macromolecular Crowding and Protein Stability. J Am Chem Soc 134:16614–16618
Wei RR, Sorger PK, Harrison SC (2005) Molecular organization of the Ndc80 complex, an essential kinetochore component. Proc Natl Acad Sci U S A 102:5363–5367
Wei RR, Al-Bassam J, Harrison SC (2007) The Ndc80/HEC1 complex is a contact point for kinetochore-microtubule attachment. Nat Struct Mol Biol 14:54–59
Weis F, Giudice E, Churcher M, Jin L, Hilcenko C, Wong CC, Traynor D, Kay RR, Warren AJ (2015) Mechanism of eIF6 release from the nascent 60S ribosomal subunit. Nat Struct Mol Biol 22:914–919
Williams BC, Karr TL, Montgomery JM, Goldberg ML (1992) The Drosophila l(1)ZW10 gene product, required for accurate mitotic chromosome segregation, is redistributed at anaphase onset. J Cell Biol 118:759–773
Williams BC, Li Z, Liu S, Williams EV, Leung G, Yen TJ, Goldberg ML (2003) ZWILCH, a New Component of the ZW10/ROD Complex Required for Kinetochore Functions. Mol Biol Cell 14:1379–1391
Xue B, Dunbrack RL, Williams RW, Dunker AK, Uversky VN (2010) PONDR-FIT: a meta-predictor of intrinsically disordered amino acids. Biochim Biophys Acta 180:996–1010
Zaoui K, Benseddik K, Daou P, Salaün D, Badache A (2010) ErbB2 receptor controls microtubule capture by recruiting ACF7 to the plasma membrane of migrating cells. Proc Natl Acad Sci U S A 107:18517–18522
Zhang J, Wan L, Dai X, Sun Y, Wei W (2014) Functional characterization of Anaphase Promoting Complex/Cyclosome (APC/C) E3 ubiquitin ligases in tumorigenesis. Biochim Biophys Acta 1845:277–293
Zhang S, Chang L, Alfieri C, Zhang Z, Yang J, Maslen S, Skehel M, Barford D (2016) Molecular mechanism of APC/C activation by mitotic phosphorylation. Nature 533:260–264
Zhonghua Liu, Vong QP, Yixian Zheng (2007) CLASPing Microtubules at the trans-Golgi Network. Dev Cell 12:839–840
Zhou W, Wei W, Sun Y (2013) Genetically engineered mouse models for functional studies of SKP1-CUL1-F-box-protein (SCF) E3 ubiquitin ligases. Cell Res 23:599–619
Zhou Z, He M, Shah AA, Wan Y (2016) Insights into APC/C: from cellular function to diseases and therapeutics. Cell Div 11:9
Zich J, May K, Paraskevopoulos K, Sen O, Syred HM, van der Sar S, Patel H, Moresco JJ, Sarkeshik A, Yates JR 3rd, Rappsilber J, Hardwick KG (2016) Mps1Mph1 Kinase Phosphorylates Mad3 to Inhibit Cdc20Slp1-APC/C and Maintain Spindle Checkpoint Arrests. PLoS Genet 12:e1005834
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Bolanos-Garcia, V.M. (2017). Protein Complexes in the Nucleus: The Control of Chromosome Segregation. In: Harris, J., Marles-Wright, J. (eds) Macromolecular Protein Complexes. Subcellular Biochemistry, vol 83. Springer, Cham. https://doi.org/10.1007/978-3-319-46503-6_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-46503-6_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-46501-2
Online ISBN: 978-3-319-46503-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)