Abstract
Over the past decade, dental tissues have become an attractive source of mesenchymal stem cells (MSCs). Dental stem cells (DSCs) are not only able to differentiate into adipogenic, chondrogenic and osteogenic lineanges, but an increasing amount of research also pointed out their potential applicability in numerous clinical disorders, such as myocardial infarction, neurodegenerative diseases and diabetes. Together with their multilineage differentiation capacity, their easy availability from extracted third molars makes these stem cells a suitable alternative for bone marrow-derived MSCs. More importantly, DSCs appear to retain their stem cell properties following cryopreservation, a key aspect in their long-term preservation and upscale production. However, the vast number of different cryopreservation protocols makes it difficult to draw definite conclusions regarding the behavior of these stem cells. The routine application and banking of DSCs is also associated with some other pitfalls, such as interdonor variability, cell culture-induced changes and the use of animal-derived culture medium additives. Only thorough assessment of these challenges and the implementation of standardized, GMP procedures will successfully lead to better treatment options for patients who no longer benefit from current stem cell therapies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Dental stem cells
- Mesenchymal stem cells
- Paracrine effects
- Multilineage differentiation
- Cryopreservation
- Dental stem cell banking
- Good manufacturing practice
17.1 Introduction
Mesenchymal stem cells (MSCs) are derived from adult tissues and have been extensively studied as therapeutic application for tissue regeneration in a wide range of diseases. This is due to their multipotent ability to transdifferentiate into other cell types such as chondrocytes, osteocytes and cardiac myocytes, which have high potential to replace damaged tissue. Sources for MSC isolation include the bone marrow (BM-MSCs) [1], adipose tissue (ASCs ) [2], Wharton’s Jelly in the umbilical cord (UMSCs) [3], umbilical cord blood (UCBC) [4] and as discussed in this chapter, dental tissues.
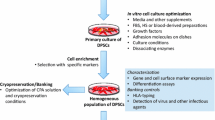
In 2000, Gronthos et al. discovered an alternative source of MSCs, i.e. odontogenic progenitor cells, which were retrieved from the dental pulp. This finding counted as a starting point for the discovery of a heterogeneous population of dental stem cells (DSCs) which are defined by their localization in the developing tooth and its associated tissues (Fig. 17.1). These populations comprise dental pulp stem cells (DPSCs) [5], stem cells from the apical papilla (SCAPs) [6], periodontal ligament stem cells (PDLSCs) [7] and dental follicle precursor cells (FSCs) [8]. The periodontal ligament (PDL) is believed to contain another source of DSCs residing in the epithelial cell rests of Malassez (ERM) which are present in the periodontal ligament matrix [9]. Full-grown or developing teeth are therefore a valuable source of DSCs. In addition, several studies reported that deciduous teeth can be utilized to isolate stem cells from the pulp of human exfoliated deciduous teeth (SHEDs) [10] and deciduous periodontal ligament (DePDL) [11]. DSCs originate from the neural crest [12, 13] however, a large fraction of these cells are of glial origin [14].
Schematic representation of the different sources of DSCs . FSCs dental follicle precursor cells, DPSCs dental pulp stem cells, SCAPs stem cells from the apical papilla, PDLSCs periodontal ligament stem cells (Figure was adapted with permission from [15])
The application of DSCs offers several advantages over more conventionally used MSC populations, such as BM-MSCs. Not only are these cells easily obtained with minimally invasive surgery but once in culture they display a high proliferative capacity, making them a suitable candidate for cell banking. The heterogeneity of DSCs encouraged numerous researchers to explore their multilineage differentiation potential, which resulted in a broad range of differentiation protocols for various cell types. Emerging therapeutic strategies are focusing on the integration of pre-differentiated and preconditioned DSCs in the diseased host tissue exerting a beneficial effect through either intercellular contacts or via paracrine mediated interactions. Autologous as well as allogeneic transplantation of DSCs in clinical studies thus requires the need for a quality control system which provides a detailed assessment of the specific DSC phenotype and its matching paracrine profile. Upscaling of DSCs and the establishment of a dental stem cell banking system should follow standard Good Manufacturing Practice (GMP) regulations . In this chapter we will provide in-depth knowledge on the heterogeneity of DSCs and their concomitant differentiation potential in vitro and in vivo. We will focus specifically on the current strategies of cryopreservation to maintain phenotypic stability and to prevent risks of cell death and contamination. An overview of the pitfalls which could arise in creating a dental stem cell bank is also given as well as practical guidelines and prospects for application in clinical studies.
17.2 Dental Stem Cells
In order to be classified as MSCs , several requirements need to be fulfilled. DSCs are characterized by the expression of the surface markers CD73, CD90 and CD105 but lack the expression of CD34, CD14, CD45 and human leukocyte antigen (HLA)-D. Moreover, they adhere to plastic and are capable of multilineage differentiation into bone-, cartilage- and fat producing cells [5–8, 10, 16] which classifies them as MSCs as proposed by the International Society for Cellular Therapy [17].
17.2.1 Dental Stem Cells: A Heterogenous Pool of Stem Cells with Multilineage (trans)Differentiation Potential
DSCs form a very heterogenous cell population in culture. High interdonor variability exists in terms of protein marker expression including CD31, CD117 (c-kit), Stro-1 and the low affinity growth factor receptor p75 (LANGFRp75) [18–21]. Remarkably, DSC variants have different stem cell characteristics as shown by studies that used subsets of DSCs for differentiation experiments. For example, Stro-1+/CD146+ SCAPs show an enhanced colony forming unit capacity and osteo/odontogenic mineralization potential [22], a feature which was previously shown to enhance the cementoblastic differentiation of PDLSCs [7]. Stro-1+ DPSCs, but also CD34+/CD117+ DPSCs have been shown to enhance bone formation [23].
In addition to multilineage differentiation towards classical mesodermal lineages, DSCs have also been subjected to myogenic-, neural- and Schwann cell differentiation experiments to provide an exogenous alternative to these slowly or not regenerating cells or to aid endogenous repair. Additionally, DSCs have been differentiated towards endothelial cells (ECs) to stimulate revascularization directly or indirectly, which will be discussed in the following sections.
17.2.1.1 Myogenic Differentiation of DSCs
Studies reporting and supporting the differentiation potential of DSCs towards myogenic cells are scarce and are mainly focused on the expression of myogenic proteins such as myosin heavy chain, titin, desmin and α-smooth muscle actin. Zhang et al. were one of the first to investigate the differentiation potential of DPSCs towards myogenic lineages in vitro and in vivo [24, 25]. To induce myogenic differentiation, DPSCs were kept in culture with 0.1 μM dexamethasone, 50 μM hydrocortisone, 50 μg/ml gentamycin and with both fetal calf and horse serum. The differentiated cells showed immunoreactivity for myosin heavy chain in vitro as well as in DPSC/collagen scaffolds after transplantation into subcutaneous tissue of immunocompromised mice. Additional research by Nakatsuka et al. showed that administration of 0.5 mM 5-Aza-20-deoxycytidine induced myotube formation and myosin heavy chain expression in mouse DPSCs [26]. A similar protocol promoted myogenic differentiation in PDLSCs, which expressed desmin [27]. While DPSCs were investigated first, the myogenic differentiation potential of SHEDs was studied into more detail. Kerkis et al. reported the expression of myogenic proteins in SHEDs such as titin and actin but they also observed the formation of Z-discs [28]. Unfortunately, none of these studies performed a functional assessment of the myogenic differentiated cells and experiments were solely restricted to evaluate expression levels of myogenic protein markers.
17.2.1.2 Endothelial Differentiation of DSCs
Due to the need for new clinically applicable revascularization strategies, the differentiation potential of DSCs towards ECs was investigated by several research groups as other MSC sources showed great promise in endothelial differentiation experiments (i.e. [29–32], reviewed in [33]). An early report described the differentiation of DPSCs towards EC-like cells through incubation of DPSCs for 40 days in high serum conditions [34]. The outcome of the study was that the majority of DPSCs differentiated towards bone-producing cells. Surprisingly, a fraction of the cells differentiated towards vascular endothelial growth factor receptor 1 (VEGFR1)+/CD44+/CD54+ EC-like cells which also expressed von Willebrand factor (vWF), CD31 and angiotensin-converting enzyme. By following the protocol described in [29], Marchionni et al. were able to obtain EC-like cells from DPSCs which expressed CD54, CD34 and vWF. Furthermore, these cells were able to form tubes in vitro [35]. Remarkably, a CD31-/CD146- subset of DPSCs highly expressed CD34 and VEGFR2 and was able to form extensive networks and had a high proliferative and migratory capacity. Moreover, these cells displayed functional endothelial activity as they gained capacity to take up acetylated low density lipoprotein (Ac-LDL) and release vWF upon histamine stimulation after being cultured in 10 ng/ml vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) for 14 days [36]. Similar to DPSCs, SHEDs formed tubes in vitro and expressed VEGFR2, CD31 and VE-cadherin after exposure to 50 ng/ml VEGF in endothelial growth medium [37]. Amorim et al. recently showed that differentiation of PDLSCs in endothelial cells resulted in expression of VEGFR2 and lectin and the formation of tube-like structures [38]. A report by Bakopoulou et al. demonstrated the endothelial-like differentiation of SCAPs under serum and oxygen/glucose deprivation as evidenced by their capillary forming capacity [39]. To date, only limited evidence for the in vivo endothelial differentiation potential of DSCs is available. A recent study of Zhang et al., for example, reported the presence of human CD31+ blood vessels following the transplantation of DPSCs and SHEDs in a tooth slice model [40]. However, transplanted DSCs mainly appear to act as pericytes as they are often found in juxtaposition to host endothelium [36, 41, 42].
17.2.1.3 Neural Differentiation Potential of DSCs
The neuroectodermal and/or glial origin of DSCs makes these cells interesting candidates to study their neurogenic differentiation potential. Although DPSCs are the most meticulously studied subtype of DSCs, the neurogenic differentiation potential of SCAPs, SHEDs, FSCs and PDLSCs has also been a focus of investigation. Remarkably, DSCs are able to differentiate both towards neuronal-like cells and peripheral glial cells with Schwann cell-like properties. Multiple approaches have been envisaged to differentiate DSCs towards neuronal cell lineages. The two most often used methods in vitro are either the application of neuro-inductive medium which contains specific neuronal inducing chemicals and/or cytokines or through neurosphere formation. Arthur et al. were one of the first to demonstrate that exposure of DPSCs to epidermal growth factor (EGF) and bFGF, increased the expression of neuronal-related markers such as neural cell adhesion molecule (NCAM), neurofilament-M (NF-M) and NF-H. Contrarily, the expression of early neuronal markers i.e. nestin and beta-III tubulin were markedly decreased. Moreover, patch-clamp recordings revealed that differentiated DPSCs acquired functional neuronal characteristics such as inward sodium currents but lacked action potential firing [43]. Follow-up research aimed to optimize the differentiation protocol of Arthur et al. to evoke generation of action potentials. Kiràly et al. enhanced neurogenic induction via epigenetic reprogramming whereas Gervois et al. used neurosphere formation in the presence of EGF and bFGF signaling. Both procedures were followed by a maturation period based on cyclic AMP and growth factor signaling [44, 45]. The outcome of these studies was similar to the study of Arthur et al., showing decreased expression of nestin and a marked increase in expression of neuronal markers. In addition, these studies revealed the existence of tetrodotoxin- and tetraethylammonium- sensitive sodium and potassium currents. The study of Gervois et al. was also able to demonstrate the generation of a single action potential. Other studies which focused on the neurogenic differentiation potential of DPSCs used neurosphere formation [46] or commercially available differentiation media [47].
Neurogenic differentiation of SHEDs is based on neuronal induction with EGF and bFGF [48], sonic hedgehog, bFGF and FGF-8 [49, 50] and/or neurosphere formation [50, 51]. Jarmalaviciute et al. were able to differentiate SHEDs towards peripheral sensory neurons after neuronal maturation by exposing the neuronally induced SHEDs to elevated cyclic AMP levels, glial cell derived neurotrophic factor (GDNF), brain- derived neurotrophic factor (BDNF) and nerve growth factor (NGF). PDLSCs were also differentiated towards neuronal cells with commercially available medium [47], EGF and bFGF [52] supplemented with glucose, progesteron, insulin, transferrin and selenite [53] or neurosphere formation [54]. SCAPs were differentiated towards neuronal cells by Lee et al. [47] with commercially available medium while both SCAPs and FSCs could be differentiated by neurosphere formation [51, 55]. In addition to directed differentiation towards neuronal cells, DPSCs were successfully differentiated towards Schwann cells with a multistep protocol. The differentiated cells expressed the glial markers CD104, glial fibrillary acidic protein (GFAP), p75 and laminin but lacked nestin expression. Moreover, these cells were able to myelinate and guide neurites of dorsal root ganglia in vitro [56]. PDLSCs from dogs were differentiated towards Schwann cell-like cells by Li et al. using different protocols [57]. These differentiated cells expressed GFAP and S100, but also nestin. SHEDs were differentiated towards Schwann cells after exposure to a mixture of growth factors such as bFGF, EGF, BDNF, GDNF, NGF and cyclic AMP.
17.2.2 DSCs as an Ambulant Growth Factor Delivery System: Paracrine Mediated Actions of DSCs
In addition to direct cell replacement strategies, DSCs have the potential to stimulate endogenous repair mechanisms through paracrine interactions. Current research is focusing on the pro-angiogenic, neuroregenerative and neuroprotective properties of DSCs which are all attributed to their rich secretome. To induce angiogenesis, pro-angiogenic growth factors promote ECs to migrate, proliferate and form hollow tubes. Each of these prerequisites are evaluated in vitro on commercially available EC-lines such as human umbilical cord vein endothelial cells (HUVECs) or human microvascular endothelial cells (HMECs). The secretome of DSCs contains a significant amount of pro-angiogenic growth factors including platelet derived growth factor, angiopoietin, bFGF, colony stimulating factors, VEGF and endothelin-1 [58–61]. On the other hand, DSCs also secrete anti-angiogenic proteins such as plasminogen activator inhibitor-1, endostatin, thrombospondin-1, tissue inhibitor of matrix metalloproteinase -1/4 and pentraxin-3 [58, 59]. Iohara et al. used the secretome of CD31-/CD146- DPSCs to investigate the proliferative effect on HUVECs and they showed a significant increase in HUVEC proliferation [36]. However, when HMECs were used, the same effect could not be repeated with the secretome of either DPSCs, SCAPs or FSCs [58]. The second most commonly investigated paracrine effect of DSCs on EC function is the migration of ECs along a gradient of chemotactic proteins. Endothelial migration, evaluated by means of a transwell assay, was found to be significantly stimulated by DPSCs and SCAPs, whereas FSCs had no apparent effect [39, 58]. The tube formation assay is a third assay which evaluates the influence of angiogenesis-promoting substances. Tube-promoting effects have been observed for all DSCs, excluding FSCs, when they were in direct co-culture with ECs [41, 60, 62]. Although these results support the pericyte-like role for DSCs in angiogenesis, paracrine mediated tube formation has also been observed. An indirect co-culture of DPSCs and HUVECs demonstrated tubular network formation [63]. Similar results were achieved when HMECs were incubated with the secretome of DPSCs [58] or HUVECs with the secretome of SCAPs [39] or SHEDS [64].
Preconditioning of DSCs with hypoxia-mimicking agents such as deferoxamine (DFO) or oxygen/glucose deprivation are current strategies to enhance the in vitro angiogenic properties of DSCs. Hypoxic preconditioning enhanced VEGF expression but did not affect DPSC proliferation . By applying the secretome of hypoxic preconditioned DPSCs to HMEC cultures, proliferation and sprouting of HMECs was evident [65]. Bakopoulou et al. demonstrated that deprivation of SCAPs from nutrients and oxygen induced the secretion of pro-angiogenic growth factors, which had an additional positive influence on EC migration and tube formation [39]. Moreover, oxygen and nutrient deprivation also seemed to enhance SCAP differentiation towards EC [39, 66]. Hypoxic preconditioning of PDLSCs increased VEGF and IL-6 secretion. Hypoxia-mimetic agents have also been used to precondition DSCs. prolyl hydroxylase inhibitors (PHD) such as cobalt chloride (CoCl2), DFO, L-mimosine and dimethyloxalglycine promoted the secretion of VEGF and increased the expression of HIF-1α in DPSCs [67], PDLSCs [68] and SCAPs [62]. When SCAPs and HUVECs were treated with CoCl2, increased tube formation was observed [62]. Similar to PHD inhibitors, the iron chelator hinokitiol upregulated HIF-1α expression and VEGF secretion in DPSCs. Moreover, the conditioned medium of these hinokitiol-exposed DPSCs enhanced angiogenesis in vitro and in vivo [69]. Together these data indicate a promising future for the use of hypoxia and hypoxia-mimicking agents to increase the angiogenic properties of DSCs.
Similar to the paracrine mediated effect of DSCs on angiogenesis, the paracrine effects of DSCs on neuroprotection, neurogenesis and neuritogenesis have been evaluated. Human DSCs display a rich secretome including BDNF, NGF, neurotrophin-3 (NT-3) and GDNF, which are considered hallmark factors involved in neurotrophic and neuroregulatory signaling pathways [45, 56, 61, 64, 70]. Moreover, DPSCs and SCAPs secrete a significantly higher amount of these factors in vitro compared to bone marrow-derived and adipose-derived MSCs [61, 71]. The DPSC secretome has been shown to enhance the survival of axotomized retinal ganglion cells and stimulate their neurite outgrowth [71]. DPSCs themselves and their secretome were able to protect astrocytes from ischemia [72]. Apel et al. observed a neuroprotective effect of DPSCs when co-cultured with amyloid beta peptide 1–42 and 6-hydroxy-dopamine (6-OHDA) treated neurons which are in vitro models for Alzheimer’s and Parkinson’s disease respectively [73]. Furthermore, neuroprotection by DPSCs was observed in cultures of dopaminergic neurons treated with either 6-OHDA [74] or a combination of MPP+ and rotenone [75]. Finally, the secretome of DPSCs and SHEDs enhanced Schwann cell survival, proliferation and migration in vitro [64, 76].
17.2.3 Immunomodulatory Properties of DSCs
DPSCs have the ability to modulate the immune response [77, 78]. Demircan et al. showed that the suppressive actions of DPSCs on T-cells were mediated via paracrine effects by means of a transwell and mixed lymphocyte reaction (MLR) assay. Increased levels of hepatocyte growth factor (HGF), HLA-G, transforming growth factor beta (TGF-β), CD54, IL-6, IL-10, CD106, and VEGF were found in DPSC/T-cell co-cultures. Moreover, in the transwell system, the expression of pro-inflammatory cytokines by T-cells such as interferon-gamma (IFN-γ), IL-2, IL-6 receptor, IL-12, IL-17A and tumor necrosis factor- alpha (TNF-α) were decreased whereas the expression of the anti-inflammatory cytokine inducible protein-10 was upregulated. Interestingly, DPSCs induced the expression of the regulatory T-cell markers CD4, CD25 and Foxp3. Apoptosis of T-cells was increased after 24 h incubation with DPSCs [79]. A similar paracrine mediated immunosuppression was exerted by the secretome of porcine and human DPSCs [21, 80, 81]. Additional insight into the immunomodulatory properties of DSCs has arisen from a study by Wada et al. who showed that PDLSCs suppressed peripheral blood mononuclear cell (PBMCs) proliferation after stimulation with a mitogen or in a MLR. Moreover, they were able to demonstrate that IFN-γ, produced by activated PBMCs, was partially responsible for the immunomodulatory effects of PDLSCs. PDLSCs cultured in the presence of activated PBMCs increased the expression of HGF, TGF-β and indoleamine 2,3- dioxygenase (IDO) [82]. In addition to these factors, Fas ligand (FasL) also appears to play a role in DSC-mediated immunosuppressive effects, as knockdown of FasL reduced the capacity of DPSCs to induce T-cell apoptosis [83]. Similarly, Toll-like receptor 3 (TLR3) agonists improved the immunosuppressive effect of DPSCs and FSCs, by upregulating TGF-β and IL-6 production. Interestingly, TLR4 agonists only improved the immunosuppressive effect of FSCs and completely abolished the effect of DPSCs by decreasing TG-β and IDO production [84]. Ding et al. confirmed that TGF-β is a key regulatory molecule in the immunomodulatory properties of DPSCs [85]. SCAPs were also found to secrete immunomodulatory proteins although in lower levels compared to BM-MSCs [61].
17.3 Applications in Regenerative Medicine
Increasing evidence indicates that stem cells represent a potential therapeutic tool for tissue engineering and regeneration in a wide variety of diseases and disorders [86, 87]. The main goal of stem cell-based therapies in regenerative medicine is to replace, repair or to enhance endogenous repair processes of injured tissues and organs [88, 89]. Until now, only limited stem cell-based therapies have been established as a clinical standard, including hematopoietic stem cell transplantation for leukaemia and epithelial stem cell-based interventions for burn wounds and corneal disorders [90, 91]. DSCs have gained great interest during the last decade because of their accessibility, (trans)differentiation potential and high proliferative capacity. Elaborate preclinical research has highlighted the underlying biological and cellular properties of different DSCs, as well as their potential application in the treatment of medical conditions [92–97], such as myocardial infarction [98], ischemic disease [99], nerve injury and other neurodegenerative disorders [47, 50, 70, 100–105], inflammatory diseases [106, 107], diabetes [108–112], muscular dystrophy [26, 113, 114], bone/cartilage defects [115–119], hair follicle loss [120], skin injuries [121], salivary gland defects [122], corneal epithelial defects [123–126], and the regeneration of dental tissues [15, 127–132].
17.3.1 Dentin, Pulp and Periodontal Tissue Regeneration
The main applications for DSCs are in the regeneration of damaged tooth-related structures such as dentin, pulp and periodontal ligament [15, 128–135]. A significant number of studies in animal models have accomplished regeneration of dentin/pulp tissue and of the cementum/periodontal complex by applying DSCs in combination with an appropriate scaffold or supporting matrix [5, 7, 134, 136, 137]. DPSCs have a potent dentinogenic potential and they promote repair and reconstruction of a dentin-pulp-like complex when they are transplanted alone or in combination with growth factors (e.g. bone morphogenetic protein-2 (BMP-2)) in the pulp cavity [138, 139]. Several growth factors such as TGF-β, bFGF and dentin matrix protein 1 (DMP1) can induce odontoblast differentiation of DPSCs [140], and Almushayt et al. demonstrated that these differentiated DPSCs form reparative dentin that covers pulp tissue [141]. Prescott et al. transplanted dentin slices containing DPSCs, collagen and DMP1 subcutaneously in nude mice, which resulted in the formation of a well-organized matrix similar to pulp tissue [142]. Tran and Doan recently achieved regeneration of dentin-like tissue in vivo by culturing human DPSCs onto dentin-derived scaffolds [143]. In line with these findings, Huang et al. reported that a vascularized dentin-pulp-like complex can be regenerated de novo in an emptied root canal space by DPSCs and SCAPs after subcutaneous transplantation in mice [132]. Additionally, also SHEDs combined with a scaffold formed dental pulp-like structures within human tooth slices [144]. Furthermore, an ongoing phase 1 clinical trial aims to repair immature permanent teeth with necrotic pulps by using SHEDs (ClinicalTrials.gov NCT01814436).
New therapeutic strategies for periodontal tissue diseases (e.g. periodontitis) focus on the addition of exogenous growth factors (e.g. bone morphogenetic proteins, platelet derived growth factor) and stem cell therapy [128, 145]. Extensive research studies have explored the use of PDLSCs to improve periodontal tissue regeneration. PDLSCs showed the best capacity to regenerate periodontium compared to other DSC populations [146, 147]. Transplantation of autologous PDLSCs combined with tricalcium phosphate/hydroxyapatite (TCP-HA) into periodontal defects improved bone formation and regeneration of cementum and periodontal ligament [148, 149]. Seo et al. also transplanted PDLSCs loaded onto TCP-HA into immunocompromised rodents, which resulted in the formation of a cementum/periodontal ligament-like structure and contributed to periodontal tissue repair [7]. Moreover, PDLSCs applied together with titanium dental implants improved periodontal ligament regeneration in a rat molar implant model [150], but also in humans [151]. In addition to the abovementioned animal models, a retrospective pilot study performed in humans provided evidence that autologous transplantation of periodontal ligament stem/progenitor cells might provide therapeutic improvement for periodontal defects without adverse effects [152]. Apart from PDLSCs, also implantation of DPSCs into periodontal defects of dogs results in bone formation with neovascularization [153, 154]. A clinical study is currently ongoing in chronic periodontal disease patients which receive a local injection of allogeneic human DPSCs in order to improve periodontal tissue regeneration (ClinicalTrials.gov NCT02523651).
The ultimate goal in dentistry is the regeneration of a functional and living tooth. Recent advances in DSC biotechnology explored the use of stem cells and scaffolds to engineer teeth with the appropriate functional properties. This approach represents a promising therapeutic strategy for the replacement of a diseased or damaged tooth [133, 155, 156]. Sonoyama et al. transplanted both human SCAPs and PDLSCs with TCP-HA as a carrier in a minipig model, resulting in the formation of a viable root/periodontal complex formation that is capable of supporting a porcelain crown [155]. Moreover, Khorsand et al. succeeded to regenerate peridontium using autologous DPSCs loaded onto Bio-Oss scaffolds in dogs that received an experimental defect [157]. Furthermore, Nakashima and coworkers were able to induce whole pulp regeneration after pulpectomy in a dog model using a combination of autologous DPSCs and stromal cell derived factor-1 loaded onto a 3D collagen scaffold [130, 158]. Implantation of FSCs combined with a treated dentin matrix in the alveolar fossa of rats also generated root-like tissues with a pulp–dentin complex and a periodontal ligament that connected the cementum-like layer to host alveolar bone [159, 160].
17.3.2 Osseous Regeneration
Tooth-derived stem cells have also been extensively used in several preclinical studies of osseous regeneration, such as craniofacial and alveolar bone healing [92, 161]. DSCs represent a promising cell-based therapy for repair of bone-related diseases and orthopedic surgeries due to their osteoregenerative capacities. Especially DPSCs are considered to be a potent cell source to enhance bone regeneration [115]. Because of their ectomesenchymal origin, DPSCs express bone-specific markers and they exhibit an osteogenic differentiation profile [34, 162]. Moreover, DPSCs can differentiate into (pre)osteoblasts and they deposit extracellular matrix that forms mineralized woven bone [163–166]. Graziano et al. showed that CD34+ DPSCs transplanted subcutaneously in rats forms several bone nodules [167]. Another study in rats demonstrated that human DPSCs promote repair of a large-scale cranial bone defects [115–117]. Clinical studies performed by d’Aquino et al. demonstrated that a construct composed of DPSCs and a collagen sponge scaffold promotes bone repair following oro-maxillofacial defects [168]. Several studies have indicated that DPSCs gain osteogenic differentiation potential when combined with different biomaterials, such as collagen and titanium [169–173]. Another important factor that stimulates the osteogenic properties of DSCs is BMP-2 [23, 174, 175]. Liu et al. showed that DPSCs expressing BMP-2 have better mineralization capacities, and they generate more bone after alveolar bone defects in a rabbit model [176]. Other DSC populations, such as PDLSCs and SHEDs also represent a promising tool for bone regeneration [177–180]. As reported by Seo et al., SHEDs combined with TCP-HA can repair critical-sized calvarial defects in mice with substantial new bone formation [180]. In line with these findings, Zheng et al. demonstrated that stem cells from miniature pig deciduous teeth were able to engraft and induce bone to repair critical size mandibular defects [181]. A clinical trial is currently ongoing that uses SHEDs for alveolar bone tissue engineering for cleft lip and palate patients (ClinicalTrials.gov – NCT01932164). Another study showed that human PDLSCs expanded ex vivo and seeded in three-dimensional scaffolds (e.g. fibrin sponge) also generated new bone [147]. When SCAPs are combined with HA scaffolds and implanted subcutaneously in immunocompromised rats, both bone- and dentin-like mineralized tissues are formed [182].
17.3.3 Neural Tissue Regeneration
Several DSC populations such as DPSCs and PDLSCs originate from ectomesenchyme, indicating its interaction with the neural crest during embryonic development [183–186]. This implicates that DSCs may possess distinct functions compared to other MSC populations (e.g. BM-MSCs) [183, 187]. As already mentioned, DSCs also have potent neural characteristics besides their classical MSC-properties. This indicates that DSCs may have a therapeutic benefit for the treatment of neurological diseases and disorders. Studies describing the effect of DSCs in animal models of central- or peripheral nervous system pathology are scarce and mainly focused on DPSCs. Arthur et al., pioneers in the use of DPSCs for neuronal regeneration, showed that DPSCs acquired a neuronal morphology, expressed neuronal markers and were able to attract trigeminal neurons after transplantation into chicken embryos [43, 188]. Moreover, neurogenic pre-differentiated DPSCs were able to migrate to the host brain after injection in the cerebrospinal fluid where they integrated in the host circuitry [189]. The therapeutic effect of DPSCs is also studied in various animal models of neurological dysfunction. These animal models include hypoxic ischemic encephalopathy [100], spinal cord injury [101] and ischemic stroke [190], in which DPSCs ameliorated the outcome of the disease. Preclinical evidence in a rodent model of middle cerebral artery ischemic stroke indicates improvement in neurobehavioral function with adult human DSC therapy [190–192]. Recently, the “first-in-human” autologous DPSC therapy clinical trial , TOOTH – The Open study Of dental pulp stem cell Therapy in Humans, was initiated and it evaluates the safety and feasibility of autologous human adult DPSC therapy in patients with chronic disability after stroke [193]. SHEDs have also been successfully applied in heatstroke animals [194], cerebral ischemia [102], perinatal hypoxia/ischemia [103], spinal cord injury [104, 105] and Parkinson’s disease [50] with an improved disease outcome after transplantation. Research of PDLSCs in in vivo models is lacking, but Bueno et al. were able to observe engraftment of PDLSCs in the host brain after transplantation [53]. FSCs were used in a spinal cord injury model after being seeded on aligned poly(\( \varepsilon \)caprolactone)/ poly-DL-lactide-co-glycolide (PCL/PLGA) fibers which supported nerve fiber outgrowth. Unfortunately, no functional improvement was observed after transplantation of these constructs [195]. In addition, SCAPs were transplanted in a hydrogel or in the apical pad of a rat spinal cord injury model [196]. Remarkably, functional improvement was only observed in the experimental group which received the entire tissue graft [197]. The proposed mechanism of disease amelioration by the transplanted cells in these studies was mainly based on paracrine effects of the transplanted cells which stimulated proliferation and differentiation of endogenous neural stem cells [102, 198] or stimulated host repair mechanisms [100–102, 190].
In vivo studies that apply DSCs for peripheral nerve injury were previously limited to reports that used artificial tubes seeded with DSCs in animal models of facial nerve injury. Sasaki et al. indicated that rat DPSCs promoted remyelination, blood vessel formation and normal nerve regeneration when applied in combination with silicon or PLGA tubes as nerve conduits. Paracrine factors secreted by the DPSCs were believed to be responsible for this effect [199, 200]. Later, Sasaki et al. showed that a silicon tube containing a DPSCs/type I collagen gel mixture improved the electrophysiological and functional characteristics of the facial nerve, with an outcome similar to nerve autografts [201]. More recently, the paracrine effects of DPSCs and SHEDs on Schwann cells and peripheral nerve function were evaluated in a sciatic nerve model [64, 76]. The study by Yamamato et al. showed that transplanted DPSCs promoted regeneration of myelinated fibers with a larger axon-to fiber ratio (G-ratio) and revascularization. Direct differentiation into Schwann cells was not observed, but endogenous Schwann cells showed reduced apoptosis and increased proliferation [76]. Similarly, Sugimura-Wakayama demonstrated that silicon conduits containing the SHED secretome significantly increased the number of myelinated axons and G-ratio. Moreover, the sciatic functional index and gastrocnemius wet weight ratio suggested reinnervation of the target muscle with functional recovery [64].
17.3.4 Diabetes
Another important research area in which DSCs may be successful is diabetes, a chronic endocrinal disease that is associated with pancreatic islet dysfunction. Transplantation of insulin-producing pancreatic islet cells represents an important therapeutic strategy for this condition. Several preclinical studies indicate that DSCs can differentiate towards a pancreatic cell lineage [108–112]. Govindasamy et al. showed that differentiated DPSCs resemble islet-like cell aggregates, which release insulin and C-peptide in a glucose-dependent manner [108]. Other studies confirmed these findings, and transplantation of islet-like cell clusters derived from human DPSCs and SHEDs restored normoglycemia in diabetic mice [109, 111, 112]. As shown by Lee et al., PDLSCs can also transdifferentiate into functional pancreatic islet-like cells and therefore represent an alternative stem cell population for pancreatic repair [110]. These preclinical studies implicate that DSCs offer a source of human tissue that can be used as an autologous stem cell therapy for diabetes in order to improve islet regeneration.
17.3.5 Angiogenesis: A Role in Myocardial Infarction and Ischemic Disease
As DSCs display a pronounced angiogenic potential, they are also utilized in clinically relevant disease models, such as myocardial infarction (MI), hindlimb ischemia and a pulpectomized tooth model. Intracardial injection of GFP-positive DPSCs in a rat model of MI resulted in increased capillary density, a reduction in infarct size and a reduced thickening of the anterior ventricular wall. Interestingly, these effects were thought to be caused by paracrine effects of the transplanted DPSCs, as no GFP-positive cells were observed in the tissue [98]. The study by Iohara et al. in which a sub-fraction of CD31−/CD146− porcine DPSCs was injected in a mouse model of hindlimb ischemia, demonstrated a higher capillary density. The transplanted cells were found in proximity to the newly formed vessels, supporting the pericyte and paracrine function of DPSCs [36]. Interestingly, the same subpopulation of DPSCs was capable of increasing vasculogenesis and neurogenesis after transplantation in a rat model of focal cerebral ischemia [21, 192]. Finally, vasculogenesis was enhanced in a pulpectomized tooth model after activation of chemotaxis in DPSCs by granulocyte-colony stimulating factor [80, 202].
17.4 Cryopreservation of Dental Stem Cells
As stated above, DSCs are an attractive source of mesenchymal stem cells for multilineage differentiation and regenerative medicine . However, long-term culture of these stem cells may be accompanied with deleterious effects such as phenotypic instability, cell death, senescence or contamination [203]. Therefore, adequate long term storage procedures are required that not only keep the cells viable, but also safeguard the phenotypic stability and differentiation capacity. The advances made in rapidly growing fields such as (stem) cell therapy , personalized medicine and cancer research, further drive the need to overcome this hurdle in order to move forward towards clinical applications [204, 205]. For allogeneic patients, cryopreservation allows the transport of cellular products and provides a time window to screen the cells prior to transplantation. For autologous patients, cells can simply be stored for later clinical use [206]. Currently a vast number of protocols are being tested for the cryopreservation of DSCs, each with their own criteria to evaluate the potential effect on the intrinsic behavior of these stem cells (Table 17.1).
During cryopreservation, the ultra-low temperatures bring an indefinite halt to the cell’s metabolism. However, cryopreservation represents a physical insult on the cells and is currently only effective for single cell suspensions and a few simple tissues [204, 205]. Since cryopreservation aims at preserving living cells, it is essential to design protocols that minimize the injuries that are associated with the freeze-thaw process in order to ensure maximum recovery of viable and functional cells [206]. One of the most important aspects of freezing cells is the rate of cooling. When cells are cooled too slowly they will dehydrate and shrink due to osmotic stress, whereas rapid cooling results in intracellular ice formation [206, 207]. However, while intracellular ice formation is lethal to cells in suspension, it could be innocuous to cell monolayers due to its propagation via gap junctions. When exposed to 100 % intracellular ice formation, DPSC monolayers have been shown to retain membrane integrity, however they lost the ability to proliferate [208]. Despite the fact that different cell types have a different membrane permeability, cooling rates of 1 °C/min are often applied for several mammalian cell types [205, 206]. Besides carefully controlling cooling rates, media can also be supplemented with cryoprotectant agents in order to minimize cryoinjury. Cryopreservation solutions for MSCs are most commonly supplemented with 10 % dimethyl sulfoxide (DMSO) [205, 209]. DMSO is a permeating cryoprotectant as it is able to penetrate the cell membrane, thereby preventing cell rupture. Several DMSO-based cryopreservation protocols have been tested for the preservation of DPSCs [203, 204, 210–212], SCAPs [213] and PDLSCs [214] (Table 17.1). Woods et al. reported that 7.8–11.6 % DMSO was optimal for DPSCs cooled at −1 °C/min in an isopropanol bath to −85 °C for 24 h followed by storage in liquid nitrogen [212]. DPSCs have been shown to retain their stem cell markers [203, 204, 210, 212] as well as their multilineage differentiation potential post thawing [25, 203]. Ding et al. reported no difference in cell viability , colony forming efficiency, proliferation rate, multilineage differentiation potential, MSC marker expression, karyotype anomalies and immunomodulatory capacities between freshly isolated SCAPs and cryopreserved (10 % DMSO + 90 % fetal bovine serum (FBS)) SCAPs [213]. Cryopreservation of PDLSCs using 10 % DMSO did not affect their proliferative capacity [214].
Despite the widespread use of DMSO as a cryoprotectant, it is known to be potentially cytotoxic. DMSO-preserved bone marrow cells have been shown to cause adverse effects post transplantation [215–217]. Since MSCs have shown great resilience during cryopreservation many other strategies have been explored to reduce cryoprotectant toxicity, minimize cryoinjuries and to develop xeno-free freezing solutions [206]. Hoping to lower or even completely remove DMSO from the freezing solutions, several other compounds have been investigated. Boron is a micro-mineral that is involved in membrane integrity and is important for membrane structure and function and could therefore minimize/inhibit intracellular ice formation. Demirci and colleagues reported an increased viability when tooth germ- derived stem cells from a single donor were preserved in a solution containing 20 μg/mL borate and 5 % DMSO. Furthermore, this solution did not affect surface antigen expression nor did it alter the osteogenic and chondrogenic differentiation potential of these cells [218]. Umemura et al. developed a DMSO-, serum- and xeno-free cryopreservation method by encapsulating DPSCs in an alginate gel. Cryopreserved DPSCs displayed a normal morphology, showed high proliferative capacity and maintained MSC marker expression [219]. Furthermore, Ducret and colleagues recently described a medicinal manufacturing approach for producing DPSCs. In this study, DPSCs were cultured under xeno-free conditions and were cryopreserved for 510 days. Cryopreserved DPSCs were reported to be free of karyotype abnormalities and to have stable doubling times, comparable to those of fresh DPSCs [220].
Using a freezing protocol involving a magnetic field, DPSCs can be frozen in a serum-free freezing solution containing only 3 % DMSO [221]. Magnetic cryopreservation (Cells Alive System, CAS) was originally designed by the ABI Corporation for the preservation of food, but has now found its way into the scientific world as well. By using magnetically induced non-thermal vibrations this freezing method prevents the formation of ice crystals [222] (Table 17.1). Within the field of dentistry, tooth auto-transplantation is a useful technique for replacing missing teeth with several advantages such as maintaining bone volume and PDL regeneration capacity as well as allowing dentofacial development [223]. It occurs that patients no longer possess a suitable donor tooth, due to prior extractions. In these cases tooth banking could greatly expand the usage of auto-transplantation [224] (Table 17.1). The survival and regeneration of the PDL is crucial for the success of the transplantation and the prognosis. Kaku et al. reported an increased survival rate of PDL cells after they were frozen with a magnetic field compared to those without a magnetic field. A magnetic field of 0.01 mT, hold time of 15 min and plunging temperature of −30 °C were determined as the optimal cryopreservation parameters for PDL cells [224]. Furthermore, no difference in mRNA expression or collagen type I was demonstrated and only a minor decrease in alkaline phosphatase was reported between cryopreserved PDL cells and control groups [222]. More importantly, there was no progressive root resorption after re-implantation of cryopreserved teeth into Wistar rats, in contrast to the widespread root resorption and ankyloses in dried teeth [222]. When intact teeth were frozen for 5 years using the CAS freezer, Abedini et al. showed no difference in collagen type I, alkaline phosphatase and VEGF both at mRNA level as well as at protein level [225]. Moreover, a clinical case illustrated PDL regeneration 1 month after the transplantation of a third molar which was cryopreserved for 3 months. Sixteen months post-surgery there was sufficient bone regeneration and complete apexogenesis was achieved [225]. Abedini and colleagues also investigated dental pulp cells isolated from either mature or immature fresh third molars or mature or immature 3-month-cryopreserved molars. Cryopreservation resulted in a delay in cellular outgrowth and pulp cells from cryopreserved molars with complete root formation could not survive properly until confluence was reached. These effects could probably be ascribed to the difficulty of the cryoprotectant to reach the pulp chamber through the narrow tooth apex. Dental pulp cells from cryopreserved immature teeth showed a decreased protein expression of VEGF and NGF compared to their fresh immature counterparts [225].
As demonstrated by the case study of Abedini et al., the field of dentistry would greatly benefit from the ability to cryopreserve intact teeth or at least dental tissues prior to stem cell isolation (Table 17.1). Studies investigating cryopreservation of teeth have mainly focused on the transplantation of permanent teeth and therefore questioned the survival of the periodontal ligament and pulp tissue of the cryopreserved teeth [225–228]. Oh and colleagues reported no significant difference in the viability and osteogenic differentiation capacity of PDL cells from 1-week-cryopreserved teeth [226]. Min et al. found a decrease in bFGF receptor expression in PDL cells isolated from 1-week-cryopreserved teeth [227]. However, after cryopreservation of intact teeth, Woods et al. could only establish DPSC cultures from 20 % of the frozen teeth [212]. The low yield could be correlated to the dimensions of the apical foramen, as Temmerman et al. obtained maximal pulp viability after cryopreservation of teeth with an apical opening of at least 9.42 mm2 [229]. For this reason, cryopreservation of pulp tissue could offer a suitable alternative. DPSCs isolated from cryopreserved pulp tissues showed no difference in CD marker expression and no loss in osteogenic and adipogenic differentiation potential compared to their fresh counterparts [230]. Gioventu and colleagues tried to circumvent the issue of a limited apical foramen by laser piercing deciduous teeth prior to cryopreservation and found that DPSCs from cryopreserved teeth showed similar growth rates and marker expression than DPSCs isolated from fresh tissue [228]. Since deciduous teeth cannot be replanted, the ability to cryopreserve them for future stem cell isolation would greatly decrease costs and efforts associated with stem cell banking from deciduous teeth [231]. Lindemann et al. only reported a 30 % isolation success rate of 1-week-cryopreserved teeth. Despite the lower culture rates and morphological aberrations, no changes in immunophenotype and differentiation potential were seen [232]. Increasing serum levels could possibly improve the culture rate after cryopreservation as Bressan and colleagues found that increasing serum concentrations from 10 % to 20 % resulted in a more than 50 % increase in culture rate [233]. The abovementioned studies only cryopreserved teeth for a short period of time and according to Ji et al. the period of cryopreservation also plays a crucial role in the viability of the resulting pulp cells. They reported a reduction in proliferation rate and increased apoptosis when teeth were cryopreserved for over 3 months [231]. Perry et al. reported a 70 % success rate of isolation from 1-month cryopreserved whole teeth [211]. Dental follicle tissue has also been shown to yield viable DSCs after 3 months of tissue storage. Tissue samples were stored in phosphate-buffered saline (PBS) supplemented with glucose, sucrose and ethylene glycol for cryoprotection. DSCs isolated from cryopreserved dental follicle showed no difference in the expression of stem cell markers or the ability for multilineage differentiation . However, cells from cryopreserved tissues did show a higher intensity of p53 protein, indicating damage from the freeze-thaw process [234]. Furthermore, upon transplantation into mandibular defects in miniature pigs, both FSCs from both fresh and cryopreserved dental follicle significantly enhanced new bone formation after 8 weeks [235]. Freezing of the intact PDL, only yielded 40 % of single-colony derived PDLSCs compared to the number of PDLSC colonies recovered from fresh periodontal ligament tissue. These cells showed a high proliferation rate and maintained their multilineage differentiation potential. Moreover, upon transplantation, PDLSCs derived from cryopreserved PDL generated a typical cementum/periodontal ligament-like structure [236]. The possibility of storing tissue samples for longer periods of time without significant damage to the inherent stem cell population offers great perspectives for regenerative medicine and stem cell banking.
17.5 From Bench to Bedside
Stem cell research holds great promise for the development of treatment strategies for various injuries and diseases. The scope of potential stem cell therapies is expanding due to advances in stem cell research. The emerging demand of stem cell therapeutics necessitates the establishment and collaboration of centralized biobanks at an international level. Initiatives like the International Stem Cell Banking Initiative (ISCBI) and the International Society for Stem Cell Research (ISSCR) strive for the creation of such centralized biobanks to provide high-quality stem cells for therapeutic purposes in a standardized approach [237, 238]. Though, this seems challenging given the heterogeneous ethical, regulatory and legal setting. In order to provide a standardized stem cell-based therapy , strict rules have to be followed regarding the different processes that are performed from isolation to transplantation [239, 240]. These processes vary highly depending on the stem cell source, type and clinical application .
Standardized procedures are essential to ensure optimal reproducibility, safety and efficacy when DSCs are used for clinical applications (Fig. 17.2). However, current manufacturing procedures for DSC-based products are not fully in line with the international guidelines for GMP for stem cell therapies . GMP requires quality control regarding donor eligibility, isolation procedure, labeling, transportation, processing, storage, lab equipment, reagents, distribution to the patient and documentation [241–243]. Briefly, the manufacturing steps of DSC-based products can be generally defined as (1) tooth extraction and pulp tissue collection, (2) DSC isolation, (3) cell culture and expansion, (4) cryobanking (5) safety control and quality testing, (6) transplantation [244]. Extraction of third molars for the isolation of DSCs is being performed in donors of different ages and with third molar in different stages of development [72, 190, 211, 245, 246]. This impairs the comparison of the experimental results and makes it difficult to provide a standardized therapy. There are no guidelines that specify the developmental stage of the tooth for the isolation of DSCs and DPSCs in particular. However, it has been suggested to use impacted third molars between Nolla developmental stage 5 (almost completed dental crown) and stage 7 (root completed for one third) in young donors, since the use of impacted molars in this developmental stage reduces the risk for contamination and avoids the use of mechanical separation [244]. Dental pulp tissue, for example, can be collected in PBS for transportation to the lab, as DPSCs remain viable for up to 5 days in this medium [212]. For the isolation of DSCs two widely used methods are available, namely enzymatic digestion and explant culture. Considering translation to clinical applications the explant method seems to be the preferred approach. Explant culture is easier, safer, less expensive and more compliant with the GMP guidelines [246]. Cell culture and expansion is arguably the most important process in providing a cell-based therapy , as it is essential for the stem cell products to be free of any microbiological contamination. A major pitfall of standard DSC culture is the continuing use of animal culture medium additives, which will be elaborately discussed in the following section. Therefore, the development of xeno-free, serum-free media is a critical objective for standardized manufacturing of DSC-based products. Additionally, the use of xeno-free dissociation buffers such as TrypLe® or Accutase® is highly recommended for passaging the cells. Nevertheless, further research is necessary to optimize the culture properties and to ensure the phenotype of the cells is not negatively altered.
To provide an adequate amount of cells for clinical trials and commercialization of the cell-based product, efficient expansion or ‘upscaling’ is crucial for the establishment of a novel successful therapy. For autologous applications, the goal is to produce an appropriate number of cells for the treatment of one patient, mostly in the range of one to five billion cells per manufacturing process [239]. In this context, the use of conventional cell culture using culture flasks or small bioreactors is feasible. However, in the case of allogenic applications where multiple patients are targeted, the upscaling has to be performed to a much higher extent. Here, the use of cell factories with large scale bioreactors becomes inevitable. Providing a cell-based therapy often involves the storage of the cell product or an intermediate form of the cell product for future use. Cryopreservation and long-term storage of cells permits completion of safety control, quality testing and stable transportation to the clinical site [216]. Optimal cryopreservation conditions are necessary to maximize cell stability, recovery and function, as extensively discussed in the previous section. Furthermore, it is required to phenotypically characterize the cells and perform additional tests to ensure the safety of the cell-based product. Flow cytometry is an ideal technique to analyze various phenotypic markers simultaneously on the cellular level. It is advised to use a panel of markers that provides a high level of specificity for DSCs. However, this seems to be challenging since there is no unique marker for DSCs and many markers that were proposed by the ISCT to identify MSCs are also expressed in other cell types, including mature fibroblasts [247, 248]. Supplementary testing of the trilineage differentiation capacity should be performed to guarantee the stem cell properties of the isolated cells.
Despite the current lack of standardization in the upscale production and long-term storage of DSCs, there are already several commercial tooth banking services available. In Japan, for example, both the Hiroshima University and Nagoya University have founded their own tooth bank. In 2008, a collaboration between the Norwegian Institute of Public Health and the University of Bergen led to the first European tooth bank [249]. Commercial tooth banking companies can also be found in the United States, such as BioEden (Austin, Texas, USA), Store-A-Tooth (Provia Laboratories, Littleton, Massachusetts, USA) and StemSave (Stemsave Inc, New York, USA).
It has to be emphasized that all variables in the manufacturing process of DSC products have to be optimized for the specific application before continuing into clinical trials , since these can affect the properties of the cell-based product. Consequently, if the product successfully passes clinical trials, it can be produced without altering the manufacturing process.
17.6 Pitfalls Associated with Dental Stem Cell Banking
When contemplating the long-term cryopreservation as well as the upscale production of (dental) stem cells, one certainly needs to take into account certain pitfalls associated with these procedures. Although considered to be common nutritional components, the use of animal-derived culture medium additives, for example, has been the subject of debate for quite some time. Next to animal welfare concerns and the high costs associated with the use of FBS and other animal-derived proteins, the addition of these cell nutrients to culture media has some other drawbacks as well as they are considered to be potentially hazardous [250–253]. More specifically, not only are these additives a potential source of endotoxins and other infectious agents such as bacteria, viruses, fungi and prions, but the xenogeneic antigens could also evoke a severe immunological response within the host [250–252]. Together with the high batch-to-batch variety in protein content, these disadvantages urged the need for alternative, serum-free cell culture systems [250, 252, 254].
With regard to the culturing of DSCs in serum-free conditions, Tarle et al. developed a chemically defined serum-free culture medium for SHEDs and PDLSCs. There were no significant differences in comparison to the stem cells cultured in FBS-containing medium; both stem cell populations were able to expand and maintained their multipotent capacity [255]. Comparable observations were made by Hirata et al. and others, showing both (sub-optimal) proliferation and expression of stem cell markers by DPSCs, PDLSCs and FSCs when cultured in serum-free media containing a range of different growth factors [256–259]. Depending on the specific growth factor supplement, however, stem cell differentiation can also be induced. Bonnamain et al., for example, indicated the expression of neuronal and oligodendrocyte markers when incubating DPSCs with EGF and bFGF [260]. Neurogenic differentiation of DPSCs was also reported by Xiao and Tsutsui, showing the expression of neural markers after using a commercially available serum replacement [261]. In addition, a CD117+ subset of DPSCs differentiated into pancreatic cells following incubation with a specific combination of growth factors and chemical substances [111]. An increase in the expression of endothelial markers was also mentioned by Karbanova et al., after incubating a serum-free DPSC culture with VEGF and insulin-transferrin-sodium selenite (ITS) [262]. In the search for appropriate alternatives for animal-derived culture medium additives, numerous publications have reported on the use of human serum or other blood-derived products in stem cell cultures [252, 254, 257, 263–271]. With respect to DSCs in particular, a recent study of Pisciolaro et al. indicated a significantly improved proliferation rate and mineralization potential when incubating DPSCs with human autologous serum [268]. A more prominent osteogenic and adipogenic differentiation of DPSCs following incubation with allogenic human serum was also found by Khanna-Jain et al., while Govindasamy et al. demonstrated the pronounced expansion of DPSCs in medium containing human platelet lysate [257, 266]. However, the use of human blood derivatives still holds several disadvantages. As with any donor-derived product, inherent differences between samples and batch-to-batch variability cannot be excluded. Besides the extensive amount that would be needed for the upscale production of (dental) stem cells, the current lack of consistent study set-ups demands for further analysis of the precise composition as well as the potential impact of these human-derived blood products on the intrinsic properties and behavior of the stem cells [252, 264, 265]. Further research is thus required before any decision can be taken by scientists as well as regulatory agencies regarding the future application of alternative culture medium additives.
Another important aspect one definitely has to keep in mind when considering the application of autologous and/or allogenic DSCs is potential donor-related variability, as the inherent behavior of these cells can be affected by a range of different factors. The pressure and tension generated during the application of orthodontic force, for example, causes the release of various growth factors and cytokines and, subsequently, the creation of a supportive microenvironment for bone remodeling, root resorption and differentiation of residing (stem) cells [272–275]. With regard to the effect of the age of the donor at the time of stem cell isolation, contrasting results are described in literature. Iohora et al., for instance, reported an age-related decline of stem cell-mediated dental pulp regeneration in dogs [276]. Similar observations were made by Feng et al. and others, showing an age-dependent decrease in proliferation , migration and osteogenic differentiation potential of DPSCs and PDLSCs [277–280]. Atari et al., on the other hand, successfully isolated DPSCs from 14 to 60-year-old donors, with no significant differences in gene expression [281]. These results were also confirmed by Kellner et al. and others, stating that age is not a determining factor for the stem cell properties and regenerative potential of DPSCs [127, 282]. Next to orthodontic tooth movement and age, the (oral) health of the patient also seems to significantly influence the biological properties of DSCs. Nicotine, for example, does not only influence their osteogenic differentiation potential, but also alters the viability, growth and migration rate of PDLSCs in particular [283–286]. In 2010, Cooper et al., already described the complex interaction between inflammation and the natural regenerative potential of dental pulp, as inflammatory mediators can modulate the repair processes within the tissue [287]. Since then, a number of papers have been published reporting on the effects of caries and/or inflammatory processes on DSCs. Alongi et al., for example, showed a decreased expression of MSC markers as well as a diminished proliferation rate and osteogenic differentiation potential in vitro by DPSCs originating from inflamed dental pulps. However, no notable differences were observed when comparing the formation of pulp/dentin complexes in immunocompromised mice [288]. Similar results were found by Liu et al. and others, indicating an altered proliferation and migration rate as well as a differential protein expression profile and immunomodulatory properties for carious and/or inflamed DPSCs and MSCs from periapical lesions [289–293]. In contrast, several papers mentioned no significantly different behavior of DPSCs isolated from carious deciduous and/or permanent teeth, thereby suggesting their potential use in cell-based therapies [294–296]. When considering the therapeutic application of autologous stem cells in cancer patients, the potential detrimental effects caused by previous treatment with radiotherapy certainly need to be taken into account. With regard to the effects of ionizing radiation (IR) on the behavior of DSCs, a couple of studies reported the induction of cell cycle arrest, premature senescence and differentiation of DPSCs following exposure to different dosages of IR [297, 298]. In contrast, Abe et al., demonstrated a radio-resistant phenotype for SCAPs, albeit with a lower hard tissue forming capacity in vivo [299]. Before any therapeutic application is possible, the implementation of a dental stem cell banking system not only demands extensive evaluation at scientific as well as regulatory level but also requires additional characterization of its associated pitfalls such as culture medium additives and donor-related confounding factors.
17.7 Conclusion and Future Perspectives
Taken together, DSCs seem to hold great promise for various applications. One of the major advances made in the field of regenerative medicine is 3D-printing. This technique evolved from a simple 3D- printed construct towards the fabrication of a complex structure containing multiple cell types and biological constitutes/components [300–302]. These constructs can ultimately serve as a 3D-scaffold in which DSCs can be studied for multilineage differentiation or more specifically for dental pulp regeneration. However, to ensure future therapeutic applications, one should strive to maintain a cost-effective DSC cell banking system which guarantees phenotypic stability of the heterogeneous populations of DSCs. Some hurdles have to be overcome to establish clinical translation of DSC-based products, since current manufacturing methods are not in line with the GMP guidelines for stem cell therapy . Developing a more standardized and compliant manufacturing protocol will result in an increased efficacy, reproducibility and safety.
Abbreviations
- MSCs:
-
Mesenchymal stem cells
- DSCs:
-
Dental stem cells
- DPSCs:
-
Dental pulp stem cells
- BM-MSCs:
-
Bone marrow-derived MSCs
- ASCs:
-
Adipose tissue-derived stem cells
- FSCs:
-
Follicle precursor cells
- PDL:
-
Periodontal ligament
- PDLSCs:
-
Periodontal ligament stem cells
- SCAPs:
-
Stem cells from the apical papilla
- UMSCs:
-
Umbilical cord MSCs
- UCBC:
-
Umbilical cord blood cells
- ERM:
-
Epithelial cell rests of Malassez
- DePDL:
-
Deciduous periodontal ligament
- SHEDs:
-
Stem cells from human exfoliated deciduous teeth
- GMP:
-
Good Manufacturing Practice
- HLA-D:
-
Human leukocyte antigen
- vWF:
-
von Willebrand factor
- DFO:
-
Deferoxamine
- ISCBI:
-
International Stem Cell Banking Initiative
References
Friedenstein AJ, Chailakhjan RK, Lalykina KS (1970) The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 3(4):393–403
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13(12):4279–4295. doi:10.1091/mbc.E02-02-0105
Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D (2006) Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells 24(3):781–792. doi:10.1634/stemcells.2005-0330
Erices A, Conget P, Minguell JJ (2000) Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol 109(1):235–242
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97(25):13625–13630. doi:10.1073/pnas.240309797
Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT (2008) Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 34(2):166–171. doi:10.1016/j.joen.2007.11.021
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364(9429):149–155. doi:10.1016/S0140-6736(04)16627-0
Morsczeck C, Gotz W, Schierholz J, Zeilhofer F, Kuhn U, Mohl C, Sippel C, Hoffmann KH (2005) Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. J Int Soc Matrix Biol 24(2):155–165. doi:10.1016/j.matbio.2004.12.004
Xiong J, Mrozik K, Gronthos S, Bartold PM (2012) Epithelial cell rests of Malassez contain unique stem cell populations capable of undergoing epithelial-mesenchymal transition. Stem Cells Dev 21(11):2012–2025. doi:10.1089/scd.2011.0471
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S (2003) SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 100(10):5807–5812. doi:10.1073/pnas.0937635100
Silverio KG, Rodrigues TL, Coletta RD, Benevides L, Da Silva JS, Casati MZ, Sallum EA, Nociti FH Jr (2010) Mesenchymal stem cell properties of periodontal ligament cells from deciduous and permanent teeth. J Periodontol 81(8):1207–1215. doi:10.1902/jop.2010.090729
Shi S, Gronthos S (2003) Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res 18(4):696–704. doi:10.1359/jbmr.2003.18.4.696
Waddington RJ, Youde SJ, Lee CP, Sloan AJ (2009) Isolation of distinct progenitor stem cell populations from dental pulp. Cells Tissues Organs 189(1–4):268–274. doi:10.1159/000151447
Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, An Z, Wang L, Hultman I, Ahrlund-Richter L, Blom H, Brismar H, Lopes NA, Pachnis V, Suter U, Clevers H, Thesleff I, Sharpe P, Ernfors P, Fried K, Adameyko I (2014) Glial origin of mesenchymal stem cells in a tooth model system. Nature 513(7519):551–554. doi:10.1038/nature13536
Hilkens P, Meschi N, Lambrechts P, Bronckaers A, Lambrichts I (2015) Dental stem cells in pulp regeneration: near future or long road ahead? Stem Cells Dev 24(14):1610–1622. doi:10.1089/scd.2014.0510
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317. doi:10.1080/14653240600855905
Akpinar G, Kasap M, Aksoy A, Duruksu G, Gacar G, Karaoz E (2014) Phenotypic and proteomic characteristics of human dental pulp derived mesenchymal stem cells from a natal, an exfoliated deciduous, and an impacted third molar tooth. Stem Cells Int 2014:457059. doi:10.1155/2014/457059
Pisciotta A, Carnevale G, Meloni S, Riccio M, De Biasi S, Gibellini L, Ferrari A, Bruzzesi G, De Pol A (2015) Human dental pulp stem cells (hDPSCs): isolation, enrichment and comparative differentiation of two sub-populations. BMC Dev Biol 15:14. doi:10.1186/s12861-015-0065-x
Tamaki Y, Nakahara T, Ishikawa H, Sato S (2013) In vitro analysis of mesenchymal stem cells derived from human teeth and bone marrow. Odontol Soc Nippon Dent Univ 101(2):121–132. doi:10.1007/s10266-012-0075-0
Ishizaka R, Hayashi Y, Iohara K, Sugiyama M, Murakami M, Yamamoto T, Fukuta O, Nakashima M (2013) Stimulation of angiogenesis, neurogenesis and regeneration by side population cells from dental pulp. Biomaterials 34(8):1888–1897. doi:10.1016/j.biomaterials.2012.10.045
Bakopoulou A, Leyhausen G, Volk J, Koidis P, Geurtsen W (2013) Comparative characterization of STRO-1(neg)/CD146(pos) and STRO-1(pos)/CD146(pos) apical papilla stem cells enriched with flow cytometry. Arch Oral Biol 58(10):1556–1568. doi:10.1016/j.archoralbio.2013.06.018
Yang X, Walboomers XF, van den Beucken JJ, Bian Z, Fan M, Jansen JA (2009) Hard tissue formation of STRO-1-selected rat dental pulp stem cells in vivo. Tissue Eng A 15(2):367–375. doi:10.1089/ten.tea.2008.0133
Zhang W, Walboomers XF, Van Kuppevelt TH, Daamen WF, Van Damme PA, Bian Z, Jansen JA (2008) In vivo evaluation of human dental pulp stem cells differentiated towards multiple lineages. J Tissue Eng Regen Med 2(2–3):117–125. doi:10.1002/term.71
Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA (2006) Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng 12(10):2813–2823. doi:10.1089/ten.2006.12.2813
Nakatsuka R, Nozaki T, Uemura Y, Matsuoka Y, Sasaki Y, Shinohara M, Ohura K, Sonoda Y (2010) 5-Aza-2′-deoxycytidine treatment induces skeletal myogenic differentiation of mouse dental pulp stem cells. Arch Oral Biol 55(5):350–357. doi:10.1016/j.archoralbio.2010.03.003
Song M, Kim H, Choi Y, Kim K, Chung C (2012) Skeletal myogenic differentiation of human periodontal ligament stromal cells isolated from orthodontically extracted premolars. Korean J Orthod 42(5):249–254. doi:10.4041/kjod.2012.42.5.249
Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, Pereira LV, Caplan AI, Cerruti HF (2006) Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs 184(3–4):105–116. doi:10.1159/000099617
Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, Werner C (2004) Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 22(3):377–384. doi:10.1634/stemcells.22-3-377
Takahashi M, Suzuki E, Oba S, Nishimatsu H, Kimura K, Nagano T, Nagai R, Hirata Y (2010) Adipose tissue-derived stem cells inhibit neointimal formation in a paracrine fashion in rat femoral artery. Am J Phys Heart Circ Phys 298(2):H415–H423. doi:10.1152/ajpheart.00391.2009
Bai K, Huang Y, Jia X, Fan Y, Wang W (2010) Endothelium oriented differentiation of bone marrow mesenchymal stem cells under chemical and mechanical stimulations. J Biomech 43(6):1176–1181. doi:10.1016/j.jbiomech.2009.11.030
Chen MY, Lie PC, Li ZL, Wei X (2009) Endothelial differentiation of Wharton’s jelly-derived mesenchymal stem cells in comparison with bone marrow-derived mesenchymal stem cells. Exp Hematol 37(5):629–640. doi:10.1016/j.exphem.2009.02.003
Bronckaers A, Hilkens P, Martens W, Gervois P, Ratajczak J, Struys T, Lambrichts I (2014) Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther 143(2):181–196. doi:10.1016/j.pharmthera.2014.02.013
d’Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, Papaccio G (2007) Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ 14(6):1162–1171. doi:10.1038/sj.cdd.4402121
Marchionni C, Bonsi L, Alviano F, Lanzoni G, Di Tullio A, Costa R, Montanari M, Tazzari PL, Ricci F, Pasquinelli G, Orrico C, Grossi A, Prati C, Bagnara GP (2009) Angiogenic potential of human dental pulp stromal (stem) cells. Int J Immunopathol Pharmacol 22(3):699–706
Iohara K, Zheng L, Wake H, Ito M, Nabekura J, Wakita H, Nakamura H, Into T, Matsushita K, Nakashima M (2008) A novel stem cell source for vasculogenesis in ischemia: subfraction of side population cells from dental pulp. Stem Cells 26(9):2408–2418. doi:10.1634/stemcells.2008-0393
Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado MA, Shi S, Santos CF, Nor JE (2010) SHED differentiate into functional odontoblasts and endothelium. J Dent Res 89(8):791–796. doi:10.1177/0022034510368647
Amorim BR, Silverio KG, Casati MZ, Sallum EA, Kantovitz KR, Nociti FH, Jr (2016) Neuropilin controls endothelial differentiation by mesenchymal stem cells from the periodontal ligament. J Periodontol:1–14. doi:10.1902/jop.2016.150603
Bakopoulou A, Kritis A, Andreadis D, Papachristou E, Leyhausen G, Koidis P, Geurtsen W, Tsiftsoglou A (2015) Angiogenic potential and secretome of human apical papilla mesenchymal stem cells in various stress microenvironments. Stem Cells Dev 24(21):2496–2512. doi:10.1089/scd.2015.0197
Zhang Z, Nor F, Oh M, Cucco C, Shi S, Nor JE (2016) Wnt/beta-catenin signaling determines the vasculogenic fate of post-natal mesenchymal stem cells. Stem Cells. doi:10.1002/stem.2334
Janebodin K, Zeng Y, Buranaphatthana W, Ieronimakis N, Reyes M (2013) VEGFR2-dependent angiogenic capacity of pericyte-like dental pulp stem cells. J Dent Res 92(6):524–531. doi:10.1177/0022034513485599
Barros MA, Martins JF, Maria DA, Wenceslau CV, De Souza DM, Kerkis A, Camara NO, Balieiro JC, Kerkis I (2015) Immature dental pulp stem cells showed renotropic and pericyte-like properties in acute renal failure in rats. Cell Med 7(3):95–108. doi:10.3727/215517914X680038
Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S (2008) Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells 26(7):1787–1795. doi:10.1634/stemcells.2007-0979
Kiraly M, Porcsalmy B, Pataki A, Kadar K, Jelitai M, Molnar B, Hermann P, Gera I, Grimm WD, Ganss B, Zsembery A, Varga G (2009) Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem Int 55(5):323–332. doi:10.1016/j.neuint.2009.03.017
Gervois P, Struys T, Hilkens P, Bronckaers A, Ratajczak J, Politis C, Brone B, Lambrichts I, Martens W (2014) Neurogenic maturation of human dental pulp stem cells following neurosphere generation induces morphological and electrophysiological characteristics of functional neurons. Stem Cells Dev. doi:10.1089/scd.2014.0117
Osathanon T, Sawangmake C, Nowwarote N, Pavasant P (2014) Neurogenic differentiation of human dental pulp stem cells using different induction protocols. Oral Dis 20(4):352–358. doi:10.1111/odi.12119
Lee JH, Um S, Song IS, Kim HY, Seo BM (2014) Neurogenic differentiation of human dental stem cells in vitro. J Korean Assoc Oral Maxillofac Surg 40(4):173–180. doi:10.5125/jkaoms.2014.40.4.173
Jarmalaviciute A, Tunaitis V, Strainiene E, Aldonyte R, Ramanavicius A, Venalis A, Magnusson KE, Pivoriunas A (2013) A new experimental model for neuronal and glial differentiation using stem cells derived from human exfoliated deciduous teeth. J Mol Neurosci: MN. doi:10.1007/s12031-013-0046-0
Nourbakhsh N, Soleimani M, Taghipour Z, Karbalaie K, Mousavi SB, Talebi A, Nadali F, Tanhaei S, Kiyani GA, Nematollahi M, Rabiei F, Mardani M, Bahramiyan H, Torabinejad M, Nasr-Esfahani MH, Baharvand H (2011) Induced in vitro differentiation of neural-like cells from human exfoliated deciduous teeth-derived stem cells. Int J Dev Biol 55(2):189–195. doi:10.1387/ijdb.103090nn
Wang J, Wang X, Sun Z, Wang X, Yang H, Shi S, Wang S (2010) Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells Dev 19(9):1375–1383. doi:10.1089/scd.2009.0258
Morsczeck C, Vollner F, Saugspier M, Brandl C, Reichert TE, Driemel O, Schmalz G (2010) Comparison of human dental follicle cells (DFCs) and stem cells from human exfoliated deciduous teeth (SHED) after neural differentiation in vitro. Clin Oral Investig 14(4):433–440. doi:10.1007/s00784-009-0310-4
Fortino VR, Chen RS, Pelaez D, Cheung HS (2014) Neurogenesis of neural crest-derived periodontal ligament stem cells by EGF and bFGF. J Cell Physiol 229(4):479–488. doi:10.1002/jcp.24468
Bueno C, Ramirez C, Rodriguez-Lozano FJ, Tabares-Seisdedos R, Rodenas M, Moraleda JM, Jones JR, Martinez S (2013) Human adult periodontal ligament-derived cells integrate and differentiate after implantation into the adult mammalian brain. Cell Transplant 22(11):2017–2028. doi:10.3727/096368912X657305
Techawattanawisal W, Nakahama K, Komaki M, Abe M, Takagi Y, Morita I (2007) Isolation of multipotent stem cells from adult rat periodontal ligament by neurosphere-forming culture system. Biochem Biophys Res Commun 357(4):917–923. doi:10.1016/j.bbrc.2007.04.031
Abe S, Hamada K, Miura M, Yamaguchi S (2012) Neural crest stem cell property of apical pulp cells derived from human developing tooth. Cell Biol Int 36(10):927–936. doi:10.1042/CBI20110506
Martens W, Sanen K, Georgiou M, Struys T, Bronckaers A, Ameloot M, Phillips J, Lambrichts I (2014) Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J 28(4):1634–1643. doi:10.1096/fj.13-243980
Li X, Gong P, Liao D (2010) In vitro neural/glial differentiation potential of periodontal ligament stem cells. AMS 6(5):678–685. doi:10.5114/aoms.2010.17080
Hilkens P, Fanton Y, Martens W, Gervois P, Struys T, Politis C, Lambrichts I, Bronckaers A (2014) Pro-angiogenic impact of dental stem cells in vitro and in vivo. Stem Cell Res 12(3):778–790. doi:10.1016/j.scr.2014.03.008
Bronckaers A, Hilkens P, Fanton Y, Struys T, Gervois P, Politis C, Martens W, Lambrichts I (2013) Angiogenic properties of human dental pulp stem cells. PLoS One 8(8), e71104. doi:10.1371/journal.pone.0071104
Yeasmin S, Ceccarelli J, Vigen M, Carrion B, Putnam AJ, Tarle SA, Kaigler D (2014) Stem cells derived from tooth periodontal ligament enhance functional angiogenesis by endothelial cells. Tissue Eng A 20(7–8):1188–1196. doi:10.1089/ten.TEA.2013.0512
Yu S, Zhao Y, Ma Y, Ge L (2016) Profiling the secretome of human stem cells from dental apical papilla. Stem Cells Dev 25(6):499–508. doi:10.1089/scd.2015.0298
Yuan C, Wang P, Zhu L, Dissanayaka WL, Green DW, Tong EH, Jin L, Zhang C (2015) Coculture of stem cells from apical papilla and human umbilical vein endothelial cell under hypoxia increases the formation of three-dimensional vessel-like structures in vitro. Tissue Eng A 21(5–6):1163–1172. doi:10.1089/ten.TEA.2014.0058
Dissanayaka WL, Hargreaves KM, Jin L, Samaranayake LP, Zhang C (2015) The interplay of dental pulp stem cells and endothelial cells in an injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo. Tissue Eng A 21(3–4):550–563. doi:10.1089/ten.TEA.2014.0154
Sugimura-Wakayama Y, Katagiri W, Osugi M, Kawai T, Ogata K, Sakaguchi K, Hibi H (2015) Peripheral nerve regeneration by secretomes of stem cells from human exfoliated deciduous teeth. Stem Cells Dev 24(22):2687–2699. doi:10.1089/scd.2015.0104
Aranha AM, Zhang Z, Neiva KG, Costa CA, Hebling J, Nor JE (2010) Hypoxia enhances the angiogenic potential of human dental pulp cells. J Endod 36(10):1633–1637. doi:10.1016/j.joen.2010.05.013
Vanacker J, Viswanath A, De Berdt P, Everard A, Cani PD, Bouzin C, Feron O, Diogenes A, Leprince JG, des Rieux A (2014) Hypoxia modulates the differentiation potential of stem cells of the apical papilla. J Endod 40(9):1410–1418. doi:10.1016/j.joen.2014.04.008
Muller HD, Cvikl B, Gruber R, Watzek G, Agis H (2012) Prolyl hydroxylase inhibitors increase the production of vascular endothelial growth factor in dental pulp-derived cells. J Endod 38(11):1498–1503. doi:10.1016/j.joen.2012.08.003
Agis H, Watzek G, Gruber R (2012) Prolyl hydroxylase inhibitors increase the production of vascular endothelial growth factor by periodontal fibroblasts. J Periodontal Res 47(2):165–173. doi:10.1111/j.1600-0765.2011.01415.x
Kim MK, Park HJ, Kim YD, Ryu MH, Takata T, Bae SK, Bae MK (2014) Hinokitiol increases the angiogenic potential of dental pulp cells through ERK and p38MAPK activation and hypoxia-inducible factor-1alpha (HIF-1alpha) upregulation. Arch Oral Biol 59(2):102–110. doi:10.1016/j.archoralbio.2013.10.009
Nosrat IV, Widenfalk J, Olson L, Nosrat CA (2001) Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev Biol 238(1):120–132. doi:10.1006/dbio.2001.0400
Mead B, Logan A, Berry M, Leadbeater W, Scheven BA (2014) Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One 9(10), e109305. doi:10.1371/journal.pone.0109305
Song M, Jue SS, Cho YA, Kim EC (2015) Comparison of the effects of human dental pulp stem cells and human bone marrow-derived mesenchymal stem cells on ischemic human astrocytes in vitro. J Neurosci Res 93(6):973–983. doi:10.1002/jnr.23569
Apel C, Forlenza OV, de Paula VJ, Talib LL, Denecke B, Eduardo CP, Gattaz WF (2009) The neuroprotective effect of dental pulp cells in models of Alzheimer’s and Parkinson’s disease. J Neural Transm 116(1):71–78. doi:10.1007/s00702-008-0135-3
Nosrat IV, Smith CA, Mullally P, Olson L, Nosrat CA (2004) Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. Eur J Neurosci 19(9):2388–2398. doi:10.1111/j.0953-816X.2004.03314.x
Nesti C, Pardini C, Barachini S, D’Alessandro D, Siciliano G, Murri L, Petrini M, Vaglini F (2011) Human dental pulp stem cells protect mouse dopaminergic neurons against MPP+ or rotenone. Brain Res 1367:94–102. doi:10.1016/j.brainres.2010.09.042
Yamamoto T, Osako Y, Ito M, Murakami M, Hayashi Y, Horibe H, Iohara K, Takeuchi N, Okui N, Hirata H, Nakayama H, Kurita K, Nakashima M (2016) Trophic effects of dental pulp stem cells on schwann cells in peripheral nerve regeneration. Cell Transplant 25(1):183–193. doi:10.3727/096368915X688074
Uccelli A, Moretta L, Pistoia V (2008) Mesenchymal stem cells in health and disease. Nat Rev Immunol 8(9):726–736. doi:10.1038/nri2395
Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, Chirumbolo G, Becchetti E, Marchionni C, Alviano F, Fossati V, Staffolani N, Franchina M, Grossi A, Bagnara GP (2005) Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 80(6):836–842
Demircan PC, Sariboyaci AE, Unal ZS, Gacar G, Subasi C, Karaoz E (2011) Immunoregulatory effects of human dental pulp-derived stem cells on T cells: comparison of transwell co-culture and mixed lymphocyte reaction systems. Cytotherapy 13(10):1205–1220. doi:10.3109/14653249.2011.605351
Murakami M, Horibe H, Iohara K, Hayashi Y, Osako Y, Takei Y, Nakata K, Motoyama N, Kurita K, Nakashima M (2013) The use of granulocyte-colony stimulating factor induced mobilization for isolation of dental pulp stem cells with high regenerative potential. Biomaterials 34(36):9036–9047. doi:10.1016/j.biomaterials.2013.08.011
Tang R, Ding G (2011) Swine dental pulp stem cells inhibit T-cell proliferation. Transplant Proc 43(10):3955–3959. doi:10.1016/j.transproceed.2011.08.102
Wada N, Menicanin D, Shi S, Bartold PM, Gronthos S (2009) Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol 219(3):667–676. doi:10.1002/jcp.21710
Zhao Y, Wang L, Jin Y, Shi S (2012) Fas ligand regulates the immunomodulatory properties of dental pulp stem cells. J Dent Res 91(10):948–954. doi:10.1177/0022034512458690
Tomic S, Djokic J, Vasilijic S, Vucevic D, Todorovic V, Supic G, Colic M (2011) Immunomodulatory properties of mesenchymal stem cells derived from dental pulp and dental follicle are susceptible to activation by toll-like receptor agonists. Stem Cells Dev 20(4):695–708. doi:10.1089/scd.2010.0145
Ding G, Niu J, Liu Y (2015) Dental pulp stem cells suppress the proliferation of lymphocytes via transforming growth factor-beta1. Hum Cell 28(2):81–90. doi:10.1007/s13577-014-0106-y
Bianco P, Robey PG (2001) Stem cells in tissue engineering. Nature 414(6859):118–121. doi:10.1038/35102181
Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF (2013) Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin 34(6):747–754. doi:10.1038/aps.2013.50
Nakashima M, Iohara K, Murakami M (2013) Dental pulp stem cells and regeneration. Endod Top 28(1):38–50
Buzhor E, Leshansky L, Blumenthal J, Barash H, Warshawsky D, Mazor Y, Shtrichman R (2014) Cell-based therapy approaches: the hope for incurable diseases. Regen Med 9(5):649–672. doi:10.2217/rme.14.35
Wayne AS, Baird K, Egeler RM (2010) Hematopoietic stem cell transplantation for leukemia. Pediatr Clin N Am 57(1):1–25. doi:10.1016/j.pcl.2009.11.005
Ghieh F, Jurjus R, Ibrahim A, Geagea AG, Daouk H, El Baba B, Chams S, Matar M, Zein W, Jurjus A (2015) The use of stem cells in burn wound healing: a review. Biomed Res Int 2015:684084. doi:10.1155/2015/684084
Park YJ, Cha S, Park YS (2016) Regenerative applications using tooth derived stem cells in other than tooth regeneration: a literature review. Stem Cells Int 2016:9305986. doi:10.1155/2016/9305986
Daltoe FP, Mendonca PP, Mantesso A, Deboni MC (2014) Can SHED or DPSCs be used to repair/regenerate non-dental tissues? A systematic review of in vivo studies. Braz Oral Res 28: 1–7
Estrela C, Alencar AH, Kitten GT, Vencio EF, Gava E (2011) Mesenchymal stem cells in the dental tissues: perspectives for tissue regeneration. Braz Dent J 22(2):91–98
Potdar PD, Jethmalani YD (2015) Human dental pulp stem cells: applications in future regenerative medicine. World J Stem Cells 7(5):839–851. doi:10.4252/wjsc.v7.i5.839
La Noce M, Paino F, Spina A, Naddeo P, Montella R, Desiderio V, De Rosa A, Papaccio G, Tirino V, Laino L (2014) Dental pulp stem cells: state of the art and suggestions for a true translation of research into therapy. J Dent 42(7):761–768. doi:10.1016/j.jdent.2014.02.018
Su-Min Lee QZ, Le AD (2014) Dental stem cells: sources and potential applications. Curr Oral Health Rep 1(1):34–42
Gandia C, Arminan A, Garcia-Verdugo JM, Lledo E, Ruiz A, Minana MD, Sanchez-Torrijos J, Paya R, Mirabet V, Carbonell-Uberos F, Llop M, Montero JA, Sepulveda P (2008) Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells 26(3):638–645. doi:10.1634/stemcells.2007-0484
Shen CY, Li L, Feng T, Li JR, Yu MX, Lu Q, Li H (2015) Dental pulp stem cells derived conditioned medium promotes Angiogenesis in Hindlimb Ischemia. Tissue Eng Regen Med 12(1):59–68. doi:10.1007/s13770-014-9053-7
Fang CZ, Yang YJ, Wang QH, Yao Y, Zhang XY, He XH (2013) Intraventricular injection of human dental pulp stem cells improves hypoxic-ischemic brain damage in neonatal rats. PLoS One 8(6), e66748. doi:10.1371/journal.pone.0066748
Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, Sakamoto K, Tauchi R, Wakao N, Imagama S, Hibi H, Kadomatsu K, Ishiguro N, Ueda M (2012) Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest 122(1):80–90. doi:10.1172/JCI59251
Inoue T, Sugiyama M, Hattori H, Wakita H, Wakabayashi T, Ueda M (2013) Stem cells from human exfoliated deciduous tooth-derived conditioned medium enhance recovery of focal cerebral ischemia in rats. Tissue Eng A 19(1–2):24–29. doi:10.1089/ten.TEA.2011.0385
Yamagata M, Yamamoto A, Kako E, Kaneko N, Matsubara K, Sakai K, Sawamoto K, Ueda M (2013) Human dental pulp-derived stem cells protect against hypoxic-ischemic brain injury in neonatal mice. Stroke 44(2):551–554. doi:10.1161/STROKEAHA.112.676759
Taghipour Z, Karbalaie K, Kiani A, Niapour A, Bahramian H, Nasr-Esfahani MH, Baharvand H (2012) Transplantation of undifferentiated and induced human exfoliated deciduous teeth-derived stem cells promote functional recovery of rat spinal cord contusion injury model. Stem Cells Dev 21(10):1794–1802. doi:10.1089/scd.2011.0408
Yamamoto A, Sakai K, Matsubara K, Kano F, Ueda M (2014) Multifaceted neuro-regenerative activities of human dental pulp stem cells for functional recovery after spinal cord injury. Neurosci Res 78:16–20. doi:10.1016/j.neures.2013.10.010
Yamaza T, Kentaro A, Chen C, Liu Y, Shi Y, Gronthos S, Wang S, Shi S (2010) Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther 1(1):5. doi:10.1186/scrt5
Wada N, Gronthos S, Bartold PM (2013) Immunomodulatory effects of stem cells. Periodontol 2000 63(1):198–216. doi:10.1111/prd.12024
Govindasamy V, Ronald VS, Abdullah AN, Nathan KR, Ab Aziz ZA, Abdullah M, Musa S, Kasim NH, Bhonde RR (2011) Differentiation of dental pulp stem cells into islet-like aggregates. J Dent Res 90(5):646–652. doi:10.1177/0022034510396879
Kanafi MM, Rajeshwari YB, Gupta S, Dadheech N, Nair PD, Gupta PK, Bhonde RR (2013) Transplantation of islet-like cell clusters derived from human dental pulp stem cells restores normoglycemia in diabetic mice. Cytotherapy 15(10):1228–1236. doi:10.1016/j.jcyt.2013.05.008
Lee JS, An SY, Kwon IK, Heo JS (2014) Transdifferentiation of human periodontal ligament stem cells into pancreatic cell lineage. Cell Biochem Funct 32(7):605–611. doi:10.1002/cbf.3057
Ishkitiev N, Yaegaki K, Kozhuharova A, Tanaka T, Okada M, Mitev V, Fukuda M, Imai T (2013) Pancreatic differentiation of human dental pulp CD117(+) stem cells. Regen Med 8(5):597–612. doi:10.2217/rme.13.42
Carnevale G, Riccio M, Pisciotta A, Beretti F, Maraldi T, Zavatti M, Cavallini GM, La Sala GB, Ferrari A, De Pol A (2013) In vitro differentiation into insulin-producing beta-cells of stem cells isolated from human amniotic fluid and dental pulp. Dig Liver Dis 45(8):669–676. doi:10.1016/j.dld.2013.02.007
Kerkis I, Ambrosio CE, Kerkis A, Martins DS, Zucconi E, Fonseca SA, Cabral RM, Maranduba CM, Gaiad TP, Morini AC, Vieira NM, Brolio MP, Sant’Anna OA, Miglino MA, Zatz M (2008) Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs: local or systemic? J Transl Med 6:35. doi:10.1186/1479-5876-6-35
Yang R, Chen M, Lee CH, Yoon R, Lal S, Mao JJ (2010) Clones of ectopic stem cells in the regeneration of muscle defects in vivo. PLoS One 5(10), e13547. doi:10.1371/journal.pone.0013547
Graziano A, d’Aquino R, Laino G, Papaccio G (2008) Dental pulp stem cells: a promising tool for bone regeneration. Stem Cell Rev 4(1):21–26. doi:10.1007/s12015-008-9013-5
Graziano A, d’Aquino R, Cusella-De Angelis MG, De Francesco F, Giordano A, Laino G, Piattelli A, Traini T, De Rosa A, Papaccio G (2008) Scaffold’s surface geometry significantly affects human stem cell bone tissue engineering. J Cell Physiol 214(1):166–172. doi:10.1002/jcp.21175
De Mendonca Costa A, Bueno DF, Martins MT, Kerkis I, Kerkis A, Fanganiello RD, Cerruti H, Alonso N, Passos-Bueno MR (2008) Reconstruction of large cranial defects in nonimmunosuppressed experimental design with human dental pulp stem cells. J Craniofac Surg 19(1):204–210. doi:10.1097/scs.0b013e31815c8a54
Fecek C, Yao D, Kacorri A, Vasquez A, Iqbal S, Sheikh H, Svinarich DM, Perez-Cruet M, Chaudhry GR (2008) Chondrogenic derivatives of embryonic stem cells seeded into 3D polycaprolactone scaffolds generated cartilage tissue in vivo. Tissue Eng A 14(8):1403–1413. doi:10.1089/tea.2007.0293
Iohara K, Zheng L, Ito M, Tomokiyo A, Matsushita K, Nakashima M (2006) Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells 24(11):2493–2503. doi:10.1634/stemcells.2006-0161
Reynolds AJ, Jahoda CA (2004) Cultured human and rat tooth papilla cells induce hair follicle regeneration and fiber growth. Differentiation 72(9–10):566–575. doi:10.1111/j.1432-0436.2004.07209010.x
Nishino Y, Ebisawa K, Yamada Y, Okabe K, Kamei Y, Ueda M (2011) Human deciduous teeth dental pulp cells with basic fibroblast growth factor enhance wound healing of skin defect. J Craniofac Surg 22(2):438–442. doi:10.1097/SCS.0b013e318207b507
Yamamura Y, Yamada H, Sakurai T, Ide F, Inoue H, Muramatsu T, Mishima K, Hamada Y, Saito I (2013) Treatment of salivary gland hypofunction by transplantation with dental pulp cells. Arch Oral Biol 58(8):935–942. doi:10.1016/j.archoralbio.2013.02.015
Gomes JA, Geraldes Monteiro B, Melo GB, Smith RL, Cavenaghi Pereira da Silva M, Lizier NF, Kerkis A, Cerruti H, Kerkis I (2010) Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Invest Ophthalmol Vis Sci 51(3):1408–1414. doi:10.1167/iovs.09-4029
Syed-Picard FN, Du Y, Lathrop KL, Mann MM, Funderburgh ML, Funderburgh JL (2015) Dental pulp stem cells: a new cellular resource for corneal stromal regeneration. Stem Cells Transl Med 4(3):276–285. doi:10.5966/sctm.2014-0115
Huang H, Wang Y, Pan B, Yang X, Wang L, Chen J, Ma D, Yang C (2013) Simple bipolar hosts with high glass transition temperatures based on 1,8-disubstituted carbazole for efficient blue and green electrophosphorescent devices with “ideal” turn-on voltage. Chemistry 19(5):1828–1834. doi:10.1002/chem.201202329
Mead B, Logan A, Berry M, Leadbeater W, Scheven BA (2013) Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest Ophthalmol Vis Sci 54(12):7544–7556. doi:10.1167/iovs.13-13045
Horibe H, Murakami M, Iohara K, Hayashi Y, Takeuchi N, Takei Y, Kurita K, Nakashima M (2014) Isolation of a stable subpopulation of mobilized dental pulp stem cells (MDPSCs) with high proliferation, migration, and regeneration potential is independent of age. PLoS One 9(5), e98553. doi:10.1371/journal.pone.0098553
Pejcic A, Kojovic D, Mirkovic D, Minic I (2013) Stem cells for periodontal regeneration. Balkan J Med Genet 16(1):7–12. doi:10.2478/bjmg-2013-0012
Bansal R, Jain A, Mittal S (2015) Current overview on challenges in regenerative endodontics. J Conserv Dent 18(1):1–6. doi:10.4103/0972-0707.148861
Nakashima M, Iohara K (2011) Regeneration of dental pulp by stem cells. Adv Dent Res 23(3):313–319. doi:10.1177/0022034511405323
Demarco FF, Conde MC, Cavalcanti BN, Casagrande L, Sakai VT, Nor JE (2011) Dental pulp tissue engineering. Braz Dent J 22(1):3–13
Huang GT, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, Shi S (2010) Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng A 16(2):605–615. doi:10.1089/ten.TEA.2009.0518
Casagrande L, Cordeiro MM, Nor SA, Nor JE (2011) Dental pulp stem cells in regenerative dentistry. Odontol Soc Nippon Dent Univ 99(1):1–7. doi:10.1007/s10266-010-0154-z
Mantesso A, Sharpe P (2009) Dental stem cells for tooth regeneration and repair. Expert Opin Biol Ther 9(9):1143–1154. doi:10.1517/14712590903103795
Volponi AA, Pang Y, Sharpe PT (2010) Stem cell-based biological tooth repair and regeneration. Trends Cell Biol 20(12):715–722. doi:10.1016/j.tcb.2010.09.012
Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S (2005) The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofacial Res 8(3):191–199. doi:10.1111/j.1601-6343.2005.00331.x
Nakashima M, Akamine A (2005) The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod 31(10):711–718
Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A (2004) Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res 83(8):590–595
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S (2002) Stem cell properties of human dental pulp stem cells. J Dent Res 81(8):531–535
He H, Yu J, Liu Y, Lu S, Liu H, Shi J, Jin Y (2008) Effects of FGF2 and TGFbeta1 on the differentiation of human dental pulp stem cells in vitro. Cell Biol Int 32(7):827–834. doi:10.1016/j.cellbi.2008.03.013
Almushayt A, Narayanan K, Zaki AE, George A (2006) Dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene Ther 13(7):611–620. doi:10.1038/sj.gt.3302687
Prescott RS, Alsanea R, Fayad MI, Johnson BR, Wenckus CS, Hao J, John AS, George A (2008) In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J Endod 34(4):421–426. doi:10.1016/j.joen.2008.02.005
Tran Hle B, Doan VN (2015) Human dental pulp stem cells cultured onto dentin derived scaffold can regenerate dentin-like tissue in vivo. Cell Tissue Bank 16(4):559–568. doi:10.1007/s10561-015-9503-z
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nor JE (2008) Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 34(8):962–969. doi:10.1016/j.joen.2008.04.009
Taba M Jr, Jin Q, Sugai JV, Giannobile WV (2005) Current concepts in periodontal bioengineering. Orthod Craniofac Res 8(4):292–302. doi:10.1111/j.1601-6343.2005.00352.x
Park JY, Jeon SH, Choung PH (2011) Efficacy of periodontal stem cell transplantation in the treatment of advanced periodontitis. Cell Transplant 20(2):271–285. doi:10.3727/096368910X519292
Trubiani O, Orsini G, Zini N, Di Iorio D, Piccirilli M, Piattelli A, Caputi S (2008) Regenerative potential of human periodontal ligament derived stem cells on three-dimensional biomaterials: a morphological report. J Biomed Mater Res A 87(4):986–993. doi:10.1002/jbm.a.31837
Liu Y, Zheng Y, Ding G, Fang D, Zhang C, Bartold PM, Gronthos S, Shi S, Wang S (2008) Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells 26(4):1065–1073. doi:10.1634/stemcells.2007-0734
He H, Yu J, Cao JEL, Wang D, Zhang H, Liu H (2011) Biocompatibility and osteogenic capacity of periodontal ligament stem cells on nHAC/PLA and HA/TCP scaffolds. J Biomater Sci Polym Ed 22(1–3):179–194. doi:10.1163/092050609X12587018007767
Lin Y, Gallucci GO, Buser D, Bosshardt D, Belser UC, Yelick PC (2011) Bioengineered periodontal tissue formed on titanium dental implants. J Dent Res 90(2):251–256. doi:10.1177/0022034510384872
Gault P, Black A, Romette JL, Fuente F, Schroeder K, Thillou F, Brune T, Berdal A, Wurtz T (2010) Tissue-engineered ligament: implant constructs for tooth replacement. J Clin Periodontol 37(8):750–758. doi:10.1111/j.1600-051X.2010.01588.x
Feng F, Akiyama K, Liu Y, Yamaza T, Wang TM, Chen JH, Wang BB, Huang GT, Wang S, Shi S (2010) Utility of PDL progenitors for in vivo tissue regeneration: a report of 3 cases. Oral Dis 16(1):20–28
Yamada Y, Ito K, Nakamura S, Ueda M, Nagasaka T (2011) Promising cell-based therapy for bone regeneration using stem cells from deciduous teeth, dental pulp, and bone marrow. Cell Transplant 20(7):1003–1013. doi:10.3727/096368910X539128
Ji YM, Jeon SH, Park JY, Chung JH, Choung YH, Choung PH (2010) Dental stem cell therapy with calcium hydroxide in dental pulp capping. Tissue Eng A 16(6):1823–1833. doi:10.1089/ten.TEA.2009.0054
Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, Shi S (2006) Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One 1, e79. doi:10.1371/journal.pone.0000079
Nor JE (2006) Tooth regeneration in operative dentistry. Oper Dent 31(6):633–642. doi:10.2341/06-000
Khorsand A, Eslaminejad MB, Arabsolghar M, Paknejad M, Ghaedi B, Rokn AR, Moslemi N, Nazarian H, Jahangir S (2013) Autologous dental pulp stem cells in regeneration of defect created in canine periodontal tissue. J Oral Implantol 39(4):433–443. doi:10.1563/AAID-JOI-D-12-00027
Iohara K, Imabayashi K, Ishizaka R, Watanabe A, Nabekura J, Ito M, Matsushita K, Nakamura H, Nakashima M (2011) Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng A 17(15–16):1911–1920. doi:10.1089/ten.TEA.2010.0615
Guo W, Gong K, Shi H, Zhu G, He Y, Ding B, Wen L, Jin Y (2012) Dental follicle cells and treated dentin matrix scaffold for tissue engineering the tooth root. Biomaterials 33(5):1291–1302. doi:10.1016/j.biomaterials.2011.09.068
Guo W, Chen L, Gong K, Ding B, Duan Y, Jin Y (2012) Heterogeneous dental follicle cells and the regeneration of complex periodontal tissues. Tissue Eng A 18(5–6):459–470. doi:10.1089/ten.TEA.2011.0261
Shen YY, Chen K, Xu N (2010) Osteogenic capacity of human deciduous dental pulp stem cells in vitro. Nan Fang Yi Ke Da Xue Xue Bao 30(1):96–99
Hwang YC, Hwang IN, Oh WM, Park JC, Lee DS, Son HH (2008) Influence of TGF-beta1 on the expression of BSP, DSP, TGF-beta1 receptor I and Smad proteins during reparative dentinogenesis. J Mol Histol 39(2):153–160. doi:10.1007/s10735-007-9148-8
Laino G, d’Aquino R, Graziano A, Lanza V, Carinci F, Naro F, Pirozzi G, Papaccio G (2005) A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Miner Res 20(8):1394–1402. doi:10.1359/JBMR.050325
Ueno A, Yamashita K, Miyoshi K, Horiguchi T, Ruspita I, Abe K, Noma T (2006) Soluble matrix from osteoblastic cells induces mineralization by dental pulp cells. J Med Invest 53(3–4):297–302
Hosoya A, Nakamura H, Ninomiya T, Hoshi K, Yoshiba K, Yoshiba N, Takahashi M, Okabe T, Sahara N, Yamada H, Kasahara E, Ozawa H (2007) Hard tissue formation in subcutaneously transplanted rat dental pulp. J Dent Res 86(5):469–474
Otaki S, Ueshima S, Shiraishi K, Sugiyama K, Hamada S, Yorimoto M, Matsuo O (2007) Mesenchymal progenitor cells in adult human dental pulp and their ability to form bone when transplanted into immunocompromised mice. Cell Biol Int 31(10):1191–1197. doi:10.1016/j.cellbi.2007.04.001
Graziano A, d’Aquino R, Laino G, Proto A, Giuliano MT, Pirozzi G, De Rosa A, Di Napoli D, Papaccio G (2008) Human CD34+ stem cells produce bone nodules in vivo. Cell Prolif 41(1):1–11. doi:10.1111/j.1365-2184.2007.00497.x
d’Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, Desiderio V, Laino G, Papaccio G (2009) Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater 18:75–83
Akkouch A, Zhang Z, Rouabhia M (2014) Engineering bone tissue using human dental pulp stem cells and an osteogenic collagen-hydroxyapatite-poly (L-lactide-co-epsilon-caprolactone) scaffold. J Biomater Appl 28(6):922–936. doi:10.1177/0885328213486705
Annibali S, Bellavia D, Ottolenghi L, Cicconetti A, Cristalli MP, Quaranta R, Pilloni A (2014) Micro-CT and PET analysis of bone regeneration induced by biodegradable scaffolds as carriers for dental pulp stem cells in a rat model of calvarial “critical size” defect: preliminary data. J Biomed Mater Res B Appl Biomater 102(4):815–825. doi:10.1002/jbm.b.33064
Mangano C, De Rosa A, Desiderio V, d’Aquino R, Piattelli A, De Francesco F, Tirino V, Mangano F, Papaccio G (2010) The osteoblastic differentiation of dental pulp stem cells and bone formation on different titanium surface textures. Biomaterials 31(13):3543–3551. doi:10.1016/j.biomaterials.2010.01.056
Maraldi T, Riccio M, Pisciotta A, Zavatti M, Carnevale G, Beretti F, La Sala GB, Motta A, De Pol A (2013) Human amniotic fluid-derived and dental pulp-derived stem cells seeded into collagen scaffold repair critical-size bone defects promoting vascularization. Stem cell Res Ther 4(3):53. doi:10.1186/scrt203
Kolind K, Kraft D, Boggild T, Duch M, Lovmand J, Pedersen FS, Bindslev DA, Bunger CE, Foss M, Besenbacher F (2014) Control of proliferation and osteogenic differentiation of human dental-pulp-derived stem cells by distinct surface structures. Acta Biomater 10(2):641–650. doi:10.1016/j.actbio.2013.11.006
Ikeda H, Sumita Y, Ikeda M, Ikeda H, Okumura T, Sakai E, Nishimura M, Asahina I (2011) Engineering bone formation from human dental pulp- and periodontal ligament-derived cells. Ann Biomed Eng 39(1):26–34. doi:10.1007/s10439-010-0115-2
Yang X, van der Kraan PM, Bian Z, Fan M, Walboomers XF, Jansen JA (2009) Mineralized tissue formation by BMP2-transfected pulp stem cells. J Dent Res 88(11):1020–1025. doi:10.1177/0022034509346258
Liu HC, Ling-Ling E, Wang DS, Su F, Wu X, Shi ZP, Lv Y, Wang JZ (2011) Reconstruction of alveolar bone defects using bone morphogenetic protein 2 mediated rabbit dental pulp stem cells seeded on nano-hydroxyapatite/collagen/poly(L-lactide). Tissue Eng A 17(19–20):2417–2433. doi:10.1089/ten.TEA.2010.0620
Ge S, Zhao N, Wang L, Yu M, Liu H, Song A, Huang J, Wang G, Yang P (2012) Bone repair by periodontal ligament stem cellseeded nanohydroxyapatite-chitosan scaffold. Int J Nanomedicine 7:5405–5414. doi:10.2147/IJN.S36714
Su F, Liu SS, Ma JL, Wang DS, Ling-Ling E, Liu HC (2015) Enhancement of periodontal tissue regeneration by transplantation of osteoprotegerin-engineered periodontal ligament stem cells. Stem Cell Res Ther 6:22. doi:10.1186/s13287-015-0023-3
Yu BH, Zhou Q, Wang ZL (2014) Periodontal ligament versus bone marrow mesenchymal stem cells in combination with Bio-Oss scaffolds for ectopic and in situ bone formation: a comparative study in the rat. J Biomater Appl 29(2):243–253. doi:10.1177/0885328214521846
Seo BM, Sonoyama W, Yamaza T, Coppe C, Kikuiri T, Akiyama K, Lee JS, Shi S (2008) SHED repair critical-size calvarial defects in mice. Oral Dis 14(5):428–434
Zheng Y, Liu Y, Zhang CM, Zhang HY, Li WH, Shi S, Le AD, Wang SL (2009) Stem cells from deciduous tooth repair mandibular defect in swine. J Dent Res 88(3):249–254. doi:10.1177/0022034509333804
Abe S, Yamaguchi S, Watanabe A, Hamada K, Amagasa T (2008) Hard tissue regeneration capacity of apical pulp derived cells (APDCs) from human tooth with immature apex. Biochem Biophys Res Commun 371(1):90–93. doi:10.1016/j.bbrc.2008.04.016
Martens W, Bronckaers A, Politis C, Jacobs R, Lambrichts I (2013) Dental stem cells and their promising role in neural regeneration: an update. Clin Oral Investig 17(9):1969–1983. doi:10.1007/s00784-013-1030-3
Miletich I, Sharpe PT (2004) Neural crest contribution to mammalian tooth formation. Birth Defects Res C Embryo Today 72(2):200–212. doi:10.1002/bdrc.20012
Chai Y, Jiang X, Ito Y, Bringas P Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM (2000) Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127(8):1671–1679
Ibarretxe G, Crende O, Aurrekoetxea M, Garcia-Murga V, Etxaniz J, Unda F (2012) Neural crest stem cells from dental tissues: a new hope for dental and neural regeneration. Stem Cells Int 2012:103503. doi:10.1155/2012/103503
Graham A, Begbie J, McGonnell I (2004) Significance of the cranial neural crest. Dev Dyn 229(1):5–13. doi:10.1002/dvdy.10442
Arthur A, Shi S, Zannettino AC, Fujii N, Gronthos S, Koblar SA (2009) Implanted adult human dental pulp stem cells induce endogenous axon guidance. Stem Cells 27(9):2229–2237. doi:10.1002/stem.138
Kiraly M, Kadar K, Horvathy DB, Nardai P, Racz GZ, Lacza Z, Varga G, Gerber G (2011) Integration of neuronally predifferentiated human dental pulp stem cells into rat brain in vivo. Neurochem Int 59(3):371–381. doi:10.1016/j.neuint.2011.01.006
Leong WK, Henshall TL, Arthur A, Kremer KL, Lewis MD, Helps SC, Field J, Hamilton-Bruce MA, Warming S, Manavis J, Vink R, Gronthos S, Koblar SA (2012) Human adult dental pulp stem cells enhance poststroke functional recovery through non-neural replacement mechanisms. Stem Cells Transl Med 1(3):177–187. doi:10.5966/sctm.2011-0039
Yang KL, Chen MF, Liao CH, Pang CY, Lin PY (2009) A simple and efficient method for generating Nurr1-positive neuronal stem cells from human wisdom teeth (tNSC) and the potential of tNSC for stroke therapy. Cytotherapy 11(5):606–617. doi:10.1080/14653240902806994
Sugiyama M, Iohara K, Wakita H, Hattori H, Ueda M, Matsushita K, Nakashima M (2011) Dental pulp-derived CD31(-)/CD146(-) side population stem/progenitor cells enhance recovery of focal cerebral ischemia in rats. Tissue Eng A 17(9–10):1303–1311. doi:10.1089/ten.TEA.2010.0306
Nagpal A, Kremer KL, Hamilton-Bruce MA, Kaidonis X, Milton AG, Levi C, Shi S, Carey L, Hillier S, Rose M, Zacest A, Takhar P, Koblar SA (2016) TOOTH (The Open study Of dental pulp stem cell Therapy in Humans): study protocol for evaluating safety and feasibility of autologous human adult dental pulp stem cell therapy in patients with chronic disability after stroke. Int J Stroke. doi:10.1177/1747493016641111
Tseng LS, Chen SH, Lin MT, Lin YC (2015) Transplantation of human dental pulp-derived stem cells protects against heatstroke in mice. Cell Transplant 24(5):921–937. doi:10.3727/096368914X678580
Li X, Yang C, Li L, Xiong J, Xie L, Yang B, Yu M, Feng L, Jiang Z, Guo W, Tian W (2015) A therapeutic strategy for spinal cord defect: human dental follicle cells combined with aligned PCL/PLGA electrospun material. Biomed Res Int 2015:197183. doi:10.1155/2015/197183
De Berdt P, Vanacker J, Ucakar B, Elens L, Diogenes A, Leprince JG, Deumens R, des Rieux A (2015) Dental apical papilla as therapy for spinal cord injury. J Dent Res 94(11):1575–1581. doi:10.1177/0022034515604612
De Berdt P, Vanacker J, Ucakar B, Elens L, Diogenes A, Leprince JG, Deumens R, des Rieux A (2015) Dental apical papilla as therapy for spinal cord injury. J Dent Res 94(11):1575–1581. doi:10.1177/0022034515604612
Huang AH, Snyder BR, Cheng PH, Chan AW (2008) Putative dental pulp-derived stem/stromal cells promote proliferation and differentiation of endogenous neural cells in the hippocampus of mice. Stem Cells 26(10):2654–2663. doi:10.1634/stemcells.2008-0285
Sasaki R, Aoki S, Yamato M, Uchiyama H, Wada K, Ogiuchi H, Okano T, Ando T (2011) PLGA artificial nerve conduits with dental pulp cells promote facial nerve regeneration. J Tissue Eng Regen Med 5(10):823–830. doi:10.1002/term.387
Sasaki R, Aoki S, Yamato M, Uchiyama H, Wada K, Okano T, Ogiuchi H (2008) Tubulation with dental pulp cells promotes facial nerve regeneration in rats. Tissue Eng A 14(7):1141–1147. doi:10.1089/ten.tea.2007.0157
Sasaki R, Matsumine H, Watanabe Y, Takeuchi Y, Yamato M, Okano T, Miyata M, Ando T (2014) Electrophysiologic and functional evaluations of regenerated facial nerve defects with a tube containing dental pulp cells in rats. Plast Reconstr Surg 134(5):970–978. doi:10.1097/PRS.0000000000000602
Iohara K, Murakami M, Takeuchi N, Osako Y, Ito M, Ishizaka R, Utunomiya S, Nakamura H, Matsushita K, Nakashima M (2013) A novel combinatorial therapy with pulp stem cells and granulocyte colony-stimulating factor for total pulp regeneration. Stem Cells Transl Med 2(7):521–533. doi:10.5966/sctm.2012-0132
Kumar A, Bhattacharyya S, Rattan V (2015) Effect of uncontrolled freezing on biological characteristics of human dental pulp stem cells. Cell Tissue Bank 16(4):513–522. doi:10.1007/s10561-015-9498-5
Davies OG, Smith AJ, Cooper PR, Shelton RM, Scheven BA (2014) The effects of cryopreservation on cells isolated from adipose, bone marrow and dental pulp tissues. Cryobiology 69(2):342–347. doi:10.1016/j.cryobiol.2014.08.003
Baust JM, Corwin WL, VanBuskirk R, Baust JG (2015) Biobanking: the future of cell preservation strategies. Adv Exp Med Biol 864:37–53. doi:10.1007/978-3-319-20579-3_4
Marquez-Curtis LA, Janowska-Wieczorek A, McGann LE, Elliott JA (2015) Mesenchymal stromal cells derived from various tissues: biological, clinical and cryopreservation aspects. Cryobiology 71(2):181–197. doi:10.1016/j.cryobiol.2015.07.003
Mazur P, Leibo SP, Chu EH (1972) A two-factor hypothesis of freezing injury. Evidence from Chinese hamster tissue-culture cells. Exp Cell Res 71(2):345–355
Zhurova M, Woods EJ, Acker JP (2010) Intracellular ice formation in confluent monolayers of human dental stem cells and membrane damage. Cryobiology 61(1):133–141. doi:10.1016/j.cryobiol.2010.06.007
Fleming KK, Hubel A (2006) Cryopreservation of hematopoietic and non-hematopoietic stem cells. Transfus Apher Sci 34(3):309–315. doi:10.1016/j.transci.2005.11.012
Papaccio G, Graziano A, d’Aquino R, Graziano MF, Pirozzi G, Menditti D, De Rosa A, Carinci F, Laino G (2006) Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. J Cell Physiol 208(2):319–325. doi:10.1002/jcp.20667
Perry BC, Zhou D, Wu X, Yang FC, Byers MA, Chu TM, Hockema JJ, Woods EJ, Goebel WS (2008) Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods 14(2):149–156. doi:10.1089/ten.tec.2008.0031
Woods EJ, Perry BC, Hockema JJ, Larson L, Zhou D, Goebel WS (2009) Optimized cryopreservation method for human dental pulp-derived stem cells and their tissues of origin for banking and clinical use. Cryobiology 59(2):150–157. doi:10.1016/j.cryobiol.2009.06.005
Ding G, Wang W, Liu Y, An Y, Zhang C, Shi S, Wang S (2010) Effect of cryopreservation on biological and immunological properties of stem cells from apical papilla. J Cell Physiol 223(2):415–422. doi:10.1002/jcp.22050
Vasconcelos RG, Ribeiro RA, Vasconcelos MG, Lima KC, Barboza CA (2012) In vitro comparative analysis of cryopreservation of undifferentiated mesenchymal cells derived from human periodontal ligament. Cell Tissue Bank 13(3):461–469. doi:10.1007/s10561-011-9271-3
Harel A (2013) Cryopreservation and cell banking for autologous mesenchymal stem cell-based therapies. Cell Tissue Transpl Ther 5:1–7. doi:10.4137/CTTT.S11249
Hunt CJ (2011) Cryopreservation of human stem cells for clinical application: a review. Transfusion Med Hemother 38(2):107–123. doi:10.1159/000326623
Rowley SD, Feng Z, Yadock D, Holmberg L, Macleod B, Heimfeld S (1999) Post-thaw removal of DMSO does not completely abrogate infusional toxicity or the need for pre-infusion histamine blockade. Cytotherapy 1(6):439–446. doi:10.1080/0032472031000141303
Demirci S, Dogan A, Sisli B, Sahin F (2014) Boron increases the cell viability of mesenchymal stem cells after long-term cryopreservation. Cryobiology 68(1):139–146. doi:10.1016/j.cryobiol.2014.01.010
Umemura E, Yamada Y, Nakamura S, Ito K, Hara K, Ueda M (2011) Viable cryopreserving tissue-engineered cell-biomaterial for cell banking therapy in an effective cryoprotectant. Tissue Eng Part C Methods 17(8):799–807. doi:10.1089/ten.tec.2011.0003
Ducret M, Fabre H, Farges JC, Degoul O, Atzeni G, McGuckin C, Forraz N, Mallein-Gerin F, Perrier-Groult E (2015) Production of human dental pulp cells with a medicinal manufacturing approach. J Endod 41(9):1492–1499. doi:10.1016/j.joen.2015.05.017
Lee SY, Huang GW, Shiung JN, Huang YH, Jeng JH, Kuo TF, Yang JC, Yang WC (2012) Magnetic cryopreservation for dental pulp stem cells. Cells Tissues Organs 196(1):23–33. doi:10.1159/000331247
Kamada H, Kaku M, Kawata T, Koseki H, Abedini S, Kojima S, Sumi A, Motokawa M, Fujita T, Ohtani J, Ohwada N, Tanne K (2011) In-vitro and in-vivo study of periodontal ligament cryopreserved with a magnetic field. Am J Orthod Dentofac Orthop 140(6):799–805. doi:10.1016/j.ajodo.2011.04.024
Amos MJ, Day P, Littlewood SJ (2009) Autotransplantation of teeth: an overview. Dent Update 36(2):102–104, 107–110, 113
Kaku M, Kamada H, Kawata T, Koseki H, Abedini S, Kojima S, Motokawa M, Fujita T, Ohtani J, Tsuka N, Matsuda Y, Sunagawa H, Hernandes RA, Ohwada N, Tanne K (2010) Cryopreservation of periodontal ligament cells with magnetic field for tooth banking. Cryobiology 61(1):73–78. doi:10.1016/j.cryobiol.2010.05.003
Abedini S, Kaku M, Kawata T, Koseki H, Kojima S, Sumi H, Motokawa M, Fujita T, Ohtani J, Ohwada N, Tanne K (2011) Effects of cryopreservation with a newly-developed magnetic field programmed freezer on periodontal ligament cells and pulp tissues. Cryobiology 62(3):181–187. doi:10.1016/j.cryobiol.2011.03.001
Oh YH, Che ZM, Hong JC, Lee EJ, Lee SJ, Kim J (2005) Cryopreservation of human teeth for future organization of a tooth bank – a preliminary study. Cryobiology 51(3):322–329. doi:10.1016/j.cryobiol.2005.08.008
Min KS, Lee HW, Lee HS, Lee JH, Park SH (2010) Comparison of gene expression in human periodontal ligament cells cultured from teeth immediately after extraction and from teeth cryopreserved for 1 week. Cryobiology 60(3):326–330. doi:10.1016/j.cryobiol.2010.02.008
Gioventu S, Andriolo G, Bonino F, Frasca S, Lazzari L, Montelatici E, Santoro F, Rebulla P (2012) A novel method for banking dental pulp stem cells. Transfus Apher Sci 47(2):199–206. doi:10.1016/j.transci.2012.06.005
Temmerman L, Beele H, Dermaut LR, Van Maele G, De Pauw GA (2010) Influence of cryopreservation on the pulpal tissue of immature third molars in vitro. Cell Tissue Bank 11(3):281–289. doi:10.1007/s10561-009-9148-x
Malekfar A, Valli KS, Kanafi MM, Bhonde RR (2016) Isolation and characterization of human dental pulp stem cells from cryopreserved pulp tissues obtained from teeth with irreversible pulpitis. J Endod 42(1):76–81. doi:10.1016/j.joen.2015.10.001
Ji EH, Song JS, Kim SO, Jeon M, Choi BJ, Lee JH (2014) Viability of pulp stromal cells in cryopreserved deciduous teeth. Cell Tissue Bank 15(1):67–74. doi:10.1007/s10561-013-9375-z
Lindemann D, Werle SB, Steffens D, Garcia-Godoy F, Pranke P, Casagrande L (2014) Effects of cryopreservation on the characteristics of dental pulp stem cells of intact deciduous teeth. Arch Oral Biol 59(9):970–976. doi:10.1016/j.archoralbio.2014.04.008
Bressan WS, Lindemann D, Machado J, Pranke P, Casagrande L (2014) Serum-containing medium effect on isolation rate of dental pulp cells from cryopreserved intact deciduous teeth. J Clin Pediatr Dent 38(4):345–348
Park BW, Jang SJ, Byun JH, Kang YH, Choi MJ, Park WU, Lee WJ, Rho GJ (2014) Cryopreservation of human dental follicle tissue for use as a resource of autologous mesenchymal stem cells. J Tissue Eng Regen Med. doi:10.1002/term.1945
Kang YH, Lee HJ, Jang SJ, Byun JH, Lee JS, Lee HC, Park WU, Lee JH, Rho GJ, Park BW (2015) Immunomodulatory properties and in vivo osteogenesis of human dental stem cells from fresh and cryopreserved dental follicles. Differentiation 90(1–3):48–58. doi:10.1016/j.diff.2015.10.001
Seo BM, Miura M, Sonoyama W, Coppe C, Stanyon R, Shi S (2005) Recovery of stem cells from cryopreserved periodontal ligament. J Dent Res 84(10):907–912
Crook JM, Hei D, Stacey G (2010) The International Stem Cell Banking Initiative (ISCBI): raising standards to bank on. In Vitro Cell Dev Biol Animal 46(3–4):169–172. doi:10.1007/s11626-010-9301-7
ISSCR (2008) Guidelines for the clinical translation of stem cells
Eaker S, Armant M, Brandwein H, Burger S, Campbell A, Carpenito C, Clarke D, Fong T, Karnieli O, Niss K, Van’t Hof W, Wagey R (2013) Concise review: guidance in developing commercializable autologous/patient-specific cell therapy manufacturing. Stem Cells Transl Med 2(11):871–883. doi:10.5966/sctm.2013-0050
Ahrlund-Richter L, De Luca M, Marshak DR, Munsie M, Veiga A, Rao M (2009) Isolation and production of cells suitable for human therapy: challenges ahead. Cell Stem Cell 4(1):20–26. doi:10.1016/j.stem.2008.11.012
Alici E, Blomberg P (2010) GMP facilities for manufacturing of advanced therapy medicinal products for clinical trials: an overview for clinical researchers. Curr Gene Ther 10(6):508–515
Sensebe L, Gadelorge M, Fleury-Cappellesso S (2013) Production of mesenchymal stromal/stem cells according to good manufacturing practices: a review. Stem Cell Res Ther 4(3):66. doi:10.1186/scrt217
Wuchter P, Bieback K, Schrezenmeier H, Bornhauser M, Muller LP, Bonig H, Wagner W, Meisel R, Pavel P, Tonn T, Lang P, Muller I, Renner M, Malcherek G, Saffrich R, Buss EC, Horn P, Rojewski M, Schmitt A, Ho AD, Sanzenbacher R, Schmitt M (2015) Standardization of good manufacturing practice-compliant production of bone marrow-derived human mesenchymal stromal cells for immunotherapeutic applications. Cytotherapy 17(2):128–139. doi:10.1016/j.jcyt.2014.04.002
Ducret M, Fabre H, Degoul O, Atzeni G, McGuckin C, Forraz N, Alliot-Licht B, Mallein-Gerin F, Perrier-Groult E, Farges JC (2015) Manufacturing of dental pulp cell-based products from human third molars: current strategies and future investigations. Front Physiol 6:213. doi:10.3389/fphys.2015.00213
Takeda T, Tezuka Y, Horiuchi M, Hosono K, Iida K, Hatakeyama D, Miyaki S, Kunisada T, Shibata T, Tezuka K (2008) Characterization of dental pulp stem cells of human tooth germs. J Dent Res 87(7):676–681
Hilkens P, Gervois P, Fanton Y, Vanormelingen J, Martens W, Struys T, Politis C, Lambrichts I, Bronckaers A (2013) Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell Tissue Res 353(1):65–78. doi:10.1007/s00441-013-1630-x
Alt E, Yan Y, Gehmert S, Song YH, Altman A, Gehmert S, Vykoukal D, Bai X (2011) Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell 103(4):197–208. doi:10.1042/BC20100117
Lv FJ, Tuan RS, Cheung KM, Leung VY (2014) Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells 32(6):1408–1419. doi:10.1002/stem.1681
Bansal R, Jain A (2015) Current overview on dental stem cells applications in regenerative dentistry. J Nat Sci Biol Med 6(1):29–34. doi:10.4103/0976-9668.149074
Gottipamula S, Muttigi MS, Kolkundkar U, Seetharam RN (2013) Serum-free media for the production of human mesenchymal stromal cells: a review. Cell Prolif 46(6):608–627. doi:10.1111/cpr.12063
Halme DG, Kessler DA (2006) FDA regulation of stem-cell-based therapies. N Engl J Med 355(16):1730–1735. doi:10.1056/NEJMhpr063086
Hemeda H, Giebel B, Wagner W (2014) Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy 16(2):170–180. doi:10.1016/j.jcyt.2013.11.004
van der Valk J, Mellor D, Brands R, Fischer R, Gruber F, Gstraunthaler G, Hellebrekers L, Hyllner J, Jonker FH, Prieto P, Thalen M, Baumans V (2004) The humane collection of fetal bovine serum and possibilities for serum-free cell and tissue culture. Toxicol In Vitro: Int J Published Assoc BIBRA 18(1):1–12
Mannello F, Tonti GA (2007) Concise review: no breakthroughs for human mesenchymal and embryonic stem cell culture: conditioned medium, feeder layer, or feeder-free; medium with fetal calf serum, human serum, or enriched plasma; serum-free, serum replacement nonconditioned medium, or ad hoc formula? All that glitters is not gold! Stem Cells 25(7):1603–1609. doi:10.1634/stemcells.2007-0127
Tarle SA, Shi S, Kaigler D (2011) Development of a serum-free system to expand dental-derived stem cells: PDLSCs and SHEDs. J Cell Physiol 226(1):66–73. doi:10.1002/jcp.22304
Hirata TM, Ishkitiev N, Yaegaki K, Calenic B, Ishikawa H, Nakahara T, Mitev V, Tanaka T, Haapasalo M (2010) Expression of multiple stem cell markers in dental pulp cells cultured in serum-free media. J Endod 36(7):1139–1144. doi:10.1016/j.joen.2010.03.002
Khanna-Jain RVS, Vuorinen A, Sandor GK, Suuronen R, Mannerstrom B, Miettinen S (2012) Growth and differentiation of human dental pulp stem cells maintained in fetal bovine serum, human serum and serum-free/xeno-free culture media. J Stem Cell Res Ther 2(4)
Morsczeck C, Ernst W, Florian C, Reichert TE, Proff P, Bauer R, Muller-Richter U, Driemel O (2008) Gene expression of nestin, collagen type I and type III in human dental follicle cells after cultivation in serum-free medium. Oral Maxillofac Surg 12(2):89–92. doi:10.1007/s10006-008-0111-y
Okamoto T, Yatsuzuka N, Tanaka Y, Kan M, Yamanaka T, Sakamoto A, Takata T, Akagawa Y, Sato GH, Sato JD, Takada K (1997) Growth and differentiation of periodontal ligament-derived cells in serum-free defined culture. In Vitro Cell Dev Biol Animal 33(4):302–309
Bonnamain V, Thinard R, Sergent-Tanguy S, Huet P, Bienvenu G, Naveilhan P, Farges JC, Alliot-Licht B (2013) Human dental pulp stem cells cultured in serum-free supplemented medium. Front Physiol 4:357. doi:10.3389/fphys.2013.00357
Xiao L, Tsutsui T (2013) Characterization of human dental pulp cells-derived spheroids in serum-free medium: stem cells in the core. J Cell Biochem 114(11):2624–2636. doi:10.1002/jcb.24610
Karbanova J, Soukup T, Suchanek J, Pytlik R, Corbeil D, Mokry J (2011) Characterization of dental pulp stem cells from impacted third molars cultured in low serum-containing medium. Cells Tissues Organs 193(6):344–365. doi:10.1159/000321160
Bernardo ME, Avanzini MA, Perotti C, Cometa AM, Moretta A, Lenta E, Del Fante C, Novara F, de Silvestri A, Amendola G, Zuffardi O, Maccario R, Locatelli F (2007) Optimization of in vitro expansion of human multipotent mesenchymal stromal cells for cell-therapy approaches: further insights in the search for a fetal calf serum substitute. J Cell Physiol 211(1):121–130. doi:10.1002/jcp.20911
Bieback K (2013) Platelet lysate as replacement for fetal bovine serum in mesenchymal stromal cell cultures. Transfus Med Hemother 40(5):326–335. doi:10.1159/000354061
Burnouf T, Strunk D, Koh MB, Schallmoser K (2016) Human platelet lysate: replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 76:371–387. doi:10.1016/j.biomaterials.2015.10.065
Govindasamy V, Ronald VS, Abdullah AN, Ganesan Nathan KR, Aziz ZA, Abdullah M, Zain RB, Kasim NH, Musa S, Bhonde RR (2011) Human platelet lysate permits scale-up of dental pulp stromal cells for clinical applications. Cytotherapy 13(10):1221–1233. doi:10.3109/14653249.2011.602337
Iudicone P, Fioravanti D, Bonanno G, Miceli M, Lavorino C, Totta P, Frati L, Nuti M, Pierelli L (2014) Pathogen-free, plasma-poor platelet lysate and expansion of human mesenchymal stem cells. J Transl Med 12:28. doi:10.1186/1479-5876-12-28
Pisciolaro RL, Duailibi MT, Novo NF, Juliano Y, Pallos D, Yelick PC, Vacanti JP, Ferreira LM, Duailibi SE (2015) Tooth tissue engineering: the importance of blood products as a supplement in tissue culture medium for human pulp dental stem cells. Tissue Eng A 21(21–22):2639–2648. doi:10.1089/ten.TEA.2014.0617
Rauch C, Feifel E, Amann EM, Spotl HP, Schennach H, Pfaller W, Gstraunthaler G (2011) Alternatives to the use of fetal bovine serum: human platelet lysates as a serum substitute in cell culture media. Altex 28(4):305–316
Shahdadfar A, Fronsdal K, Haug T, Reinholt FP, Brinchmann JE (2005) In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells 23(9):1357–1366. doi:10.1634/stemcells.2005-0094
Shih DT, Burnouf T (2015) Preparation, quality criteria, and properties of human blood platelet lysate supplements for ex vivo stem cell expansion. New Biotechnol 32(1):199–211. doi:10.1016/j.nbt.2014.06.001
Cardaropoli DG, L. (2007) The influence of orthodontic movement on periodontal tissues level. Semin Orthod 13(4):234–245
Chen Y, Mohammed A, Oubaidin M, Evans CA, Zhou X, Luan X, Diekwisch TG, Atsawasuwan P (2015) Cyclic stretch and compression forces alter microRNA-29 expression of human periodontal ligament cells. Gene 566(1):13–17. doi:10.1016/j.gene.2015.03.055
Krishnan V, Davidovitch Z (2006) Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofac Orthop 129(4):469. doi:10.1016/j.ajodo.2005.10.007, e461–432
Meeran NA (2013) Cellular response within the periodontal ligament on application of orthodontic forces. J Indian Soc Periodontol 17(1):16–20. doi:10.4103/0972-124X.107468
Iohara K, Murakami M, Nakata K, Nakashima M (2014) Age-dependent decline in dental pulp regeneration after pulpectomy in dogs. Exp Gerontol 52:39–45. doi:10.1016/j.exger.2014.01.020
Feng X, Xing J, Feng G, Huang D, Lu X, Liu S, Tan W, Li L, Gu Z (2014) p16(INK4A) mediates age-related changes in mesenchymal stem cells derived from human dental pulp through the DNA damage and stress response. Mech Ageing Dev 141–142:46–55. doi:10.1016/j.mad.2014.09.004
Lossdorfer S, Kraus D, Jager A (2010) Aging affects the phenotypic characteristics of human periodontal ligament cells and the cellular response to hormonal stimulation in vitro. J Periodontal Res 45(6):764–771. doi:10.1111/j.1600-0765.2010.01297.x
Ma D, Ma Z, Zhang X, Wang W, Yang Z, Zhang M, Wu G, Lu W, Deng Z, Jin Y (2009) Effect of age and extrinsic microenvironment on the proliferation and osteogenic differentiation of rat dental pulp stem cells in vitro. J Endod 35(11):1546–1553. doi:10.1016/j.joen.2009.07.016
Zhang J, An Y, Gao LN, Zhang YJ, Jin Y, Chen FM (2012) The effect of aging on the pluripotential capacity and regenerative potential of human periodontal ligament stem cells. Biomaterials 33(29):6974–6986. doi:10.1016/j.biomaterials.2012.06.032
Atari M, Gil-Recio C, Fabregat M, Garcia-Fernandez D, Barajas M, Carrasco MA, Jung HS, Alfaro FH, Casals N, Prosper F, Ferres-Padro E, Giner L (2012) Dental pulp of the third molar: a new source of pluripotent-like stem cells. J Cell Sci 125(Pt 14):3343–3356. doi:10.1242/jcs.096537
Kellner M, Steindorff MM, Strempel JF, Winkel A, Kuhnel MP, Stiesch M (2014) Differences of isolated dental stem cells dependent on donor age and consequences for autologous tooth replacement. Arch Oral Biol 59(6):559–567. doi:10.1016/j.archoralbio.2014.02.014
James JA, Sayers NM, Drucker DB, Hull PS (1999) Effects of tobacco products on the attachment and growth of periodontal ligament fibroblasts. J Periodontol 70(5):518–525. doi:10.1902/jop.1999.70.5.518
Kim SY, Kang KL, Lee JC, Heo JS (2012) Nicotinic acetylcholine receptor alpha7 and beta4 subunits contribute nicotine-induced apoptosis in periodontal ligament stem cells. Mol Cells 33(4):343–350. doi:10.1007/s10059-012-2172-x
Ng TK, Carballosa CM, Pelaez D, Wong HK, Choy KW, Pang CP, Cheung HS (2013) Nicotine alters MicroRNA expression and hinders human adult stem cell regenerative potential. Stem Cells Dev 22(5):781–790. doi:10.1089/scd.2012.0434
Yanagita M, Kashiwagi Y, Kobayashi R, Tomoeda M, Shimabukuro Y, Murakami S (2008) Nicotine inhibits mineralization of human dental pulp cells. J Endod 34(9):1061–1065. doi:10.1016/j.joen.2008.06.005
Cooper PR, Takahashi Y, Graham LW, Simon S, Imazato S, Smith AJ (2010) Inflammation-regeneration interplay in the dentine-pulp complex. J Dent 38(9):687–697. doi:10.1016/j.jdent.2010.05.016
Alongi DJ, Yamaza T, Song Y, Fouad AF, Romberg EE, Shi S, Tuan RS, Huang GT (2010) Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen Med 5(4):617–631. doi:10.2217/rme.10.30
Dokic J, Tomic S, Cerovic S, Todorovic V, Rudolf R, Colic M (2012) Characterization and immunosuppressive properties of mesenchymal stem cells from periapical lesions. J Clin Periodontol 39(9):807–816. doi:10.1111/j.1600-051X.2012.01917.x
Liu Y, Gao Y, Zhan X, Cui L, Xu S, Ma D, Yue J, Wu B, Gao J (2014) TLR4 activation by lipopolysaccharide and Streptococcus mutans induces differential regulation of proliferation and migration in human dental pulp stem cells. J Endod 40(9):1375–1381. doi:10.1016/j.joen.2014.03.015
Ma D, Cui L, Gao J, Yan W, Liu Y, Xu S, Wu B (2014) Proteomic analysis of mesenchymal stem cells from normal and deep carious dental pulp. PLoS One 9(5), e97026. doi:10.1371/journal.pone.0097026
Ma D, Gao J, Yue J, Yan W, Fang F, Wu B (2012) Changes in proliferation and osteogenic differentiation of stem cells from deep caries in vitro. J Endod 38(6):796–802. doi:10.1016/j.joen.2012.02.014
Yazid FB, Gnanasegaran N, Kunasekaran W, Govindasamy V, Musa S (2014) Comparison of immunodulatory properties of dental pulp stem cells derived from healthy and inflamed teeth. Clin Oral Investig 18(9):2103–2112. doi:10.1007/s00784-014-1207-4
Pereira LO, Rubini MR, Silva JR, Oliveira DM, Silva IC, Pocas-Fonseca MJ, Azevedo RB (2012) Comparison of stem cell properties of cells isolated from normal and inflamed dental pulps. Int Endod J 45(12):1080–1090. doi:10.1111/j.1365-2591.2012.02068.x
Sun HH, Chen B, Zhu QL, Kong H, Li QH, Gao LN, Xiao M, Chen FM, Yu Q (2014) Investigation of dental pulp stem cells isolated from discarded human teeth extracted due to aggressive periodontitis. Biomaterials 35(35):9459–9472. doi:10.1016/j.biomaterials.2014.08.003
Werle SB, Lindemann D, Steffens D, Demarco FF, de Araujo FB, Pranke P, Casagrande L (2016) Carious deciduous teeth are a potential source for dental pulp stem cells. Clin Oral Investig 20(1):75–81. doi:10.1007/s00784-015-1477-5
Havelek R, Soukup T, Cmielova J, Seifrtova M, Suchanek J, Vavrova J, Mokry J, Muthna D, Rezacova M (2013) Ionizing radiation induces senescence and differentiation of human dental pulp stem cells. Folia Biol 59(5):188–197
Muthna D, Soukup T, Vavrova J, Mokry J, Cmielova J, Visek B, Jiroutova A, Havelek R, Suchanek J, Filip S, English D, Rezacova M (2010) Irradiation of adult human dental pulp stem cells provokes activation of p53, cell cycle arrest, and senescence but not apoptosis. Stem Cells Dev 19(12):1855–1862. doi:10.1089/scd.2009.0449
Abe S, Hamada K, Yamaguchi S, Amagasa T, Miura M (2011) Characterization of the radioresponse of human apical papilla-derived cells. Stem Cell Res Ther 2(1):2. doi:10.1186/scrt43
Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32(8):773–785. doi:10.1038/nbt.2958
Jung JW, Lee JS, Cho DW (2016) Computer-aided multiple-head 3D printing system for printing of heterogeneous organ/tissue constructs. Sci Rep 6:21685. doi:10.1038/srep21685
Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A (2016) A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 34(3):312–319. doi:10.1038/nbt.3413
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hilkens, P. et al. (2016). Cryopreservation and Banking of Dental Stem Cells. In: Karimi-Busheri, F., Weinfeld, M. (eds) Biobanking and Cryopreservation of Stem Cells. Advances in Experimental Medicine and Biology, vol 951. Springer, Cham. https://doi.org/10.1007/978-3-319-45457-3_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-45457-3_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-45455-9
Online ISBN: 978-3-319-45457-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)